The ptxD Gene Confers Rapeseed the Ability to Utilize Phosphite and a Competitive Advantage against Weeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
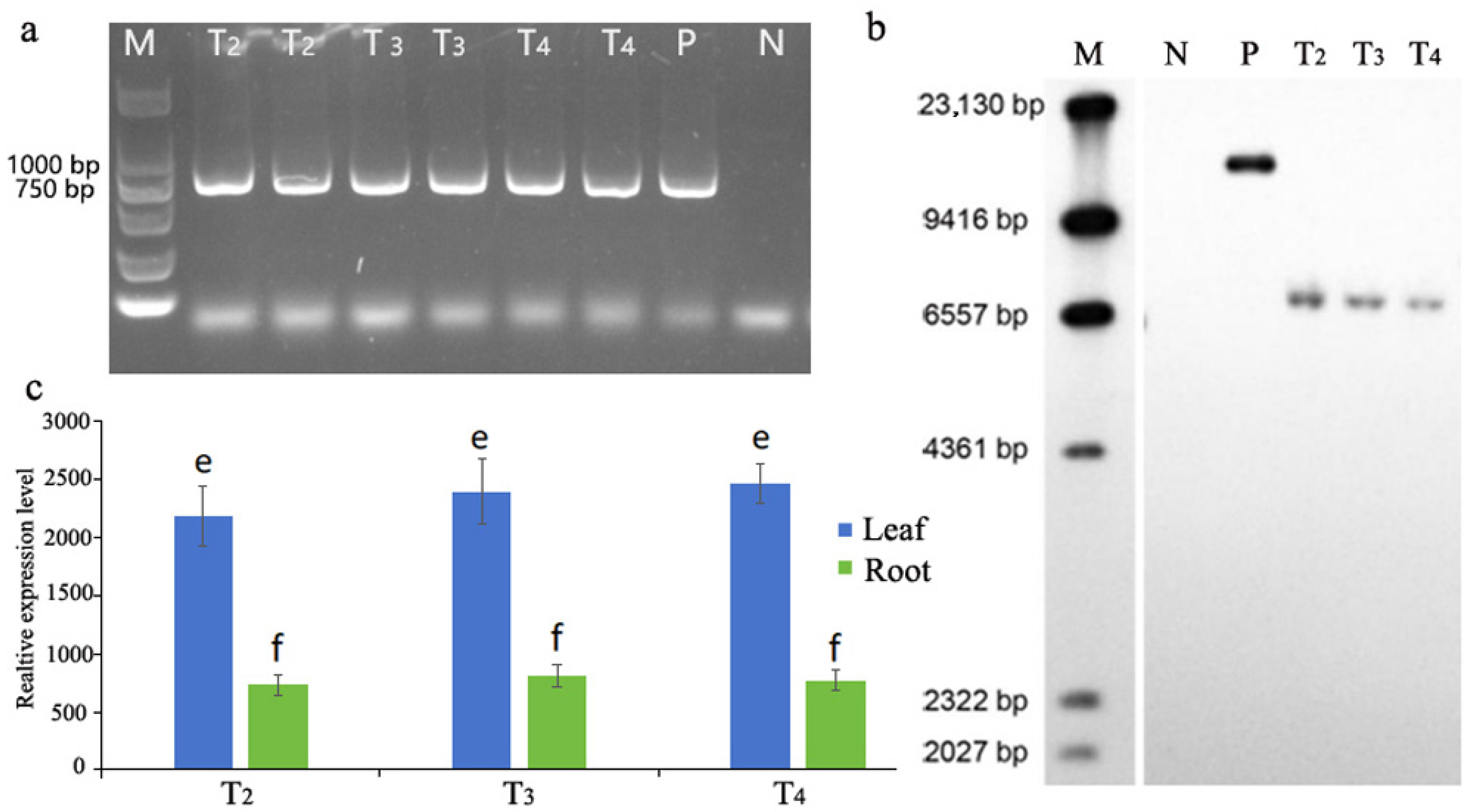
2.2. Genomic DNA Extraction, PCR, and Southern Blotting
2.3. Quantitative Real-Time RT-PCR
2.4. Assessment of ptxD-Expressing Rapeseed Plants’ Ability to Utilize Phi as a Source of P Using Sand Culture Experiments
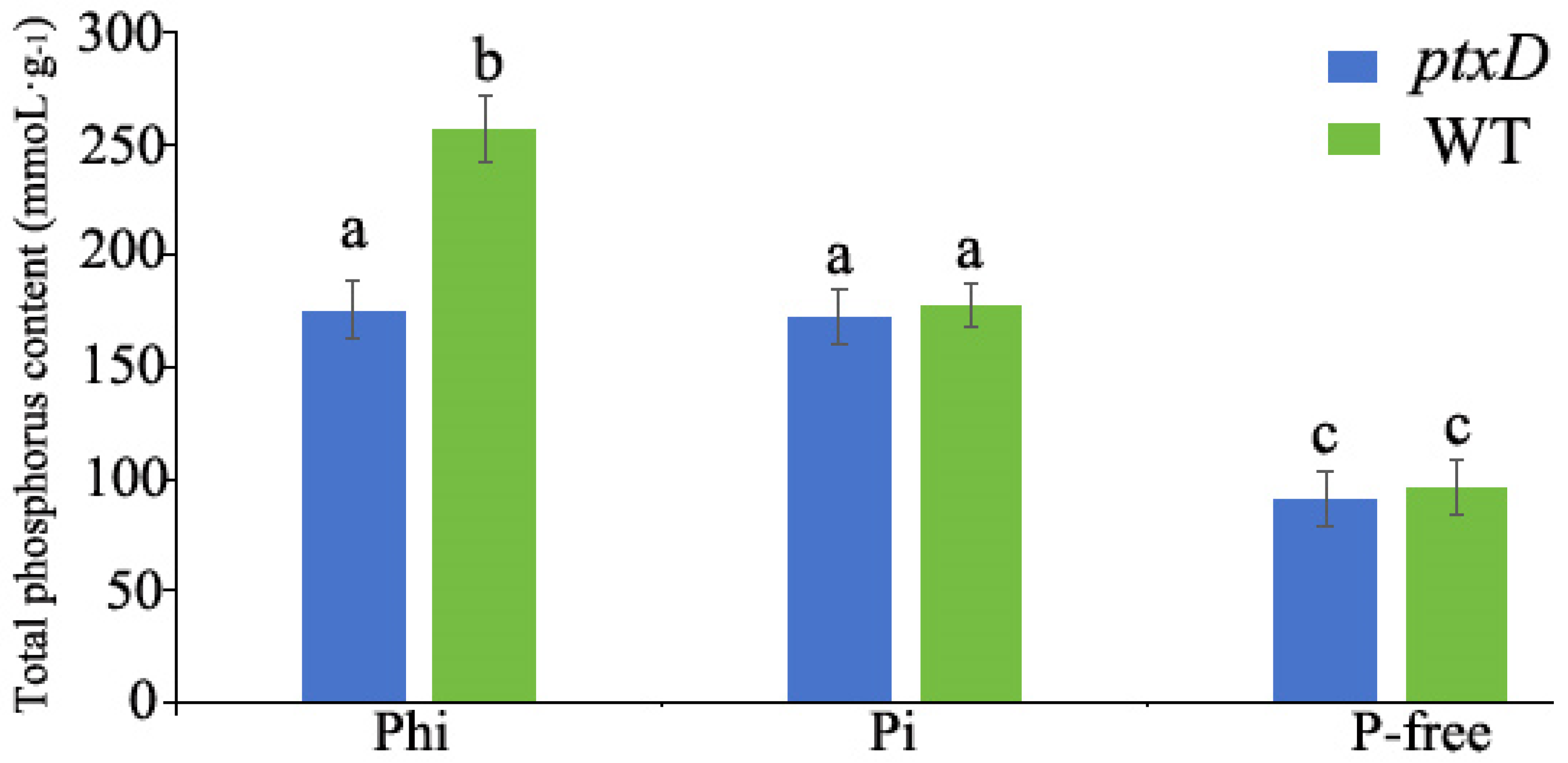
2.5. Determination of Total Phosphorus Content
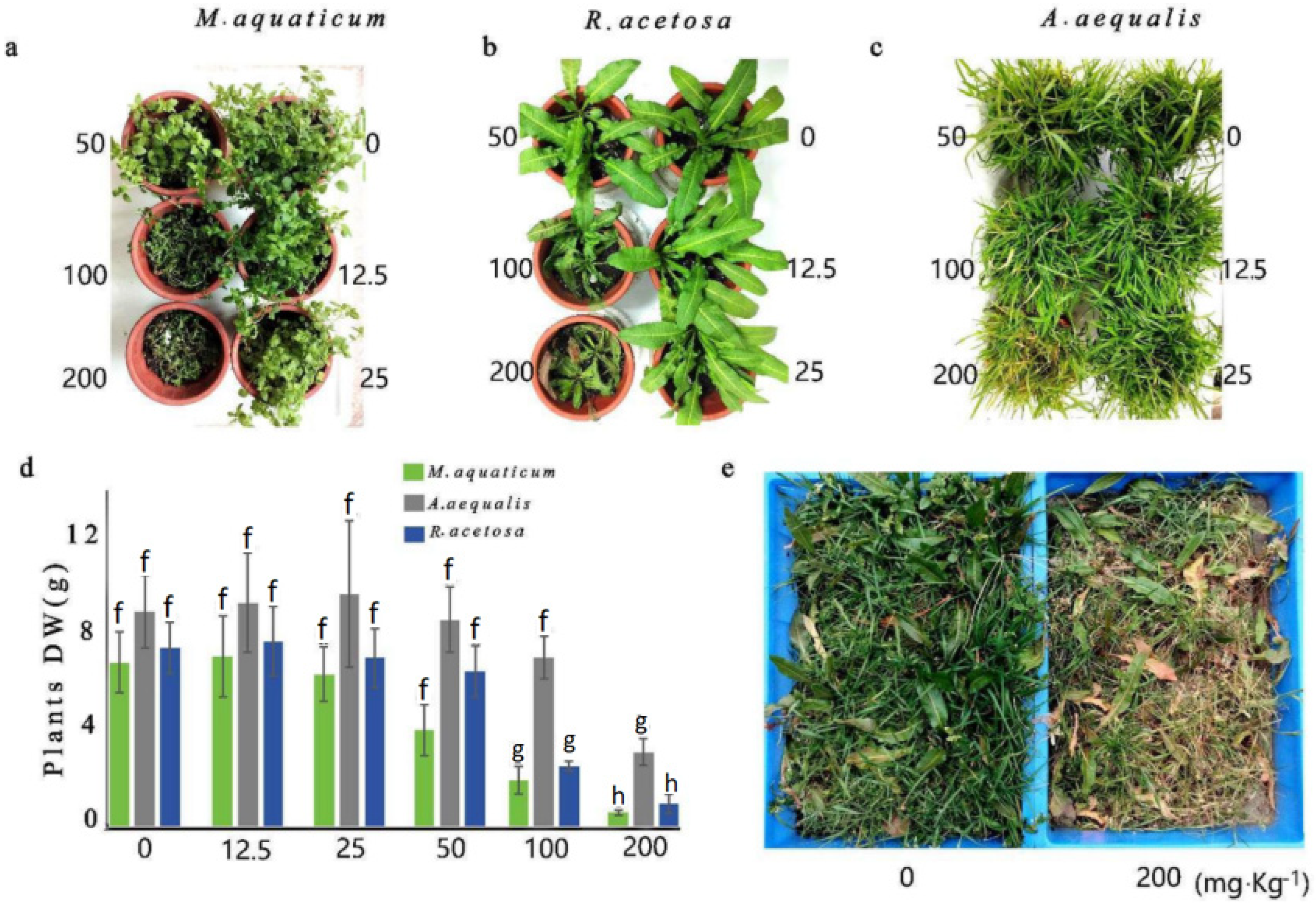
2.6. Evaluation of Weed Control Efficacy of Phi Using Pot Experiments
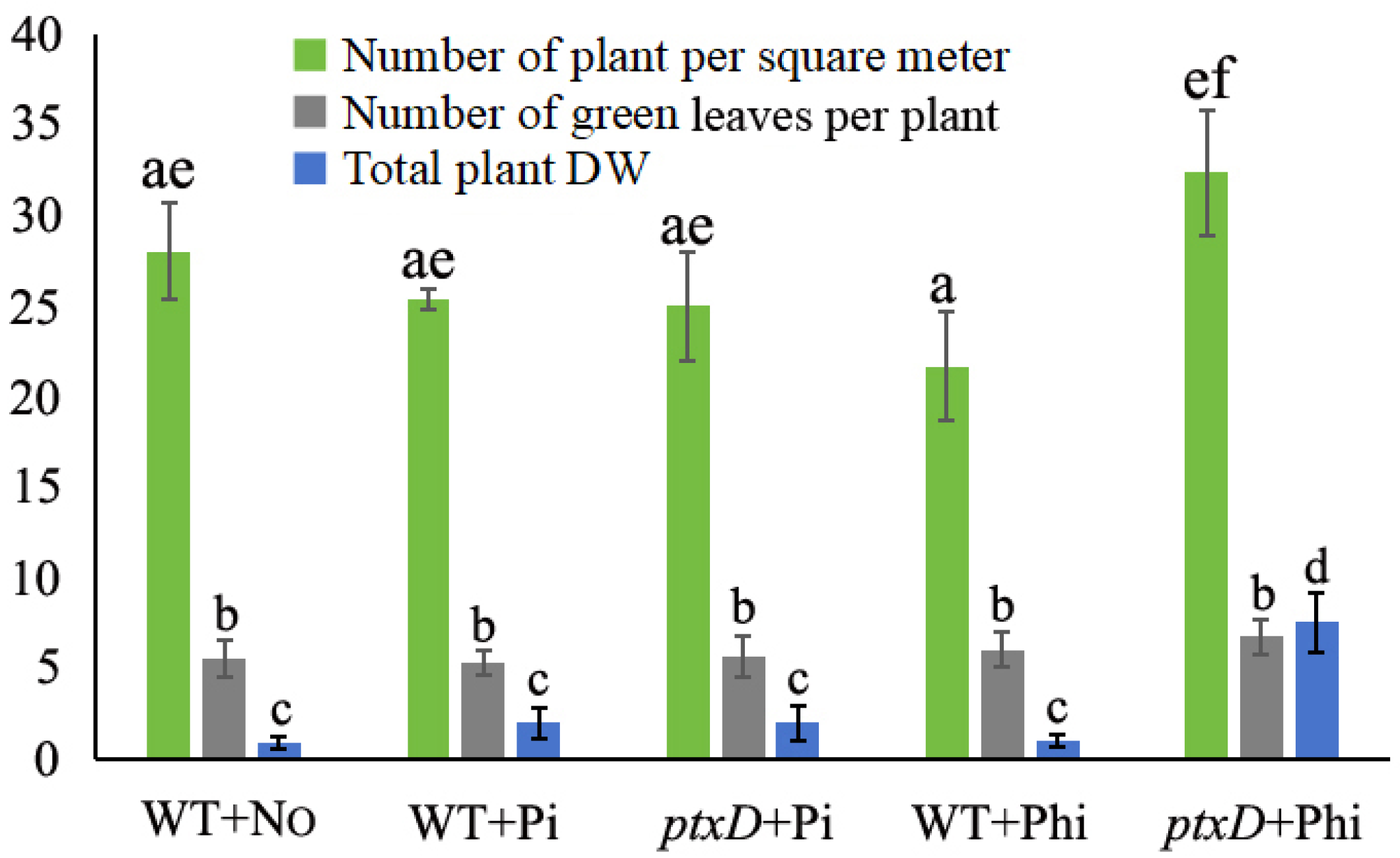
2.7. Competition Experiments with Field Weeds
2.8. Effects of Phosphite on Soil Microorganisms
3. Results
3.1. The Stable Inheritance of the ptxD Gene in Transgenic Offsprings
3.2. ptxD-Expressing Rapeseed Can Utilize Phi as an Exclusive Source of Phosphorus
3.3. Phi Accumulates in Non-Transgenic WT Rapeseed Plants
3.4. Phi Effectively Suppresses Weeds in Rapeseed Fields
3.5. ptxD/Phi Is Highly Effective in Suppressing Weeds in Rapeseed Fields
