Impact of Duckweed (Lemna minor L.) Growing in Paddy Fields on Rice Yield and Its Underlying Causes
Abstract
1. Introduction
2. Materials and Methods
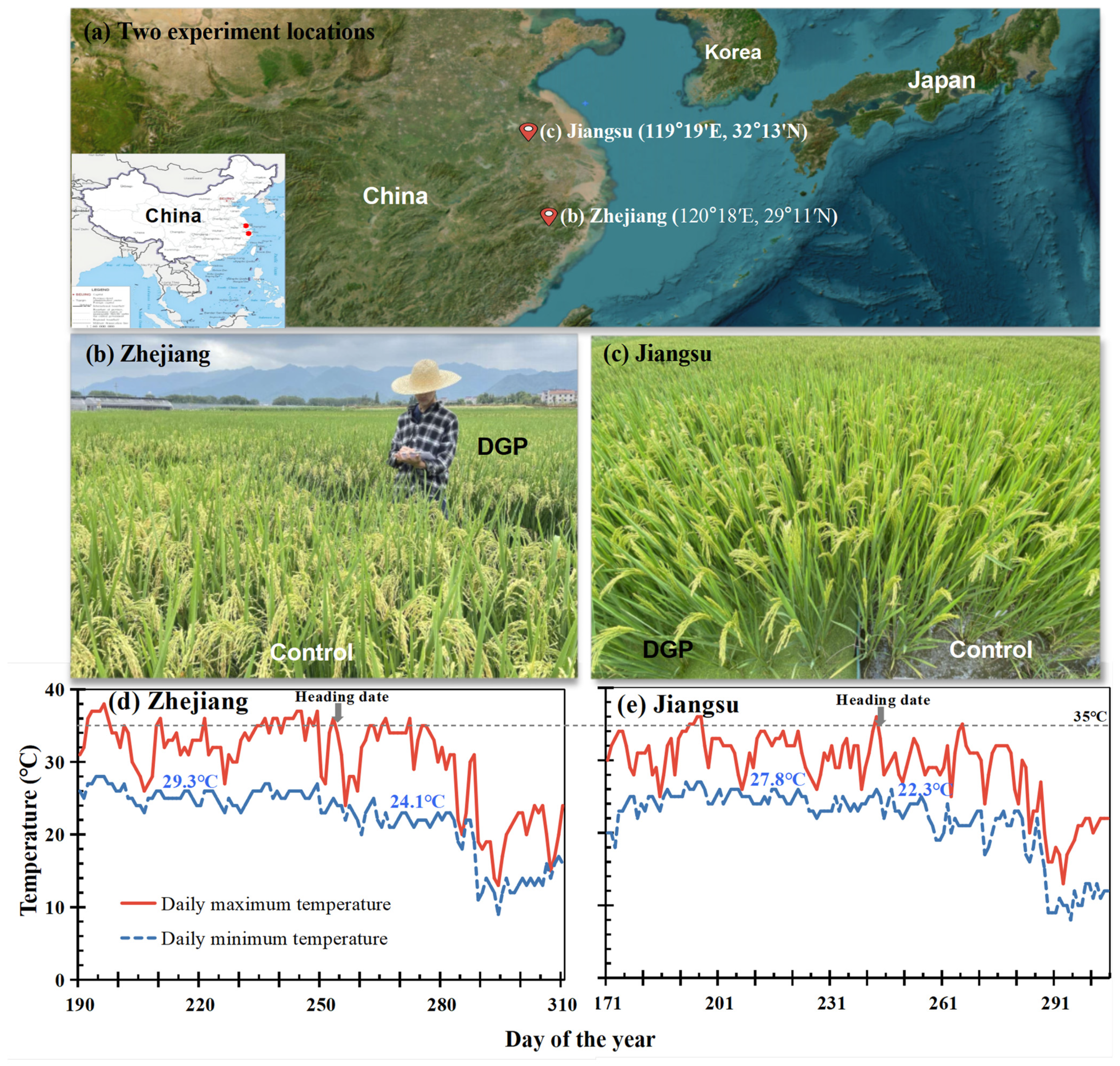
2.1. Study Site
2.2. Experimental Setup and Rice Cultivation
2.3. Parameter Measurements
2.3.1. Temperature and pH
2.3.2. Leaf Area Indices of Duckweed Plants
2.3.3. Photosynthesis Indices
2.3.4. ASD Canopy Spectrum
2.3.5. Accumulation of Dry Matter and Theoretical Yields
2.4. Statistical Analysis
3. Results
3.1. Ecological Factors of the Paddy Fields
3.2. Photosynthesis Traits and SPAD Values
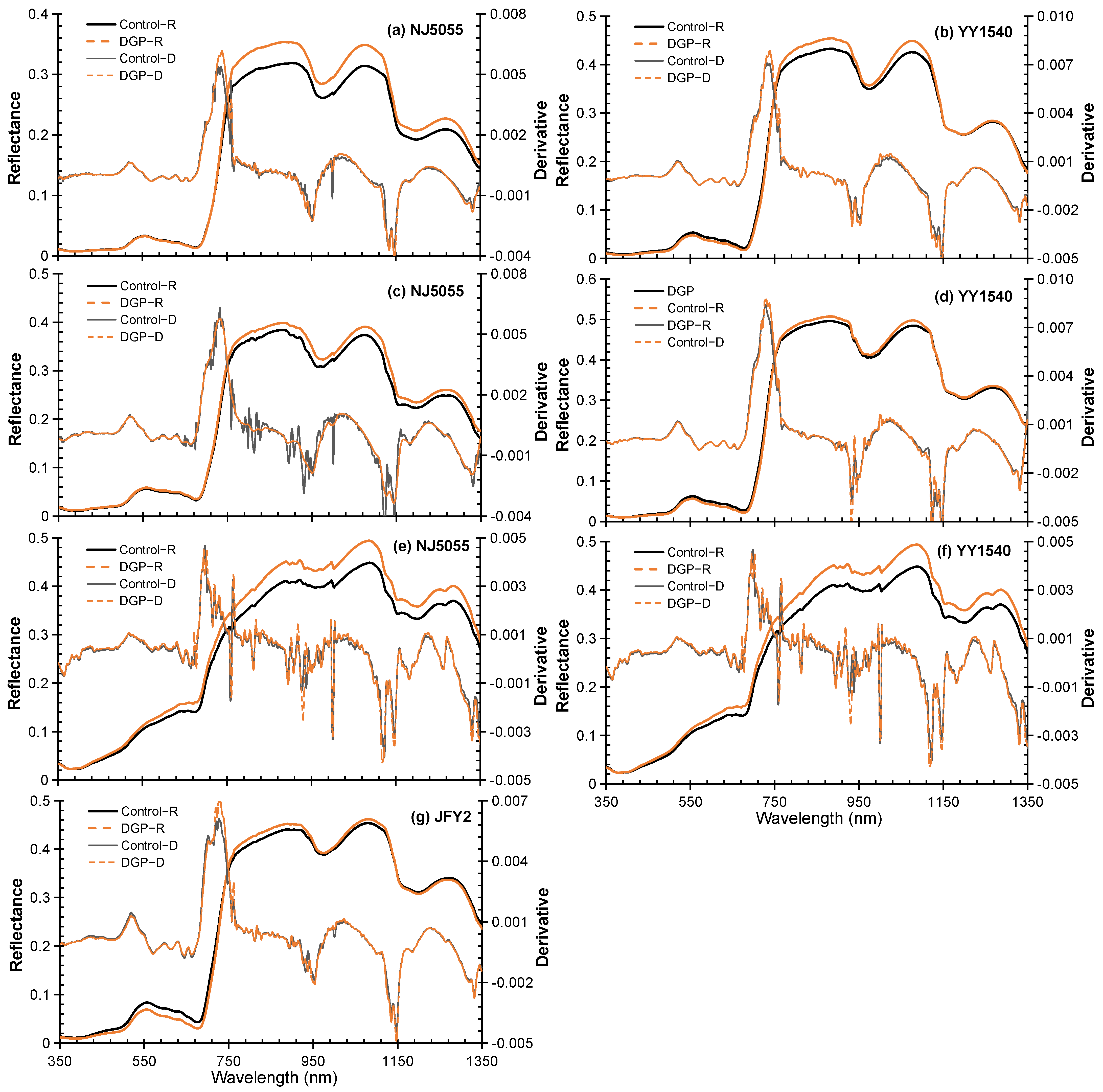
3.3. Spectral Characteristics of the Rice Canopy
3.4. Grain Yield and Structural Components
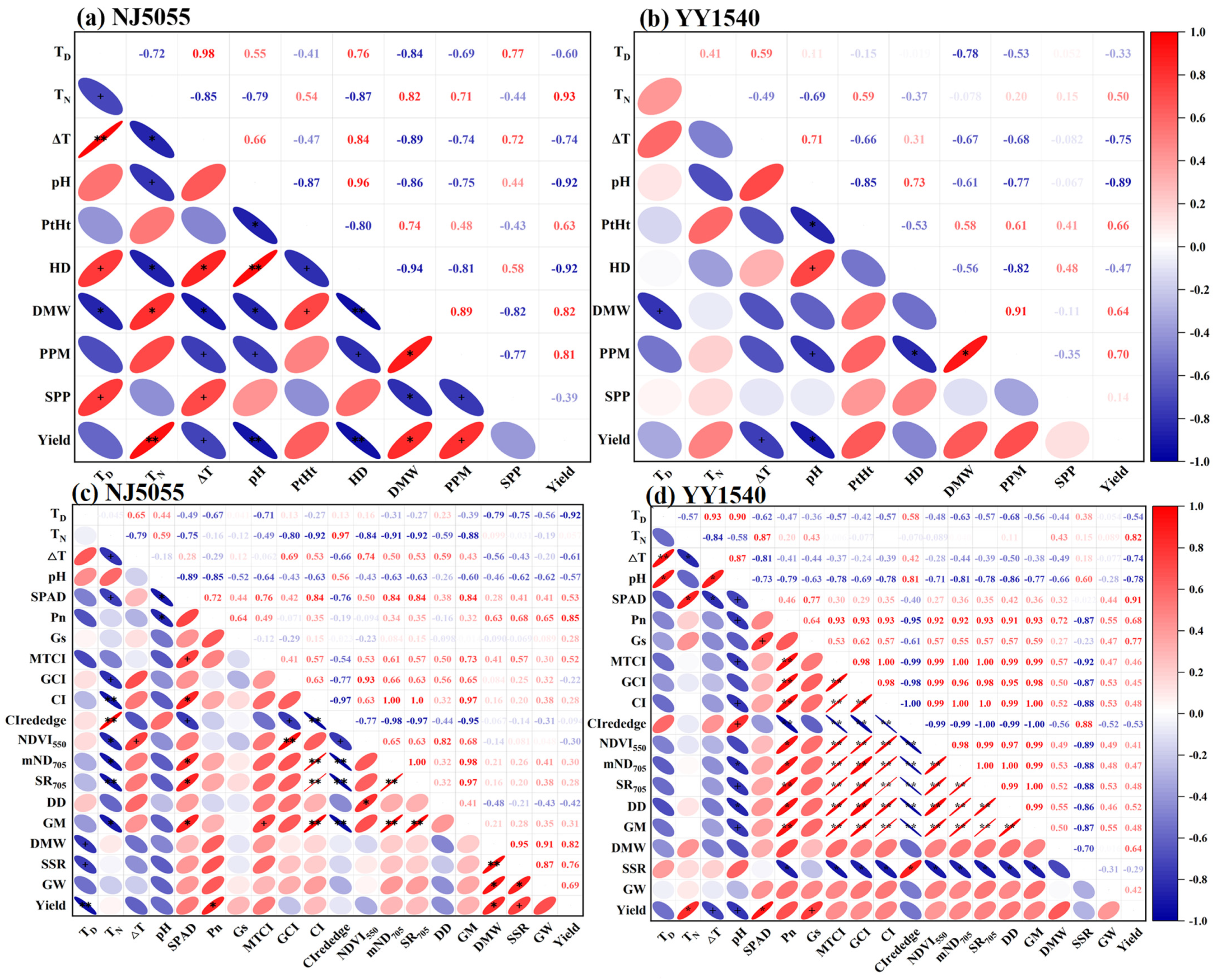
3.5. Correlation Analysis Results for Each Index under DGP Conditions
4. Discussion
4.1. DGP-Induced Changes in Environmental Factors Are Important Causes of Increases in Rice Production
4.2. DGP Enhanced the Photosynthetic Capacities of Leaves at the Filling Stage, Laying the Foundation for Increased Rice Yield
4.3. DGP Might Optimize the Canopy Spectral Architectures of Rice
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- An, D.; Zhou, Y.; Li, C.S.; Xiao, Q.; Wang, T.; Zhang, Y.T.; Wu, Y.R.; Li, Y.B.; Chao, D.Y.; Messing, J.; et al. Plant evolution and environmental adaptation unveiled by long-read whole-genome sequencing of spirodela. Proc. Natl. Acad. Sci. USA 2019, 116, 18893–18899. [Google Scholar] [CrossRef]
- Zaffer, B.; Sheikh, I.U.; Banday, T.M.; Adil, S. Effect of inclusion of different levels of duckweed (lemna minor) on the performance of broiler chicken. Indian J. Anim. Res. 2020, 55, 1200–1205. [Google Scholar] [CrossRef]
- Ziegler, P.; Appenroth, K.J.; Sree, K.S. Survival strategies of duckweeds, the world’s smallest angiosperms. Plants 2023, 12, 2215. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.Q.; Lian, Y.F.; Xu, L.; Chen, Y. Reduction of ammonia volatilization from urea by a floating duckweed in flooded rice fields. Soil Biol. Biochem. 2009, 73, 1890–1895. [Google Scholar] [CrossRef]
- Jing, L.Q.; Li, F.; Zhao, Y.H.; Wang, X.K.; Zhao, F.C.; Lai, S.K.; Sun, X.L.; Wang, Y.X.; Yang, L.X. Research progress on the carbon and nitrogen sink of duckweed growing in paddy and its effects on rice yield. Sci. Agric. Sin. 2023, 56, 4717–4728. [Google Scholar]
- Jing, L.Q.; Chen, C.; Lu, Q.; Wang, Y.X.; Zhu, J.G.; Lai, S.K.; Wang, Y.L.; Yang, L.X. How do elevated atmosphere CO2 and temperature alter the physiochemical properties of starch granules and rice taste? Sci. Total Environ. 2021, 766, 142592. [Google Scholar] [CrossRef]
- Jing, L.Q.; Chen, C.; Hu, S.W.; Dong, S.P.; Pan, Y.; Wang, Y.X.; Lai, S.K.; Wang, Y.L.; Yang, L.X. Effects of elevated atmosphere CO2 and temperature on the morphology, structure and thermal properties of starch granules and their relationship with cooked rice taste. Food Hydrocoll. 2021, 112, 106360. [Google Scholar] [CrossRef]
