A Base Layer of Ferrous Sulfate-Amended Pine Bark Reduces Phosphorus Leaching from Nursery Containers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Effect of FeSO4 Rate and FSB Depth
2.1.1. Substrate Characteristics
2.1.2. Substrate Treatments
2.1.3. Leachate Collection
2.1.4. P and Fe Remaining in Pine Bark
2.2. Experiment 2: Effect of Dolomite and FS Form
2.2.1. Substrate Treatments
2.2.2. Leachate Collection
2.2.3. P remaining in Pine Bark
2.3. Statistical Analysis
3. Results
3.1. Experiment 1
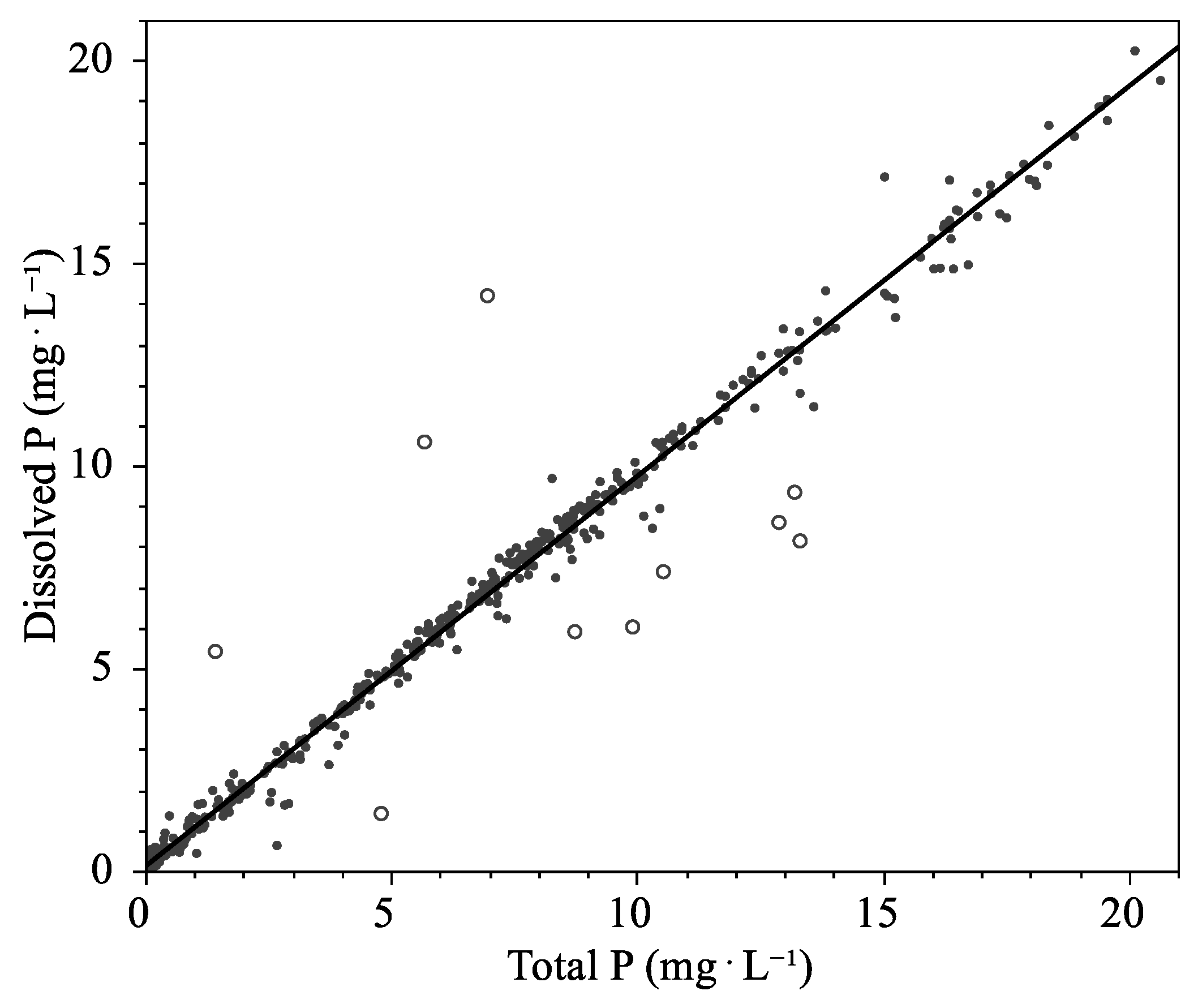
3.1.1. Leachate Phosphorus
3.1.2. Substrate Phosphorus
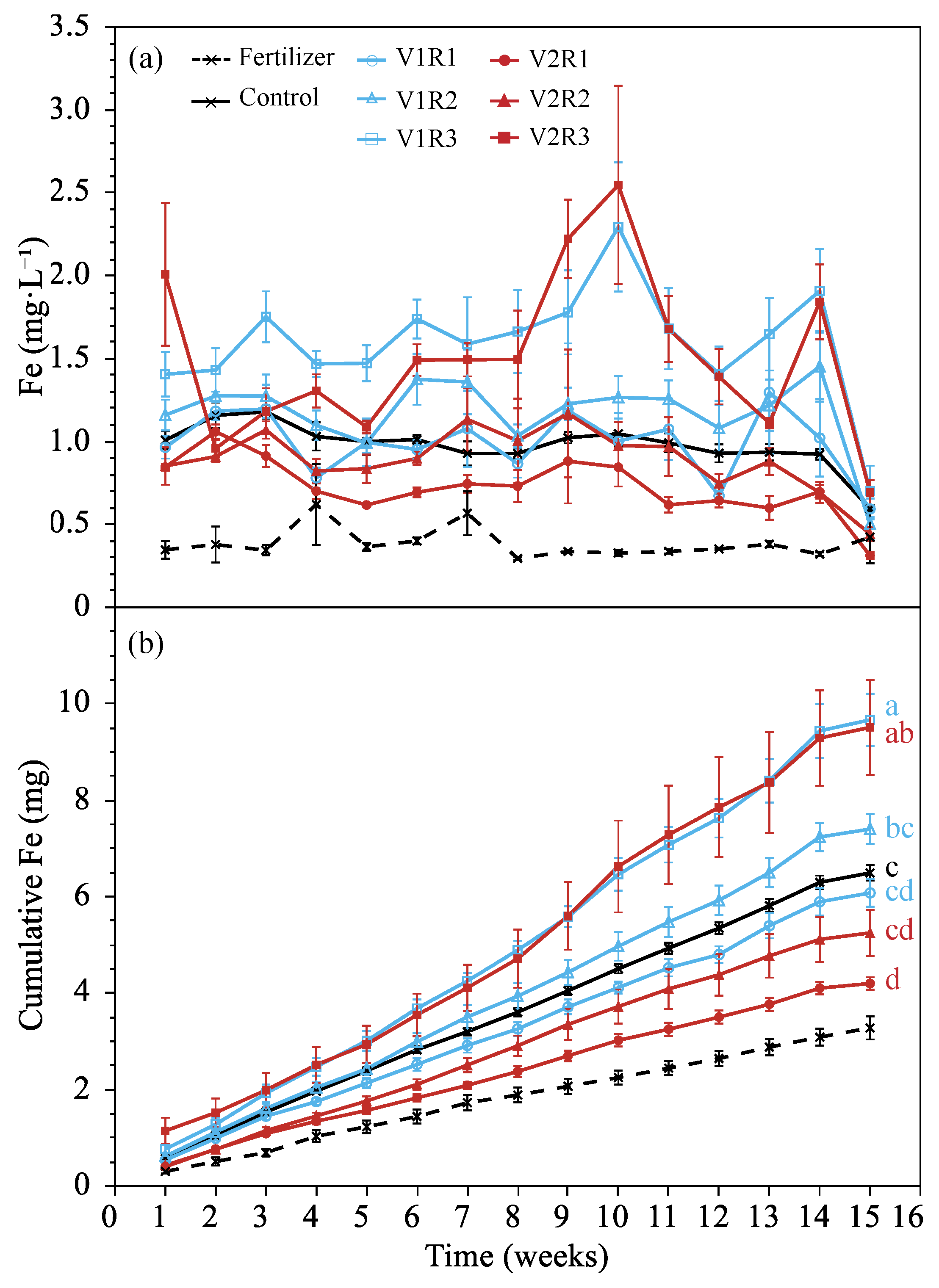
3.1.3. Leachate Iron
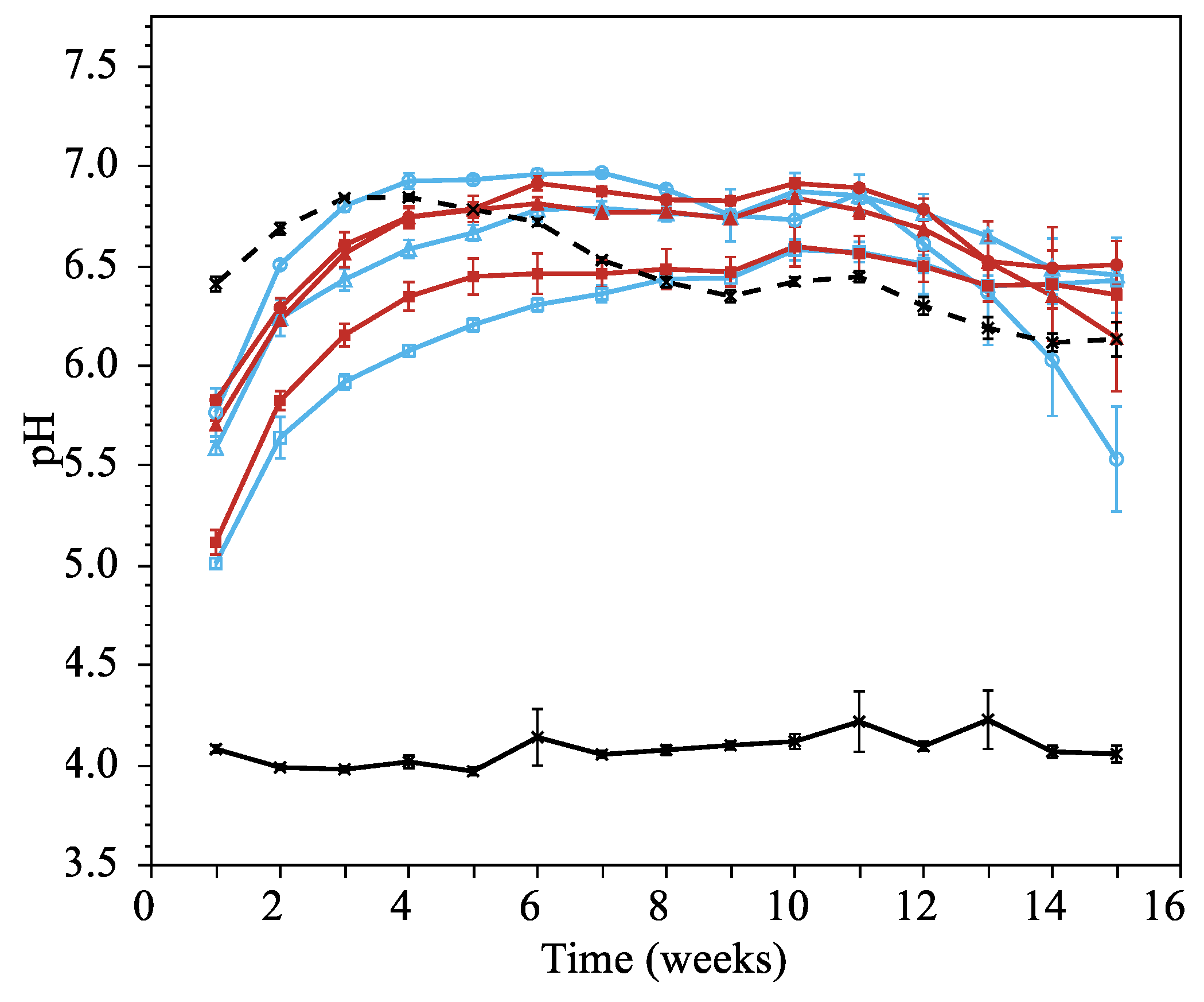
