Abstract
Drought stress significantly hampers plant growth and productivity. Strigolactones (SLs), a class of carotenoid-derived plant hormones, are recognized for their pivotal role in modulating plant morphology and enhancing drought resistance. Nonetheless, the underlying mechanisms through which SLs influence drought tolerance in tall fescue remain largely unexplored. In this study, we employed TIS108 to inhibit SL biosynthesis under drought conditions and assessed a range of morphological and physiological parameters in tall fescue, including biomass both above and below ground, antioxidase activities, proline and soluble sugar contents, and survival rates, across treatments of drought and drought coupled with TIS108 inhibition. Our findings demonstrate that the suppression of SL synthesis detrimentally affects the drought resilience of tall fescue. Through comprehensive transcriptome sequencing and subsequent qRT-PCR analyses of samples subjected to drought with and without TIS108 treatment, we identified a marked downregulation of genes involved in auxin metabolism and root development. This downregulation correlated with significant reductions in total root length, root surface area, and the number of root tips under drought stress conditions. Collectively, our research elucidates that the inhibition of SL synthesis impairs drought tolerance in tall fescue by constraining root growth and development, mediated through the modulation of auxin metabolism.
1. Introduction
Strigolactones (SLs), a novel class of carotenoid-derived plant hormones, have emerged as pivotal regulators of plant growth, development, and stress responses. Initially characterized as germination stimulants for parasitic plants like Striga, Orobanche, and Phelipanche [1,2], SLs have also been recognized for their crucial role in sculpting plant architecture and fostering symbiosis with arbuscular mycorrhizal fungi [3]. The intricate biosynthetic and signaling pathways of SLs involve a series of key genes responsible for carotenoid and SL biosynthesis, as well as SL signal transduction: (1) the β-carotene biosynthesis genes, namely, those encoding phytoene synthase (CrtB) and phytoene desaturase (PDS); (2) the SL biosynthesis genes DWARF27 (D27), more axillary growth 1 (MAX1), MAX3 (also known as carotenoid cleavage dioxygenase 7 (CCD7), and MAX4 (also known as CCD8); and (3) the SL signal transduction genes α/β-hydrolase receptor DWARF14 (D14) and MAX2, as well as D53 [4,5,6,7,8,9,10,11,12,13].
Thus far, many studies have focused on the role of SLs in regulating plant architecture, and mutations in key genes involved in SLs synthesis lead to the formation of dwarf plants with increased lateral branch tillers [6,14,15]. Studies conducted on maize [16], potato [17], wheat [18], sorghum [19], and apples [20] have revealed a negative correlation between SL content and tiller number in mutant dwarfs. Likewise, entirely blocking the SL synthesis promoted dwarfing [6,21]. Nevertheless, in addition to regulating plant appearance, SLs is also an important drought-resistance-conferring hormone in plants, and SLs and their pathways that play positive regulatory roles in drought stress have been verified in some plants, such as Arabidopsis [22], soybean [23], grape [24], and apple [25]. Recent advancements have shed light on the interactive network between SLs and other phytohormones, revealing complex synergistic and antagonistic relationships that play a significant role in plant adaptation to environmental stresses. Specifically, the cross-talk between SLs and auxin has been identified as a critical mechanism in root development and architecture. SLs have been shown to modulate auxin transport and sensitivity, impacting root formation and growth patterns [26,27]. Moreover, the relationship between SLs and jasmonic acid (JA) points towards a synergistic mechanism mediating plant responses to abiotic and biotic stresses. SLs and JA together have been implicated in enhancing plant resilience against pathogen attacks and facilitating stress responses, including drought tolerance [28,29]. The intricate balance between SLs, auxin, and JA underscores the complexity of hormonal regulation in plants.
Tall fescue, a vital forage and turf grass known for its resilience to abiotic stresses, offers an excellent model for investigating SLs’ regulatory functions under drought conditions [30,31]. A previous study has already shown that the SL synthesis pathway exists in tall fescue and appears to play a positive role in drought stress [32]. In our previous transcription data analysis [33], we also found that key SL synthesis genes were induced by drought, indicating that SLs function in drought. However, the specific molecular mechanisms underlying SL-mediated drought tolerance in tall fescue demand further elucidation.
This study aims to dissect the role of SLs in modulating drought resistance in tall fescue, hypothesizing that reduced SL synthesis weakens the plant’s drought tolerance by impeding root development. We propose that SLs act as critical modulators of root architecture and function under drought stress, thereby influencing the overall drought resilience of tall fescue. Through comprehensive morphological, physiological, and transcriptomic analyses, this research endeavors to unravel the intricate dynamics between SL regulation and drought resistance, contributing novel insights into the adaptive strategies of tall fescue and other cereals in water-limited environments.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The species under study is a commercial-type tall fescue, ‘Qiancao No. 1’, provided by Guizhou Institute of Prataculture, Guizhou Academy of Agricultural Sciences, China. Seedlings, established in petri dishes for three days, were subsequently transferred to a growth chamber for hydroponic cultivation in half-strength Hoagland’s nutrient solution. Growth conditions included a 14/10 h light/dark cycle, photosynthetically active radiation of 450 μmol m−2 s−1 at canopy level, temperatures of 22/18 °C (day/night), and 70% relative humidity.
2.2. Experimental Design
This experiment was divided into two treatment groups (TGs), each comprising thirty plants. TG1 was subjected to drought stress without SL synthesis inhibition, whereas TG2 experienced both drought stress and SL synthesis inhibition. An optimally irrigated control group (CG), grown under identical conditions minus the drought stress, was included to serve as a baseline for evaluating the impacts of drought and SL synthesis inhibition on plant growth and physiological responses.
2.3. SL-Biosynthesis Inhibition Treatment and Drought Treatment
TIS108 (StrigoLab, Turin, Italy) was used at a concentration of 5 μM to inhibit SL biosynthesis [34]. This method was applied to each TG2 plant. Polyethylene glycol 6000 (PEG6000) was purchased from Guangdong Guanghua Sci-Tech Co., Ltd. (Shantou, China) and added to the hydroponic solution of TGs 1 and 2 at a concentration of 20% (w/v) to simulate drought stress. Seedlings treated for approximately three weeks were used to observe root physiological indices and for transcriptome sequencing. Drought treatment for soil cultivation: Seeds were sown in a seedling tray (perlite: nutrient soil = 1:1) (seedling tray size: 54 cm × 28 cm, 32 holes), and the seedlings were thinned 3 days after germination. Twelve days post-thinning, the water supply was stopped to induce drought treatment naturally. One group was retained as the control, while the other group underwent treatment with 5 μM TIS108. The plants were re-watered after 21 d of drought treatment. Seedlings treated for approximately three weeks were used to measure the root:shoot ratio, above- and below-ground biomass, antioxidase activity, and survival rate.
2.4. 5-DS Content Measurement
5-Deoxystrigo (5DS) was the first strigolactone produced and the precursor of other strigolactones [7]. The 5-DS content was determined by ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). The samples were pretreated as described by Flokova [35]. The protocol for chromatographic and mass spectroscopic methods was as previously described by Xiao [36] and Pan [37], respectively.
2.5. Measurement of Biomass, Survival Rate, and Physiological Index
The aboveground parts and the cleaned roots of plants from each treatment group were weighed for 30 samples before the drought treatment (0 d) and after the drought treatment (14 d, 21 d, and 21 d rehydration), and measurements for each treatment were repeated thrice. Following 21 d of rehydration, the root architecture was observed, and the survival rate of plants from each treatment group was calculated and repeated thrice.
For determining the activities of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT), as well as the contents of the osmoregulatory substances proline (Pro) and soluble sugar (SS), the samples were prepared using a kit (Solarbio, Beijing, China) and were detected using an enzyme marker following the manufacturer’s instructions. Three biological replicates were used in each experiment.