- IEA. Global Energy Review: CO2 Emissions in 2021. 2022. Available online: www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 22 February 2024).
- National Climate Center. China Climate Bulletin 2022. 2023. Available online: http://www.ncc-cma.net/channel/news/newsid/100060 (accessed on 6 February 2023).
- Radha, B.; Sunitha, N.C.; Sah, R.P.; TP, M.A.; Krishna, G.K.; Umesh, D.K.; Thomas, S.; Anilkumar, C.; Upadhyay, S.; Kumar, A.; et al. Physiological and molecular implications of multiple abiotic stresses on yield and quality of rice. Front. Plant Sci. 2023, 13, 996514. [Google Scholar] [CrossRef]
- Kollah, B.; Patra, A.K.; Mohanty, S.R. Aquatic Microphylla Azolla: A perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. Pollut. Res. 2016, 23, 4358–4369. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, S.; Xu, S.H.; Shen, J.Y.; Cao, L.K.; Sha, Z.M.; Chu, Q.N. A non-chemical weed control strategy, introducing duckweed into the paddy field. Pest Manag. Sci. 2022, 78, 3654–3663. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zheng, X.Q.; Peng, C.F.; Li, J.; Xu, Y. Driving forces and future trends on total nitrogen loss of planting in China. Environ. Pollut. 2021, 267, 115660.1–115660.6. [Google Scholar]
- Lu, Y.F.; Kronzucker, H.J.; Shi, W.M. Stigmasterol root exudation arising from Pseudomonas inoculation of the duckweed rhizosphere enhances nitrogen removal from polluted waters. Environ. Pollut. 2021, 287, 117587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Pan, C.; Borisjuk, N. The dynamics of NO3− and NH4+ uptake in duckweed are oordinated with the expression of major nitrogen assimilation genes. Plants 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.D.; Guo, B.; Liu, C.; Liu, J.L.; Qiu, G.Y.; Fu, Q.L.; Li, H. Alleviation of aqueous nitrogen loss from paddy fields by growth and decomposition of duckweed (Lemna minor L.) after fertilization. Chemosphere 2023, 311, 137073. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Landesman, L.; Bergmann, B.A.; Classen, J.J.; Howard, J.W.; Yamamoto, Y.T. Nutrient removal from swine lagoon liquid by Lemna minor 8627. Trans. ASAE 2002, 45, 1003–1010. [Google Scholar] [CrossRef]
- Pagliuso, D.; Grandis, A.; Fortirer, J.S.; Camargo, P.; Floh, E.I.S.; Buckeridge, M.S. Duckweeds as Promising Food Feedstocks Globally. Agronomy 2022, 12, 796. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wang, J.; Meng, L.; Zhu, Q.L.; El-Sawy, M.M.; Chen, Z.X.; Tu, X.S.; El-Saadony, M.T.; Zhang, Y.H.; Zhang, J.B.; et al. Integrative knowledge-based nitrogen management practices can provide positive effects on ecosystem nitrogen retention. Nat. Food 2024, 4, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Amirahmadi, E.; Moudrý, J.; Konvalina, P.; Hörtenhuber, S.J.; Ghorbani, M.; Neugschwandtner, R.W.; Jiang, Z.; Krexner, T.; Kopecký, M. Environmental life cycle assessment in organic and conventional rice farming systems: Using a cradle to farm gate approach. Sustainability 2022, 14, 15870. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Erisman, J.W.; Abrol, Y.P.; Brkunda, M.; Datta, A.; Davidson, E.; Vries, W.D.; Oenema, O.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution; Center for Ecology and Hydrology: Edinburgh, UK, 2013; p. 249094. [Google Scholar]
- Chen, X.D.; Guo, B.; Liu, J.L.; Wang, H.Y.; Li, N.Y.; Ma, J.; Fu, Q.L.; Li, H. Effects of duckweed on changes of nitrogen content in rice floodwater with variable nitrogen fertilizer application. J. Nucl. Agric. Sci. 2021, 35, 2674–2679. [Google Scholar]
- Sun, H.J.; Dan, A.; Feng, Y.F.; Vithanage, M.; Mandal, S.; Shaheen, S.M.; Rinklebe, J.; Shi, W.M.; Wang, H.L. Floating duckweed mitigated ammonia volatilization and increased grain yield and nitrogen use efficiency of rice in biochar amended paddy soils. Chemosphere 2019, 237, 124532. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lai, Y.C.; Tang, Z.X.; Zheng, M.M.; Shi, J.; Gu, M.Y.; Shen, J.Y.; Cao, L.K.; Sha, Z.M. Effects of duckweed mulching on composition and diversity of weed communities in paddy fields. China J. Eco-Agri. 2021, 29, 672–682. [Google Scholar]
- Yao, Y.L.; Zhang, M.; Tian, Y.H.; Zhao, M.; Zeng, K.; Zhang, B.W.; Zhao, M.; Yin, B. Azolla biofertilizer for improving low nitrogen use efficiency in an intensive rice cropping system. Field Crops Res. 2018, 216, 158–164. [Google Scholar] [CrossRef]
- Yao, Y.L.; Zhang, M.; Tian, Y.H.; Zhao, M.; Zhang, B.W.; Zeng, K.; Zhao, M.; Yin, B. Urea deep placement in combination with Azolla for reducing nitrogen loss and improving fertilizer nitrogen recovery in rice field. Field Crops Res. 2018, 218, 141–149. [Google Scholar] [CrossRef]
- Luo, J.S.; Hu, S.W.; Li, T.; He, F.H.; Tian, C.; Han, Y.; Mao, Y.L.; Jing, L.Q.; Yang, L.X.; Wang, Y.X. A preliminary study of the impacts of duckweed coverage during rice growth on grain yield and quality. Plants 2024, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS): Analgorithm for the remote estimation of the concentrations of chlorophyll A, chlorophyll B, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gitelson, A.; Arkebauer, T.; Solovchenko, A.; Nguy-Robertson, A.; Inoue, Y. An insight into spectral composition of light available for photosynthesis via remotely assessed absorption coefcient at leaf and canopy levels. Photosynth. Res. 2022, 151, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhu, L.J.; Feng, Z.Z. Hyperspectral characteristics and chlorophyll content estimation of winter wheat under ozone stress. Acta Ecol. Sin. 2023, 43, 3213–3223. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Yao, Y.L.; Zhang, M.; Tian, Y.H.; Zhao, M.; Zhang, B.W.; Zhao, M.; Zeng, K.; Yin, B. Duckweed (Spirodela polyrhiza) as green manure for increasing yield and reducing nitrogen loss in rice production. Field Crops Res. 2017, 214, 273–282. [Google Scholar] [CrossRef]
