Abstract
Corn (Zea mays L.) is an important cereal for food sovereignty, extensively planted due to its adaptation to various agroecological conditions. Climatic conditions and pests can affect its production. Concerning the latter, natural enemies could be considered in biological control programs. The objective of this study was to estimate the influence of the presence of insects, damage to plants and cobs, and their incidence on corn yield in two planting seasons. During the rainy (February to May) and dry seasons (Jun to October) of the year 2023, in Calceta, Lodana, and Quevedo, three important corn-growing areas on the Ecuadorian coast, corn plots of 1126 m2 were planted. The diversity of phytophagous and natural enemies was estimated. Damage to young plants and cobs was observed, and corn yield was determined. Of the 9073 insects observed, 44.2% and 55.8% constituted phytophagous and natural enemies, respectively. A moderate diversity (Shannon-H Index = 2.474–2.629 and Margalef Index = 2.734–3.110) of insects associated with corn was determined. Yield (range: 6.9 to 15.3 t) was negatively correlated with rainfall and cob damage (p < 0.05). Although precipitation is necessary for planting in rainy season, frequent and intense rains could be affect corn yield.
1. Introduction
Corn (Zea mays L.) (Poaceae) is one of the most widespread and cultivated cereals in the world because it represents an important source of energy for human consumption [1,2]. By the year 2022, production was estimated at 1163 million tons from 203.4 million hectares [3]. In 2021, corn ranked 86th among the most traded products in the world [4]. The main producing countries are the United States, China, Brazil, the European Union, Argentina, India, Ukraine, Mexico, Russia, and South Africa [1,5]. In Ecuador, corn is grown in all provinces, with a production of 1,736,397 tons harvested from 416,091 hectares, mainly planted in Los Ríos and Manabí, provinces that account for 70% of national production [6].
Although this crop adapts to a variety of agroecological conditions that favor its cultivation [2], yields can be limited by specific climatic conditions [7] such as drought and extreme temperatures [8]. In Ecuador, corn-producing provinces are affected by climatic conditions, which, coupled with climate change, have led to a decrease in crop productivity, with Manabí being one of the provinces showing low yields apparently related to temperature increases [9].
Issues caused by weeds, diseases, and pests also affect corn cultivation [10]. Among these, damage caused by pests can occur throughout the corn’s phenological cycle. The fall armyworm (FAW), Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae), can attack any part of the plant, but the most damage has been recorded in the whorl of young plants [11]. Attacking the foliage, other important pests include sap-sucking insects such as the western flower thrips, Frankliniella occidentalis Pergande (Thysanoptera: Thripidae), corn aphid, Rhopalosiphum maidis Fitch (Hemiptera: Aphididae), corn leafhopper, Peregrinus maidis (Ashmead) (Hemiptera: Delphacidae), and corn leafhopper, Dalbulus maidis (De Long & Wolcott) (Hemiptera: Cicadellidae) [12,13]. Chewing pests also cause damage to foliage and roots, with notable species of leaf beetles (Coleoptera: Chrysomelidae) and grasshoppers (Orthoptera) [12,14]. The cob is attacked by corn earworms, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) [15], and by corn ear fly, Euxesta spp. (Diptera: Ulidiidae) [16], whose presence has recently become much more noticeable in cornfields in Ecuador.
On the other hand, associated with these pests is a diversity of natural enemies including predators, entomopathogens, and parasitoids. In Ecuador, 33 taxa of parasitoids attacking corn pests have been reported [17]. Centeno-Parrales et al. [18] reported nine taxa of natural enemies derived from the trophic chain starting from the phytophagous R. maidis. A study by Hernández-Trejo et al. [10] mentioned about ten taxa of predators, parasitoids, and entomopathogens attacking corn pests. Among the main natural enemies of corn pests, the predatory families Carabidae [19], Cocinellidae (Coleoptera), Syrphidae (Diptera), Pentatomidae, Reduviidae (Hemiptera), Chrysopidae (Neuroptera) [10,12] have been mentioned, while among the parasitoids, Tachinidae, Sarcophagidae (Diptera) [12,19], Braconidae, Icheumonidae, and Platygasteridae (Hymenoptera) [10,12] stand out, among others.
This suggests the potential of natural enemies in biological control programs that could reduce corn pests with lower environmental impact [10,20]. Unfortunately, ignoring them has largely caused ecological and economic disasters in the world in the context of agricultural pest management, which has sometimes led to crises since the second half of the last century [21].
Knowledge of arthropods associated with a crop is the first step towards rationalizing entomological problems within the framework of Integrated Pest Management. For phytophagous arthropods, it is important to determine the influence of biotic factors and climatic variables that condition their population fluctuations and consequent levels of damage. On the other hand, the diagnosis of natural enemies of corn phytophagous arthropods is fundamental to establish the role they could play in regulating populations of the organisms they consume [10,20,22].
This research aimed to study the entomofauna associated with corn to estimate the diversity and abundance of phytophagous insects as well as natural enemies (parasitoids and predators) for considering their potential use in biological control programs. Additionally, it sought to establish the importance of the damage caused by some insects on corn production in three important planting areas along the Ecuadorian coast according to the planting season.
2. Materials and Methods
2.1. Location and Study Period
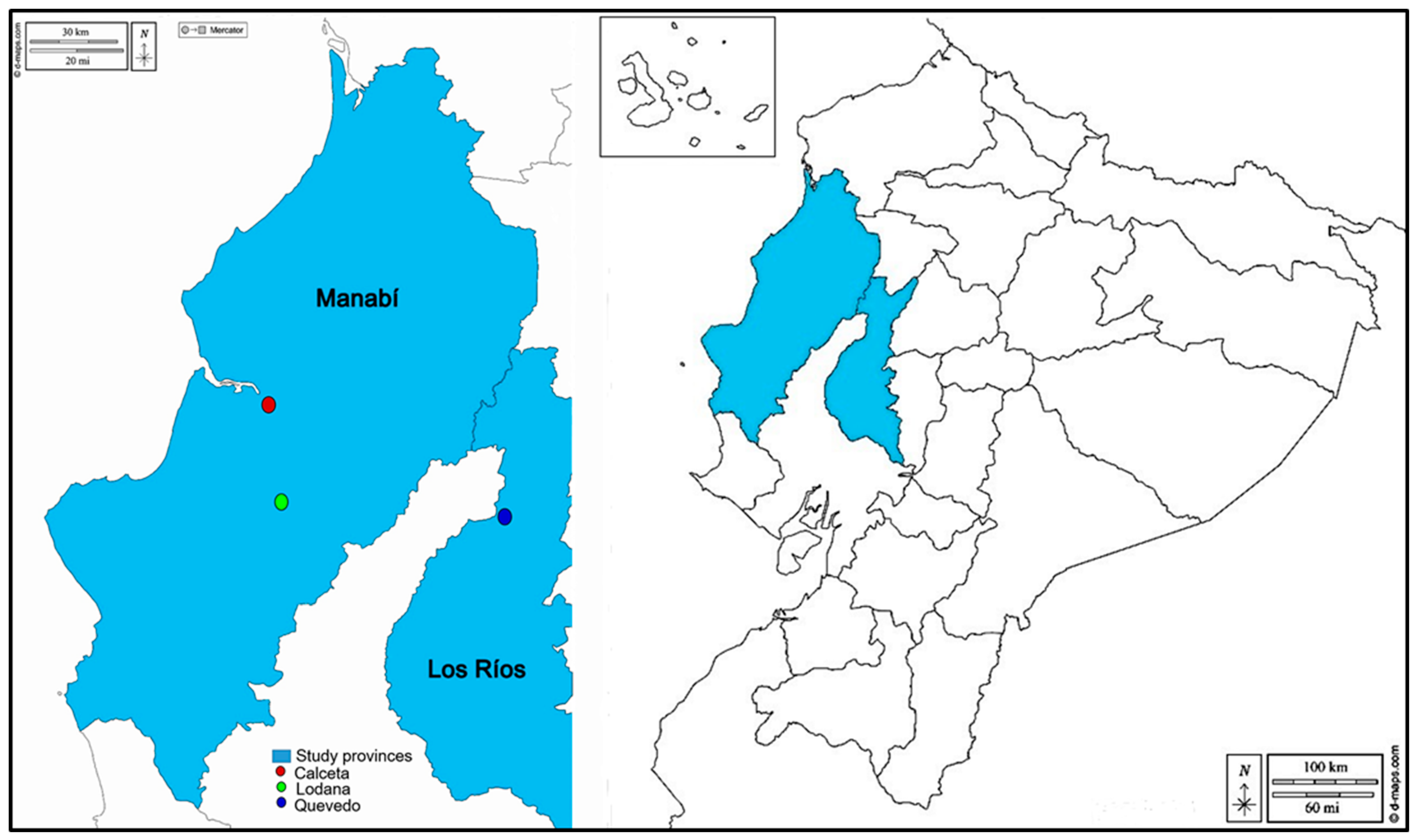
During the months of February to October 2023, two corn plots (the rainy season and the dry season) were planted in the producing distinct areas, Calceta (coordinates: 0°49′23″ S; 80°11′01″ W; altitude, 15 m), Lodana (coordinates 01°09′51″ S and 80°23′24″ W, altitude 60 m) (Manabí province), and Quevedo (1°10′37″ S and 79°29′39″ W; altitude; 80 m) (Los Ríos province) whose locations are shown on the map in Figure 1. It is worth noting that in the three sites where the corn plots were planted, they constitute experimental areas where, in addition to corn, other short-cycle crops such as peppers, tomatoes, melons, and beans are also planted in the surrounding areas.
Figure 1.
Map of Ecuador showing provinces and studies areas.
The Ecuadorian coast is characterized by having two well-defined climatic seasons, a rainy season from December to May and a dry season from June to November [23]. According to the life zones established by Holdridge, Calceta and Lodana belong to a tropical dry forest while Quevedo corresponds to a tropical humid forest [24]. Precipitation, relative humidity (RH, %) and temperature (°C) were obtained from the meteorological stations of the National Institute of Meteorology and Hydrology of Ecuador (INAMHI, https://www.inamhi.gob.ec/ (accessed on 15 February 2024)), for Calceta (code: M1230), Lodana (code: M1208), and Quevedo (code: M006) (Figure S1).
Calceta, Lodana and Quevedo are rural localities whose main economic activity is agriculture. Corn is cultivated throughout the year, with one cycle being planted at the beginning of the rainy season (January–February) and the other during the dry season (June). In Calceta, corn is the second most planted crop (28.57%) after rice, with cassava, cacao, coffee, and banana also being cultivated [25]. In Lodana, corn is the main crop, with additional plantations of peanuts, watermelon, and peppers, among others [26]. Finally, in Quevedo, corn is the second most planted crop (18.57%) after cacao, with banana, cassava, and passion fruit also being grown [27].
2.2. Management of Corn Plots
Plots of approximately 1126 m2 (70 × 16 m) were planted with hybrid corn ADV 9139®; in each of the studied locations during the two seasons of the year (Figure S2). The plots were planted at a distance of 0.8 m between rows and 0.20 m between plants for a density of 62,500 plants per hectare. The vegetation surrounding the plots was removed (Figure S2). Each plot was divided into four blocks separated by 2 m. Within each block, four plots were delimited randomly in different locations, including eight rows of four meters long where the respective evaluations were carried out. All cultural practices were applied to the plots for optimal corn development and production, except for insecticide sprays which were suspended to observe, without interference from these agrochemicals, the diversity of associated insects, the magnitude of damage from some pests, and the yields achieved. For fertilization, three applications were made: the first, five days after plant emergence (DDE), applying 30 kg/ha of a complete N-P-K formula (12-24-12).
The second application was carried out 20 days after emergence (DDE) with 25.5 kg/ha of CH4N2O; a third application was made with a mixture of 6.2 kg/ha of KCL, 12.5 kg of (NH4)2SO4, and 25.5 kg of CH4N2O. Subsequently, at 46 DDE, a concentrated liquid solution containing N (6.5%), K2O (2.7%), B (1.3%), and Zn (1.3%) at a dose of 1 L/ha was applied. Finally, at 51 DDE, another concentrated solution was applied at a dose of 2.5 L/ha, which included N (10%), P2O5 (4%), K2O (7%), B (0.02%), Zn (0.07%), Mn (0.13%), and Mo (0.003%). For weed control, the plots were treated with a pre-emergent herbicide, mesotrione, at a dose of 0.45 L/ha, and then weed control was done mechanically at 20, 50, 75, and 100 DDE. During the rainy season, fungicides based on a mixture of azoxystrobin + difenoconazole at a dose of 0.4 L/ha and a mixture of propiconazole + difenoconazole were sprayed at 51 DDE at a dose of 0.20 L/ha. Irrigation was applied through a drip system three times a week in the dry season in Calceta and Lodana, while no irrigation was carried out in Quevedo in either season.