2.6. Sample Preparation for Generation of Tall Fescue Transcriptome
Due to the unavailability of a complete tall fescue genome, a full-length transcriptome was generated using single-molecule real-time (SMRT) sequencing in conjunction with isoform sequencing (Iso-Seq) analysis (Pacific Biosciences (PacBio), Palo Alto, CA, USA). Total RNA was extracted from the samples using the easy-spin total RNA extraction kit (iNtRON Biotechnology, Seongnam, Republic of Korea). Full-length cDNA was synthesized from purified poly(A) RNAs using the SMARTer PCR cDNA synthesis kit (Clontech Laboratories Inc., Mountain View, CA, USA). The cDNA was size-selected with size ranges of 1–2, 2–3, 3–6, and >6 kb using BluePippin to minimize the bias that favored the sequencing of shorter transcripts. Further amplification and size selection were performed to create SMRTbell template libraries as recommended by PacBio. Three Iso-Seq libraries were sequenced from the five SMRT cell lines using the PacBio RS II platform. The raw data were processed into reads of interest (ROIs). Full-length non-chimeric (FLNC) transcripts were detected by searching for the poly(A) tail and the 5′ and 3′ cDNA primer sequences in the ROIs. Iterative clustering for error correction (ICE) and the Quiver algorithm, as implemented in the RS_IsoSeq module of the SMRT analysis software (Version 2.3), were used to obtain consensus isoforms and full-length consensus sequences with a post-correction accuracy above 99%. The consensus isoforms were annotated based on the following databases: Gene Ontology (GO), Clusters of Orthologous Groups of proteins (KOG/COG/eggNOG), NCBI’s non-redundant protein sequences (NR), Protein Family (Pfam), a manually annotated and reviewed protein sequence database (Swiss-Prot), and Kyoto Encyclopedia of Genes and Genomes (KEGG).
2.7. Sample Preparation for Illumina Sequencing
To evaluate the expression of target genes in tall fescue under varying conditions for each TG, samples were collected and prepared for Illumina sequencing. Total RNA was isolated from each sample using TRIzol reagent (Ambion, Austin, TX, USA) and purified using an RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). Biotin-oligo (dT) magnetic beads were used to isolate mRNA from each total RNA sample (Illumina, San Diego, CA, USA). These mRNAs were then randomly cleaved into small fragments using a fragmentation buffer. The fragmented mRNAs were used to synthesize first-strand cDNA using random hexamer primers and M-MuLV reverse transcriptase (RNase H). Twelve libraries were sequenced using the Illumina HiSeq 2000 platform (Illumina, USA).
2.8. Identification and Validation of Differentially Expressed Isoforms (DEIs)
Clean paired-end reads obtained via Illumina sequencing were mapped to a newly generated transcriptome to identify all differentially expressed isoforms. The RSEM software package (Version 1.10.1) [38] was used to count the number of reads mapped onto each isoform. Based on the isoform length and read count, the fragments per kilobase of transcript per million fragments mapped (FPKM) for each isoform were calculated. Finally, a differential expression analysis of the two TGs was performed using the DESeq R package (version 1.10.1). Isoforms with an adjusted p-value < 0.05, found by DESeq, were considered differentially expressed.
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was used to understand the importance and the role of SLs in regulating root development under drought stress. Expression of known root growth and development genes; RPs, AMT, GRAS, LOX2, RBOHs, DMAS, FBL, PSMD, P5CS, and SAHH were selected for further analysis. The expression levels of known auxin synthesis, transport, and signaling genes YUCC6, YUCC8, YUCAL, PIN1, PIN2, PIN3, AUX, and ARF were selected for further analysis.
Total RNA was isolated from the samples using two different treatments, as previously described. First-strand cDNA was synthesized using a one-step SYBR1 Prime Script1 RT-PCR kit (Takara Bio Inc., Dalian, China). Using a CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and SYBR Premix Ex Taq (Takara Bio Inc., Dalian, China), the cycle threshold (CT) values of triplicate samples were recorded and further calculated using the 2−∆∆Ct method, with FaTublin as the reference gene. The designed primers are listed in Supplementary Table S1.
2.9. Data Availability
The sequence data generated for this study are accessible from the NCBI Sequence Read Archive under the accession numbers (SRA: SRR8202096, SRR8202097, SRR8202098, SRR8202099, and SRR8202100).
2.10. Statistical Analyses
Each experiment was repeated at least three times. Statistical analyses were performed using analysis of variance (ANOVA) followed by Duncan’s multiple range tests. Means were considered significantly different at a p-value of <0.05.
3. Results
3.1. TIS108 Treatment Inhibits SL Synthesis under Drought Conditions in Tall Fescue
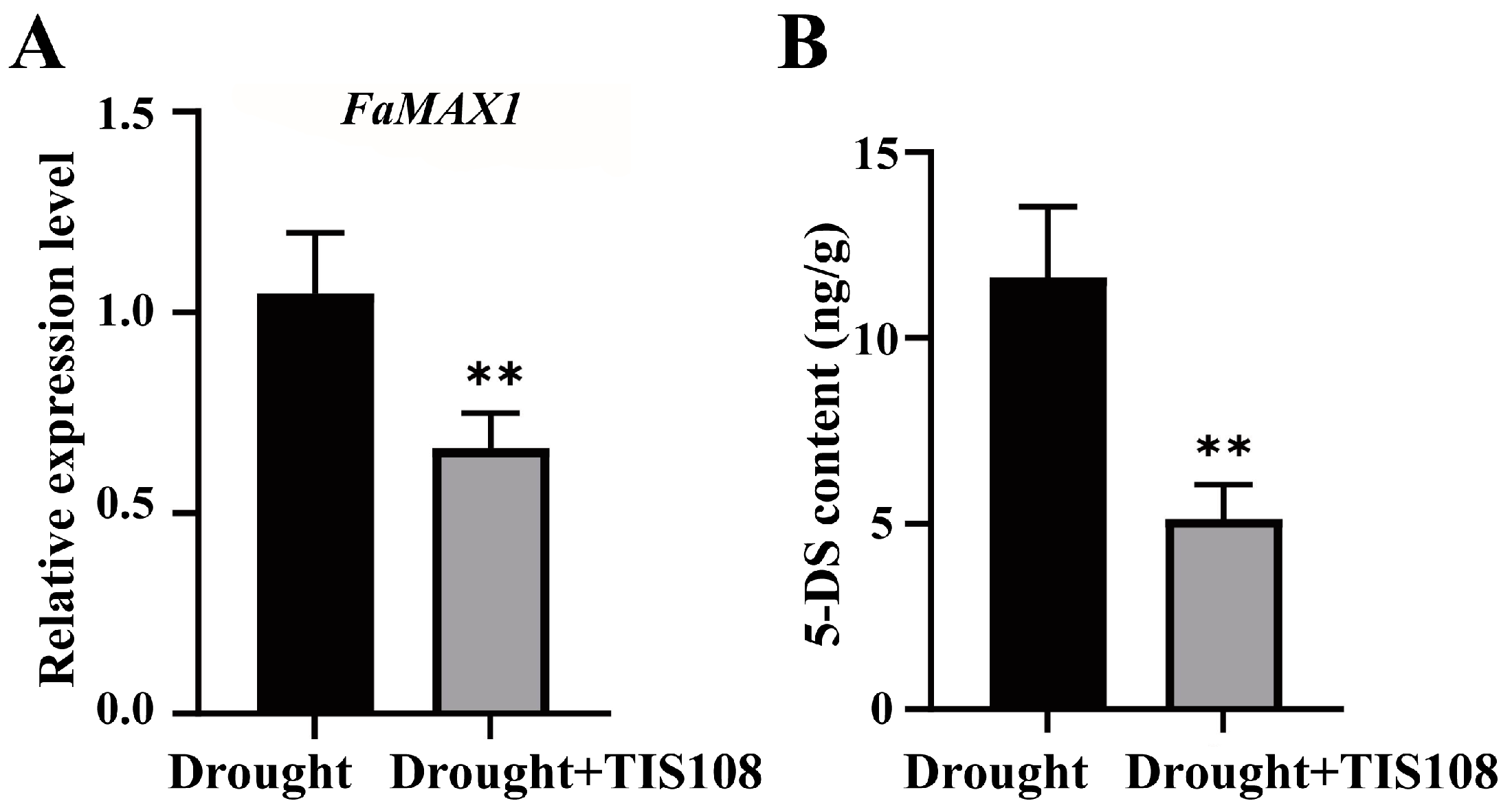
We examined the expression of FaMAX1 and 5-DS levels to characterize the expression levels of SLs under drought conditions. The results indicated that, following TIS108 treatment, FaMAX1 expression decreased significantly compared to the control (Figure 1A), and the 5-DS content also showed a similar trend (Figure 1B), indicating that TIS108 reduced SL synthesis in tall fescue plants under drought conditions.