- Cai, W.W.; Ai, T.C.; Li, R.; Jin, Z.Y.; Xu, J.G.; Cao, K.K. Effect of controlled release fertilizer and urea additive on photosynthetic characteristics and yield of double cropping rice. Soil Fert. Sci. Chin. 2018, 3, 54–60. [Google Scholar]
- Xiong, D.; Flexas, J. Leaf economics spectrum in rice: Leaf anatomical, biochemical, and physiological trait trade-offs. J. Exp. Bot. 2018, 69, 5599–5609. [Google Scholar] [CrossRef]
- Wang, L.H.; Sun, F.; Chen, C.M.; Wang, Y.Y.; Liu, Y.S.; Zhou, Q. Combined effects of aluminum and cadmium in acidified soil on root growth in rice Seedlings. J. Agro-Environ. Sci. 2013, 32, 2511–2512. [Google Scholar]
- Ning, Q.; Chen, L.; Jia, Z.; Zhang, C.; Ma, D.; Li, F.; Zhang, J.; Li, D.; Han, X.; Cai, Z.; et al. Multiple long-term observations reveal a strategy for soil pH-dependent fertilization and fungal communities in support of agricultural production. Agric. Ecosyst. Environ. 2020, 293, 106837. [Google Scholar] [CrossRef]
- Zhou, J.J.; Tang, S.; Pan, W.K.; Xu, M.; Liu, X.; Ni, L.; Mao, X.L.; Sun, T.; Fu, H.R.; Han, K.F.; et al. Long-term application of controlled-release fertilizer enhances rice production and soil quality under non-flooded plastic film mulching cultivation conditions. Agric. Ecosyst. Environ. 2023, 358, 108720. [Google Scholar] [CrossRef]
- Huang, W.J.; Gilbert, S.; Poulev, A.; Acosta, K.; Lebeis, S.; Long, C.L.; Lam, E. Host-specific and tissue-dependent orchestration of microbiome community structure in traditional rice paddy ecosystems. Plant Soil 2020, 452, 379–395. [Google Scholar] [CrossRef]
- Cui, T.; Zhou, X.F.; Huang, Y.F.; Guo, Y.T.; Lin, Y.R.; Song, L.Y.; Zhang, J.C. Retrieval of winter wheat canopy carotenoid content with ground-and airborne-based hyperspectral data. Phyton.-Int. J. Exp. Bot. 2023, 92, 2633–2648. [Google Scholar] [CrossRef]
- Cheng, W.G.; Sakai, H.; Yagi, K.; Hasegawa, T. Combined effects of elevated [CO2] and high night temperature on carbon assimilation, nitrogen absorption, and the allocations of C and N by rice (Oryza sativa L.). Agric. For. Meteorol. 2010, 150, 1174–1181. [Google Scholar] [CrossRef]
- Zhen, B.; Guo, R.Q.; Zhou, X.G.; Li, H.Z.; Niu, Q.L.; Qiu, H.S.; Tian, G.L.; Li, S.J. The effects of thermal and waterlogging stresses at booting stage on photosynthesis and yield of rice. J. Irrig. Drain 2021, 40, 45–51. [Google Scholar]
- Gates, D.M. Heat transfer in plants. Sci. Am. 1965, 213, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Madonsela, S.; Cho, M.A.; Naidoo, L.; Main, R.; Majozi, N.P. Exploring the utility of Sentinel-2 for estimating maize chlorophyll content and leaf area index across different growth stages. J. Spat. Sci. 2023, 68, 339–351. [Google Scholar] [CrossRef]