3.1.4. Leachate pH
3.2. Experiment 2
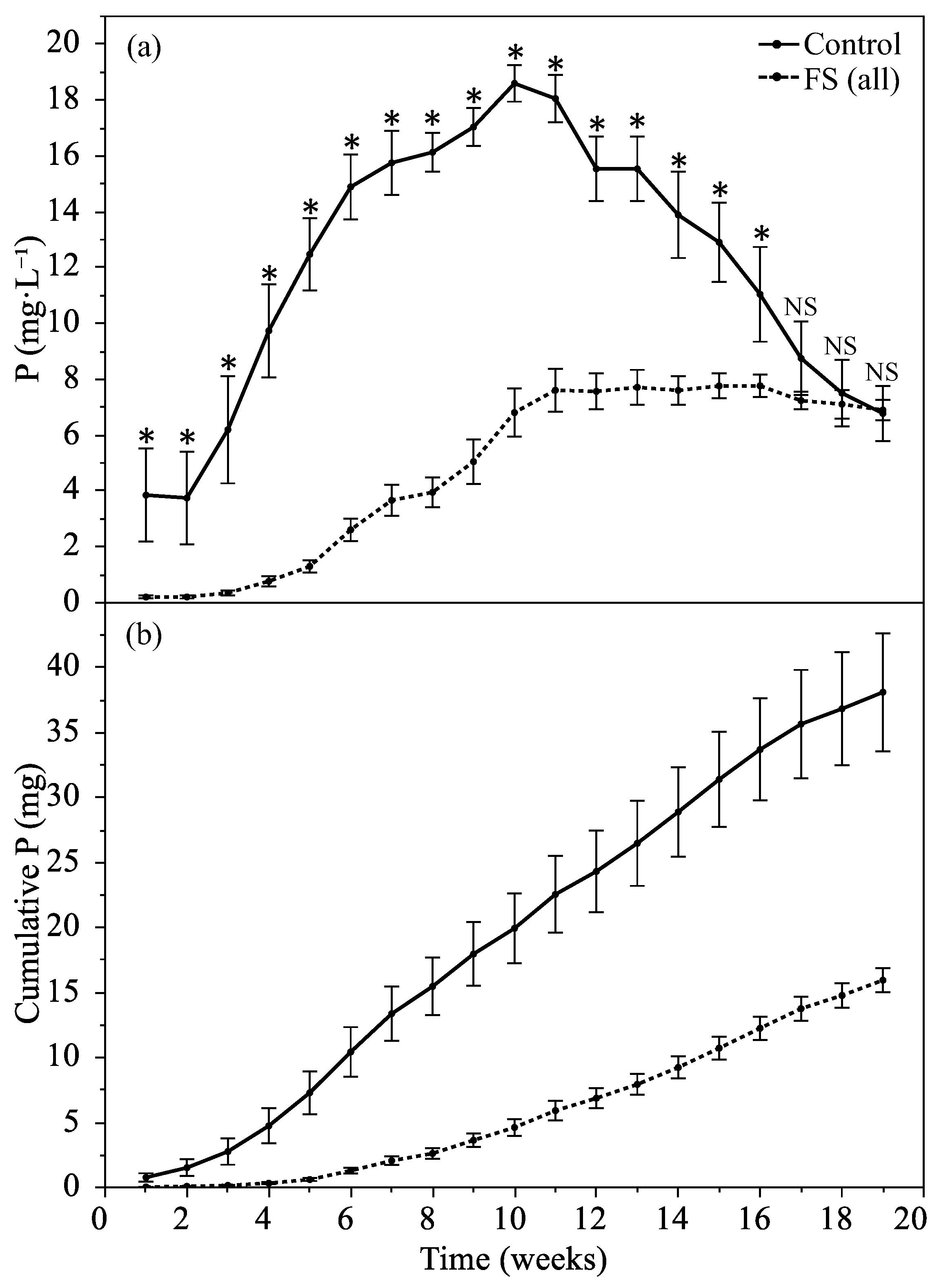
3.2.1. Leachate Phosphorus
3.2.2. Substrate Phosphorus
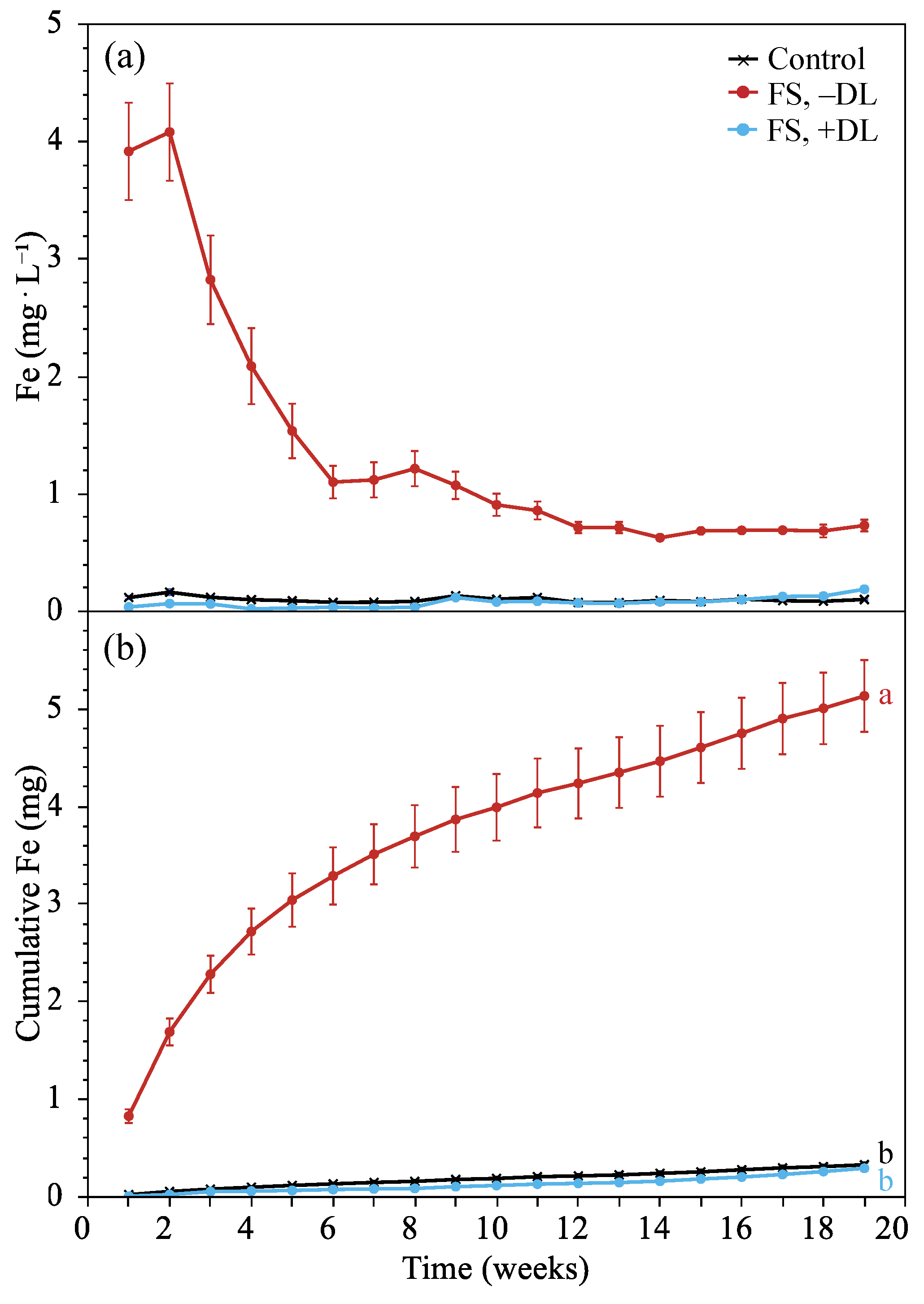
3.2.3. Leachate Iron
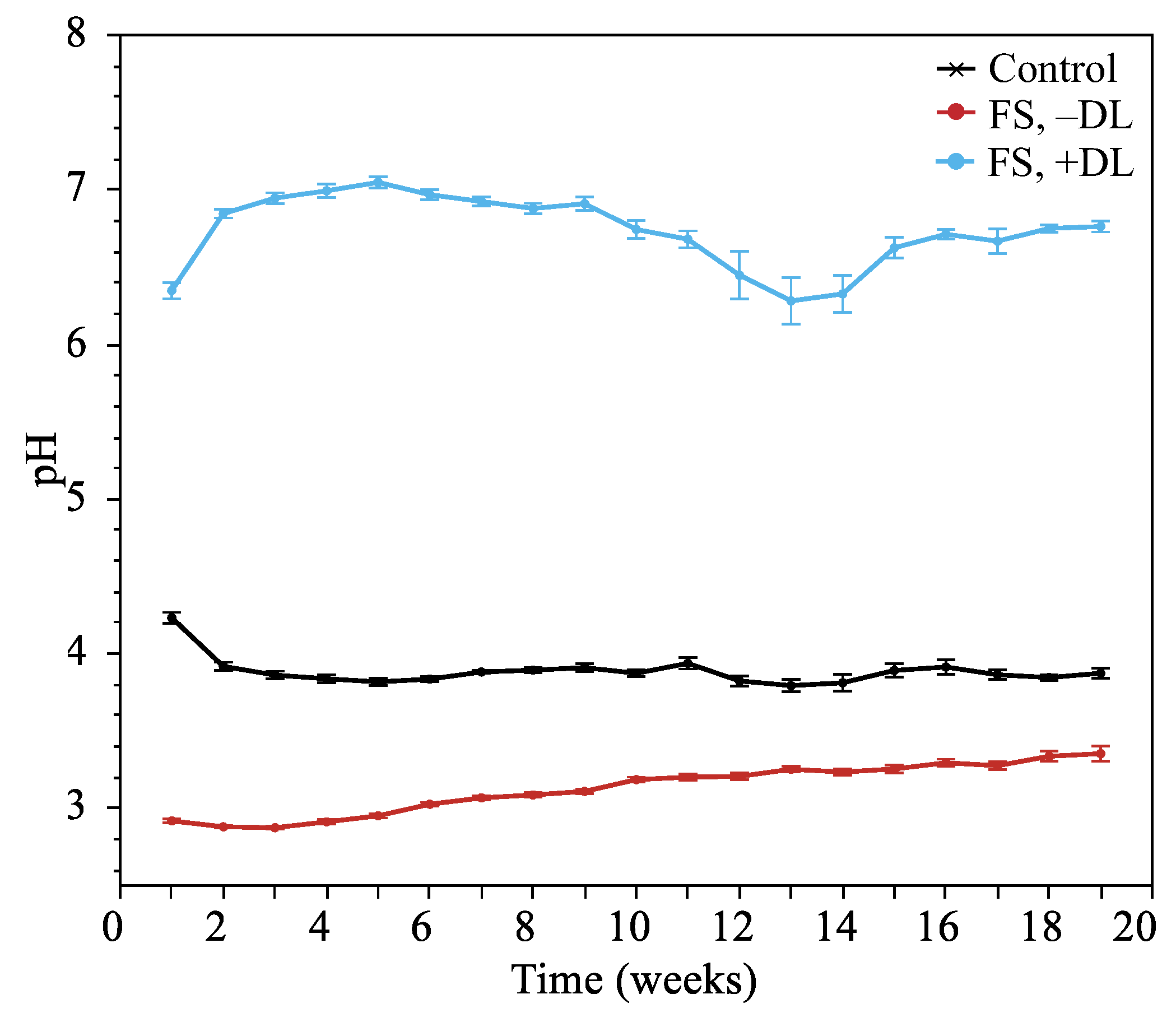
Leachate pH
4. Discussion
4.1. Effect of FS on P Leaching
4.2. Effect of FSB Volume and Fe Rate on P Leaching
4.3. Effect of FS Form and Dolomite on P Leaching
4.4. P in Post-Experiment Pine Bark
4.5. Treatment Effects on Fe Leaching
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Agriculture, N.A.S.S. 2019 Census of Horticultural Specialties. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/HORTIC.pdf (accessed on 11 November 2022).