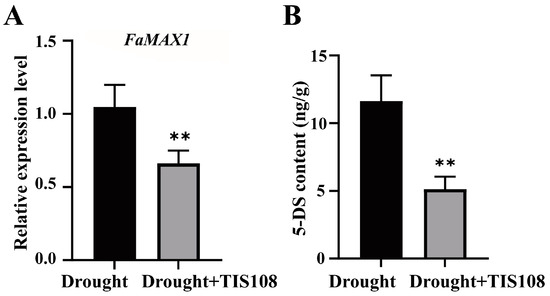
Figure 1.
FaMAX1 expression and 5-DS content after TIS108 treatment. (A) Expression of FaMAX1 in drought and drought + TIS108 treatment groups; (B) 5-DS content in drought and drought + TIS108 treatment groups. Data represent mean ± SEM; ** p < 0.01.
3.2. Inhibiting SL Synthesis Decreased Drought Tolerance of Tall Fescue
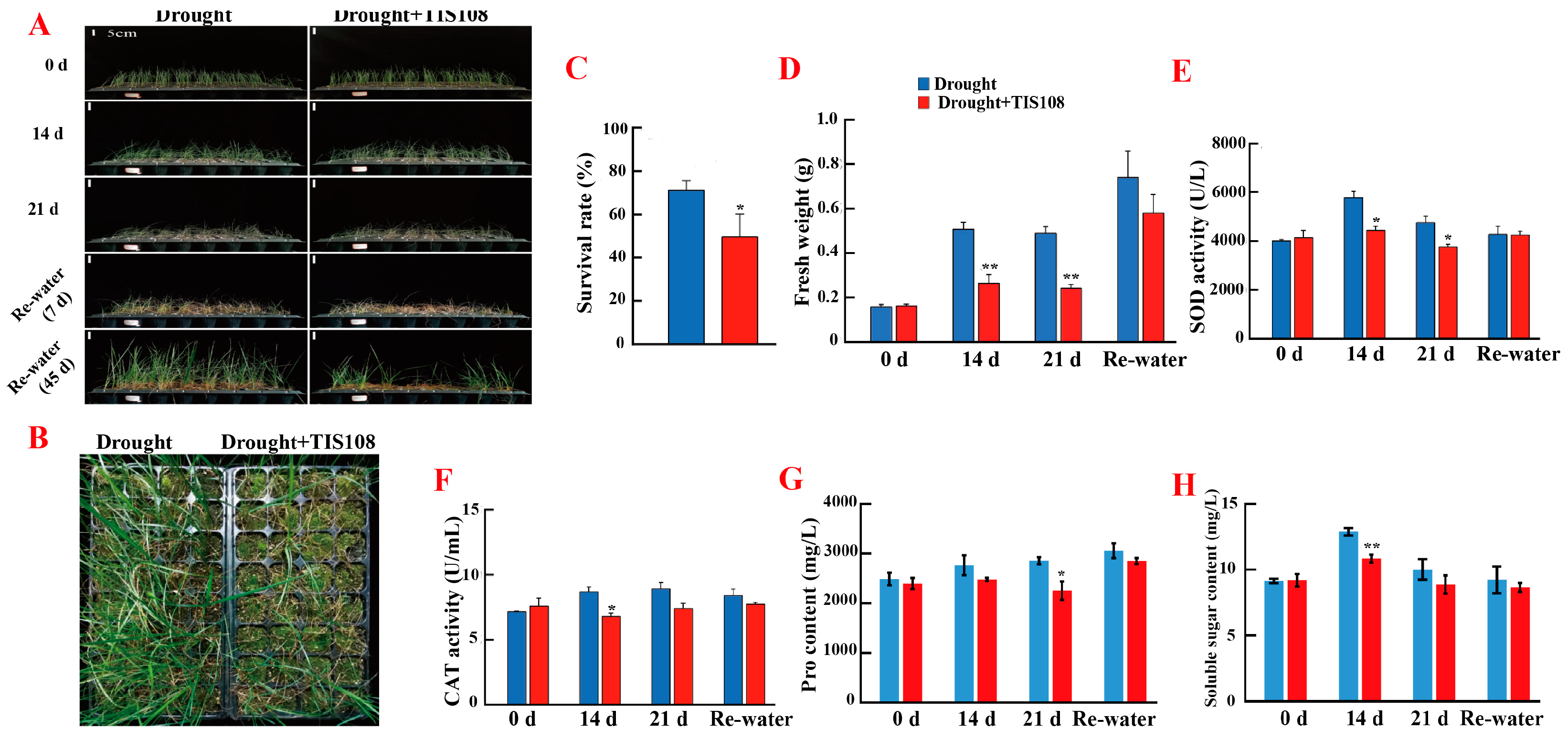
As shown in Figure 2A,B, when treated with TIS108 under drought conditions, the fresh weight decreased significantly with prolonged drought (Figure 2D). The survival rate after rehydration 45 d following drought stress was also analyzed. Statistical analysis showed that, when treated with TIS108, the survival rate of tall fescue after re-watering significantly reduced. This implies that a decrease in SLs causes tall fescue to withstand more drought-stress damage (Figure 2C).
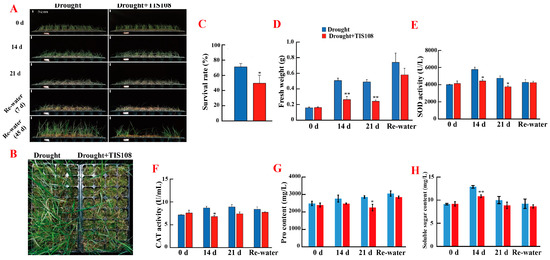
Figure 2.
Inhibiting SL synthesis under drought decreased drought tolerance of tall fescue. (A,B) Photograph of the drought- and drought + TIS108-treated tall fescues subjected to drought stress for different times and then rewatered for one week and 45 d. (C) Survival rate statistic under drought and drought + TIS108. (D) Fresh weight under drought and drought + TIS108. SOD and CAT activity (E,F) and proline and soluble sugar content (G,H) under drought and drought + TIS108. Dates represent mean ± SEM; asterisks indicate significant differences as determined by t test (* p < 0.05, ** p < 0.01). Each experiment was repeated at least three times.
We further examined the antioxidase activity of tall fescue and found that SOD activity significantly decreased after TIS108 treatment under drought conditions (Figure 2E), whereas CAT activity significantly decreased only at 14 d (Figure 2F); the proline content shown significantly decreased at 21 d (Figure 2G), while the soluble sugar content shown significantly decreased at 14 d (Figure 2H), indicating that inhibiting SL biosynthesis escalated the stress on tall fescue.
