Classification and Identification of Pinecone Mulching in Blueberry Cultivation Based on Crop Leaf Characteristics and Hyperspectral Data
Abstract
:1. Introduction
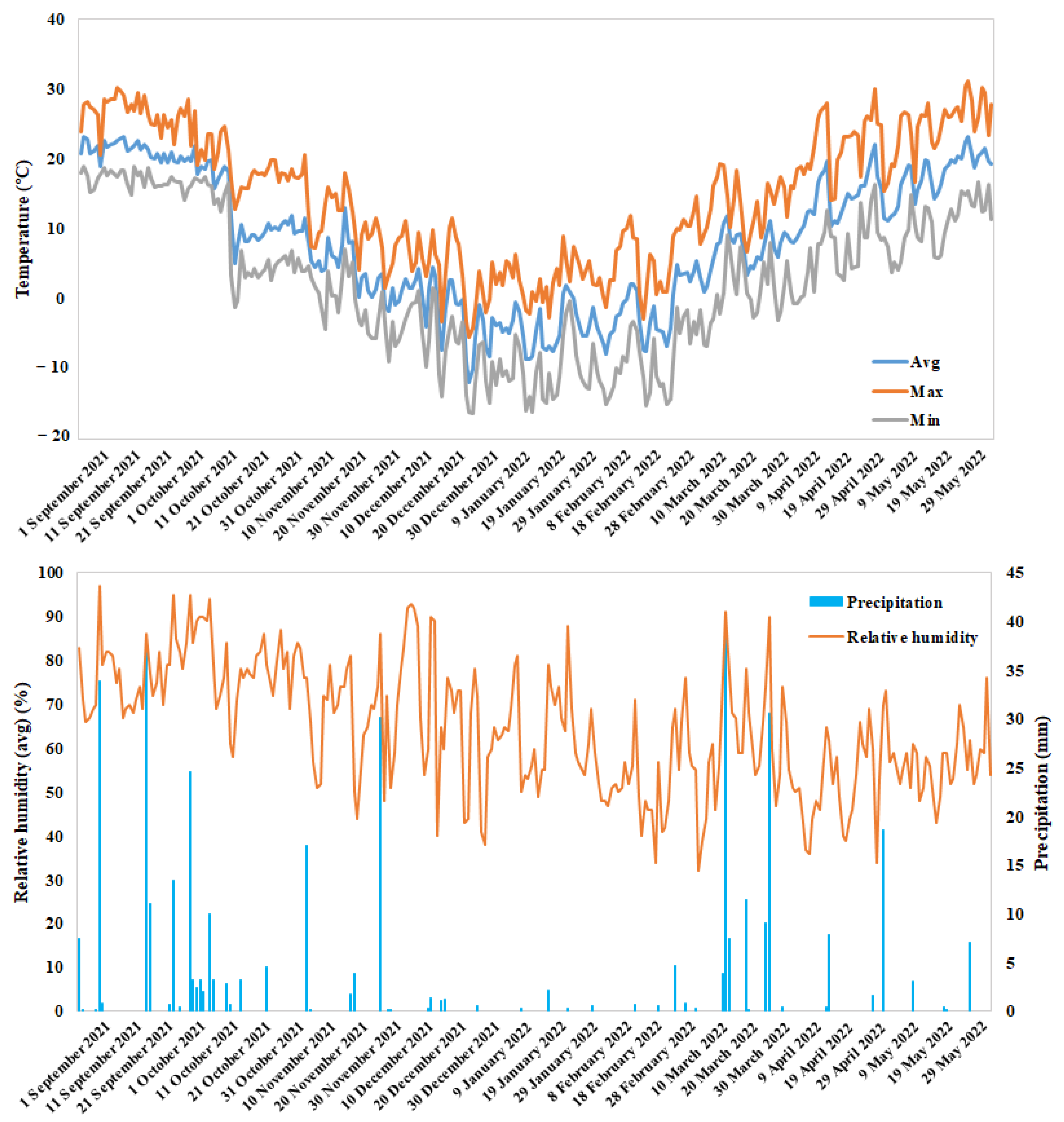
2. Materials and Methods
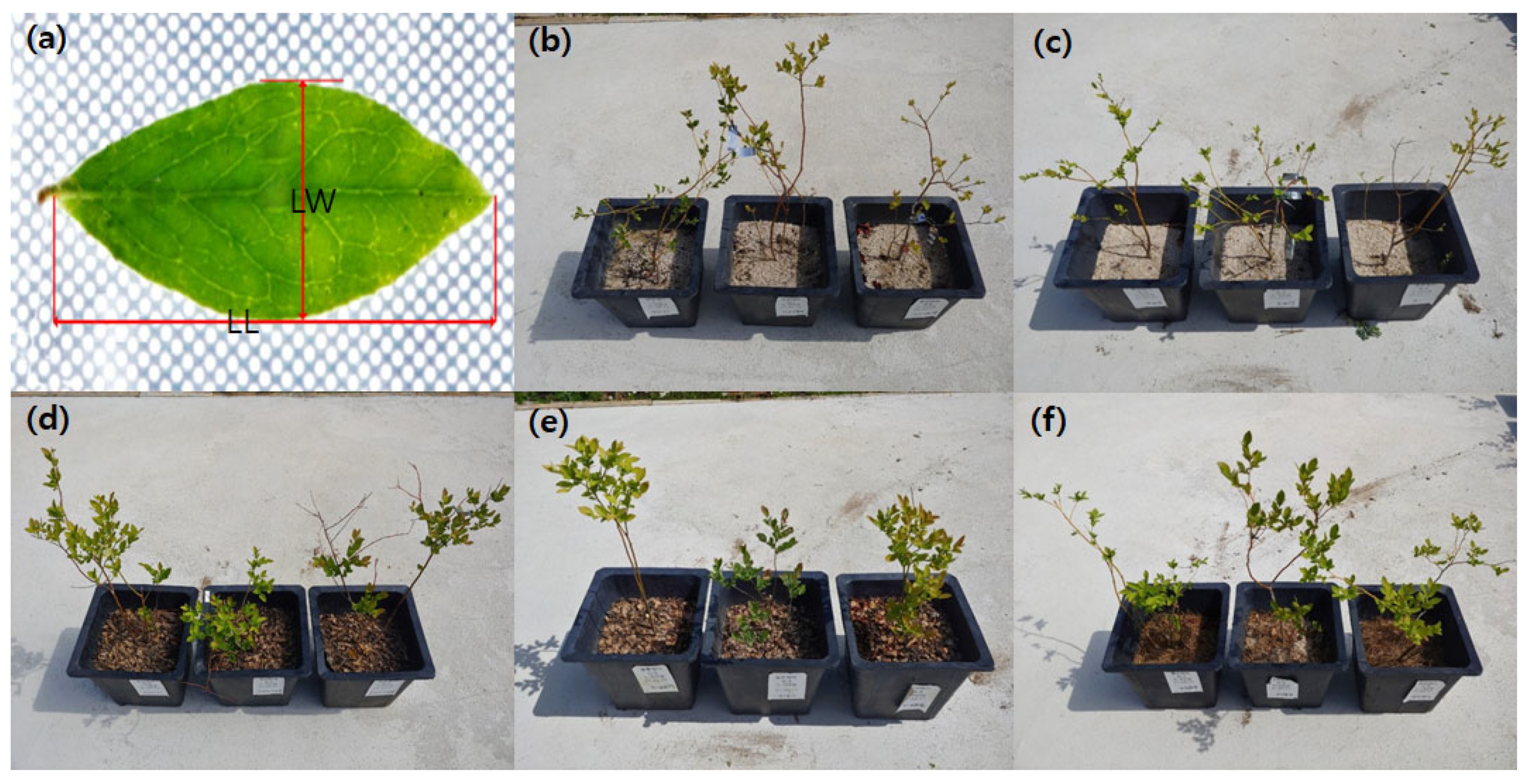
2.1. Plant Materials and Treatments
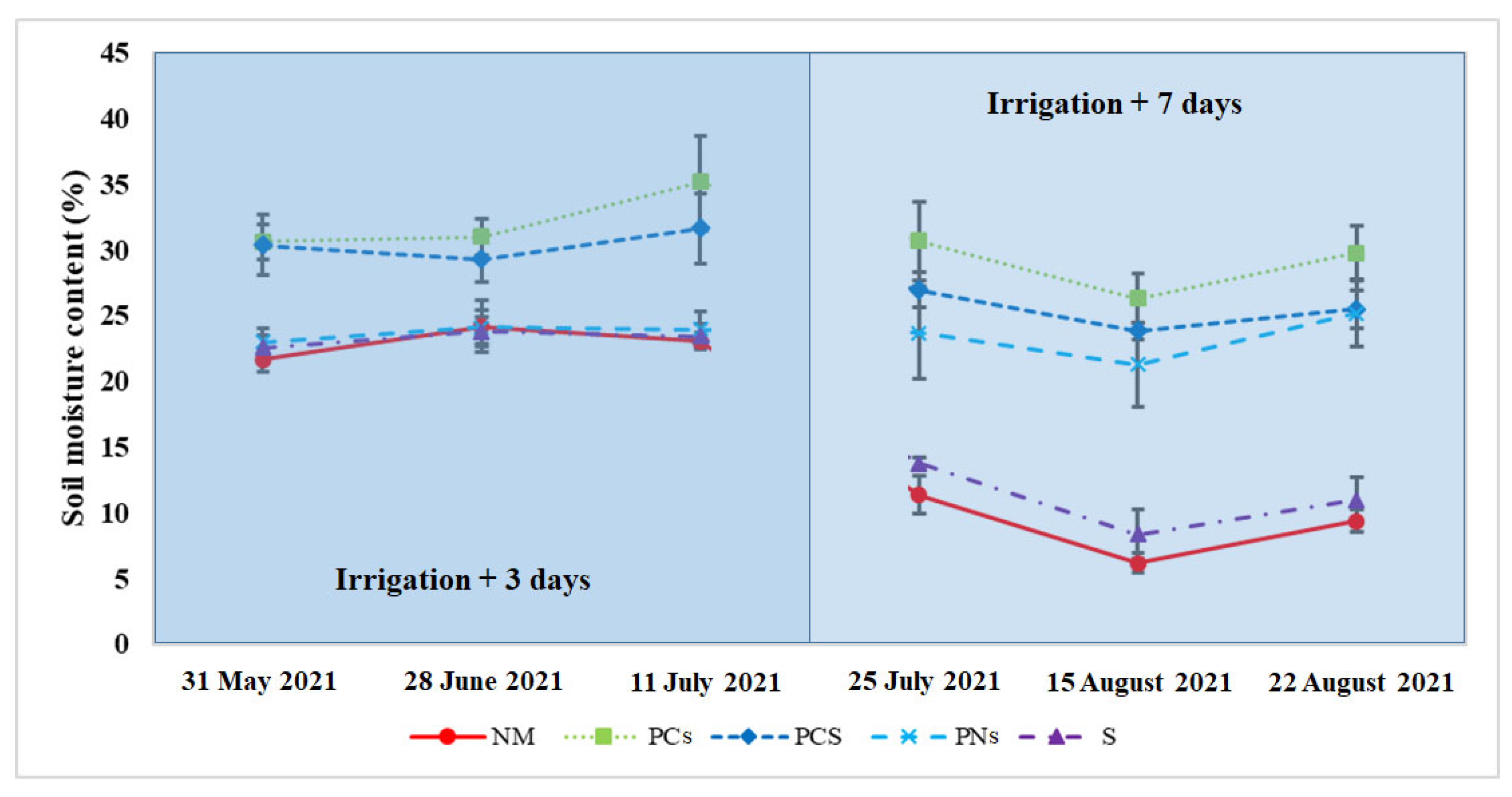