- Majsztrik, J.C.; Ristvey, A.G.; Lea-Cox, J.D. Water and Nutrient Management in the Production of Container-Grown Ornamentals. Hortic. Rev. 2011, 38, 253–297. [Google Scholar] [CrossRef]
- Cole, J.C.; Dole, J.M. Temperature and Phosphorus Source Affect Phosphorus Retention by a Pine Bark-Based Container Medium. HortScience 1997, 32, 236–240. [Google Scholar] [CrossRef]
- Godoy, A.; Cole, J.C. Phosphorus Source Affects Phosphorus Leaching and Growth of Containerized Spirea. HortScience 2000, 35, 1249–1252. [Google Scholar] [CrossRef]
- Yeager, T.; Barrett, J. Phosphorus Leaching from 32P-Superphosphate-Amended Soilless Container Media. HortScience 1984, 19, 216–217. [Google Scholar] [CrossRef]
- Yeager, T.H.; Barrett, J.E. Phosphorus and Sulfur Leaching from an Incubated Superphosphate-Amended Soilless Container Medium. HortScience 1985, 20, 671–672. [Google Scholar] [CrossRef]
- Yeager, T.H.; Barrett, J.E. Influence of Incubation Time on Phosphorus Leaching from a Container Medium. J. Environ. Hortic. 1985, 3, 186–187. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, Eutrophication and Harmful Algal Blooms along the Freshwater to Marine Continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Lea-Cox, J.D. Water Quality Regulations in the Chesapeake Bay: Working to More Precisely Estimate Nutrient Loading Rates and Incentivize Best Management Practices in the Nursery and Greenhouse Industry. HortScience 2013, 48, 1097–1102. [Google Scholar] [CrossRef]
- Fain, G.B.; Gilliam, C.H.; Tilt, K.M.; Olive, J.W.; Wallace, B. Survey of Best Management Practices in Container Production Nurseries. J. Environ. Hortic. 2000, 18, 142–144. [Google Scholar] [CrossRef]
- Mack, R.; Owen, J.S.; Niemiera, A.X.; Latimer, J. Virginia Nursery and Greenhouse Grower Survey of Best Management Practices. HortTechnology 2017, 27, 386–392. [Google Scholar] [CrossRef]
- Million, J.B.; Yeager, T.H. Leaching Fraction-Based Microirrigation Schedule Reduced Water Use but Not N and P Loss during Production of a Container-Grown Shrub. HortScience 2021, 56, 147–153. [Google Scholar] [CrossRef]
- Million, J.B.; Yeager, T.H. Fabric Containers Increased Irrigation Demand but Decreased Leachate Loss of Nitrogen and Phosphorus Compared With Conventional Plastic Containers During Production of Dwarf Burford Holly. HortScience 2022, 57, 743–749. [Google Scholar] [CrossRef]
- Million, J.; Yeager, T.; Albano, J. Consequences of Excessive Overhead Irrigation on Runoff during Container Production of Sweet Viburnum 1. J. Environ. Hortic. 2007, 25, 117–125. [Google Scholar] [CrossRef]
- Tyler, H.H.; Warren, S.L.; Bilderback, T.E. Cyclic Irrigation Increases Irrigation Application Efficiency and Decreases Ammonium Losses. J. Environ. Hortic. 1996, 14, 194–198. [Google Scholar] [CrossRef]
- Tyler, H.H.; Warren, S.L.; Bilderback, T.E. Reduced Leaching Fractions Improve Irrigation Use Efficiency and Nutrient Efficacy. J. Environ. Hortic. 1996, 14, 199–204. [Google Scholar] [CrossRef]
- Williams, K.A.; Nelson, P.V. Modifying a Soilless Root Medium with Aluminum Influences Phosphorus Retention and Chrysanthemum Growth. HortScience 1996, 31, 381–384. [Google Scholar] [CrossRef]
- Bugbee, G.J.; Elliott, G.C. Leaching of Nitrogen and Phosphorus from Potting Media Containing Biosolids Compost as Affected by Organic and Clay Amendments. Bull. Environ. Contam. Toxicol. 1998, 60, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.S.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Industrial Mineral Aggregate Amendment Affects Physical and Chemical Properties of Pine Bark Substrates. HortScience 2007, 42, 1287–1294. [Google Scholar] [CrossRef]
- Owen, J.S.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Phosphorus Rate, Leaching Fraction, and Substrate Influence on Influent Quantity, Effluent Nutrient Content, and Response of a Containerized Woody Ornamental Crop. HortScience 2008, 43, 906–912. [Google Scholar] [CrossRef]
- Ogutu, R.A.; Williams, K.A.; Pierzynski, G.M. Phosphate Sorption of Calcined Materials Used as Components of Soilless Root Media Characterized in Laboratory Studies. HortScience 2009, 44, 431–437. [Google Scholar] [CrossRef]
- Shreckhise, J.H.; Owen, J.S.; Eick, M.J.; Niemiera, A.X.; Altland, J.E.; Jackson, B.E. Dolomite and Micronutrient Fertilizer Affect Phosphorus Fate When Growing Crape Myrtle in Pine Bark. HortScience 2020, 55, 832–840. [Google Scholar] [CrossRef]
- Abdi, D.E.; Blanchard, J.; Fields, J.S.; Santos, L.; Beasley, L.; Beasley, J. Reducing Anion Nutrient Leaching Losses from a Short-Cycle Container-Grown Crop (Tagetes Patula) Using Activated Aluminum. Agriculture 2023, 13, 1028. [Google Scholar] [CrossRef]