2.3. Field and Laboratory Evaluations
2.3.1. Associated Entomofauna
For the study of the entomofauna, field and laboratory observations were conducted to detect the trophic relationships of insects with corn. Yellow traps were also set up to quantify the abundance. Field and laboratory observations began one week after germination and continued throughout the crop cycle, examining insects feeding on corn and the natural enemies attacking phytophagous insects (Figure S3A–F). Information was recorded, and insect samples were placed in jars with 70% ethyl alcohol (Figure S3C,D). In the laboratory, specimens were diagnosed and preserved, with some stored in alcohol and others mounted with entomological pins for identification (Figure S3G,H).
The placement of the yellow traps (21 × 29.7 cm) began one month after germination (Figure S4) (one per block). The traps were attached to the plants in the central rows using thin galvanized wire (Figure S4C). Every 15 days, the traps were changed during the crop cycle for each study area. The traps were removed and taken to the laboratory to be observed under a Stemi DV4 manufactured by Carl Zeiss®, Göttingen, Germany stereomicroscope (magnification: 8–32×) (Figure S4D–I) in order to count the taxa of phytophagous and natural enemies previously established in field and laboratory observations.
To calculate the diversity indices, the insects were identified to Family, with the exception of spiders, which were identified up to Order. Subsequently, within each family, the insects were diagnosed to the highest identifiable taxon (is a taxonomic group of any rank, such as a species, family, or class) possible (family, subfamily, genus, or species) by comparison with specimens in the entomological collection of the Ecuadorian Agency for Quality Assurance of Agriculture (Agrocalidad). The evaluation of the diversity of the entomofauna aimed to detect abundance, especially of natural enemies, to consider those that could potentially be used in biological control programs. It is important to note that soil-dwelling insects were not evaluated in this study.
2.3.2. Damage to Plants by Spodoptera frugiperda
Damage by S. frugiperda on young plants of corn (from the first days of plant emergence to seven weeks) were evaluated in the fields. These observations began approximately five days after plant emergence, coinciding with the onset of FAW attack. To assess the damage, ten plants from the central rows of each replicate were randomly selected, and damage was recorded when the whorl was damaged with the presence of the larva or fresh excrement (Figure S5). These observations were conducted weekly for seven weeks, evaluating a total of 40 plants in each assessment. This was used to calculate the percentage of plants damaged by FAW.
2.3.3. Observations of Predation and Parasitism on Spodoptera frugiperda
In the fields, it were observed if there was predation on any of the stages of FAW. To observe possible parasitism, egg masses, larvae, and pupae of FAW were collected in the fields and taken to the laboratory. The masses were placed in Petri dishes containing moistened paper towel to prevent desiccation and were observed daily to determine the number of FAW larvae or the number of parasitoids if they emerged from the eggs (Figure S6A). FAW larvae and pupae were placed in transparent plastic containers (5 × 5 cm, height × diameter) containing moistened paper towel, and the same procedure was followed to record if they were parasitized (Figure S6B–E). For each stage, a total of approximately 100 egg masses, larvae, or pupae were collected in the first five weeks of each crop cycle per zone and season studied.
2.3.4. Cob Damage
Regarding cob damage, 20 cob from the plants in the six central rows were randomly selected from each block, and the damage by insects attacking this organ was observed (Figure S7). Each damaged cob with the presence of worms or fresh excrement was recorded. This was used to calculate the percentage of cobs damaged in a similar manner to the assessment done for plants damaged by S. frugiperda.
2.3.5. Yield
The cobs of all plants were harvested from the six central rows, for which the following were recorded: cob length (CL, cm), cob diameter (CD, cm), number of rows (NR), and cob weight (CW, g). Subsequently, the cobs were shelled, and the yield of dry grain (g) (13% moisture and 1% impurities) per plot was determined, and the grain yield (GY) in tons per hectare was estimated. The Shelling index or the rate of GY relative to the total CW was also calculated.
2.4. Data Analysis
Diversity indices, Shannon-H Index, Margalef Index, as well as Dominance Index D, the number of observed taxa per locality, and Chao 1 Index were calculated. An analysis was also conducted to associate the abundance of taxa and crops per zone using the IndVal (%) value to determine the indicator taxa for each zone (p < 0.05). To compare the zones including seasons and sampling, similarity was analyzed using the Bray-Curtis similarity index. These analyses were carried out using the Past version 4.12 software [28].
Canonical analysis was performed between the present insects and zones per season. Yield component variables were analyzed through a two-way analysis between zones (3) and seasons (2), and the means were compared using the Tukey test (p < 0.05). Prior to the analysis of variance, the normality of the residuals was analyzed using the Shapiro-Wilk test (p < 0.05), and the homogeneity of variances was assessed using the Bartlett test. A Spearman correlation analysis was conducted between phytophagous families, rainfall, temperature, relative humidity, plant damage, cob damage and estimated yield per hectare (p < 0.05). Quadratic regression equations were estimated between yield and rainfall and cob damage (p < 0.05). These analyses were carried out using the R Development Core Team software [29].
3. Results
3.1. Associated Entomofauna
Some of the phytophagous insects and natural enemies were photographed in the cornfields and are shown in Figure 2 and Figure 3. Field and laboratory observations confirmed the association of the taxa counted in the traps with the corn food chain. In the traps, 9073 individuals were observed, of which 44.2% were phytophagous and 55.8% belonged to natural enemy taxa (Table 1). However, by zones, Calceta had the highest phytophagous rate at 52.1%, while Lodana and Quevedo had 44.6% and 40.4% phytophagous taxa, respectively.

Figure 2.
On corn leaves. (A): chrysomelid adult, (B): couple of chrysomelids copulating, (C): coccinellid in a colony of Rhopalosiphum maidis, (D): couple of coccinellids copulating.

Figure 3.
On corn leaves. (A): reduvid bugs adult, (B,C): reduvid bugs adult preying, (D): braconid adult, (E): Ichneumonid adult, (F): coccinelid adult.

Table 1.
Number of individuals by ecological guild per season for each area studied. Period February–October 2023.
These individuals are included in 25 arthropod taxa, eight of which are phytophagous insect families, and 17 taxa are natural enemies, distributed among 11 predator taxa and 6 parasitoid taxa (Table 2).

Table 2.
Arthropod taxa by ecological guild in the three study areas, Calceta, Lodana and Quevedo. Period February–October 2023.
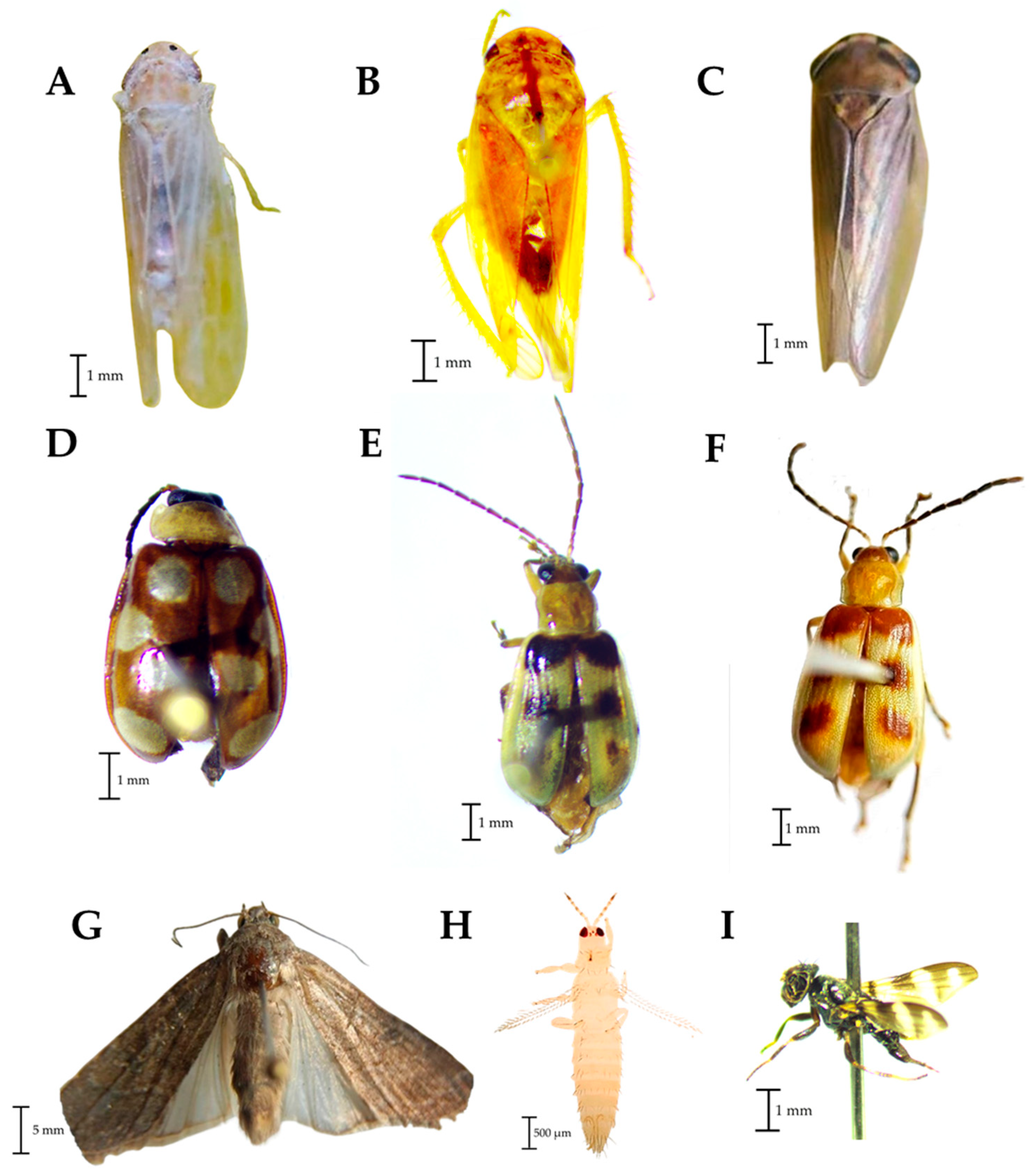
Rophalosiphum maidis was the aphid species detected in Aphididae (Figure 2C). In the family Cicadellidae, three taxa were also detected, one corresponding to D. maidis (Figure 4A) and the other two only identified up to family level (Figure 4B,C). In the family Chyrsomelidae, three taxa were found, one belonging to the genus Omophoita (Figure 4D) and the other two taxa probably belonging to Diabrotica (Figure 4E,F). In the Noctuidae family, the traps captured adults of S. frugiperda (Figure 4G) while in the Thripidae family the species corresponds to F. occidentalis (Figure 4H). Finally, in the family Ulidiidae the species Euxesta mazorca Steyskal was diagnosed (Figure 4I). Unidentified species of the families Acrididae and Tettigoniidae were captured in the traps and observed in the corn plots feeding on leaves of young plants (between a week and a month after germination).
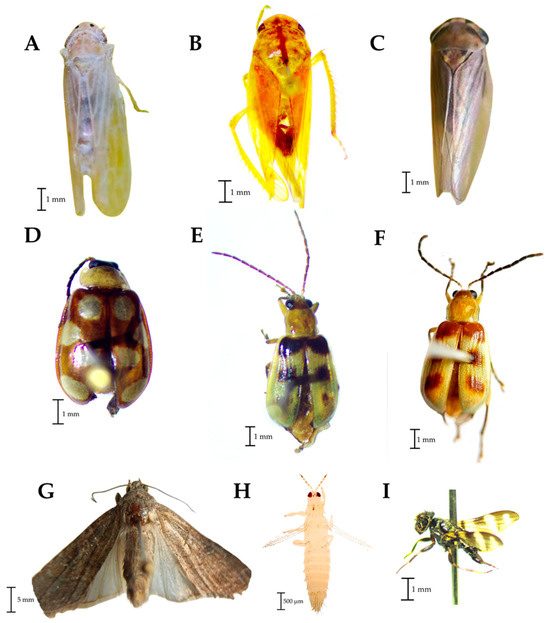
Figure 4.
Families of phytophagous found in traps placed in corn plots. (A–C): Cicadellidae, (D–F): Chrysomelidae; (G): Noctuidae, (H): Thripidae, (I): Ulidiidae.