3.3. Combined Transcriptome Analysis of the Second and Third Generations of Tall Fescue
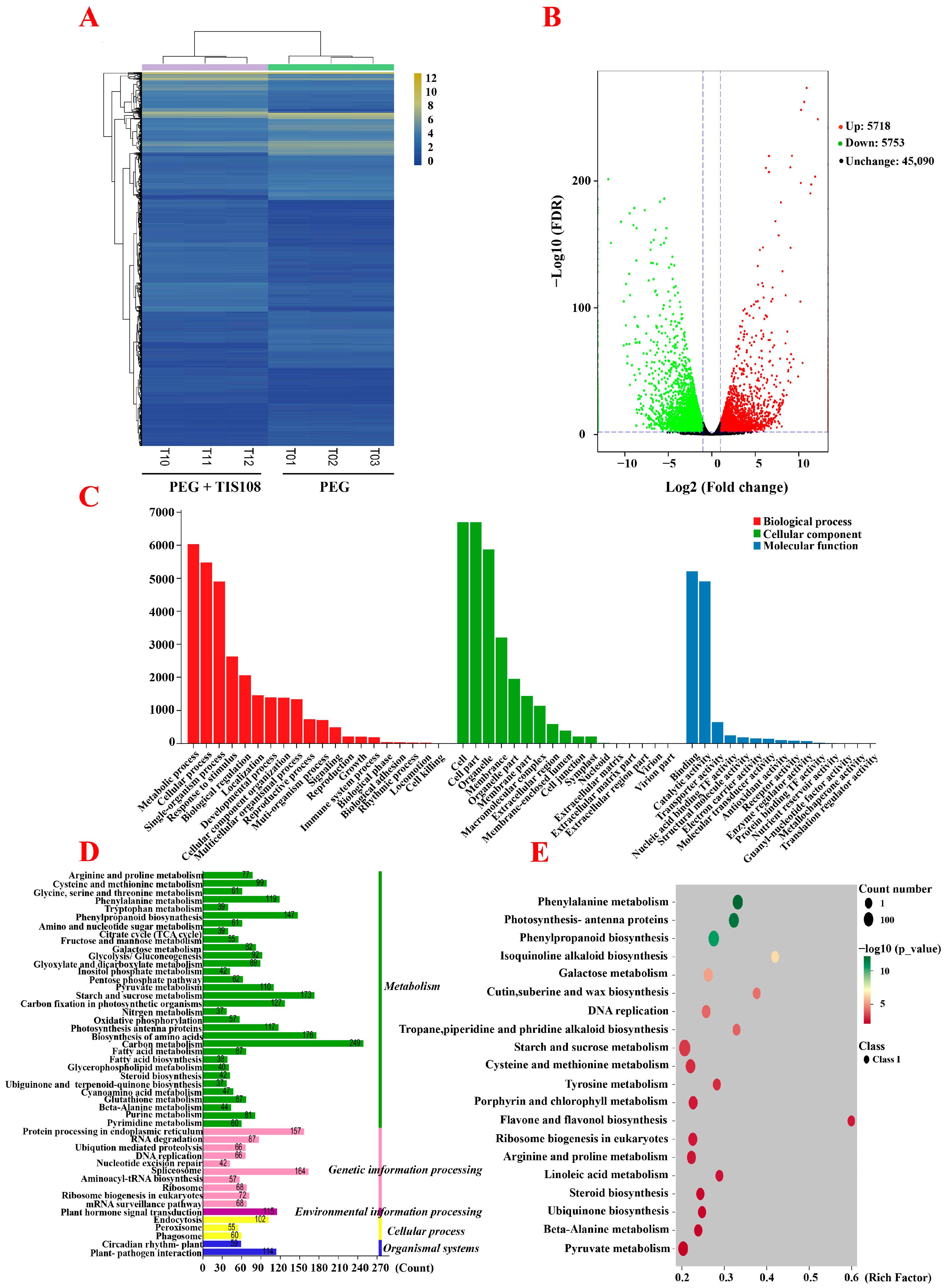
A total of six constructed cDNA libraries (PEG1, PEG2, PEG3, PEG + TIS108-1, PEG + TIS108-2, PEG + TIS108-3) were sequenced using the Illumina HiSeq™ 2000 platform. A total of 43.13 Gb of clean data were obtained from the six tall fescue samples’ cDNA libraries; the Q30 value was more than 92.98%, and the GC content was 55.94–59.32% (Table S2). These clean data were compared and matched with full-length transcripts obtained from third-generation transcriptomes for differential gene expression analyses and functional annotation (Table S3). To detect the correlation of biological duplication of the samples, the Pearson correlation coefficient was used to analyze the gene expression level of the samples in the two treatment groups. Estimation of the Pearson correlation coefficient revealed a good correlation between the samples in the group. There were significant differences between groups, confirming accurate experimental sampling, good biological duplication between samples used for transcriptome sequencing, and reliability of the sequencing data (Figure S1).
To study the specific ways in which SLs participated in the response of tall fescue to drought stress, the DEGs from the PEG + TIS108 and PEG group were clustered, and the results revealed three biological repeats of a TG clustered together, while those in distinct TG were significantly separated (Figure 3A). A total of 11,471 DEGs were identified, of which 5718 were upregulated (49.85%) and 5753 were downregulated (50.15%) (Figure 3B). In the GO annotation analysis, 9006 DEGs were annotated into 52 subcategories of biological processes, cell components, and molecular functions, among which most DEGs were annotated in the biological processes (Figure 3C). KEGG annotation analysis showed that, among the five pathways (cellular processes, environmental information processing, genetic information processing, metabolism, and organismal systems), the pathway with the most DEG annotations was metabolism (Figure 3D). KEGG enrichment analysis of DEGs showed the highest degree of enrichment in flavone and flavonol biosynthesis, followed by isoquinoline alkaloid biosynthesis, cutin, suberin and wax biosynthesis, phenylalanine metabolism, tropane, piperidine, and pyridine alkaloids piperidine and pyridine alkaloid biosynthesis, photosynthesis–antenna proteins, sesquiterpenoid and triterpenoid biosynthesis and triterpenoid biosynthesis), etc. In addition, β-alanine synthesis, thiamine metabolism, and tryptophan metabolism were also significantly enriched (Figure 3E).
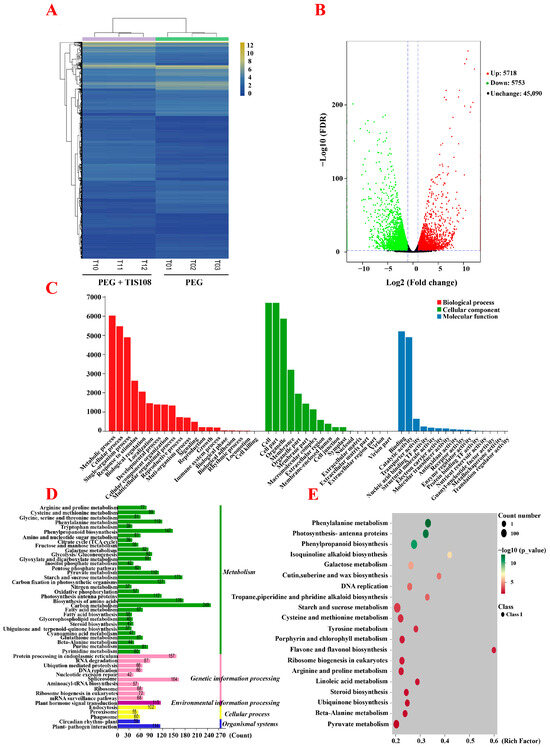
Figure 3.