- Liu, T.J.; Pan, X.Z.; Tang, J.J.; Zhu, B.; Hu, W.J.; Zhang, X.D.; Jiang, S.L.; Chen, M.Q. An analysis on hyper-spectral characteristics of rice under different nitrogen levels. Acta Agric. Univ. Jiangxiensis 2014, 36, 699–704. [Google Scholar]
- Cheng, Y.X.; Xiao, F.; Huang, D.Y.; Yang, Y.; Cheng, W.D.; Jin, S.C.; Li, G.H.; Ding, Y.F.; Paul, M.J.; Liu, Z.H. High canopy photosynthesis before anthesis explains the outstanding yield performance of rice cultivars with ideal plant architecture. Field Crop Res. 2024, 306, 109223. [Google Scholar] [CrossRef]

| Vegetation Index | Name or Meaning | Calculation Formula | Conference |
|---|---|---|---|
| RARSc | Ratio analysis of reflectance spectra for carotenoids | R760/R500 | [28] |
| MTCI | MERIS terrestrial chlorophyll index | (R750 − R710)/(R710 − R680) | [29] |
| GCI | Green chlorophyll index | R920/R500 − 1 | [30] |
| NDVI705 | Normalized difference vegetation index | (R750 − R705)/(R750 + R705) | [31] |
| CI | Chlorophyll index | (R750/R705) − 1 | [32] |
| CIrededge | Red-edge chlorophyll index | (R700 − R800)/(R690 + R720) − 1 | [32] |
| mND705 | Modified normalized difference | (R750 − R705)/(R750 + R705 − 2 × R445) | [33] |
| SR705 | Simple ratio | R750/R705 | [33] |
| GM | Relative dark green index | R750/R700 | [32] |
| Growth Stage | Location | Cultivar (CV) | Treatment | TD | TN | ΔT | Tair | Tleaf | pH | LAI |
|---|---|---|---|---|---|---|---|---|---|---|
| Jointing | Jiangsu | NJ5055 | Control | 33.2 ± 0.3 | 25.9 ± 0.1 | 7.4 ± 0.4 | 33.2 ± 0.3 | 32.8 ± 0.3 | 8.13 ± 0.04 | 0 |
| DGP | 32.7 ± 0.3 ns | 26.2 ± 0.1 ns | 6.5 ± 0.3 ns | 33.3 ± 0.4 ns | 32.8 ± 0.4 ns | 7.26 ± 0.04 ** | 2.7 ± 0.4 | |||
| YY1540 | Control | 34.1 ± 0.5 | 25.5 ± 0.4 | 8.6 ± 0.5 | 31.6 ± 0.0 | 31.7 ± 0.0 | 8.13 ± 0.04 | 0 | ||
| DGP | 33.8 ± 0.3 ns | 26.1 ± 0.3 ns | 7.7 ± 0.2 ns | 31.7 ± 0.1 ns | 31.7 ± 0.0 ns | 7.49 ± 0.07 ** | 2.3 ± 0.3 | |||
| ANOVA (p_Value) | ||||||||||
| DGP | 0.432 | 0.133 | 0.074 | 0.977 | 0.977 | <0.001 | ||||
| CV | 0.068 | 0.403 | 0.021 | 0.003 | 0.003 | 0.085 | ||||
| DGP × CV | 0.770 | 0.609 | 0.968 | 0.839 | 0.839 | 0.072 | ||||
| Mid-Filling | Zhejiang | JFY2 | Control | 33.1 ± 0.4 | 22.0 ± 0.4 | 11.1 ± 0.2 | 36.6 ± 0.2 | 35.6 ± 0.4 | ||
| DGP | 32.2 ± 0.2 ns | 22.2 ± 0.3 ns | 10.0 ± 0.4 ns | 36.4 ± 0.2 ns | 35.7 ± 0.6 ns | |||||
| Jiangsu | NJ5055 | Control | 29.1 ± 0.2 | 23.3 ± 0.5 | 5.8 ± 0.5 | 35.7 ± 0.0 | 35.5 ± 0.2 | 7.49 ± 0.09 | 0 | |
| DGP | 28.1 ± 0.2 * | 22.9 ± 0.5 ns | 5.1 ± 0.7 ns | 36.2 ± 0.0 ns | 35.9 ± 0.2 ns | 7.19 ± 0.07 + | 2.7 ± 0.4 | |||
| YY1540 | Control | 29.7 ± 0.2 | 23.2 ± 0.2 | 6.5 ± 0.2 | 32.7 ± 0.5 | 32.6 ± 0.0 | 7.54 ± 0.05 | 0 | ||
| DGP | 28.8 ± 0.3 + | 23.6 ± 0.3 ns | 5.2 ± 0.5 + | 32.6 ± 0.6 ns | 32.4 ± 0.0 + | 7.14 ± 0.04 ** | 2.4 ± 0.2 | |||
| ANOVA (p_Value) | ||||||||||
| DGP | 0.003 | 0.928 | 0.061 | 0.174 | 0.402 | 0.002 | ||||
| CV | 0.020 | 0.430 | 0.473 | <0.001 | <0.001 | 0.980 | ||||