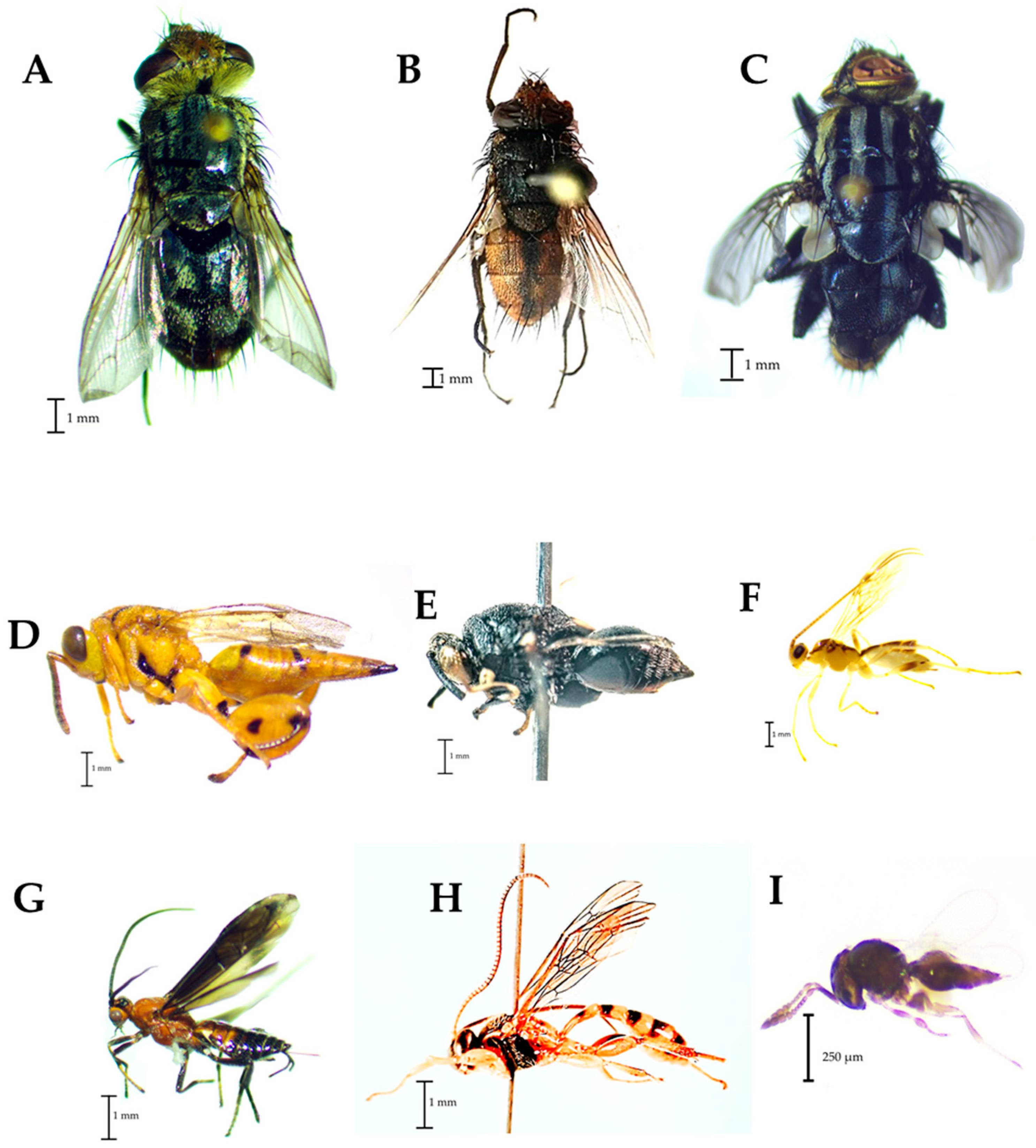
Among the parasitoids, the identified tachinids were Archytas marmoratus (Townsend) (Figure 5A) and Lespesia sp. (Figure 5B), while in the Sarcophagidae family, Sarcophaga sp. was the identified taxon (Figure 5C). Conura sp. (Figure 5D) and Brachymeria sp. (Figure 5E) were the diagnosed taxa in the Chalcididae family. In the superfamily Ichneumonoidea, the observed taxa were Meteorus sp. (Figure 5F) and Rogadinae (Figure 5G) in the Braconidae family, and Metopiinae was the taxon of the Ichneumonidae family (Figure 5H). Finally, Telenomus sp. was the taxon found in the Platygasteridae family (Figure 5I).
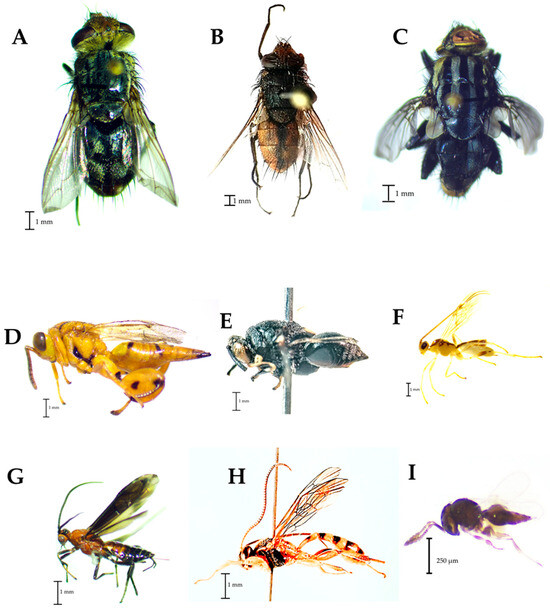
Figure 5.
Families of parasitoids found in traps placed in corn plots. (A,B): Tachinidae, (C): Sarcophagidae, (D,E): Chalcididae, (F,G): Braconidae; (H): Ichneumonidae, (I): Platygasteridae.
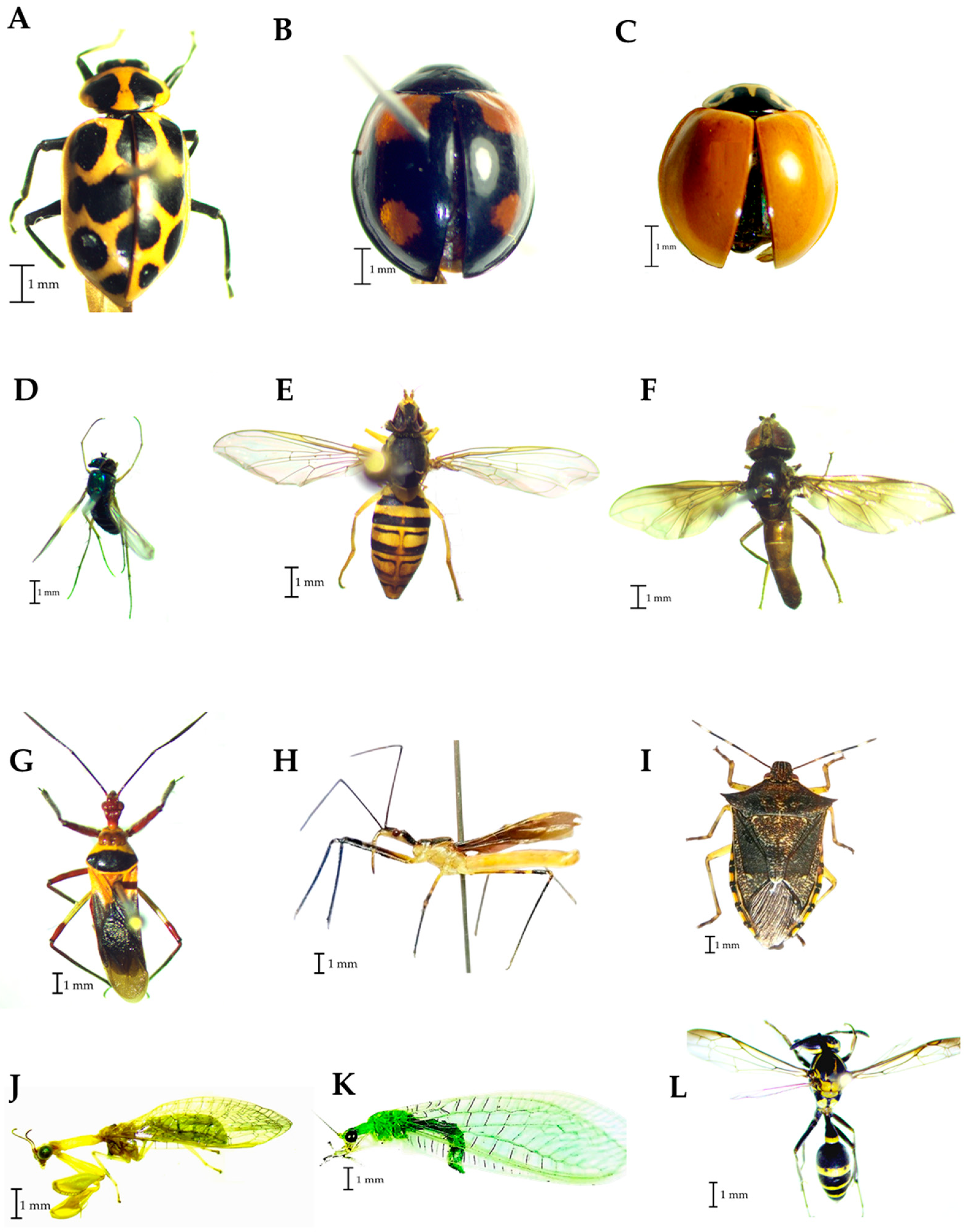
Regarding predators, three species of Coccinellidae were identified, Coleomegilla maculata (De-Gueer) (Figure 6A), Cheilomenes sexmaculata Fabricius (Figure 6B), and Cycloneda sanguinea Linnaeus (Figure 6C). Condylostylus sp. (Figure 6D) is the taxon detected belonging to the Dolichopodidae family, while the species Toxomerus politus Say (Figure 6E) and Ocyptamus dimidiatus (Fabricius) (Figure 6F) were identified in the Syrphidae family. Zelus spp. (Figure 6G,H) was the taxon identified in the Reduviidae family. Podisus sp. was determined in the Pentatomidae family (Figure 6I). Two taxa were identified at the family level in the orders Neuroptera, Mantispidae (Figure 6J) and Chrysopidae (Figure 6K), and Polybia occidentalis Olivier (Figure 6L) was the Vespidae species found in the traps.
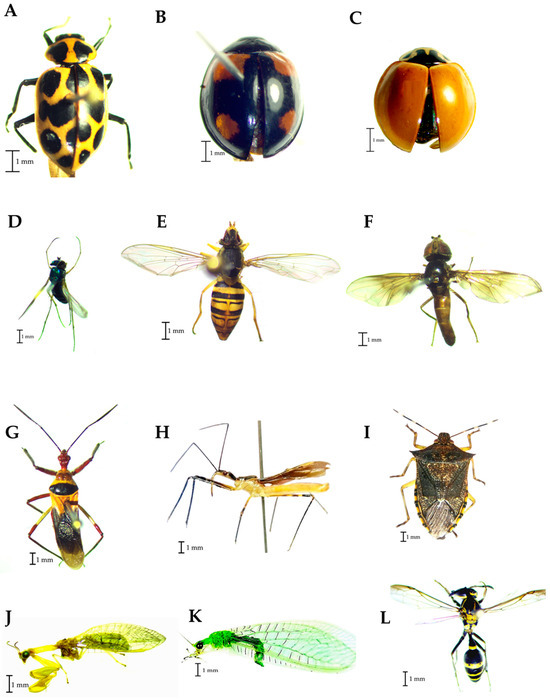
Figure 6.
Families of predators found in traps placed in corn plots. (A–C): Coccinellidae, (D): Dolichopididae, (E,F): Syrphidae; (G,H): Reduviidae, (I): Pentatomidae, (J): Mantispidae, (K): Chrysopidae, (L): Vespidae.
Calceta, Lodana, and Quevedo contributed 23.9%, 24.7%, and 51.4%, respectively, of the insects found. The number of diagnosed taxa was lower in Quevedo compared to Calceta and Lodana; the highest number of taxa was observed in the latter zone (Table 3). Shannon-H Index (2.474–2.629) and Margalef Index (2.734–3.110) estimated moderate diversity in all zones, while the Dominance D Index indicated that no taxon dominated over the rest (Table 3). Chao-1 Index suggests appropriate sampling efforts because the observed taxa are equal to the expected taxa per zone.

Table 3.
Estimated diversity indices for the three areas studied, Calceta, Lodana and Quevedo.