Transcriptome analysis of the PEG and PEG + TIS108 groups. (A) Hierarchical cluster analysis of the PEG and PEG + TIS108 groups. (B) Volcano plot of the PEG and PEG + TIS108 groups. (C) GO annotation of the PEG and PEG + TIS108 groups. (D) KEGG pathway analysis of the PEG and PEG + TIS108 groups. (E) Enrichment in KEGG pathways of the PEG and PEG + TIS108 groups.
3.4. Genes Related to Root Growth and Development and Auxin Metabolism Were Influenced by TIS108 under Drought Conditions
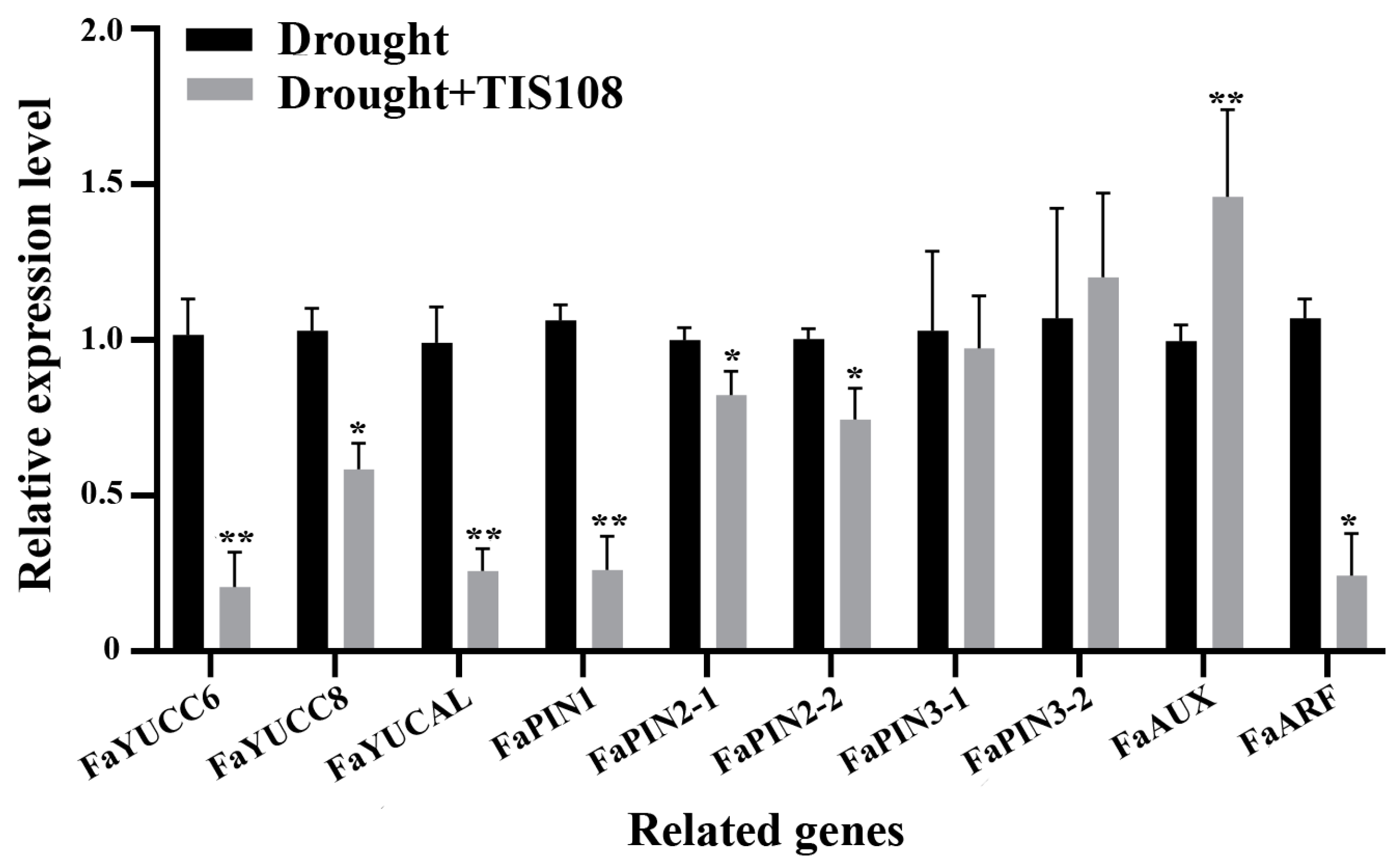
Further analysis of the DEGs revealed significant enrichment of genes related to auxin synthesis, transport, signaling, and root development, with auxin being the most important hormone regulating root development. First, we examined whether auxin synthesis-, transport-, and signaling-related genes in tall fescue were affected by drought. The results indicated that TIS108 treatment significantly inhibited the expression of auxin synthesis (YUCC6, YUCC8, and YUCAL), and auxin transport genes (PIN1 and PIN2), whereas PIN3 expression was not significantly affected. In particular, AUX (inhibitor of the auxin signaling pathway) was induced by TIS108 under drought conditions, whereas ARF (downstream transducers of the auxin traducing pathway) was also significantly inhibited by TIS108 treatment (Figure 4).
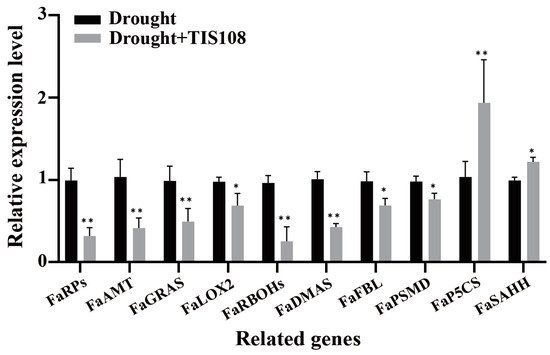
Figure 4.
Expression of auxin synthesis-, transport-, and signaling-related genes under drought and drought + TIS108 treatments. Data represent mean ± SEM, * p < 0.05, ** p < 0.01.