3.6. The Application of Phi Does Not Have a Significant Impact on the Soil’s Microbial Environment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an Analog of Phosphate, Suppresses the Coordinated Expression of Genes under Phosphate Starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Herrera-Estrella, L. Engineering phosphorus metabolism in plants to produce a dual fertilization and weed control system. Nat. Biotechnol. 2012, 30, 889–893. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Hammond, J.P. The Ecophysiology of Plant-Phosphorus Interactions; Springer: Dordrecht, The Netherlands, 2008; pp. 13–19. [Google Scholar]
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: A physiological and molecular assessment. Sci. Rep. 2019, 9, 14880. [Google Scholar] [CrossRef] [PubMed]
- Lizcano-Toledo, R.; Reyes-Martín, M.P.; Celi, L.; Fernández-Ondoño, E. Phosphorus dynamics in the soil–plant–environment relationship in cropping systems: A review. Appl. Sci. 2021, 11, 11133. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Adeux, G.; Vieren, E.; Carlesi, S.; Bàrberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating crop yield losses through weed diversity. Nat. Sustain. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Dong, X.; Liu, Y.; Lu, J. Herbicide and nitrogen rate effects on weed suppression, N uptake, use efficiency and yield in winter oilseed rape (Brassica napus L.). Glob. Ecol. Conserv. 2019, 17, e00529. [Google Scholar] [CrossRef]
- Perthame, L.; Colbach, N.; Brunel-Muguet, S.; Busset, H.; Lilley, J.M.; Matejicek, A.; Moreau, D. Quantifying the nitrogen demand of individual plants in heterogeneous canopies: A case study with crop and weed species. Eur. J. Agron. 2020, 119, 126102. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Parra-Arroyo, L.; González-González, R.B.; Castillo-Zacarías, C.; Martínez, E.M.M.; Sosa-Hernández, J.E.; Bilal, M.; Parra-Saldívar, R. Highly hazardous pesticides and related pollutants: Toxicological, regulatory, and analytical aspects. Sci. Total Environ. 2022, 807, 151879. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.; Varanasi, V.K.; Danilova, T.V.; Koo, D.-H.; Nakka, S.; Peterson, D.E.; Tranel, P.J.; Friebe, B.; Gill, B.S.; Jugulam, M. Physical mapping of amplified copies of the 5-enolpyruvylshikimate-3-phosphate synthase gene in glyphosate-resistant Amaranthus tuberculatus. Plant Physiol. 2017, 173, 1226–1234. [Google Scholar] [CrossRef]