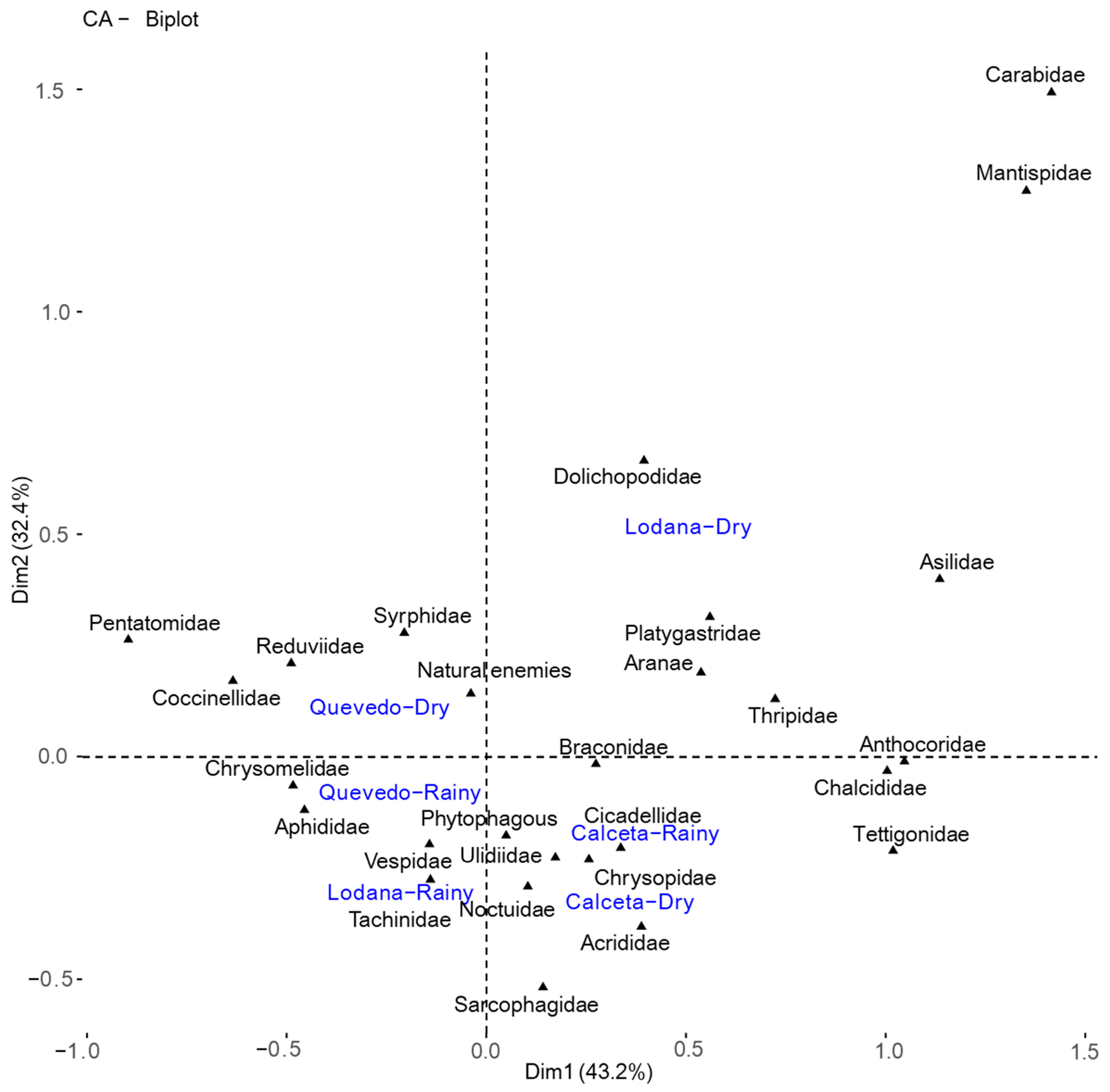
The multivariate canonical correlation analysis carried out to establish the interrelationship between the insect families and the study areas according to the planting season (Figure 7) suggests that the rainy and dry seasons in Calceta shared the same taxa, with a higher number of phytophagous, as well as a predator taxon and a parasitoid. Lodana in the rainy season presented three taxa of natural enemies and a phytophagous, while in the dry season of this zone, four predator taxa, two parasitoids, and a taxon in the phytophagous guild were present. In Quevedo during the rainy season, there was a higher presence of two phytophagous taxa, a predator, and two parasitoids, and in the dry season, four predator taxa were present (Figure 7).
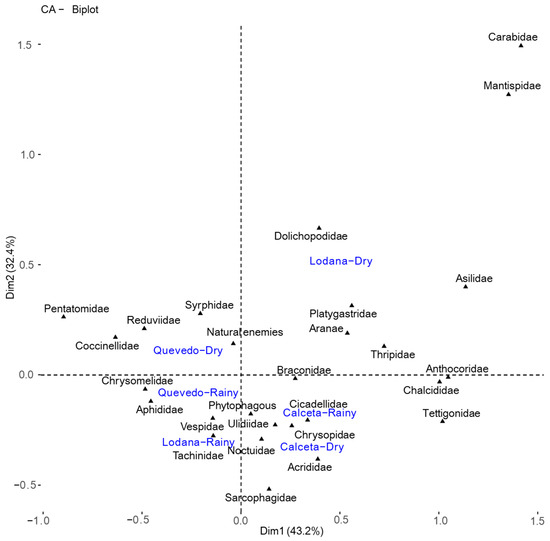
Figure 7.
Canonical analysis between phytophagous and natural enemies taxa detected for each study area in the rainy and dry seasons.
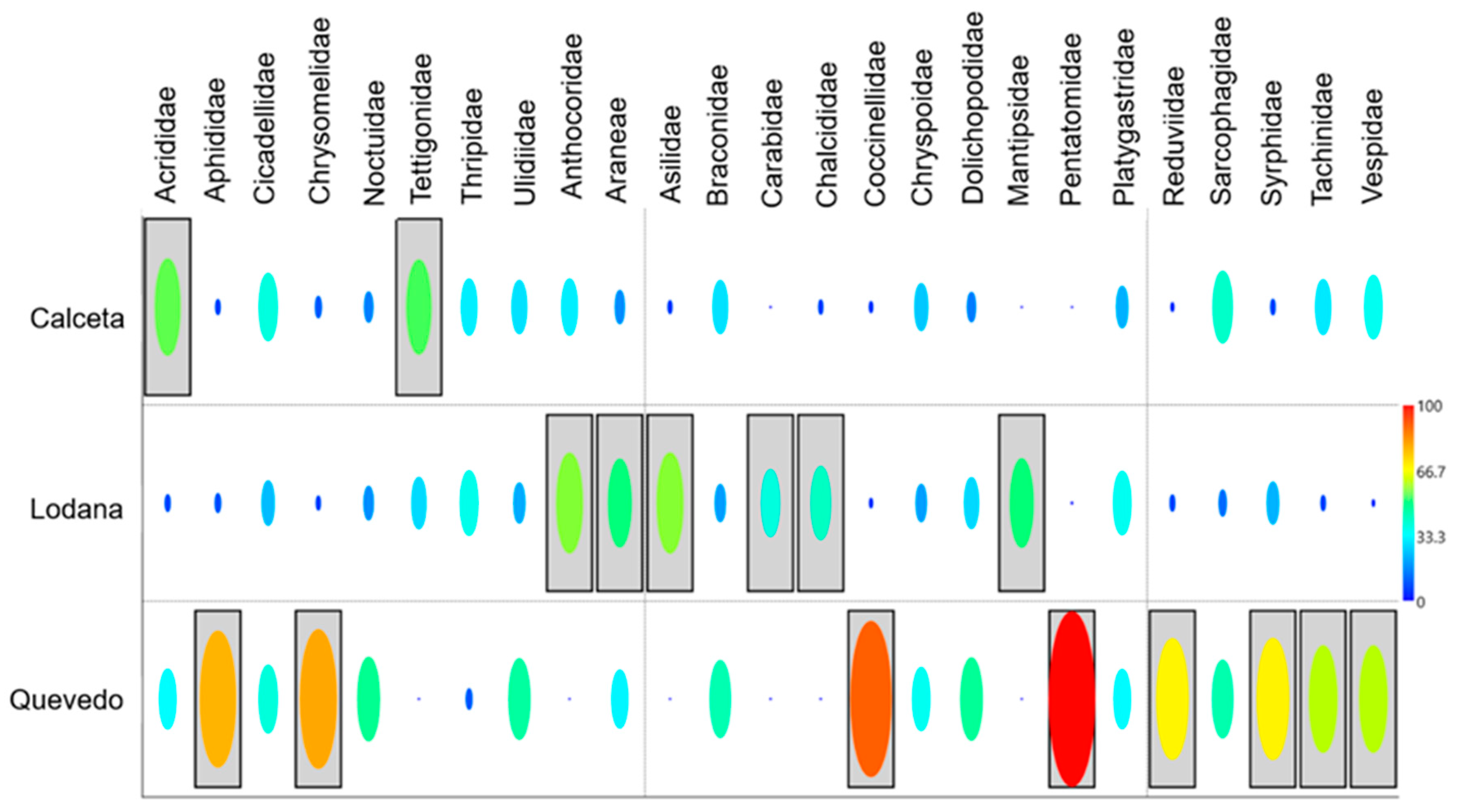
This information is complemented by the indicator species (%IndVal) that estimated the taxa with the highest preference in each zone (Figure 8). Calceta showed the lowest number of indicator taxa, corresponding to the phytophagous guild. Six taxa of natural enemies are indicators of Lodana, while Quevedo showed the highest number of indicator taxa for this zone (eight taxa, six natural enemies, and two phytophagous), where the Pentatomidae family showed the highest IndVal (98%), indicating a conspicuous preference for this zone.
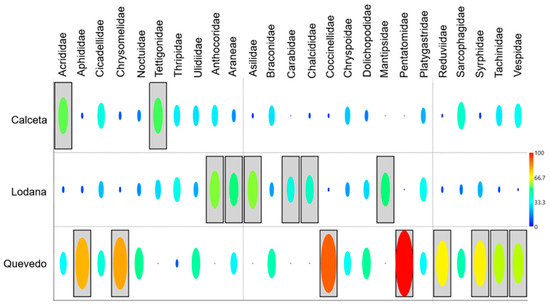
Figure 8.
Habitat preference (%IndVal) of the taxa found by study area.
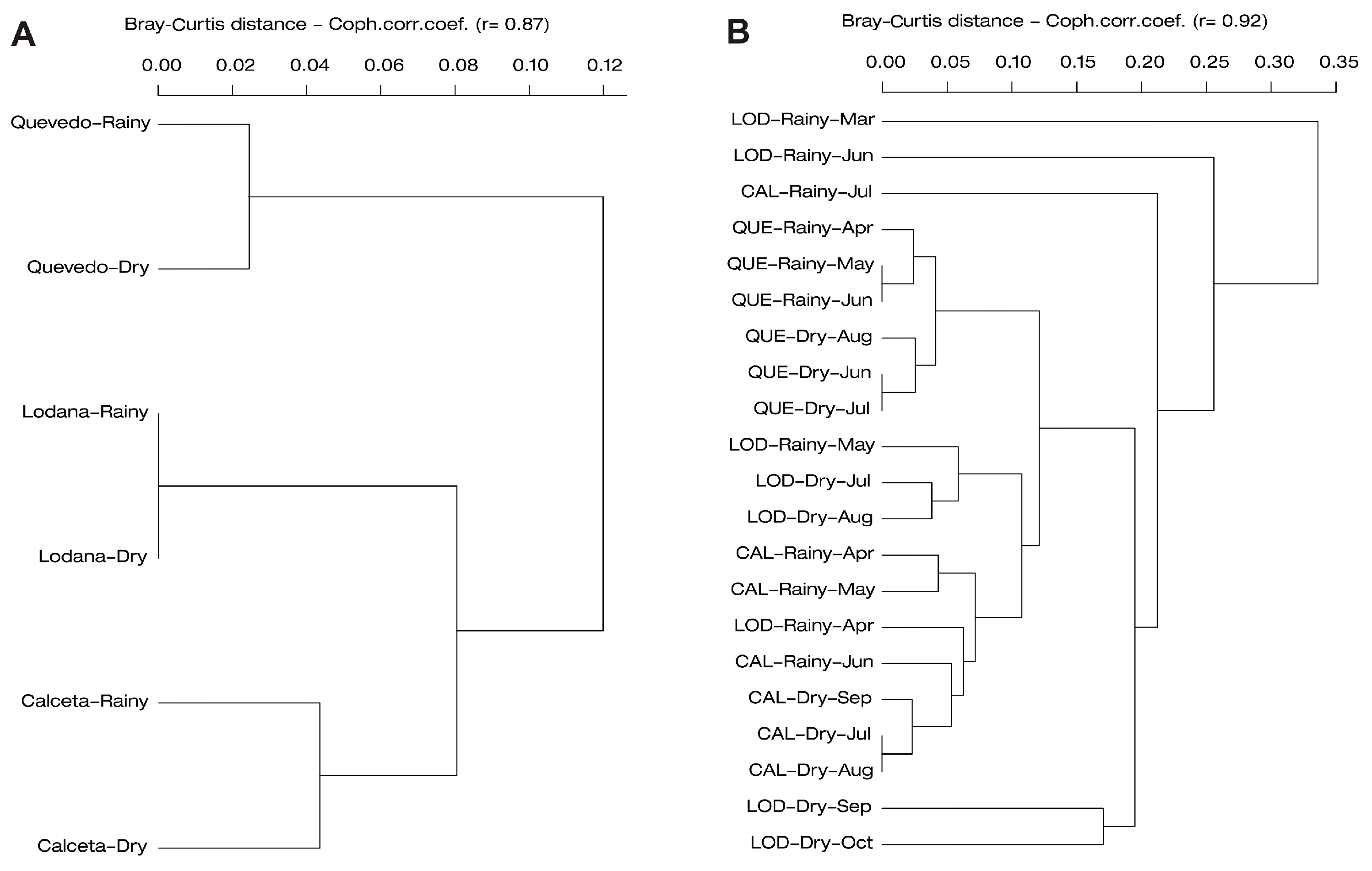
The dendrograms by area per season (Figure 9A) as well as for each sampling (Figure 9B) analyzed using the Bray-Curtis similarity index allowed the effects of the rainy and dry seasons on taxon composition to be observed. Thus, Calceta and Lodana have affinities in terms of taxon abundance and diversity in both the dry and rainy seasons, separating them from what was detected in the two seasons of Quevedo (Figure 9A). The clustering by sampling allowed the affinities in samplings by season by area to be observed (Figure 9B). In Lodana, the March sampling appears to differ, which was probably associated with the start of the counts in that area during the rainy season.