- Watts, D.B.; Runion, G.B.; Torbert, H.A. Influence of Flue Gas Desulfurization Gypsum on Phosphorus Loss from a Horticultural Growth Medium. Horticulturae 2021, 7, 199. [Google Scholar] [CrossRef]
- Bartley, P.C.; Erbrick, L.B.; Knotts, M.J.; Watts, D.B.; Torbert, H.A. Influence of Flue Gas Desulfurization Gypsum on Phosphorus Loss in Pine Bark Substrates. Agriculture 2023, 13, 283. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Lee, D.J.; Nguyen, P.D.; Bui, X.T. Modification of Agricultural Waste/by-Products for Enhanced Phosphate Removal and Recovery: Potential and Obstacles. Bioresour. Technol. 2014, 169, 750–762. [Google Scholar] [CrossRef]
- Pokhrel, M.R.; Poudel, B.R.; Aryal, R.L.; Paudyal, H.; Ghimire, K.N. Removal and Recovery of Phosphate from Water and Wastewater Using Metal-Loaded Agricultural Waste-Based Adsorbents: A Review. J. Inst. Sci. Technol. 2019, 24, 77–89. [Google Scholar] [CrossRef]
- Handreck, K.; Black, N.D. Growing Media for Ornamental Plants and Turf, 4th ed.; University of New South Wales Press Ltd.: Randwick, Sydney, 2010; ISBN 978-1-74223-082-5. [Google Scholar]
- Handreck, K.A. Iron-Phosphorus Interactions in the Nutrition of Seedling Macadamia in Organic Potting Media. Aust. J. Exp. Agric. 1992, 32, 773–779. [Google Scholar] [CrossRef]
- Fields, J.S.; Owen, J.S.; Altland, J.E. Substrate Stratification: Layering Unique Substrates within a Container Increases Resource Efficiency without Impacting Growth of Shrub Rose. Agronomy 2021, 11, 1454. [Google Scholar] [CrossRef]
- Khamare, Y.; Marble, S.C.; Altland, J.E.; Pearson, B.J.; Chen, J.; Devkota, P. Effect of Substrate Stratification on Growth of Common Nursery Weed Species and Container-Grown Ornamental Species. HortTechnology 2022, 32, 74–83. [Google Scholar] [CrossRef]
- Khamare, Y.; Marble, S.C.; Altland, J.E.; Pearson, B.J.; Chen, J.; Devkota, P. Effect of Substrate Stratification Without Fine Pine Bark Particles on Growth of Common Nursery Weed Species and Container-Grown Ornamental Species. HortTechnology 2022, 32, 491–498. [Google Scholar] [CrossRef]
- Criscione, K.S.; Fields, J.S.; Owen, J.S.; Fultz, L.; Bush, E. Evaluating Stratified Substrates Effect on Containerized Crop Growth under Varied Irrigation Strategies. HortScience 2022, 57, 400–413. [Google Scholar] [CrossRef]
- Fields, J.S.; Criscione, K.S. Stratified Substrates Can Reduce Peat Use and Improve Root Productivity in Container Crop Production. HortScience 2023, 58, 364–372. [Google Scholar] [CrossRef]
- Fonteno, W.C.; Harden, C.T. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 2003. [Google Scholar]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-1-4200-2529-3. [Google Scholar]
- Method 3051A (SW-846); Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Method 3015A (SW-846); Microwave Assisted Acid Digestion of Aqueous Samples and Extracts. U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Morgan, B.; Lahav, O. The Effect of pH on the Kinetics of Spontaneous Fe(II) Oxidation by O2 in Aqueous Solution—Basic Principles and a Simple Heuristic Description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef]
- Wolfinger, R. Covariance Structure Selection in General Mixed Models. Commun. Stat.-Simul. Comput. 1993, 22, 1079–1106. [Google Scholar] [CrossRef]
- Marini, R.P. Approaches to Analyzing Experiments with Factorial Arrangements of Treatments Plus Other Treatments. HortScience 2003, 38, 117–120. [Google Scholar] [CrossRef]
- Yeager, T.H.; Wright, R.D. Pine Bark—Phosphorus Relationships. Commun. Soil Sci. Plant Anal. 1982, 13, 57–66. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Haridas, A. Removal of Phosphate from Aqueous Solutions and Sewage Using Natural and Surface Modified Coir Pith. J. Hazard. Mater. 2008, 152, 527–535. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Min, S.-H. Biosorbents Prepared from Wood Particles Treated with Anionic Polymer and Iron Salt: Effect of Particle Size on Phosphate Adsorption. Bioresour. Technol. 2008, 99, 626–630. [Google Scholar] [CrossRef]
- Robalds, A.; Dreijalte, L.; Bikovens, O.; Klavins, M. A Novel Peat-Based Biosorbent for the Removal of Phosphate from Synthetic and Real Wastewater and Possible Utilization of Spent Sorbent in Land Application. Desalination Water Treat. 2016, 57, 13285–13294. [Google Scholar] [CrossRef]