| DGP × CV | 0.637 | 0.303 | 0.517 | 0.130 | 0.092 | 0.512 | ||||
| Maturity | Jiangsu | NJ5055 | Control | 20.7 ± 0.1 | 13.2 ± 0.6 | 7.5 ± 0.6 | 26.6 ± 0.7 | 26.2 ± 0.7 | ||
| DGP | 20.7 ± 0.1 ns | 13.2 ± 0.6 ns | 7.5 ± 0.6 ns | 26.7 ± 0.7 ns | 26.4 ± 0.9 ns | |||||
| YY1540 | Control | 20.5 ± 0.4 | 13.1 ± 0.1 | 7.4 ± 0.3 | 26.4 ± 0.0 | 26.3 ± 0.0 | ||||
| DGP | 20.0 ± 0.3 ns | 13.3 ± 0.4 ns | 6.7 ± 0.7 ns | 26.7 ± 0.0 ** | 26.7 ± 0.0 ** | |||||
| ANOVA (p_Value) | ||||||||||
| DGP | 0.133 | 0.884 | 0.230 | 0.638 | 0.506 | |||||
| CV | 0.023 | 0.961 | 0.106 | 0.803 | 0.620 | |||||
| DGP × CV | 0.180 | 0.735 | 0.190 | 0.765 | 0.833 | |||||
| Growth Stage | Location | Cultivar (CV) | Treatment | Pn | Gs | Ci | Tr | WUE | Ls | AMC | SPAD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prefilling | Jiangsu | NJ5055 | Control | 22.5 ± 1.5 | 0.44 ± 0.03 | 281.0 ± 6.3 | 7.0 ± 0.4 | 3.2 ± 0.1 | 0.26 ± 0.02 | 0.08 ± 0.01 | 50.5 ± 0.5 |
| DGP | 25.9 ± 0.5 ns | 0.48 ± 0.03 ns | 275.6 ± 7.2 ns | 7.6 ± 0.3 ns | 3.4 ± 0.2 ns | 0.27 ± 0.02 ns | 0.09 ± 0.00 ns | 54.7 ± 0.6 ** | |||
| YY1540 | Control | 24.3 ± 0.7 | 0.37 ± 0.00 | 257.0 ± 4.2 | 4.8 ± 0.0 | 5.1 ± 0.2 | 0.32 ± 0.01 | 0.09 ± 0.00 | 46.6 ± 0.2 | ||
| DGP | 25.6 ± 0.4 ns | 0.56 ± 0.00 ** | 290.7 ± 0.8 ** | 6.7 ± 0.0 ** | 3.8 ± 0.1 ** | 0.23 ± 0.00 ** | 0.09 ± 0.00 ns | 53.0 ± 1.5 * | |||
| ANOVA (p_Value) | |||||||||||
| DGP | 0.012 | 0.003 | 0.028 | 0.001 | 0.004 | 0.032 | 0.355 | 0.001 | |||
| CV | 0.286 | 0.905 | 0.400 | <0.001 | <0.001 | 0.507 | 0.274 | 0.018 | |||
| DGP × CV | 0.169 | 0.025 | 0.007 | 0.017 | 0.001 | 0.009 | 0.026 | 0.264 | |||
| Mid-Filling | Zhejiang | JFY2 | Control | 20.6 ± 1.1 | 0.90 ± 0.05 | 317.2 ± 4.0 | 19.9 ± 0.8 | 1.03 ± 0.02 | 0.16 ± 0.01 | 0.06 ± 0.00 | 33.9 ± 2.4 |
| DGP | 23.0 ± 0.7 ns | 0.79 ± 0.21 ns | 296.7 ± 25.8 ns | 17.5 ± 2.8 ns | 1.41 ± 0.29 ns | 0.21 ± 0.06 ns | 0.08 ± 0.01 ns | 40.0 ± 1.2 + | |||
| Jiangsu | NJ5055 | Control | 20.5 ± 1.2 | 0.24 ± 0.02 | 221.9 ± 1.4 | 5.5 ± 0.2 | 3.7 ± 0.1 | 0.41 ± 0.01 | 0.09 ± 0.01 | 35.9 ± 1.0 | |
| DGP | 23.9 ± 0.7 + | 0.30 ± 0.04 ns | 229.7 ± 24.1 ns | 6.7 ± 0.5 ns | 3.6 ± 0.4 ns | 0.40 ± 0.06 ns | 0.11 ± 0.01 ns | 38.7 ± 0.8 + | |||
| YY1540 | Control | 21.5 ± 0.5 | 0.41 ± 0.02 | 275.7 ± 3.3 | 6.3 ± 0.2 | 3.4 ± 0.1 | 0.27 ± 0.01 | 0.08 ± 0.00 | 35.3 ± 0.4 | ||
| DGP | 25.9 ± 1.1 * | 0.56 ± 0.07 ns | 283.7 ± 12.2 ns | 7.7 ± 0.7 ns | 3.4 ± 0.3 ns | 0.25 ± 0.03 ns | 0.09 ± 0.01 + | 36.7 ± 0.5 + | |||
| ANOVA (p_Value) | |||||||||||
| DGP | 0.010 | 0.039 | 0.575 | 0.016 | 0.814 | 0.622 | 0.149 | 0.003 | |||
| CV | 0.187 | 0.002 | 0.007 | 0.054 | 0.306 | 0.003 | 0.136 | 0.031 | |||
| DGP × CV | 0.637 | 0.304 | 0.994 | 0.823 | 0.829 | 0.922 | 0.963 | 0.159 | |||
| Maturity | Jiangsu | NJ5055 | Control | 10.1 ± 1.1 | 0.33 ± 0.02 | 316.0 ± 2.1 | 7.7 ± 0.4 | 1.3 ± 0.1 | 0.17 ± 0.01 | 0.03 ± 0.00 | 21.6 ± 1.0 |