- Ofosu, R.; Agyemang, E.D.; Márton, A.; Pásztor, G.; Taller, J.; Kazinczi, G. Herbicide resistance: Managing weeds in a changing world. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Price, A.J.; Balkcom, K.; Culpepper, S.; Kelton, J.; Nichols, R.; Schomberg, H. Glyphosate-resistant Palmer amaranth: A threat to conservation tillage. J. Soil Water Conserv. 2011, 66, 265–275. [Google Scholar] [CrossRef]
- Costas, A.M.G.; White, A.K.; Metcalf, W.W. Purification and Characterization of a Novel Phosphorus-oxidizing Enzyme from Pseudomonas stutzeri WM88*210. J. Biol. Chem. 2001, 276, 17429–17436. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Herrera-Estrella, L. A novel dominant selectable system for the selection of transgenic plants under in vitro and greenhouse conditions based on phosphite metabolism. Plant Biotechnol. J. 2013, 11, 516–525. [Google Scholar] [CrossRef]
- Manna, M.; Achary, V.M.M.; Islam, T.; Agrawal, P.K.; Reddy, M.K. The development of a phosphite-mediated fertilization and weed control system for rice. Sci. Rep. 2016, 6, 24941. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, D.; López-Arredondo, D.L.; Janga, M.R.; Campbell, L.M.; Estrella-Hernández, P.; Bagavathiannan, M.V.; Herrera-Estrella, L.; Rathore, K.S. Selective fertilization with phosphite allows unhindered growth of cotton plants expressing the ptxD gene while suppressing weeds. Proc. Natl. Acad. Sci. USA 2018, 115, E6946–E6955. [Google Scholar] [CrossRef] [PubMed]
- Changko, S.; Rajakumar, P.D.; Young, R.E.; Purton, S. The phosphite oxidoreductase gene, ptxD as a bio-contained chloroplast marker and crop-protection tool for algal biotechnology using Chlamydomonas. Appl. Microbiol. Biotechnol. 2020, 104, 675–686. [Google Scholar] [CrossRef]
- Xiong, T. Studies on Transformation of Phosphite Dehydrogenase Gene ptxD in Brassica napus. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2020. [Google Scholar]
- Parkhi, V.; Kumar, V.; Campbell, L.M.; Bell, A.A.; Shah, J.; Rathore, K.S. Resistance against various fungal pathogens and reniform nematode in transgenic cotton plants expressing Arabidopsis NPR1. Transgenic Res. 2010, 19, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Holman, W.I.M. A new technique for the determination of phosphorus by the molybdenum blue method. Biochem. J. 1943, 37, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Zhou, Z.; Yu, C.; Liu, R.; Yang, C.; Zhang, F.; Song, G.; Meng, Y. Yield