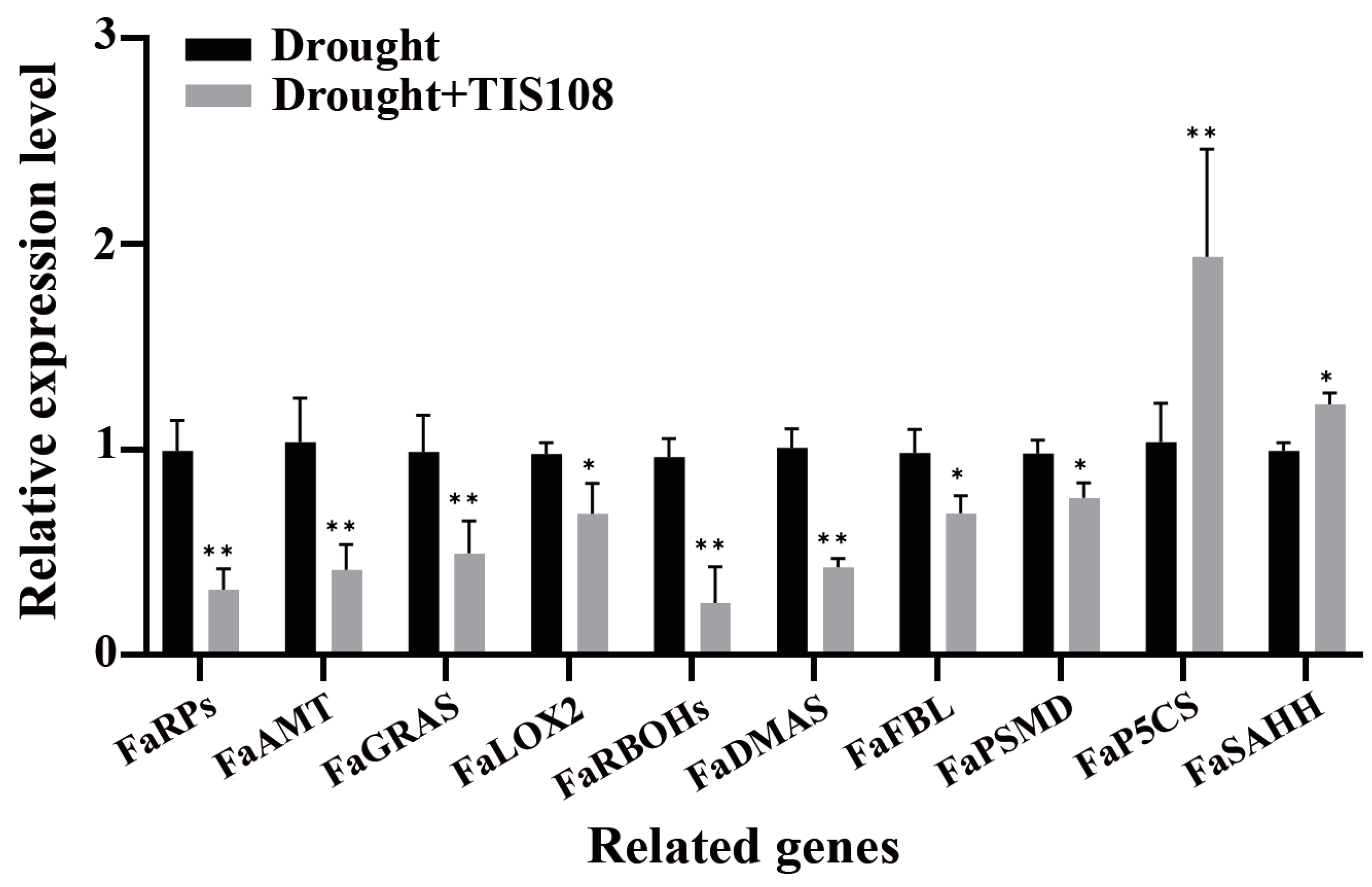
FaRPs, FaAMT, and FaGRAS play important regulatory roles in root morphogenesis and development, as well as root hair elongation and growth. Therefore, we further verified the expression patterns of these genes under drought and drought + TIS108 conditions using qRT-PCR. The results revealed that the expression of these genes significantly changed due to TIS108 treatment (Figure 5). Among these, FaRPs, FaAMT (root morphogenesis), FaGRAS (root development), FaRBOHs, FaDMA (root cell differentiation), and FaFBL (root hair development) were significantly downregulated. Nevertheless, the expressions of FaP5CS and FaSAHH, which negatively regulate root development, were significantly upregulated (Figure 5).
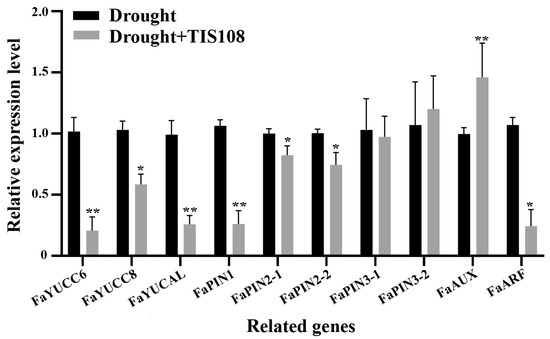
Figure 5.
Expression of root growth- and development-related genes after drought and drought + TIS108 treatments. Dates represent mean ± SEM; * p < 0.05, ** p < 0.01.
3.5. Inhibition of SL Synthesis Inhibited Root Development of Tall Fescue
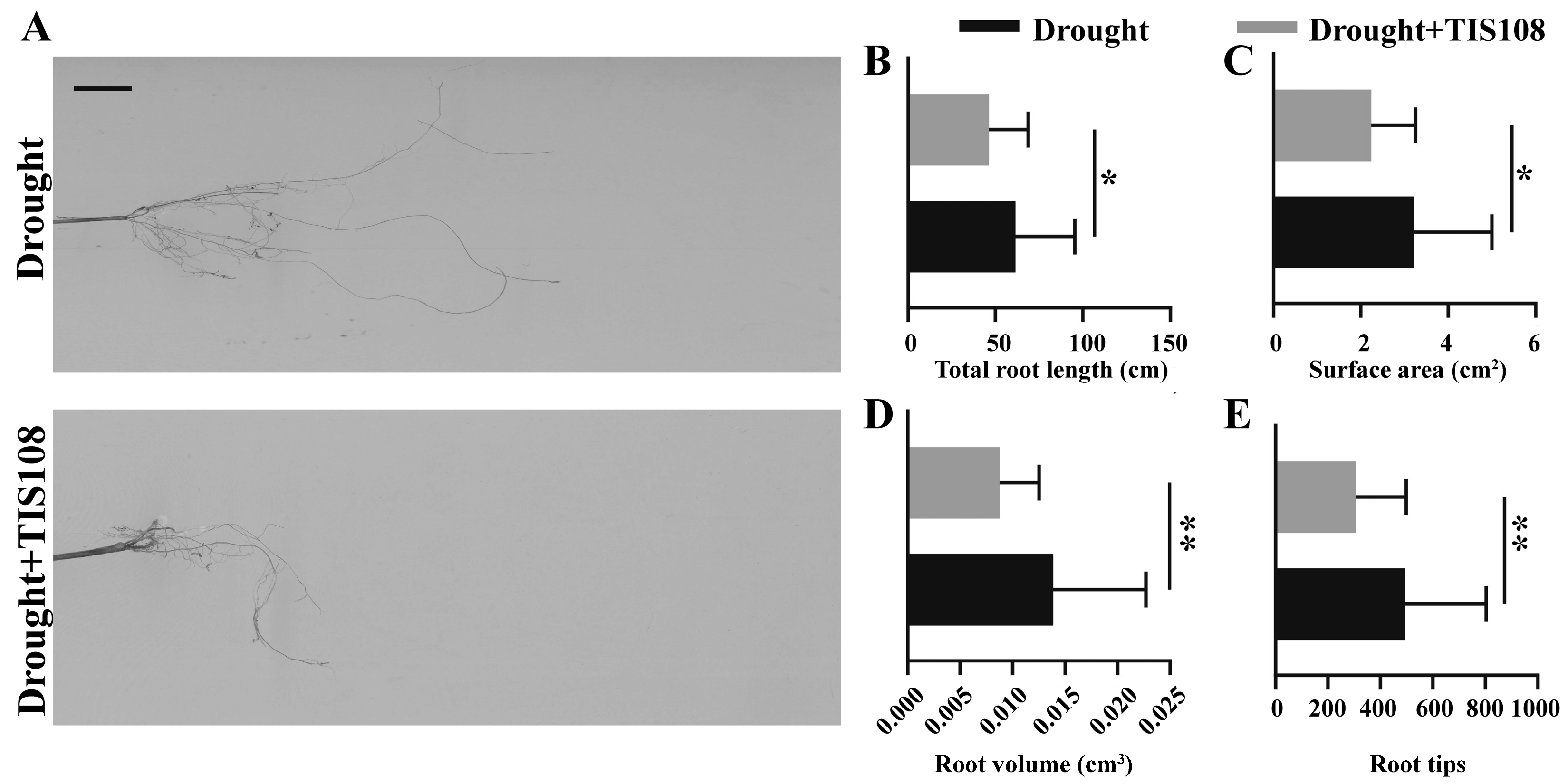
Based on the changes in the expression of auxin synthesis, transport, and signaling, as well as root development genes, we further verified the changes in root architecture, which is important for plant survival under drought stress. The total root length, root surface area, total number of root tips, and root volume of tall fescue were calculated and analyzed, and the results indicated that, when treated with TIS108 under drought conditions, the root length was significantly reduced compared to the drought treatment alone (Figure 6A,B). The root surface area (Figure 6C), root volume (Figure 6D), and root tips (Figure 6E) showed similar trends. These results indicate that obstructing SLs synthesis inhibited root development in tall fescues.