| DGP | 11.4 ± 1.9 ns | 0.27 ± 0.01 + | 296.3 ± 8.0 + | 6.7 ± 0.2 + | 1.7 ± 0.2 ns | 0.22 ± 0.02 + | 0.04 ± 0.01 ns | 25.2 ± 1.6 ns | |||
| YY1540 | Control | 10.9 ± 1.0 | 0.47 ± 0.01 | 336.4 ± 6.3 | 8.1 ± 0.1 | 1.3 ± 0.1 | 0.13 ± 0.01 | 0.03 ± 0.00 | 17.1 ± 0.6 | ||
| DGP | 13.0 ± 0.5 ns | 0.43 ± 0.01 * | 316.0 ± 0.6 * | 7.6 ± 0.1 * | 1.7 ± 0.0 * | 0.17 ± 0.00 ** | 0.04 ± 0.00 * | 18.7 ± 0.4 + | |||
| ANOVA (p_Value) | |||||||||||
| DGP | 0.237 | 0.009 | 0.005 | 0.023 | 0.041 | 0.012 | 0.135 | 0.052 | |||
| CV | 0.405 | <0.001 | 0.005 | 0.051 | 0.827 | 0.009 | 0.807 | 0.002 | |||
| DGP × CV | 0.786 | 0.518 | 0.949 | 0.315 | 0.933 | 0.559 | 0.856 | 0.383 | |||
| Growth Stage | Location | Cultivar (CV) | Treatment | RARSc | MTCI | GCI | NDVI705 | CI | CIrededge | mND705 | SR705 | GM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prefilling | Jiangsu | NJ5055 | Control | 21.5 ± 1.3 | 3.5 ± 0.1 | 8.8 ± 0.5 | 0.6 ± 0.0 | 3.7 ± 0.2 | −3.2 ± 0.1 | 0.7 ± 0.0 | 4.7 ± 0.2 | 6.0 ± 0.4 |
| DGP | 24.7 ± 1.6 ns | 3.9 ± 0.2ns | 10.0 ± 0.7 ns | 0.7 ± 0.0 ns | 4.2 ± 0.3 ns | −3.5 ± 0.1 ns | 0.7 ± 0.0 ns | 5.2 ± 0.3 ns | 7.1 ± 0.6 ns | |||
| YY1540 | Control | 19.3 ± 0.9 | 3.0 ± 0.1 | 7.1 ± 0.2 | 0.6 ± 0.0 | 3.2 ± 0.1 | −2.9 ± 0.0 | 0.7 ± 0.0 | 4.2 ± 0.1 | 5.4 ± 0.2 | ||
| DGP | 23.1 ± 0.7 * | 3.5 ± 0.1 ** | 8.5 ± 0.2 ** | 0.7 ± 0.0 * | 3.8 ± 0.1 * | −3.3 ± 0.1 ** | 0.7 ± 0.0 * | 4.8 ± 0.1 * | 6.6 ± 0.3 * | |||
| ANOVA (p_Value) | ||||||||||||
| DGP | 0.021 | 0.009 | 0.019 | 0.020 | 0.021 | 0.004 | 0.019 | 0.021 | 0.019 | |||
| CV | 0.145 | 0.011 | 0.008 | 0.044 | 0.041 | 0.057 | 0.013 | 0.041 | 0.152 | |||
| DGP × CV | 0.813 | 0.906 | 0.884 | 0.610 | 0.813 | 0.606 | 0.564 | 0.813 | 0.922 | |||
| Mid-Filling | Zhejiang | JFY2 | Control | 10.7 ± 0.3 | 1.8 ± 0.1 | 4.3 ± 0.3 | 0.5 ± 0.0 | 1.7 ± 0.1 | −2.1 ± 0.1 | 0.5 ± 0.0 | 2.7 ± 0.1 | 3.4 ± 0.1 |
| DGP | 14.3 ± 0.3 * | 2.3 ± 0.1 * | 5.6 ± 0.2 + | 0.5 ± 0.0 * | 2.4 ± 0.1 * | −2.4 ± 0.0 * | 0.6 ± 0.0 * | 3.4 ± 0.1 * | 4.3 ± 0.1 * | |||
| Jiangsu | NJ5055 | Control | 14.2 ± 1.1 | 2.5 ± 0.1 | 5.7 ± 0.3 | 0.5 ± 0.0 | 2.2 ± 0.1 | −2.4 ± 0.1 | 0.6 ± 0.0 | 3.2 ± 0.1 | 3.9 ± 0.2 | |
| DGP | 14.3 ± 0.8 ** | 2.7 ± 0.0 * | 5.8 ± 0.5 * | 0.5 ± 0.0 ** | 2.5 ± 0.2 ** | −2.5 ± 0.1 * | 0.6 ± 0.0 ** | 3.5 ± 0.2 ** | 4.3 ± 0.2 ** | |||
| YY1540 | Control | 17.5 ± 0.6 | 2.8 ± 0.1 | 6.6 ± 0.2 | 0.6 ± 0.0 | 2.9 ± 0.1 | −2.7 ± 0.1 | 0.6 ± 0.0 | 3.9 ± 0.1 | 5.2 ± 0.2 | ||
| DGP | 21.2 ± 1.1 ns | 3.4 ± 0.2 + | 7.9 ± 0.5 ns | 0.6 ± 0.0 ns | 3.7 ± 0.2 ns | −3.1 ± 0.1 ns | 0.7 ± 0.0 ns | 4.7 ± 0.2 ns | 6.3 ± 0.3 ns | |||
| ANOVA (p_Value) | ||||||||||||
| DGP | 0.052 | 0.007 | 0.073 | 0.173 | 0.019 | 0.016 | 0.024 | 0.019 | 0.012 | |||
| CV | 0.001 | 0.002 | 0.004 | 0.003 | 0.001 | 0.001 | 0.002 | 0.001 | <0.001 | |||
| DGP × CV | 0.062 | 0.081 | 0.129 | 0.159 | 0.202 | 0.085 | 0.516 | 0.202 | 0.138 | |||