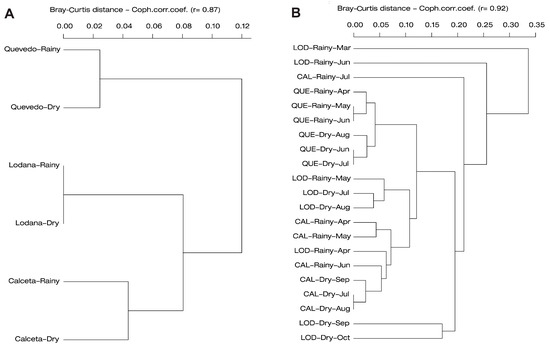
Figure 9.
Similarity dendrogram using the Bray–Curtis index showing affinities: (A) Area per planting season, (B) Area per planting season by sampling. In the three studied areas per seasons. CAL: Calceta, LOD: Lodana, QUE: Quevedo.
3.2. Plant Damage by Spodoptera frugiperda
The Tukey test did not detect significant differences for plant damage by season or for the zone by season interaction (Table 4). Differences were detected by study zone, with Calceta (p < 0.05) showing three times higher damage (70%) than that observed in Lodana and Quevedo (21–25%).

Table 4.
Analysis of variance and mean of yield variables, damage to plants and cobs. CL: Cob length (cm) was transformed by square root; CD: Cob diameter (cm); NR: Number of rows; CW: Cob weight (g); SI: Shelling index (%); GY: Grain yield (tons); CDa: Cob damage (%); PD: Plant damage (%).
3.3. Observations of Predation and Parasitism on Spodoptera frugiperda
Parasitoids belonging to the genus Telenomus (Hymenoptera: Platygastridae) emerged from S. frugiperda eggs (Figure 10A). The lowest parasitism was observed in Calceta during the rainy season and the highest in Lodana during the dry season (Table 5). Each mass consisted of approximately 150 to 200 eggs, of which not all were parasitized, with 25 to 90% of the eggs parasitized per mass being detected.

Figure 10.
Parasitism observed on the fall armyworm (FAW) Spodoptera frugiperda. (A): FAW egg mass with adults of the parasitoid Telenomus sp. (Hymenoptera: Platygastridae). (B): FAW larva with the exit hole of the parasitoid next to a pupa of Diptera: Tachinidae.

Table 5.
Percentage (%) of Spodoptera frugiperda eggs parasitized by Telenomus sp. (Hymenoptera: Platygastridae).
From parasitized larvae of S. frugiperda, adults of parasitoids from the genera Lespesia and Sarcophaga (Diptera: Tachinidae) (Figure 10B), as well as, Meteorus (Hymenoptera: Braconidae) and were obtained, while from the pupae, the species, A. marmoratus (Diptera: Tachinidae), was recovered. Parasitism in larvae and pupae ranged from 30 to 35% in all evaluated areas. Additionally, in the field, individuals of Podisus sp. (Hemiptera: Pentatomidae) were observed preying on S. frugiperda larvae. Figure 11 shows eggs and nymphs of Podisus sp. The natural enemies observed on S. frugiperda had previously been mentioned as taxa captured in the yellow traps.
Figure 11.
(A): eggs and (B): nymphs of the predator Podisus sp. (Hemiptera: Pentatomidae).
3.4. Damage on Cobs
Although the ear fly, E. mazorca, was detected in the yellow traps, no damage by this insect was observed on the cobs. The observed damage was caused by H. zea (Figure S7), which was significantly higher in the rainy season compared to the dry season (range: 9 to 21%). These damages were significant in Calceta during the rainy season (Table 4). Conversely, in the same area during the dry season, the damages only reached 4.6%.
3.5. Yield
Regarding the variables measuring yield, the Tukey test found differences only in length (CL) and cob diameter (CD) for the study areas (Table 4; p < 0.05). CL was greater in cobs harvested in Lodana and lower in those from Quevedo, while CD was significantly higher in cobs obtained from Calceta and Lodana. The NR ranged from 13.3 to 15.6, being higher in the dry season compared to the rainy season, with no significant differences between areas and in the zone by season interaction.
CW was higher in the dry season but showed no differences between areas. The zone by season interaction was significant, showing the highest values in Lodana during the dry season, with no differences from Calceta in the dry season. The lowest CW values were detected in all areas during the rainy season (Table 4). SI followed a similar pattern to CW, with no differences between areas but differences for seasons and the zone by season interaction. The dry season presented a significantly higher SI, while between areas by season, it was significantly lower in Quevedo during the dry season.
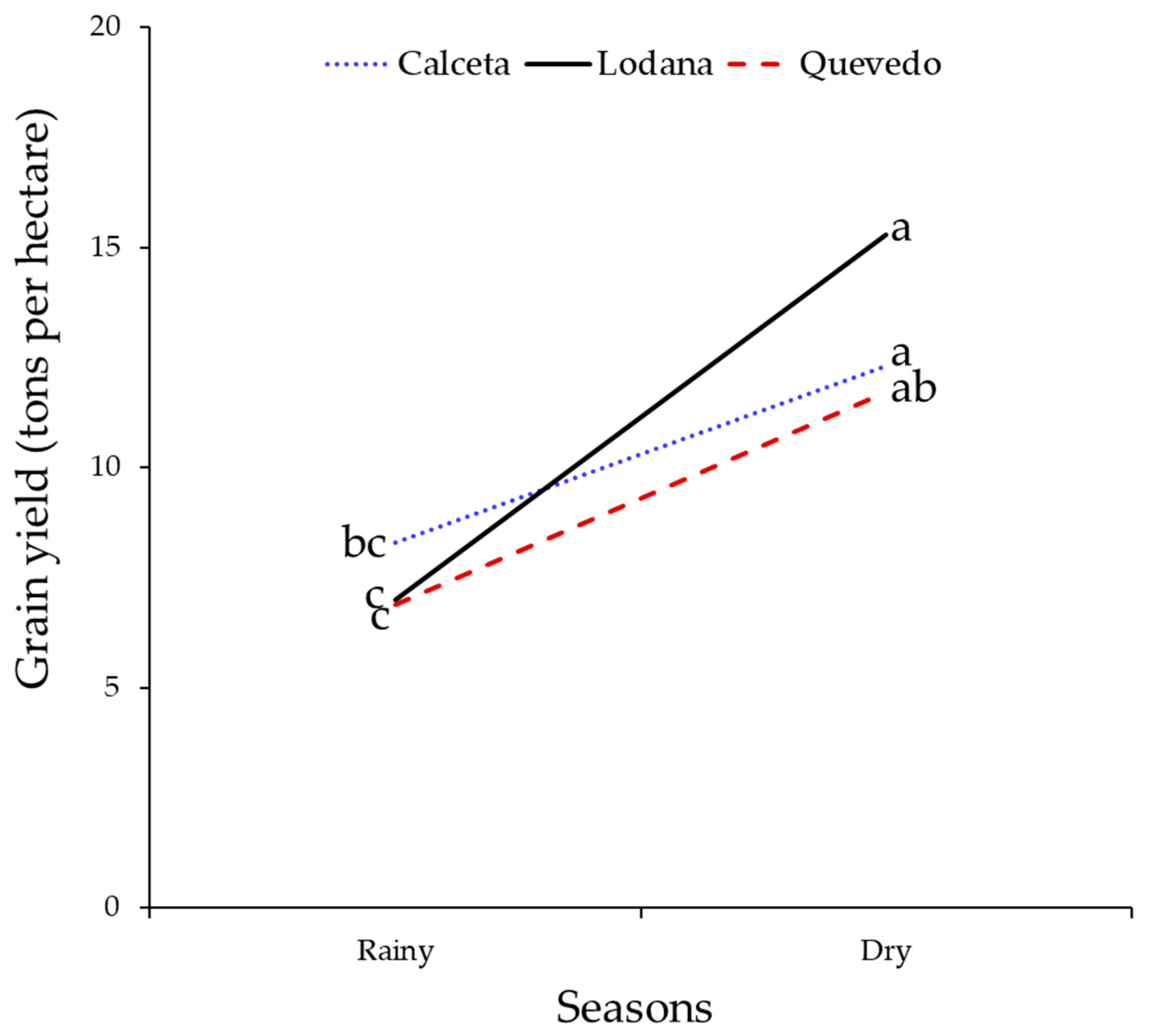
Grain yields also did not differ between areas. However, rainfall affected the yield, being higher in the dry season. These yields were higher in all areas during the dry season (Table 4, Figure 12).
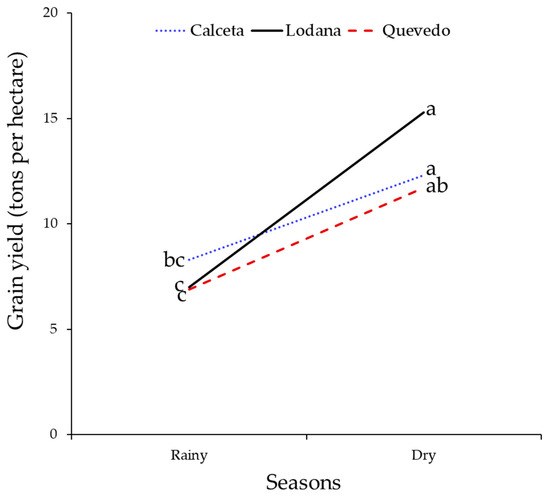
Figure 12.
Grain yield for the three study areas in the rainy and dry seasons. Means with the same letter by planting season do not differ significantly. Comparisons of means made with the test with the Tukey test (p < 0.05).
3.6. Relationship between Yield, Climatic Variables, Phytophagous Insects, and Damage
The damage to plants caused by S. frugiperda was higher in Calceta but did not differ between seasons or in the season by area interaction (Table 4). In this study, cob damage was caused by H. zea larvae, which were higher in the rainy season where Calceta showed significantly higher damages (Table 4).