and potassium use efficiency of cotton with wheat straw incorporation and potassium fertilization on soils with various conditions in the wheat-cotton rotation system. Field Crops Res. 2015, 172, 132–144. [Google Scholar] [CrossRef]
- Loera-Quezada, M.M.; Leyva-González, M.A.; López-Arredondo, D.; Herrera-Estrella, L. Phosphite cannot be used as a phosphorus source but is non-toxic for microalgae. Plant Sci. 2014, 231, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Guan, C.Y.; Chen, S.Y. Research on the occurrence patterns and control of main weeds in no till live broadcast rapeseed fields. Hunan Agric. Sci. 2011, Z1, 37–38. [Google Scholar]
- Lu, J.X.; Chen, S.Y.; Guan, C.Y.; Liu, Z.S.; Xiao, G. Occurrence regularity of weeds and Its control techniques in winter rape fields of the middle and lower reaches of the Yangtze river. Crop Res. 2012, 26, 639–642. [Google Scholar]
- Chu, Q.Y.; Yu, L.S.; Xiao, M.K.; Wang, Q.; Zhu, Z.S. Occurrence pattern of weeds in rapeseed fields and chemical control techniques. J. Anhui Agric. Sci. 2006, 34, 4066–4067. [Google Scholar]
- Hu, M.; Pu, H.; Kong, L.; Gao, J.; Long, W.; Chen, S.; Zhang, J.; Qi, C. Molecular characterization and detection of a spontaneous mutation conferring imidazolinone resistance in rapeseed and its application in hybrid rapeseed production. Mol. Breed. 2015, 35, 46. [Google Scholar] [CrossRef]
- Lu, A.X.; Zhao, Y.L.; Wang, J.H. Distribution characteristics of nitrogen and phosphorus in agricultural soil profiles under different landuse. Ecol. Sin. 2007, 27, 3920–3929. [Google Scholar]



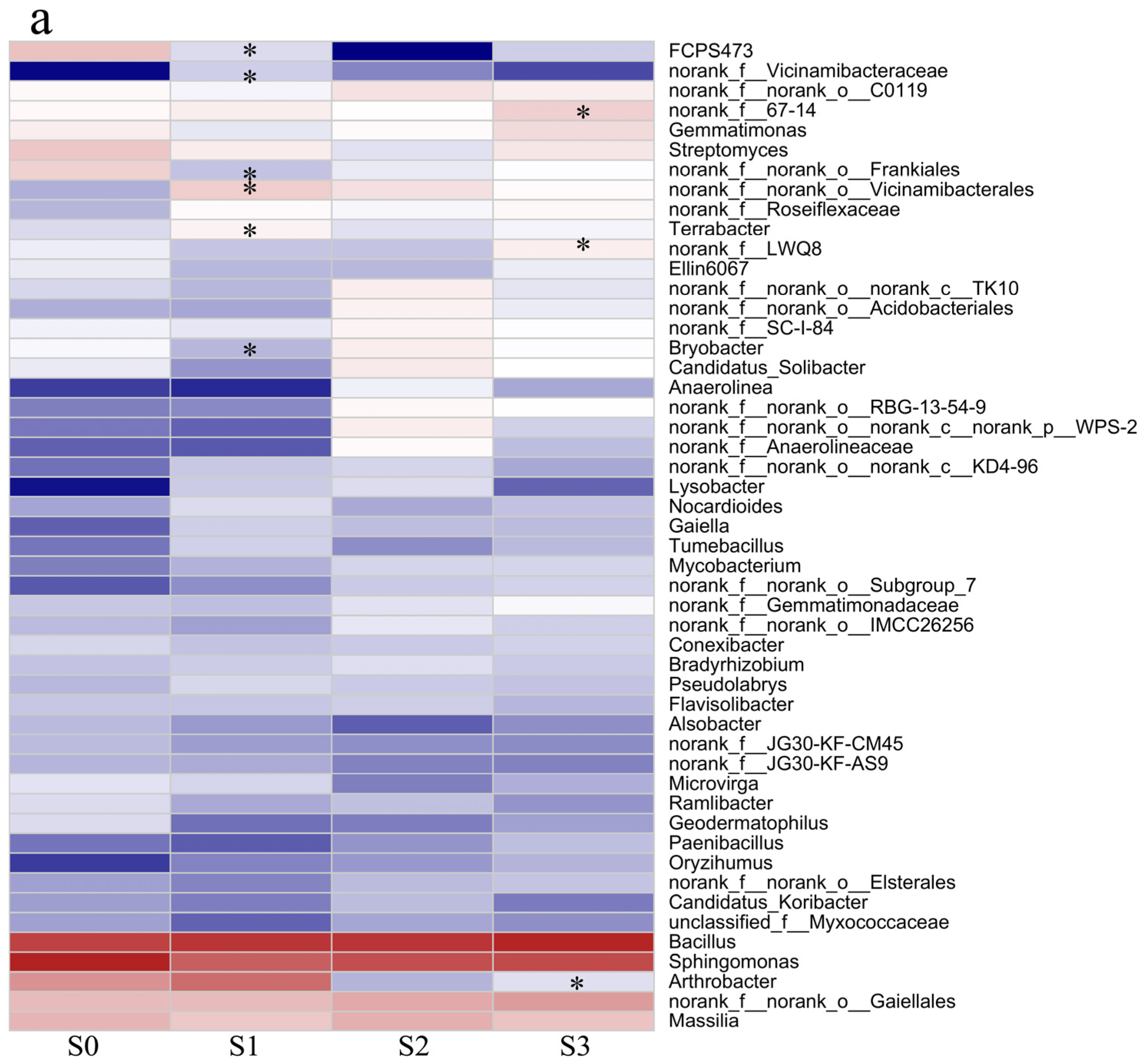
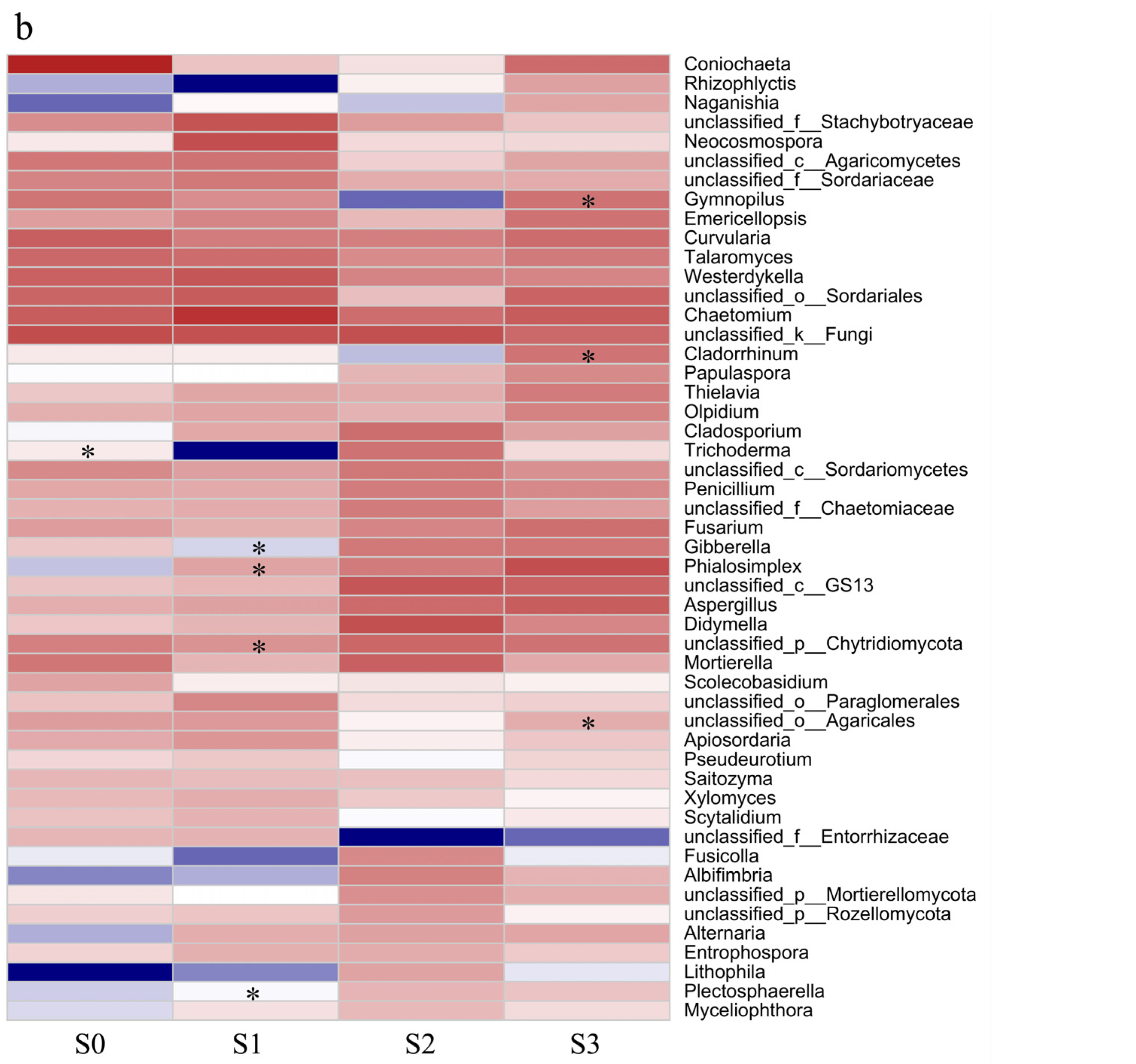
| Group | ACE | Chao | Sobs | Shannon | Coverage | Simpson |
|---|---|---|---|---|---|---|
| S0-G | 3027.623 ± 158.455 a | 2898.133 ± 130.398 a | 2537.333 ± 172.468 a | 6.002 ± 0.270 a | 0.980 ± 0.002 a | 0.0193 ± 0.006 a |
| S1-G | 2804.977 ± 83.162 a | 2701.364 ± 112.107 a | 2452.667 ± 27.501 a | 5.775 ± 0.115 a | 0.980 ± 0.001 a | 0.0190 ± 0.002 a |
| S2-G | 2899.503 ± 195.424 a | 2694.368 ± 166.002 a | 2375.000 ± 158.300 a | 5.820 ± 0.221 a | 0.981 ± 0.002 a | 0.0195 ± 0.004 a |
| S3-G | 2795.756 ± 90.496 a | 2799.957 ± 81.468 a | 2540.667 ± 117.194 a | 5.953 ± 0.175 a | 0.981 ± 0.001 a | 0.0197 ± 0.008 a |
| S0-F | 495.032 ± 130.266 a | 516.870 ± 136.257 a | 588.333 ± 122.083 a | 4.152 ± 0.258 a | 0.997 ± 0.001 a | 0.017 ± 0.009 a |