- Zhang, R.; Leiviskä, T.; Taskila, S.; Tanskanen, J. Iron-Loaded Sphagnum Moss Extract Residue for Phosphate Removal. J. Environ. Manag. 2018, 218, 271–279. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Ohta, S.; Harada, H.; Ohto, K.; Kawakita, H. The Adsorption of Phosphate from an Aquatic Environment Using Metal-Loaded Orange Waste. J. Colloid Interface Sci. 2007, 312, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gerke, J.; Hermann, R. Adsorption of Orthophosphate to Humic-Fe-Complexes and to Amorphous Fe-Oxide. J. Plant Nutrition. Soil 1992, 155, 233–236. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate from Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Gerke, J. Humic (Organic Matter)-Al(Fe)-Phosphate Complexes: An Underestimated Phosphate Form in Soils and Source of Plant-Available Phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Broschat, T.K. Nitrate, Phosphate, and Potassium Leaching from Container-Grown Plants Fertilized by Several Methods. HortScience 1995, 30, 74–77. [Google Scholar] [CrossRef]
- McGinnis, M.S.; Warren, S.L.; Bilderback, T.E. Replacing Conventional Nursery Crop Nutrient Inputs with Vermicompost for Container Production of Hibiscus moscheutos L. ‘Luna Blush’. HortScience 2009, 44, 1698–1703. [Google Scholar] [CrossRef]
- Warren, S.L.; Bilderback, T.E.; Kraus, H.H. Method of Fertilizer Application Affects Nutrient Losses of Controlled-Release Fertilizer. Acta Hortic. 2001, 548, 349–356. [Google Scholar] [CrossRef]
- Yeager, T.; Gardner, T.; Nyhuis, K. Nutrient Loading from Initial Watering of Container Plants. Acta Hortic. 2018, 1191, 183–186. [Google Scholar] [CrossRef]
- Hoskins, T.C.; Owen, J.S.; Niemiera, A.X. Water Movement through a Pine-Bark Substrate during Irrigation. HortScience 2014, 49, 1432–1436. [Google Scholar] [CrossRef]
- Manna, A.; Naskar, N.; Sen, K.; Banerjee, K. A Review on Adsorption Mediated Phosphate Removal and Recovery by Biomatrices. J. Indian Chem. Soc. 2022, 99, 100682. [Google Scholar] [CrossRef]
- Morris, A.J. Phosphate Binding to Fe and Al in Organic Matter as Affected by Redox Potential and pH. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 2011. [Google Scholar]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Tung, K.L. Feasibility of Iron Loaded ‘Okara’ for Biosorption of Phosphorous in Aqueous Solutions. Bioresour. Technol. 2013, 150, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Hesterberg, D.L. Iron(III) Coordination and Phosphate Sorption in Peat Reacted with Ferric or Ferrous Iron. Soil Sci. Soc. Amer. J. 2012, 76, 101–109. [Google Scholar] [CrossRef]
- Handreck, K.A. Available Phosphorus in Potting Media: Extractants and Interpretation of Extract Data. Commun. Soil Sci. Plant Anal. 1991, 22, 529–557. [Google Scholar] [CrossRef]
- Argo, W.R.; Biernbaum, J.A. The Effect of Irrigation Method, Water-Soluble Fertilization, Replant Nutrient Charge, and Surface Evaporation on Early Vegetative and Root Growth of Poinsettia. J. Am. Soc. Hortic. Sci. 1995, 120, 163–169. [Google Scholar] [CrossRef]
- Handreck, K.A. Assessment of Iron Availability in Soilless Potting Media. Commun. Soil Sci. Plant Anal. 1989, 20, 1297–1320. [Google Scholar] [CrossRef]
- Altland, J.E.; Buamscha, M.G. Nutrient Availability from Douglas Fir Bark in Response to Substrate pH. HortScience 2008, 43, 478–483. [Google Scholar] [CrossRef]
- Shreckhise, J.H. (U.S. Department of Agriculture, Agricultural Research Service, McMinnville, TN, USA). Unpublished work. 2021. [Google Scholar]




| Substrate Property | Mean | sd |
|---|---|---|
| Particle size distribution (% by mass) | ||
| Coarse z | 55 | 5.1 |
| Medium | 30 | 3.4 |
| Fine | 15 | 1.7 |
| Air space y (% by vol.) | 30 | 4.0 |
| Container capacity (% by vol.) | 45 | 2.0 |
| Bulk density (g·cm−3) | 0.17 | 0.002 |
| Soluble | Insoluble | |||
|---|---|---|---|---|
| Element | Mean | sd | Mean | sd |
| μg·cm−3 | ||||
| P | 0.11 | 0.01 | 24.58 | 2.94 |
| K | 18.72 | 0.69 | 158.19 | 12.02 |
| Ca | 4.40 | 0.24 | 428.82 | 33.15 |
| Mg | 1.77 | 0.08 | 108.55 | 14.32 |
| S | 0.44 | 0.02 | 48.94 | 4.37 |
| Cu | 0.01 | 0.00 | 3.57 | 0.94 |
| Fe | 0.46 | 0.02 | 508.52 | 144.40 |
| Mn | 0.17 | 0.01 | 19.63 | 1.50 |
| Mo | 0.00 | 0.00 | 0.17 | 0.09 |
| Zn | 0.95 | 0.04 | 4.48 | 0.16 |
| Na | 8.08 | 0.39 | 1.35 | 0.21 |
| Al | 1.47 | 0.08 | 490.48 | 14.97 |
| Week | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| P (mg·L−1) | |||||||||||||||
| Fertilizer | 21.9 | 22.5 | 22.6 | 22.2 | 22.3 | 22.4 | 23.1 | 23.4 | 23.0 | 22.4 | 22.4 | 22.9 | 22.9 | 22.2 | 23.0 |
| Control | 11.2 a z | 12.9 a | 18.1 a | 17.7 a | 18.8 a | 21.1 a | 20.6 a | 20.7 a | 21.2 a | 21.6 a | 21.7 a | 21.8 a | 22.7 a | 22.5 a | 25 a |
| V1R1 | 6.5 bc | 5.6 b | 9.8 b | 10.7 b | 13.4 b | 16.8 b | 17.7 ab | 18.9 a | 19.6 a | 20.7 a | 21.1 a | 21 ab | 22.5 a | 22.2 a | 24.3 ab |
| V1R2 | 4.7 cd | 5.7 b | 9.5 b | 10.4 bc | 12.5 b | 13.0 c | 14.9 abc | 15.8 b | 16.5 b | 17.9 ab | 18.4 a | 18.4 bc | 19.9 a | 20.0 a | 21.5 bc |