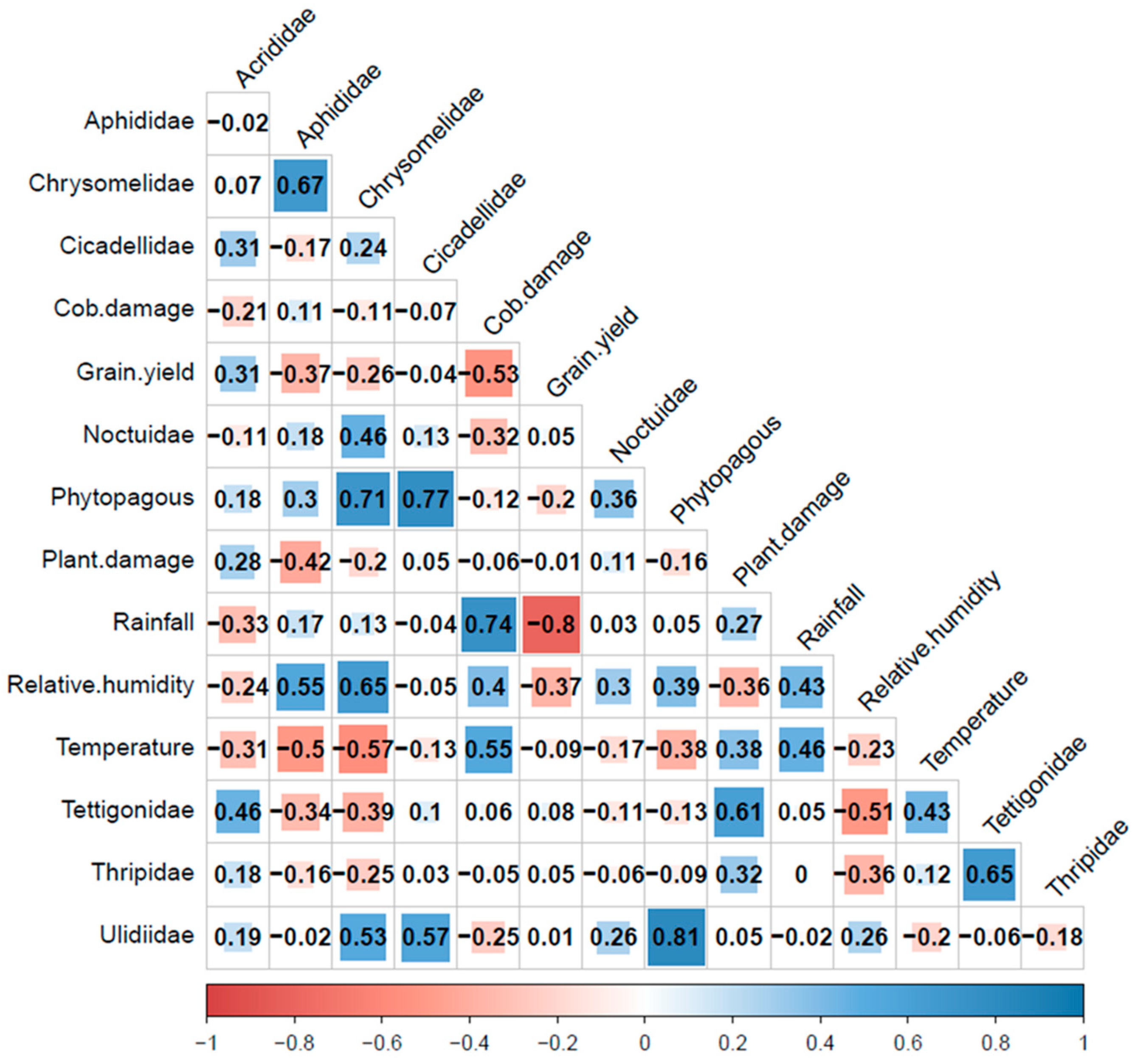
The correlation between phytophagous insects, yields, and damage (plants and cob) with climatic variables is shown in Figure 13. During the study, 1128, 1002, and 1883 phytophagous individuals were captured by the traps in Calceta, Lodana, and Quevedo, respectively. Despite this, no significant field damage caused by phytophagous families was observed except for S. frugiperda and H. zea belonging to the Noctuidae family. The traps captured some noctuids, and the abundance of these insects showed a weak negative correlation with cob damage (r: −0.32, Figure 13). This was expected because noctuids have nocturnal habits, and the chromatic traps mainly attract insects with diurnal habits. In the field, sporadic colonies of R. maidis and leaf perforations caused by chrysomelids were observed. Aphid colonies were attacked by larvae and adult predators such as ladybirds and hoverflies. The phytophagous insects were mainly composed of individuals from the Ulidiidae, Cicadellidae, and Chrysomelidae families (Figure 13). The most important findings included the high positive correlation detected between cob damage, rainfall, and temperature, along with the high negative correlation between rainfall and grain yield. Grain yield also showed a negative correlation with cob damage (Figure 13). This would indicate that corn crop production was affected by both cob damage and rainfall occurrences, as confirmed by the calculated regression equations.
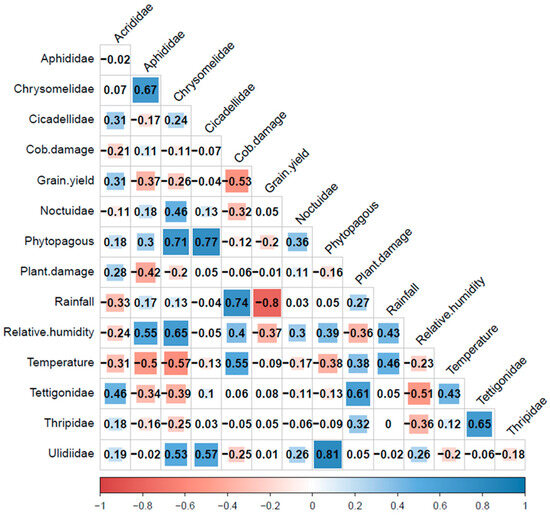
Figure 13.
Spearman correlation (p < 0.05) analysis between phytophagous insects, plant and cob damage, grain yield and climatic variables.
The quadratic regression model (3) determined with a high coefficient of determination the negative effect of rainfall on yield, while the model estimated with the damage in the cob (4) explained 41% of that variation.
where;
Y = 0.0000228819X2 − 0.085851X +15.457
X = Rainfall
Y = Grain Yield
R2 = 0.9373
p < 0.001
where;
Y = 0.013679X2 − 0.73664X + 17.282
X = Cob damage
R2 = 0.4183
p < 0.001.
4. Discussion
The results showed the highest abundance of the Cicadellidae (14.5%), Ulidiidae (13.8%), Chrysomelidae (8.3%), and Aphididae (4.9%) families. Three taxa of Cicadellidae were found, including D. maidis. Dalbulus maidis is considered an important pest affecting corn cultivation due to the transmission of various pathogens such as spiroplasmas but mainly for vectoring the Maize rayado fino virus (MRFV) [13]. This viral disease reduces corn yield and has been reported to cause losses in corn crops in the Andean region of Ecuador [30]. Although this virus has not been reported for the Ecuadorian coast, the presence of the virosis in the country, as well as the presence of its main vector, D. maidis, and other leafhoppers, make these insects potential entomological problems for corn production on the Ecuadorian coast.
Euxesta mazorca was the fly species identified in the Ulidiidae family and constitutes the second most abundant phytophagous insect in this study. In field observations, flies were detected landing on the cobs and traps also captured adults of this species. Although E. mazorca was one of the taxa detected in the phytophagous guild with the highest number of individuals captured in yellow traps, no damage was observed on the stigma, silk, or grain of the cob. This species has been reported to cause significant losses in corn production along with H. zea in other countries [16,31]. The genus Euxesta has been reported in Ecuador since two decades [32], and although adults were noticeable in corn crops, this species is not listed among the main pests in Ecuador [33]. In fact, in the two crop cycles for the three zones, we observed the presence of adults in the traps, but no larvae were detected in the cobs when they were inspected to record damage to this organ.
Chrysomelid species were abundant in this study. The importance of these beetles lies in the fact that their larvae can damage corn roots [34], and additionally, together with thrips of the Frankliniella genus (Thripidae), they can transmit the Maize chlorotic mottle virus [35]. This along with the Sugarcane mosaic virus transmitted by aphid species (Aphididae), has caused devastating epidemics in corn production in the province of Manabí in recent years [35].
A study conducted in two areas of the municipality of San José de las Lajas, Mayabeque province, Cuba, detected the phytophagous insects S. frugiperda, Diabrotica spp., and D. maidis as important corn-associated pests [36]. As for natural enemies, parasitoids belonging to the Tachinidae (6.3%), Sarcophagidae (4.1%), Platygastridae (1.6%), and Ichneumonoidea (1.1%) taxa showed the highest abundance through captures, but also, in field and laboratory studies, they were recovered as biological control agents of eggs, larvae, and pupae of S. frugiperda. Hernandez-Trejo et al. [10] emphasize the importance of species belonging to these taxa in the biological control of key pests in corn. They identified Telenomus remus Nixon (Platygastridae) and Meteorus sp. (Ichneumonoidea: Braconidae) attacking eggs and larvae of S. frugiperda, respectively. In fact, the same researchers mentioned that each corn pest could be controlled by various parasitoids or predators.
Consistent with these results, Gurrola-Pérez et al. [37] have found species of the genus Lespesia as larval parasitoids, while A. marmoratus is a larva-pupa parasitoid of S. frugiperda. For its part, Sarcophaga sp. has also been mentioned as a parasitoid of fall armyworm larvae in several Latin American countries [38]. Likewise, Santiago [19] also identified the families Ichneumonidae and Sarcophagidae as biological control agents of several Lepidoptera species attacking corn.
Regarding predators, Condylostylus sp. (Dolichopodidae, 12%), P. occidentalis (Vespidae, 6.5%), Zelus spp. (Reduviidae, 5.4%), T. politus and O. dimidiatus (Syrphidae, 5%), C. maculata, Ch. sexmaculata, and C. sanguinea (Coccinellidae, 4.5%), Podisus sp. (Pentatomidae, 3.8%), and various spider species (Araneae) were the most abundant taxa. In line with these findings, Condylostylus sp. was identified as the predator taxon with the highest number of individuals in a study conducted in Ponta Grossa, Brazil, which compared the diversity of the entomofauna associated with Bt transgenic corn and conventional corn (with and without pesticide applications) [39].
Similarly, species of Polybia (Vespidae), Carabidae, and spiders have been obtained in subsistence corn in Tegucigalpa, Honduras, demonstrating the importance of these taxa as predators [40]. Corroborating results from previous research, the reduvid bug, Zelus spp. (Reduviidae), plays an important role in the corn trophic chain by preying on the aphid, R. maidis, but it has also been reported to attack coccinellids, thus consuming at more than one trophic level [18].
The Coccinellidae species observed in this research had been mentioned in corn crops. Hernandez-Trejo et al. [41] reported two of the three ladybird species found here, C. maculata and C. sanguinea, preying on eggs of S. frugiperda. These two species, along with Ch. sexmaculata, had been recorded as part of the ladybirds associated with corn agroecosystems in the province of Manabí, Ecuador [42]. Similar to what was found in this study, the predator Podisus sp. (Pentatomidae) has been detected by Santiago [19] preying on FAW larvae. Additionally, they reported Syrphidae and Coccinellidae taxa preying on aphid species.
These results showed the presence of phytophagous insects and natural enemies (parasitoids and predators) with moderate diversity (Shannon-H Index = 2.474–2.629 and Margalef Index = 2.734–3.110) in the three study areas (Calceta, Lodana and Quevedo).
On the other hand, the affinity of the diversity and abundance of insect families present in Calceta and Lodana (dry and rainy seasons) separated from Quevedo, are likely related to the life zone. While Calceta and Lodana belong to a tropical dry forest, Quevedo is included in a tropical wet forest [24].
These results are similar to those detected in the diversity study conducted by Mirabal et al. [36] in San José de las Lajas, Mayabeque province, Cuba, who observed a higher richness of taxa in the Orders Hymenoptera, Coleoptera, Diptera, and Hemiptera, with a Shannon Index ranging from 2.23 to 2.37.
These findings also coincide with those reported by Frizzas et al. [39] in relation to the Shannon diversity index, which showed values from 2.24 to 2.33 when studying the insect fauna associated with transgenic corn based on Bacillus thuringiensis (Bt) and corn treated and untreated with insecticides.
Additionally, Sánchez et al. [22] conducted a study comparing beneficial entomofauna in a transgenic Bt corn crop and a conventional corn treated with insecticides. The researchers found a higher abundance of beneficial insects in the transgenic crop, attributed to lower insecticide applications in that crop, with the abundance sequence being Hymenoptera, followed by Coleoptera, Diptera, and Araneae, while in this study, the sequence was Diptera, followed by Hemiptera, Hymenoptera, and Coleoptera.
Regarding the damage to corn plants caused by FAW, Calceta reached the highest damage (>70%) compared to Lodana and Quevedo (21–25%). The observed damage in Calceta is higher than that reported by Santiago [19] in Barranca, Lima, Peru, who found 31.2% of corn plants with damaged whorls, and in turn, is higher than the estimates for Lodana and Quevedo. The damage estimated in Quevedo is slightly higher than that recorded in plots without pesticide application, which showed 16.6% damage, higher in the dry season compared to the rainy season in trials conducted during the rainy and dry seasons of 2021 [43]. Such differences could be attributed to the fact that during the dry season of 2023, there was heavier rainfall compared to the scarce precipitation that occurred in the dry season of 2021.