| S1-F | 448.733 ± 14.431 a | 353.795 ± 9.783 b | 517.667 ± 10.066 a | 3.487 ± 0.123 b | 0.998 ± 0.000 a | 0.014 ± 0.008 a |
| S2-F | 491.875 ± 51.733 a | 494.438 ± 49.539 a | 551.333 ± 57.813 a | 3.994 ± 0.074 a | 0.998 ± 0.001 a | 0.017 ± 0.008 a |
| S3-F | 435.564 ± 82.577 a | 443.067 ± 77.106 a | 506.333 ± 79.475 a | 3.891 ± 0.071 a | 0.998 ± 0.000 a | 0.018 ± 0.005 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Xiong, T.; Lu, W.; Zhao, J.; Zhang, Z.; Xiao, G. The ptxD Gene Confers Rapeseed the Ability to Utilize Phosphite and a Competitive Advantage against Weeds. Agronomy 2024, 14, 727. https://doi.org/10.3390/agronomy14040727
Xu D, Xiong T, Lu W, Zhao J, Zhang Z, Xiao G. The ptxD Gene Confers Rapeseed the Ability to Utilize Phosphite and a Competitive Advantage against Weeds. Agronomy. 2024; 14(4):727. https://doi.org/10.3390/agronomy14040727
Chicago/Turabian StyleXu, Dinghui, Teng Xiong, Wenbin Lu, Jinsheng Zhao, Zhenqian Zhang, and Gang Xiao. 2024. "The ptxD Gene Confers Rapeseed the Ability to Utilize Phosphite and a Competitive Advantage against Weeds" Agronomy 14, no. 4: 727. https://doi.org/10.3390/agronomy14040727