| V1R3 | 3.9 de | 3.2 bc | 5.9 bc | 6.8 bcd | 5.9 cd | 9.0 de | 11.2 c | 9.7 c | 11.2 c | 11.9 cd | 12.8 b | 13.6 d | 15.5 b | 16.2 b | 17.2 d |
| V2R1 | 7.4 b | 3.5 bc | 6.4 bc | 6.7 cd | 8.8 c | 11.4 cd | 14 bc | 14.7 b | 16.6 b | 17.3 ab | 19.6 a | 19.8 ab | 20.5 a | 20.1 a | 22.7 ab |
| V2R2 | 4.4 cde | 2.4 bc | 3.7 cd | 4.4 de | 4.8 d | 7 ef | 9.7 cd | 10.2 c | 11.7 c | 14.2 bc | 14.3 b | 15.6 cd | 16.1 b | 16.8 b | 18.5 cd |
| V2R3 | 2.3 e | 1.0 c | 0.9 d | 1.4 e | 3.7 d | 4.8 f | 4.1 d | 4.7 d | 6.2 d | 7.7 d | 7.8 c | 8.9 e | 9.8 c | 9.7 c | 11.2 e |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fert. vs. control y | <0.0001 | <0.0001 | <0.0001 | 0.0005 | <0.0001 | 0.0024 | 0.0370 | <0.0001 | 0.0001 | 0.0213 | 0.2274 | 0.0120 | 0.3913 | 0.5459 | 0.0064 |
| Substrate P Conc. | ||
|---|---|---|
| Treatment | Top | Bottom |
| μg·cm−3 | ||
| Control | 16.0 | 11.5 |
| FSB vol. pooled means | ||
| V1 | 14.0 | 7.9 *,z |
| V2 | 19.8 | 6.3 ** |
| Fe rate pooled means | ||
| R1 | 18.7 | 9.8 |
| R2 | 17.4 | 7.7 *** |
| R3 | 14.7 | 3.5 *** |
| FSB vol. × Fe rate | ||
| V1R1 | 15.5 | 11.4 |
| V1R2 | 14.2 | 8.5 ** |
| V1R3 | 12.3 | 3.9 *** |
| V2R1 | 21.9 | 8.3 *** |
| V2R2 | 20.5 | 6.9 *** |
| V2R3 | 17.1 | 3.1 *** |
| Significance y | ||
| FSB volume | *** | *** |
| Fe rate | L * | L *** |
| Interaction | NS | NS |
| Week | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Source | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| Significance | ||||||||||||||||||||
| P | Main effect (DL) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Main effect (FS form) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Interaction (DL x FS form) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Control vs. all others z | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | * | NS | NS | NS | |
| Fe | Main effect (DL) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Main effect (FS form) | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Interaction (DL × FS form) | ** | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Control vs. all others | *** | *** | *** | ** | ** | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Control vs. −DL treatments | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Control vs. +DL treatments | *** | *** | ** | *** | *** | ** | *** | *** | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | ** | |
| pH | Main effect (DL) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Main effect (FS form) | NS | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Interaction (DL × FS form) | NS | NS | NS | * | NS | NS | ** | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Control vs. all others | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Control vs. −DL treatments | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | *** | ** | ** | ** | ** | *** | |
| Control vs. +DL treatments | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Substrate | ||||
|---|---|---|---|---|
| Top Layer | Bottom Layer | |||
| Source | Leachate | Water Extract x | Water extract | DTPA Extract w |
| p-values | ||||
| Treatment | <0.0001 | 0.4271 | 0.1043 | 0.0141 |
| Contrasts | ||||
| Main effect (DL) | 0.6503 | 0.2097 | 0.0143 | 0.0145 |
| Main effect (FS form) | 0.6398 | 0.1961 | 0.9216 | 0.2435 |
| Interaction (DL × FS form) | 0.6401 | 0.8463 | 0.2377 | 0.5311 |
| Control vs. all others | <0.0001 | 0.4803 | 0.6966 | 0.0132 |
| Control vs. −DL treatments | <0.0001 | 0.8623 | 0.5222 | 0.0022 |
| Control vs. +DL treatments | <0.0001 | 0.2614 | 0.1820 | 0.1465 |
| Pooled means v | ||||
| mg P/container | mg P/layer | |||
| Control | 42.2 | 21.4 | 5.1 | 4.8 |
| −DL | 15.2 | 22.5 | 5.5 | 8.3 ** |
| +DL | 16.3 | 29.2 | 4.1 | 6.3 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shreckhise, J.H.; Altland, J.E. A Base Layer of Ferrous Sulfate-Amended Pine Bark Reduces Phosphorus Leaching from Nursery Containers. Agronomy 2024, 14, 757. https://doi.org/10.3390/agronomy14040757
Shreckhise JH, Altland JE. A Base Layer of Ferrous Sulfate-Amended Pine Bark Reduces Phosphorus Leaching from Nursery Containers. Agronomy. 2024; 14(4):757. https://doi.org/10.3390/agronomy14040757
Chicago/Turabian StyleShreckhise, Jacob H., and James E. Altland. 2024. "A Base Layer of Ferrous Sulfate-Amended Pine Bark Reduces Phosphorus Leaching from Nursery Containers" Agronomy 14, no. 4: 757. https://doi.org/10.3390/agronomy14040757
APA StyleShreckhise, J. H., & Altland, J. E. (2024). A Base Layer of Ferrous Sulfate-Amended Pine Bark Reduces Phosphorus Leaching from Nursery Containers. Agronomy, 14(4), 757. https://doi.org/10.3390/agronomy14040757