In this study, the differences in the diversity and abundance of insects observed in Quevedo are attributed to belonging to a different life zone than Calceta and Lodana. Despite the affinity between Calceta and Lodana, which are included in the same life zone, some differences in diversity and abundance were present between the two areas. Calceta showed slightly higher numbers of phytophagous insects, lower parasitism on S. frugiperda, and greater damage from this insect on plants. This could be related to the specific characteristics of each site. It could also be linked to the precipitation that occurred in the year 2023. Rainfall between February and March recorded in Calceta was three times higher than that recorded for the same period in Lodana (Figure S1). These local factors could be influencing changes in the patterns of diversity and abundance of insects associated with corn between these areas.
In this study, grain yield was negatively correlated with cob damage. Only larvae of H. zea were observed in the cob. It has been reported that larvae of this species can attack leaves, but the most significant damage occurs in the cob [15].
This would indicate the importance of cob sampling to detect the presence of this insect pest within pest management programs. This is especially important in the rainy season, as rainfall increased cob damage, which in turn adversely affected corn yields. We estimated a positive relationship between cob damage by H. zea with high rainfall, temperature, and in turn, the negative association between intense and frequent rainfall—cob damage versus grain yield.
Although in the Ecuadorian coast, the rainfall of the rainy season is utilized for planting during the first cycle of the year, in 2023 very intense rains were recorded starting in January that exceeded the annual averages. In Calceta, precipitation reached 1593.8 mm, in Lodana about 1093.5 mm, while in Quevedo it was 3168.2 mm. This would imply that frequent and intense rains could affect corn crop yields, as they affect plant rooting, decrease fertilizer utilization (losses due to leaching), and also favor the development of fungal diseases in both leaves and cobs.
Lopez et al. [9] pointed out low corn crop yields in the Manabí province due to the influence of climate change, and although they mentioned that the causes have not been studied in detail, they suggest that the decrease in yields could be associated with the temperature increase as a consequence of the mentioned climate change. In this study, temperature was positively correlated with both plant damage and cob damage. It is worth noting that on the Ecuadorian coast, the rainy season is characterized by high temperatures, while in the dry season, temperatures tend to decrease [23].
The effects of climate change on corn yield have been investigated in other areas. A study that reviewed climatic variables and corn yield in Transylvania, Romania during the period 2012–2021 showed that a 1 °C increase in seasonal average temperature can reduce corn yield by 3 to 13% [7]. Kim et al. [44] made observations about the effect of rainfall on corn dry matter yield in Suwon, South Korea. The researchers found that rainfall increased from the year 2005 onwards, leading to a decrease in yield by 4745.27 kg/ha, due to the increase in the frequency and intensity of precipitation. Kim and Lee [8] pointed out that rising temperatures and changing precipitation patterns can affect corn yield, highlighting the need for the development of corn varieties resistant to high temperatures that can adapt to unstable ecosystems as a result of climate change.
Regarding the management of pests that attack corn, the diversification of the agroecosystem such as intercropping could be considered. Recently, Pierre et al. [45] analyzed the impact of intercropping systems of corn with legumes on the diversity and abundance of entomophagous and phytophagous insects. The researchers found that these diversified systems attract a greater abundance and diversity of natural enemies, which could contribute to biological pest control in corn.
Other alternatives include improving resistant varieties, as well as using formulations derived from plant extracts. Some plant extracts have been tested for controlling S. frugiperda in corn with promising results [46].
For the development of sustainable agricultural pest management programs, several aspects must be considered. The first one involves understanding the development and production of the crop in the area, where plants represent the base of the food chain (autotrophic organisms). Plants provide food resources from the second trophic level that these consumers (heterotrophic organisms) cannot produce on their own. The pest status of any phytophagous insect depends on the possibility of increasing their populations. This is primarily defined by the biotic potential of the phytophagous insect, and secondly by the incidence of extrinsic factors such as the adaptability of the plant and the environmental conditions in which the agricultural system develops.
The climate and biotic components contextualize that environmental setting. Within the biotic components, the diversity associated with the crop and trophic interactions can play an important role in regulating the expression of the biotic potential of phytophagous insects. Thus, natural enemies can be determining factors in regulating the populations of phytophagous insects. On the other hand, dynamic interactions, crop-phytophagous insect-natural enemies framed within the environmental conditions will help define critical periods to protect production. This work included some of these aspects as a basis for pest management. The impact of climate on crop growth was investigated over two planting seasons, during which environmental factors significantly influenced corn production. Rainfall and temperatures affected the plant’s physiology, further favored damage to the cob, and consequently decreased grain yield.
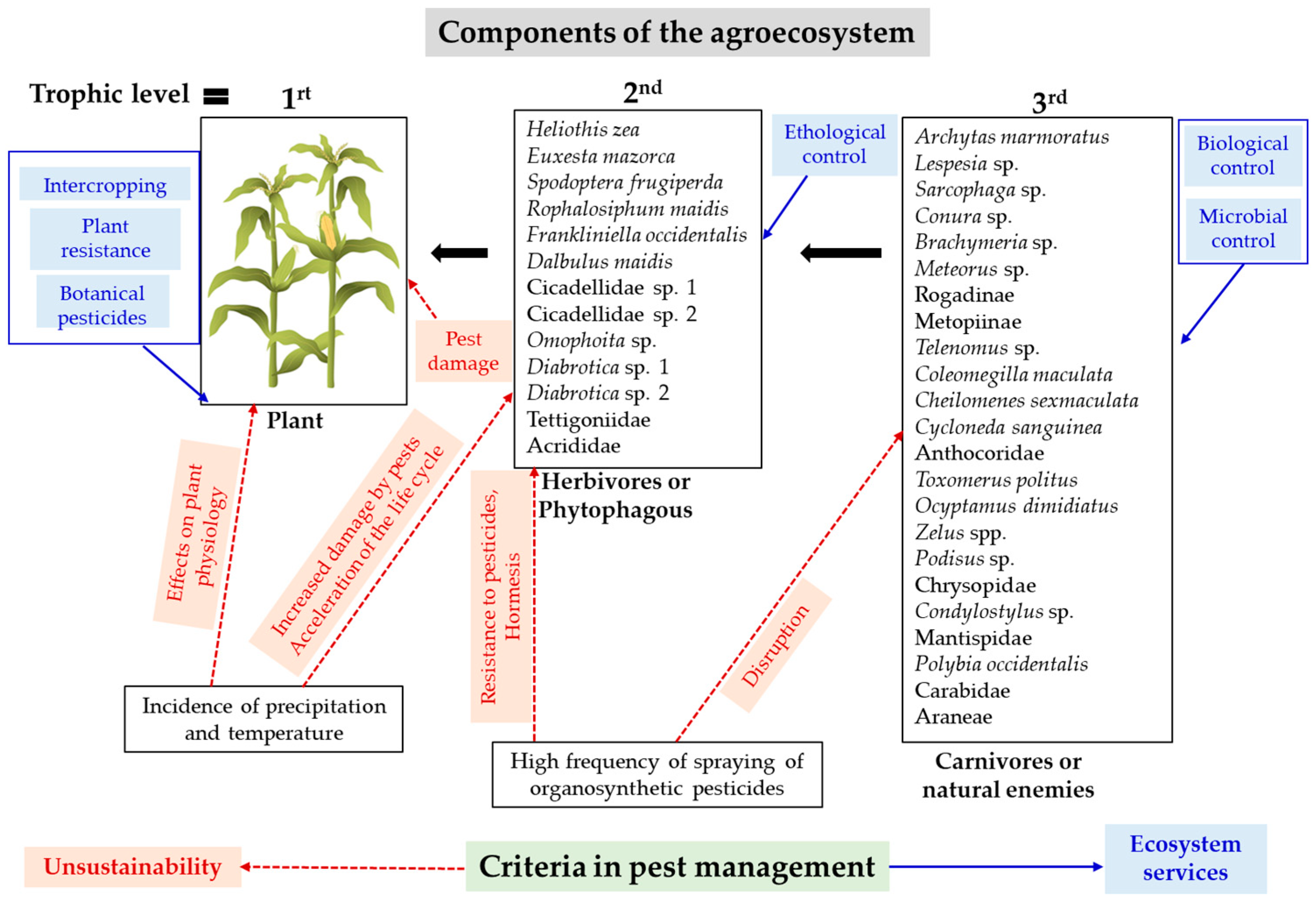
Due to the effect of climate on corn, models that integrate corn yield and rainfall and temperature patterns could be constructed to predict the effect of these climate variables on crop productivity, which is especially important in times of climate change. On the other hand, pest management in corn is based solely on chemical insecticide applications in Ecuador [33,47] as well as in other countries [12]. Since corn is a fundamental product for food sovereignty both in the Americas and in worldwide [7], it is necessary to explore other management strategies for pest control in corn. Figure 14 summarizes some criteria to be considered in pest management under a systemic approach.
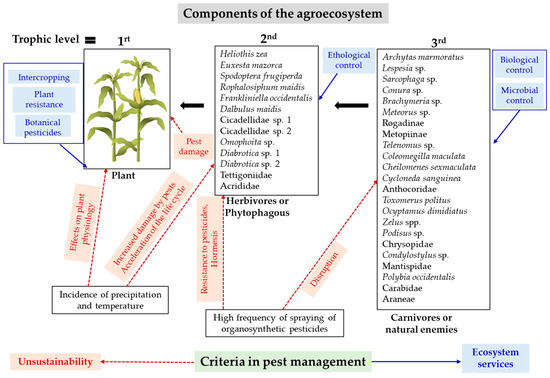
Figure 14.
Systemic approach to pest management. The blue arrows and letters indicate managament practices. The red arrows indicate the adverse effects of climate, pest damage and frequent pesticide applications on the trophic levels of corn.
In this research, we observed a moderate diversity of taxa, especially among natural enemies. This diversity can be utilized to design applied biological control programs. Podisus sp. is a good candidate as a predator of S. frugiperda, as well as taxa within the families Tachinidae, Sarcophagidae, Braconidae, and Platygasteridae. One strategy to consider is conservation biological control, which involves providing appropriate conditions to agroecosystems to attract beneficial insect fauna and their implicit ecosystem services [17]. This necessarily entails reducing sprays with organo-synthetic insecticides.
Future research will focus on evaluating corn genotypes and implementing conservation biological control practices as strategies to mitigate climate effects and the impact of pests.
5. Conclusions
A moderate diversity was determined in which natural enemies outnumbered phytophagous insects. Cob and plant damage were associated with the high temperatures recorded in the rainy season, where yields were negatively affected by rainfall and cob damage. Due to the effects of climate change, pests, and the need to increase food production, we face the great challenge of ensuring corn productivity with sustainability criteria. The beneficial insect fauna recorded forms one of the bases for the rationalization of pest management programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14040748/s1, Figure S1: Rainfall (mm), Relative Humidity (%) and Temperatures (°C) recorded during January–December 2023 in Calceta, Lodana and Quevedo., Ecuador. Source: INAMHI meteorological stations (https://www.inamhi.gob.ec/, accessed on 15 February 2024), Calceta (code: M1230), Lodana (code: M1208), and Quevedo (code: M006); Figure S2: Corn plot showing the surrounding areas; Figure S3: (A–F) Field observations and collection of insects associated with corn, (G,H) Laboratory observations and preservation of insects; Figure S4: (A–C) Placement and (D–I) review of yellow traps; Figure S5: Damage by Spodoptera frugiperda; Figure S6: Daily laboratory observations on Spodoptera frugiperda (FAW) on corn leaves. (A) FAW egg masses in Petri dishes, (B) FAW larvae in plastic containers. (C,D): Detail of FAW larvae, (E) FAW pupa. Figure S7. Evaluation of damage by earworm, Heliothis zea.
Author Contributions
Conceptualization, data curation, project administration, visualization, writing—original draft preparation, writing—review and editing D.T.C.; formal analysis, methodology, supervision, writing—review and editing, F.S.-M.; investigation, methodology, supervision, writing—review and editing, F.Z., J.C.-O., G.V. and G.C.; data curation, investigation, writing—review and editing, K.P., J.Z., V.S.-N., V.P., J.M.-M. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Corporación Ecuatoriana para el Desarrollo de la Investigación y la Academia (CEDIA), grant number No. I + D + I-XVII-2022-90.
Data Availability Statement
Data are contained within the Supplementary Materials.
Acknowledgments
The authors would like to thanks to Corporación Ecuatoriana para el Desarrollo de la Investigación y la Academia—CEDIA for the financial support given to the present research, development and innovation work through its CEPRA program, especially for the “Desarrollo de un prototipo de pesticida botánico validado en el cultivo de maíz” No. I + D + I-XVII-2022-90. The author would like to thank ESPAM, UTM, UTEQ and UTA for the administrative and academic efforts that allowed this research to be carried out.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Filipenco, D. Top Corn-Producing Countries Worldwide. Available online: https://www.developmentaid.org/news-stream/post/167740/corn-producing-countries-worldwide (accessed on 14 February 2024).