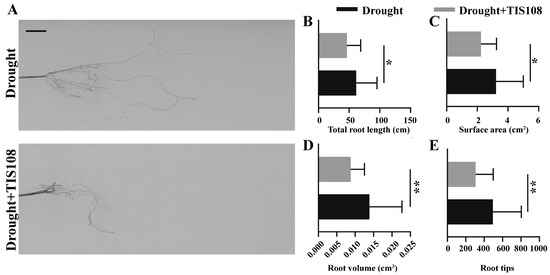
Figure 6.
Root growth under drought and drought application of TIS108. Photograph (A), total root length (B), root surface area (C), root volume (D), and root tips (E) of three-week-old tall fescues from drought and drought + TIS108 treatment groups. Data represent mean ± SEM, * p < 0.05, ** p < 0.01. Bar represents 1 cm.
4. Discussion
In recent years, strigolactones (SLs), a class of carotenoid-derived plant hormones, have emerged as key regulators of plant growth and development, influencing a myriad of physiological processes, including root architecture, shoot branching, chlorophyll synthesis, senescence, and responses to both biotic and abiotic stresses [39]. The increasing incidence of natural disasters, such as drought and salinization, has intensified the focus on SLs’ role in plant drought tolerance, underscoring the importance of elucidating the mechanisms by which strigolactones enhance drought resilience. In this context, our study reveals a significant contribution of SLs to the drought tolerance of tall fescue, an essential forage and turfgrass species. Utilizing TIS108, a strigolactone synthesis inhibitor previously applied to Arabidopsis, we observed analogous effects on tall fescue, particularly concerning the expression of the core synthesis gene MAX1 [40]. This similarity indicates the conservation of the SL synthesis pathway across species.
Our investigations into the impact of SL synthesis inhibition under drought conditions reveal that the drought tolerance of tall fescue is significantly compromised, primarily through alterations in root architecture and physiological responses. Root development, which is critically sensitive to drought stress [41], was notably impaired by TIS108 treatment, diminishing the plant’s capacity for water absorption and drought tolerance. This inhibition was accompanied by a marked downregulation of genes related to root growth and development, underscoring the pivotal role of SLs in these processes under drought conditions. Moreover, auxin plays a central role in regulating root growth and development, and we found that auxin synthesis-, transport-, and transduction-related gene expressions were significantly influenced by TIS108 treatment under drought stress (Figure 4). This indicates a possible coordination between SLs and auxin to regulate root growth and development to cope with drought stress. Notably, our results showed that the expression of the auxin transport genes PIN1 and PIN2 was inhibited by TIS108 under drought conditions. While, in a previous study, strigolactones could inhibit traffic and plasma membrane localization of the auxin transporter PIN, thereby regulating auxin flux and eventually affecting plant branches [42,43], the effects of SLs in modulating root growth and development in Arabidopsis and rice plants may be explained by their role in inhibiting PIN-mediated polar auxin transport from aerial tissues to roots [44]. However, our findings can more likely be attributed to a reduction in auxin synthesis induced by TIS108 treatment, resulting in diminished auxin content from the shoot to the root. This interplay requires further detailed investigation.
A notable aspect of our findings is the reduction in proline accumulation following TIS108 treatment, a crucial adaptation to drought stress. Proline, a significant osmolyte, plays a vital role in maintaining cell osmotic balance, protecting cellular structures, and scavenging free radicals under stress conditions [45]. Given the observed downregulation of P5CS (Δ1-pyrroline-5-carboxylate synthetase) gene expression, which is directly involved in proline biosynthesis, our results suggest that SLs may enhance drought tolerance through a mechanism that involves the stabilization of proline levels. This finding aligns with the study by Valliyodan and Nguyen, who highlighted the role of P5CS in proline accumulation under drought stress [46].
Additionally, our study extends to exploring the complex relationship between SL and jasmonic acid (JA) synthesis pathways. The LOX gene, a pivotal component in JA biosynthesis, exhibited significant transcriptional changes in response to SL synthesis inhibition. This observation suggests a possible interaction between the SL and JA pathways, which may converge in stress response and developmental processes. Previous studies have documented the synergistic effects of SL and JA on stress resistance and root architecture [47]. Thus, our data propose that the cross-talk between SL and JA pathways could be a critical factor in modulating the plant’s response to drought stress, warranting further investigation into the precise molecular interactions and signaling networks involved.
5. Conclusions
This research conclusively demonstrates that SLs play a vital role in enhancing drought resistance in tall fescue through the regulation of root development. Our findings validate the initial hypothesis that SL synthesis inhibition compromises the plant’s drought resistance, specifically by impairing root development. This underscores the importance of SLs in mediating plant responses to water scarcity, highlighting their potential as targets for enhancing drought resilience in crops.
The practical implications of our study are significant, offering insights into genetic and hormonal modulation strategies to improve crop resilience against drought. By understanding the mechanisms through which SLs influence root architecture and drought tolerance, agronomists and plant breeders can develop more drought-resistant plant varieties, contributing to agricultural sustainability under changing climatic conditions.
For future research, it is imperative to delve deeper into the molecular interplay between SL and auxin pathways in root development under stress conditions. Exploring the potential genetic manipulation of SL biosynthesis or signaling could unveil new avenues for enhancing plant adaptation to drought stress. Furthermore, extending this research to other crops could help to generalize the findings, paving the way for broad-based applications in crop improvement programs focused on drought resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14040725/s1, Table S1. Primers used in this study. Table S2. Overview of Illumina HiSeq data. Table S3. Reads from Illumina HiSeq data mapping to the PacBio Iso-Seq transcriptome. Figure S1. Sample correlation heat map of PEG + TIS108 and PEG groups.
Author Contributions
Conceptualization, L.Z. and D.Z.; methodology, L.Z., C.Y., Y.C. (Yueyu Chen) and D.Z.; validation, L.Z., C.Y., Y.C. (Yueyu Chen), J.D. and L.G.; data curation and writing—original draft preparation, L.Z., L.G. and C.Y.; writing—review and editing, L.Z. and L.G.; visualization, C.Y. and D.L.; supervision, D.Z.; project administration, L.Z., C.Y., Y.C. (Yueyu Chen), J.D., L.G., D.L., Y.X. and Y.C. (Ying Chen); funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers: 31701950 and 31960609), Innovation Project (grant number: Qian Ke Fu Qi [2022]004), Science and Technology Program of Guizhou (grant number: [2019]2856), and Program of Guizhou Academy of Agricultural Sciences (grant numbers: [2015]021, [2017]17, [2021]13, [2021]33, and [2021]47), Science and Technology Foundation of Guizhou Province ([2020]1Z027).