| Maturity | Jiangsu | NJ5055 | Control | 5.0 ± 0.0 | 0.8 ± 0.0 | 3.0 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | −1.4 ± 0.0 | 0.2 ± 0.0 | 1.4 ± 0.0 | 1.5 ± 0.0 |
| DGP | 5.1 ± 0.0 * | 0.9 ± 0.0 ns | 3.1 ± 0.1 ns | 0.2 ± 0.0 ns | 0.4 ± 0.0 ns | −1.4 ± 0.0 ns | 0.2 ± 0.0 ns | 1.4 ± 0.0 ns | 1.6 ± 0.0 ns | |||
| YY1540 | Control | 8.0 ± 0.2 | 0.6 ± 0.0 | 3.8 ± 0.1 | 0.2 ± 0.0 | 0.5 ± 0.0 | −1.4 ± 0.0 | 0.2 ± 0.0 | 1.5 ± 0.0 | 1.7 ± 0.0 | ||
| DGP | 8.5 ± 1.0 ns | 0.7 ± 0.0 * | 4.1 ± 0.4 ns | 0.2 ± 0.0 ** | 0.6 ± 0.0 ** | −1.5 ± 0.0 * | 0.3 ± 0.0 ** | 1.6 ± 0.0 ** | 1.9 ± 0.0 ** | |||
| ANOVA (p_Value) | ||||||||||||
| DGP | 0.533 | 0.022 | 0.388 | 0.019 | 0.001 | 0.017 | 0.001 | 0.001 | 0.004 | |||
| CV | 0.001 | 0.001 | 0.004 | 0.001 | <0.001 | 0.001 | 0.001 | <0.001 | <0.001 | |||
| DGP × CV | 0.689 | 0.254 | 0.733 | 0.015 | 0.020 | 0.081 | 0.023 | 0.020 | 0.055 | |||
| Location | Cultivar (CV) | Treatment | Panicles m−2 | Spikelets per Panicle | Seed Setting Rate (%) | 1000-Grain Weight (g) | Yield (m−2) |
|---|---|---|---|---|---|---|---|
| Zhejiang | JFY2 | Control | 187.9 ± 11.2 | 276.1 ± 16.1 | 80.6 ± 2.7 | 23.6 ± 0.1 | 981.1 ± 37.5 |
| DGP | 202.9 ± 17.2 ns | 287.5 ± 10.4 ns | 75.9 ± 3.3 ns | 24.2 ± 0.4 ns | 1062.3 ± 42.8 ns | ||
| Jiangsu | NJ5055 | Control | 304.0 ± 12.9 | 131.4 ± 6.0 | 90.4 ± 0.6 | 25.3 ± 0.8 | 909.8 ± 17.3 |
| DGP | 337.1 ± 6.6 + | 123.8 ± 7.3 ns | 93.8 ± 1.0 * | 26.2 ± 0.6 ns | 1021.8 ± 13.3 ** | ||
| YY1540 | Control | 186.0 ± 10.6 | 283.9 ± 8.4 | 89.1 ± 1.2 | 21.4 ± 0.4 | 1000.6 ± 23.2 | |
| DGP | 209.9 ± 3.6 + | 287.7 ± 8.6 ns | 84.8 ± 2.2 ns | 21.5 ± 0.6 ns | 1099.7 ± 19.3 * | ||
| ANOVA (p_Value) | |||||||
| DGP | 0.008 | 0.760 | 0.780 | 0.458 | 0.002 | ||
| CV | <0.001 | <0.001 | 0.012 | 0.001 | 0.005 | ||
| DGP × CV | 0.555 | 0.365 | 0.037 | 0.618 | 0.757 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, L.; Wang, X.; Zhao, Y.; Li, F.; Su, Y.; Cai, Y.; Zhao, F.; Dong, G.; Yang, L.; Wang, Y. Impact of Duckweed (Lemna minor L.) Growing in Paddy Fields on Rice Yield and Its Underlying Causes. Agronomy 2024, 14, 726. https://doi.org/10.3390/agronomy14040726
Jing L, Wang X, Zhao Y, Li F, Su Y, Cai Y, Zhao F, Dong G, Yang L, Wang Y. Impact of Duckweed (Lemna minor L.) Growing in Paddy Fields on Rice Yield and Its Underlying Causes. Agronomy. 2024; 14(4):726. https://doi.org/10.3390/agronomy14040726
Chicago/Turabian StyleJing, Liquan, Xunkang Wang, Yihan Zhao, Fan Li, Yu Su, Yang Cai, Fucheng Zhao, Guichun Dong, Lianxin Yang, and Yunxia Wang. 2024. "Impact of Duckweed (Lemna minor L.) Growing in Paddy Fields on Rice Yield and Its Underlying Causes" Agronomy 14, no. 4: 726. https://doi.org/10.3390/agronomy14040726
APA StyleJing, L., Wang, X., Zhao, Y., Li, F., Su, Y., Cai, Y., Zhao, F., Dong, G., Yang, L., & Wang, Y. (2024). Impact of Duckweed (Lemna minor L.) Growing in Paddy Fields on Rice Yield and Its Underlying Causes. Agronomy, 14(4), 726. https://doi.org/10.3390/agronomy14040726