- García-Lara, S.; Serna-Saldivar, S.O. Corn History and Culture. In Corn: Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; Woodhead Publishing and AACC International Press: Cambridge, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Food and Agriculture Data. 2024. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 February 2024).
- OEC. Corn, Maize (Corn). Available online: https://oec.world/en/profile/hs/corn (accessed on 15 February 2024).
- U.S. Deparment of Agriculture (USDA). Corn 2023 World Production: 1,232,570 (1000 MT). Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=0440000 (accessed on 15 February 2024).
- MAG (Ministerio de Agricultura y Ganadería). Cifras Agroproductivas. Available online: http://sipa.agricultura.gob.ec/index.php/cifras-agroproductivas (accessed on 15 February 2024).
- Șimon, A.; Moraru, P.I.; Ceclan, A.; Russu, F.; Chețan, F.; Bărdaș, M.; Popa, A.; Rusu, T.; Pop, A.I.; Bogdan, I. The Impact of climatic factors on the development stages of maize crop in the Transylvanian Plain. Agronomy 2023, 13, 1612. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, B.-M. Effects of climate change and drought tolerance on maize growth. Plants 2023, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Gaiser, T.; Ewert, F.; Srivastava, A. Effects of recent climate change on maize yield in Southwest Ecuador. Atmosphere 2021, 12, 299. [Google Scholar] [CrossRef]
- Hernandez-Trejo, A.; Estrada, B.; Rodríguez-Herrera, R.; García, J.M.; Patiño-Arellano, A.; Osorio-Hernández, E. Importance of biological control of pests in corn (Zea mays L.). Rev. Mex. Cienc. Agríc. 2019, 10, 803–813. [Google Scholar] [CrossRef]
- Thumar, R.; Zala, M.; Varma, H.; Dhobi, C.; Patel, B.M.; Patel, M.B.; Borad, P.K. Evaluation of insecticides against fall armyworm, Spodoptera frugiperda (J. E. Smith) infesting maize. Int. J. Chem. Stud. 2020, 8, 100–104. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, J.; Suby, S.B.; Sekhar, J.C.; Lakshmi, S.P. Pests of maize. In Pests and Their Management; Omkar, Ed.; Springer Nature: New Delhi, India, 2018; pp. 51–79. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Frizzas, M.R. Eight decades of Dalbulus maidis (DeLong & Wolcott) (Hemiptera, Cicadellidae) in Brazil: What we know and what we need to know. Neotrop. Entomol. 2022, 51, 1–17. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Mcdonald, T.; Rand, T.A.; Fausti, S.W. Spatial and numerical relationships of arthropod communities associated with key pests of maize. J. Appl. Entomol. 2015, 139, 446–456. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F. Pest status and management of corn earworm (Lepidoptera: Noctuidae) in field corn in the United States. J. Integr. Pest. Manag. 2019, 10, 19. [Google Scholar] [CrossRef]
- Lopes, S.R.; Cruz, I. Management of Euxesta spp. in sweet corn with McPhail traps. Neotrop. Entomol. 2020, 49, 139–146. [Google Scholar] [CrossRef]
- Chirinos, D.T.; Anchundia, M.; Castro, R.; Castro, J.; Geraud, J.E.; Kondo, T. Diversity of native and exotic parasitoids attacking agricultural pests in Ecuador: Are Ecuadorian biocontrol programs in decline? Anartia 2021, 33, 7–26. [Google Scholar] [CrossRef]
- Centeno-Parrales, J.A.; Chirinos, D.T.; Kondo, T. Trophic networks associated with the corn leaf aphid, Rhopalosiphum maidis (Fitch, 1856) (Hemiptera: Aphididae) in a cornfield, Manabí, Ecuador. Sci. Agropecu. 2022, 13, 327–333. [Google Scholar] [CrossRef]
- Santiago, M.H. Population fluctuation of phytophagous insects and biological controllers in maize culture (Zea mays L.), agroceres variety. Infinitum 2019, 9, 39–45. [Google Scholar]
- Agasyeva, I.; Ismailov, V.; Nefedova, M.; Nastasiy, A.; Goloborodko, E.; Petrischev, V. Application of innovative biological agents for sustainable corn farming. Nexo Rev. Cient. 2023, 36, 344–351. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Luckmann, W.H. Introduction to Insect Pest Management, 1st ed.; John Wiley and Sons: New York, NY, USA, 1975; p. 672. [Google Scholar]
- Sanchez, M.L.; Linares, J.C.; Fernandez, C.; Perez, K.D. Analysis of benefic entomofauna in transgenic and conventional corn crops, Cordoba-Colombia. Rev. Temas Agrar. 2018, 23, 121–130. Available online: https://revistas.unicordoba.edu.co/index.php/temasagrarios/article/view/1296 (accessed on 15 February 2024). [CrossRef]
- Rivadeneira, J.F.; Zambrano, Y.E.; Pérez-Martín, M.Á. Adapting water resources systems to climate change in tropical areas: Ecuadorian coast. Sci. Total Environ. 2020, 703, 135554. [Google Scholar] [CrossRef] [PubMed]
- Holdridge, L. Life Zone Ecology; Tropical Science Center: San José, Costa Rica, 1967; Available online: https://reddcr.go.cr/sites/default/files/centro-de-documentacion/holdridge_1966_-_life_zone_ecology.pdf (accessed on 29 February 2024).
- Cusme, Y.A.; Montesdeoca, M.G.M.; Cedeño, K.K.A.; Hidalgo, M.K. La gestión productiva agrícola en el sector minorista del cantón Bolívar de la provincia Manabí, Ecuador. Mikarimin. Rev. Cientif. Multidiscipl. 2017, 3, 43–58. Available online: https://revista.uniandes.edu.ec/ojs/index.php/mikarimin/article/view/797 (accessed on 15 March 2024).
- Velez, L.M.; Fuentes, M.Á.; Moreira, M.F.; Lucio, L.F. Uso hídrico en la producción agrícola de la parroquia Lodana del cantón Santa Ana. UNESUM-Ciencias. Rev. Cientif. Multidiscipl. 2021, 5, 115–128. Available online: https://doi.org/10.47230/unesum-ciencias.v4.n3.2020.251 (accessed on 15 March 2024). [CrossRef]
- MAGAP (Ministerio de Agricultura, Ganadería, Acuacultura y Pesca). Memoria Técnica Cantón Quevedo Sur. Proyecto: Generación de Geoinformación Para la Gestión del Territorio a Nivel Nacional Escala 1:25000. Sistemas Agroproductivos. 2013. Available online: https://www.geoportaligm.gob.ec/geodescargas/quevedo/mt_quevedo_sur_sistemas_productivos.pdf (accessed on 15 March 2024).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 15 February 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 31 October 2023).
- Vásquez, J.; Mora, E. Incidence of and yield loss caused by maize rayado fino virus in maize cultivars in Ecuador. Euphytica 2007, 153, 339–342. [Google Scholar] [CrossRef]
- Amancio, M.B.; Cruz, I. Population dynamics of Euxesta spp. (Diptera: Otitidae) in maize (Zea mays L.), wheat (Triticum aestivum L.) and polyculture. Rev. Bras. Milho Sorgo 2019, 18, 311–321. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/209548/1/Population-dynamics.pdf (accessed on 15 February 2024). [CrossRef]
- Tapia, I.; Bermeo, D.; Edison, S.; Racines, M. Evaluación de cuatro métodos de aplicación de aceite comestible vegetal en el control de Heliothis zea y Euxesta sp. en la sierra del Ecuador. In Proceedings of the XVIII Reunión Latinoamericana del Maiz, Mina Gerais, Brazil, 27–28 August 1999; pp. 671–676. Available online: http://181.112.143.123/bitstream/41000/2827/1/iniapsc322est.pdf (accessed on 15 February 2024).
- Estrada, M. Principales insectos plaga del maíz (Zea mays, L.) en Ecuador. Rev. Cient. Agroecosistemas 2022, 10, 182–191. Available online: https://aes.ucf.edu.cu/index.php/aes/article/view/577 (accessed on 15 February 2024).
- Calles-Torrez, V.; Knodel, J.J.; Boetel, M.A.; Wade French, B.; Fuller, B.W.; Ransom, J.K. Field-Evolved resistance of northern and western corn rootworm (Coleoptera: Chrysomelidae) populations to corn hybrids expressing single and pyramided Cry3Bb1 and Cry34/35Ab1 Bt proteins in North Dakota. J. Econ. Entomol. 2019, 112, 1875–1886. [Google Scholar] [CrossRef]
- Quito-Avila, D.F.; Alvarez, R.A.; Mendoza, A.A. Occurrence of maize lethal necrosis in Ecuador: A disease without boundaries? Eur. J. Plant Pathol. 2016, 146, 705–710. [Google Scholar] [CrossRef]
- Mirabal, L.; Gonzalez, C.; Castillo, N.; Pérez, N.; Gómez, J.; López, A.; Ceballos, M.; Amador, A. Entomofauna asociada a dos agroecosistemas de maíz (Zea mays L.) en San José de las Lajas, Mayabeque. Métodos Ecol. Sist. 2016, 11, 47–57. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=7975999 (accessed on 15 February 2024).
- Gurrola-Pérez, C.C.; Álvarez-Zagoya, R.; Hernández-Mendoza, J.L.; Correa-Ramírez, M.; Pérez-Santiago, G. Registro de Lespesia archippivora, Lespesia postica, y Archytas marmoratus parasitando larvas de Spodoptera frugiperda en Durango, México. Southwest. Entomol. 2018, 43, 505–510. [Google Scholar] [CrossRef]
- Molina-Ochoa, J.; Carpenter, J.E.; Heinrichs, E.A.; Foster, J.E. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: An inventory. Fla. Entomol. 2003, 86, 254–289. [Google Scholar] [CrossRef]
- Frizzas, M.R.; Oliveira, C.M.D.; Omoto, C. Diversity of insects under the effect of Bt maize and insecticides. Arq. Inst. Biol. 2017, 84, e0062015. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; O’Neil, R.J. Influence of extra-field characteristics to abundance of key natural enemies of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in subsistence maize production, International. J. Pest Manag. 2007, 53, 89–99. [Google Scholar] [CrossRef]
- Hernández-Trejo, A.; Osorio-Hernández, E.; López-Santillán, J.A.; Ríos-Velasco, C.; Varela-Fuentes, S.E.; Rodríguez-Herrera, R. Beneficial insects associated to control of the fall armyworm (Spodoptera frugiperda) in maize (Zea mays L.) cultivation. Rev. Agroproduct. 2018, 11, 9–14. Available online: https://revista-agroproductividad.org/index.php/agroproductividad/article/view/142 (accessed on 15 February 2024).
- Bailon, A.G.; Mendoza, F.L.; Solis, L.; Velasquez, J.; Montes, K.; Perla, D.R.; Kondo, T.; Chirinos, D.T. Endemic and invasive Coccinellidae associated with maize (Zea mays L.) fields, in Manabi province, Ecuador. Folia Oecol. 2022, 49, 35–41. [Google Scholar] [CrossRef]
- Briones, M.A.; Sánchez-Mora, F.D.; Chirinos, D.T. Can fall armyworm damage decrease depending on the season, maize hybrid, and type of pesticides? Sci. Agropecu. 2023, 14, 313–320. [Google Scholar] [CrossRef]
- Kim, M.; Chemere, B.; Sung, K. Effect of heavy rainfall events on the dry matter yield trend of whole crop maize (Zea mays L.). Agriculture 2019, 9, 75. [Google Scholar] [CrossRef]
- Pierre, J.F.; Jacobsen, K.L.; Latournerie-Moreno, L.; Torres-Cab, W.J.; Chan-Canché, R.; Ruiz-Sánchez, E. A review of the impact of maize-legume intercrops on the diversity and abundance of entomophagous and phytophagous insects. PeerJ 2023, 11, e15640. [Google Scholar] [CrossRef] [PubMed]
- López, J.J.; Chirinos, D.T.; Ponce, W.H.; Solórzano, R.F.; Alarcón, J.P. Actividad insecticida de formulados botánicos sobre el gusano cogollero, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2022, 48, e11739. [Google Scholar] [CrossRef]
- Nieto, C.A.; Corro, K.P.; Diaz, M.A.; Sánchez, K.A. Mapeo geográfico toxicológico de plaguicidas utilizados en cultivos de maíz zona norte de la provincia de Los Ríos. Código Cient. Rev. Investig. 2023, 4, 73–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).