Data Availability Statement
The sequence data generated for this study are accessible via the NCBI Sequence Read Archive under the accession numbers (SRA: SRR8202096, SRR8202097, SRR8202098, SRR8202099, and SRR8202100).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Ann. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef]
- Xu, E.; Chai, L.; Zhang, S.; Yu, R.; Zhang, X.; Xu, C.; Hu, Y. Catabolism of strigolactones by a carboxylesterase. Nat. Plants 2021, 7, 1495–1504. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Yang, T.; Lian, Y.; Wang, C. Comparing and Contrasting the Multiple Roles of Butenolide Plant Growth Regulators: Strigolactones and Karrikins in Plant Development and Adaptation to Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 6270. [Google Scholar] [CrossRef]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone Signaling and Evolution. Ann. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef]
- Waters, M.T.; Brewer, P.B.; Bussell, J.D.; Smith, S.M.; Beveridge, C.A. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 2012, 159, 1073–1085. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Zhang, W.; Li, G.; Chen, Z.; Zhai, W.; Zhao, X.; Pan, X.; Xie, Q.; Zhu, L. The rice high-tillering DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. Cell Mol. Biol. 2006, 48, 687–698. [Google Scholar] [CrossRef]
- Arite, T.; Iwata, H.; Ohshima, K.; Maekawa, M.; Nakajima, M.; Kojima, M.; Sakakibara, H.; Kyozuka, J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. Cell Mol. Biol. 2007, 51, 1019–1029. [Google Scholar] [CrossRef]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef]
- Lazar, G.; Goodman, H.M. MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Ruyter-Spira, C.; Al-Babili, S.; van der Krol, S.; Bouwmeester, H. The biology of strigolactones. Trends Plant Sci. 2013, 18, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Cur. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef]
- Drummond, R.S.; Martínez-Sánchez, N.M.; Janssen, B.J.; Templeton, K.R.; Simons, J.L.; Quinn, B.D.; Karunairetnam, S.; Snowden, K.C. Petunia hybrida carotenoid cleavage DIOXYGENASE7 is involved in the production of negative and positive branching signals in petunia. Plant Physiol. 2009, 151, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, G.; Zhao, Y.; Wang, H.H.; Dai, Z.; Xue, W.; Yang, J.; Wei, H.; Shen, R.; Wang, H. DWARF53 interacts with transcription factors UB2/UB3/TSH4 to regulate maize tillering and tassel branching. Plant Physiol. 2021, 187, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Pasare, S.A.; Ducreux, L.J.M.; Morris, W.L.; Campbell, R.; Sharma, S.K.; Roumeliotis, E.; Kohlen, W.; van der Krol, S.; Bramley, P.M.; Roberts, A.G.; et al. The role of the potato (Solanum tuberosum) CCD8 gene in stolon and tuber development. New Phytol. 2013, 198, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Colasuonno, P.; Lozito, M.L.; Marcotuli, I.; Nigro, D.; Giancaspro, A.; Mangini, G.; De Vita, P.; Mastrangelo, A.M.; Pecchioni, N.; Houston, K.; et al. The carotenoid biosynthetic and catabolic genes in wheat and their association with yellow pigments. BMC Genom. 2017, 18, 122. [Google Scholar] [CrossRef]
- Mohemed, N.; Charnikhova, T.; Bakker, E.J.; van Ast, A.; Babiker, A.G.; Bouwmeester, H.J. Evaluation of field resistance to Striga hermonthica (Del.) Benth. in Sorghum bicolor (L.) Moench. The relationship with strigolactones. Pest. Manag. Sci. 2016, 72, 2082–2090. [Google Scholar] [CrossRef]
- An, J.P.; Li, R.; Qu, F.J.; You, C.X.; Wang, X.F.; Hao, Y.J. Apple F-Box Protein MdMAX2 Regulates Plant Photomorphogenesis and Stress Response. Front. Plant Sci. 2016, 7, 1685. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Lammers, M.; Pollina, T.; Tóth, P.; Haider, I.; Pozo, M.J.; de Maagd, R.A.; Ruyter-Spira, C.; Bouwmeester, H.J.; et al. The tomato carotenoid cleavage dioxygenase8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 2012, 196, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Quain, M.D.; Makgopa, M.E.; Márquez-García, B.; Comadira, G.; Fernandez-Garcia, N.; Olmos, E.; Schnaubelt, D.; Kunert, K.J.; Foyer, C.H. Ectopic phytocystatin expression leads to enhanced drought stress tolerance in soybean (Glycine max) and Arabidopsis thaliana through effects on strigolactone pathways and can also result in improved seed traits. Plant Biotechnol. J. 2014, 12, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, J.; Wang, C.; Pang, Y.; Li, L.; Tang, X.; Li, B.; Sun, Q. Genome-wide analysis of the strigolactone biosynthetic and signaling genes in grapevine and their response to salt and drought stresses. PeerJ 2022, 10, e13551. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, Q.; Jing, G.; Ma, M.; Li, C.; Ma, F. Overexpression of MdIAA24 improves apple drought resistance by positively regulating strigolactone biosynthesis and mycorrhization. Tree Physiol. 2021, 41, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Soundappan, I.; Bennett, T.; Morffy, N.; Liang, Y.; Stanga, J.P.; Abbas, A.; Leyser, O.; Nelson, D.C. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 2015, 27, 3143–3159. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates and octadecanoids: Signals in plant stress responses and development. Prog. Nucl. Acid Res. Mol. Biol. 2013, 92, 165–221. [Google Scholar]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 2018, 504, 406–410. [Google Scholar] [CrossRef]
- Raeside, M.; Friend, M.; Behrendt, R.; Lawson, A.; Clark, S. A review of summer-active tall fescue use and management in Australia’s high-rainfall zone. N. Z. J. Agric. Res. 2012, 55, 393–411. [Google Scholar] [CrossRef][Green Version]
- Humphreys, M.W.; Thomas, H. Improved Drought Resistance in Introgression Lines Derived from Lolium multiflorum × Festuca arundinacea Hybrids. Plant Breed. 1993, 111, 155–161. [Google Scholar] [CrossRef]
- Rezaei Ghaleh, Z.; Sarmast, M.K.; Atashi, S. 6-Benzylaminopurine (6-BA) ameliorates drought stress response in tall fescue via the influencing of biochemicals and strigolactone-signaling genes. Plant Physiol. Biochem. 2020, 155, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhong, L.; Ou, E.; Tian, F.; Yao, M.; Chen, M.; Yan, X.; Li, Y.; Li, X.; He, R.; et al. Using Isoform Sequencing for De Novo Transcriptome Sequencing and the Identification of Genes Related to Drought Tolerance and Agronomic Traits in Tall Fescue (Festuca arundinacea Schreb.). Agronomy 2023, 13, 1484. [Google Scholar] [CrossRef]
- Ito, S.; Umehara, M.; Hanada, A.; Kitahata, N.; Hayase, H.; Yamaguchi, S.; Asami, T. Effects of triazole derivatives on strigolactone levels and growth retardation in rice. PLoS ONE 2011, 6, e21723. [Google Scholar] [CrossRef] [PubMed]
- Floková, K.; Tarkowská, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novák, O. UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.M.; Cai, W.J.; Ye, T.T.; Ding, J.; Feng, Y.Q. Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal. Chim. Acta 2018, 1031, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Alvi, A.F.; Sehar, Z.; Fatma, M.; Masood, A.; Khan, N.A. Strigolactone: An Emerging Growth Regulator for Developing Resilience in Plants. Plants 2022, 11, 2604. [Google Scholar] [CrossRef]
- Ito, S.; Umehara, M.; Hanada, A.; Yamaguchi, S.; Asami, T. Effects of strigolactone-biosynthesis inhibitor TIS108 on Arabidopsis. Plant Signal. Behav. 2013, 8, e24193. [Google Scholar] [CrossRef]
- Busso, C.A.; Mueller, R.J.; Richards, J.H. Effects of Drought and Defoliation on Bud Viability in Two Caespitose Grasses. Ann. Bot. 1989, 63, 477–485. [Google Scholar] [CrossRef]
- Zhang, J.; Mazur, E.; Balla, J.; Gallei, M.; Kalousek, P.; Medveďová, Z.; Li, Y.; Wang, Y.; Prát, T.; Vasileva, M.; et al. Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat. Commun. 2020, 11, 3508. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Taylor, C.; Leyser, O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013, 11, e1001474. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H. Cellular events of strigolactone signalling and their crosstalk with auxin in roots. J. Exp. Bot. 2015, 66, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- De Ollas, C.; Dodd, I.C. Physiological impacts of ABA–JA interactions under water-limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).