2.2. Soil Characteristic Analysis
2.3. Leaf Growth and Physiological Characteristics
2.4. Hyperspectral Data Collection
2.5. Datasets for Classification
2.6. Statistical Analysis and Machine Learning Methods
3. Results
3.1. Soil Characteristics
3.2. Leaf Growth and Physiological Characteristics
3.3. Vegetation Indices with the Hyperspectral Measurement
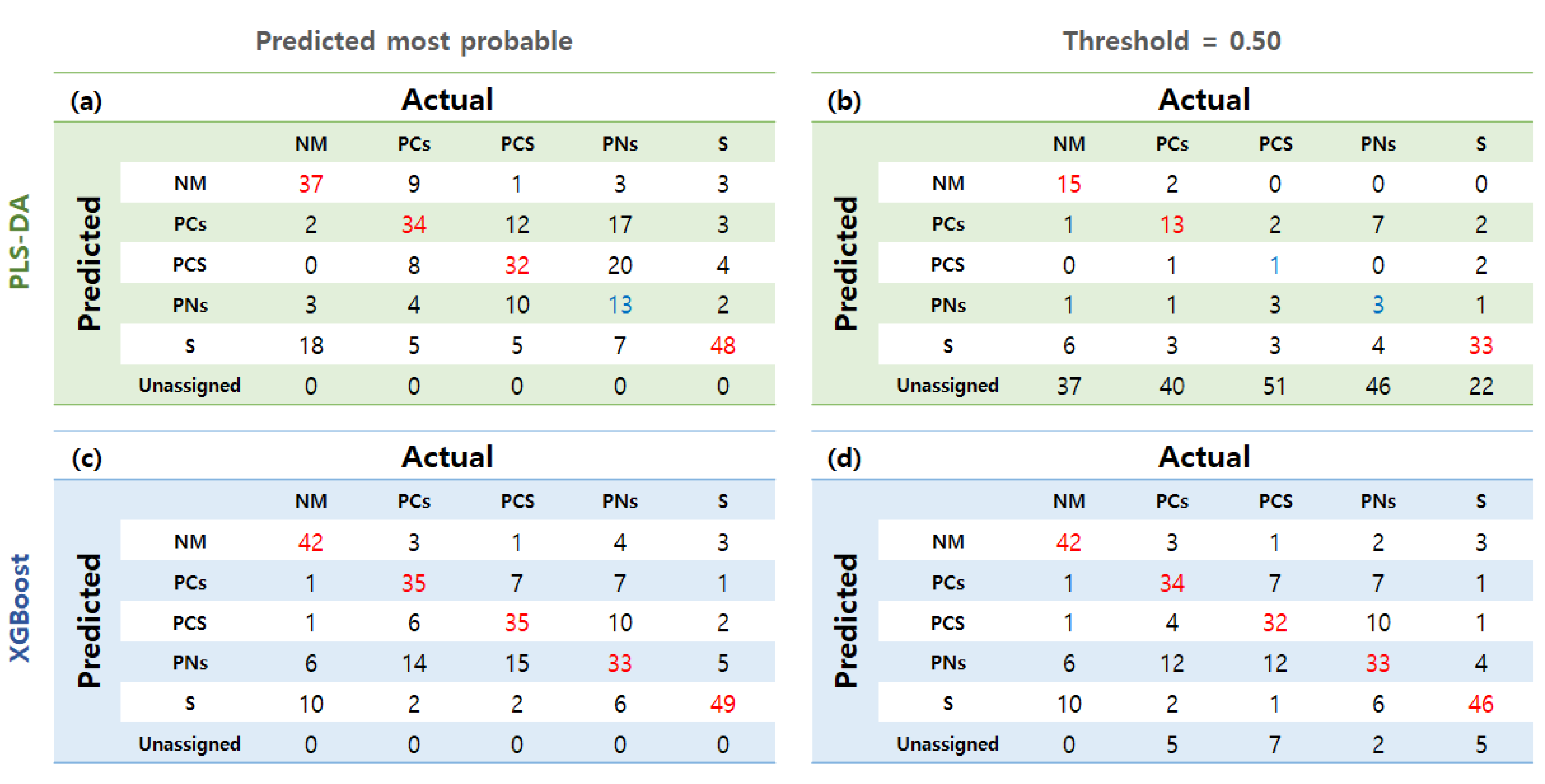
3.4. Classification Modeling with Leaf Growth and Physiological Data
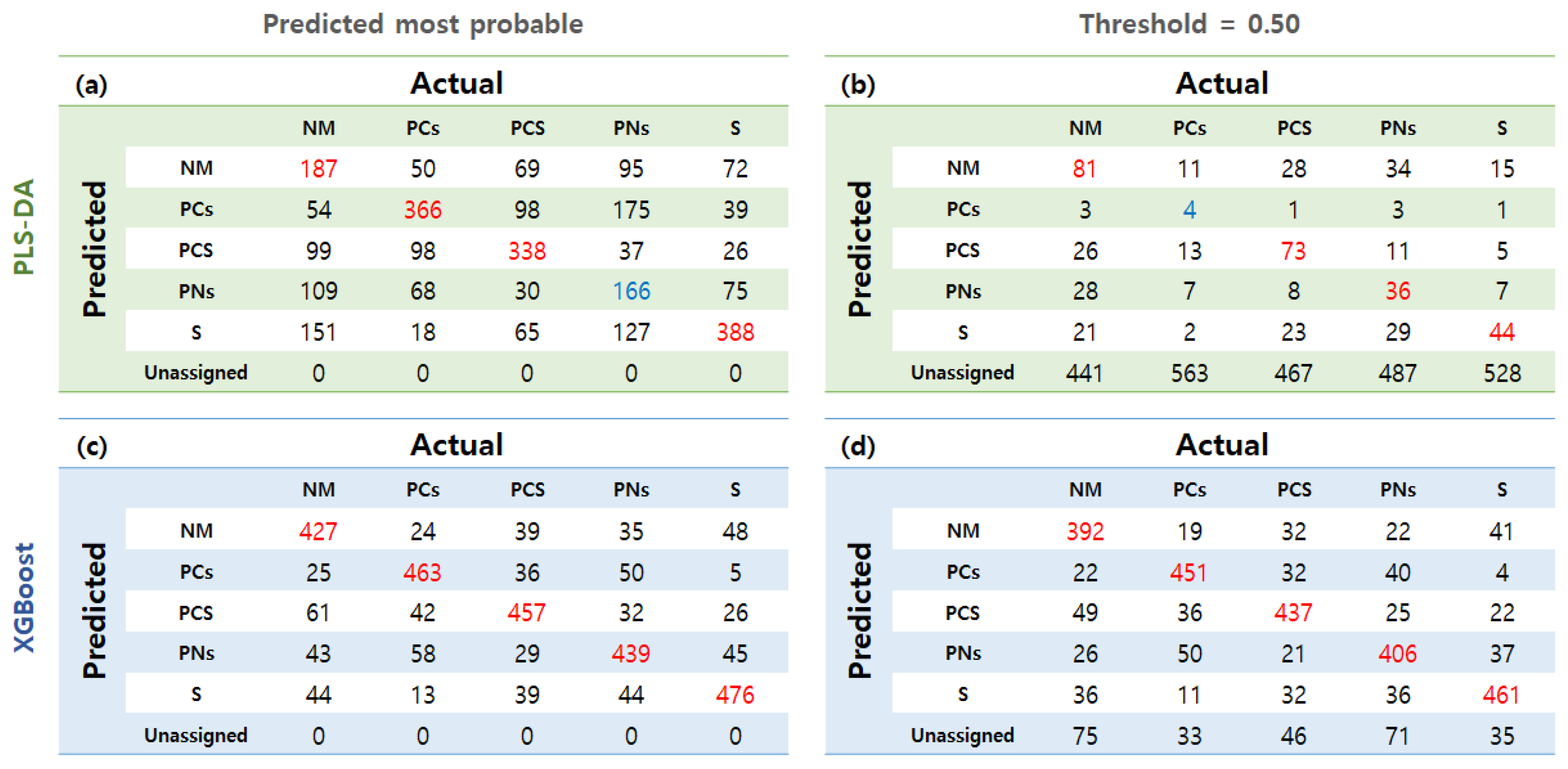
3.5. Classification Modeling with VI Data
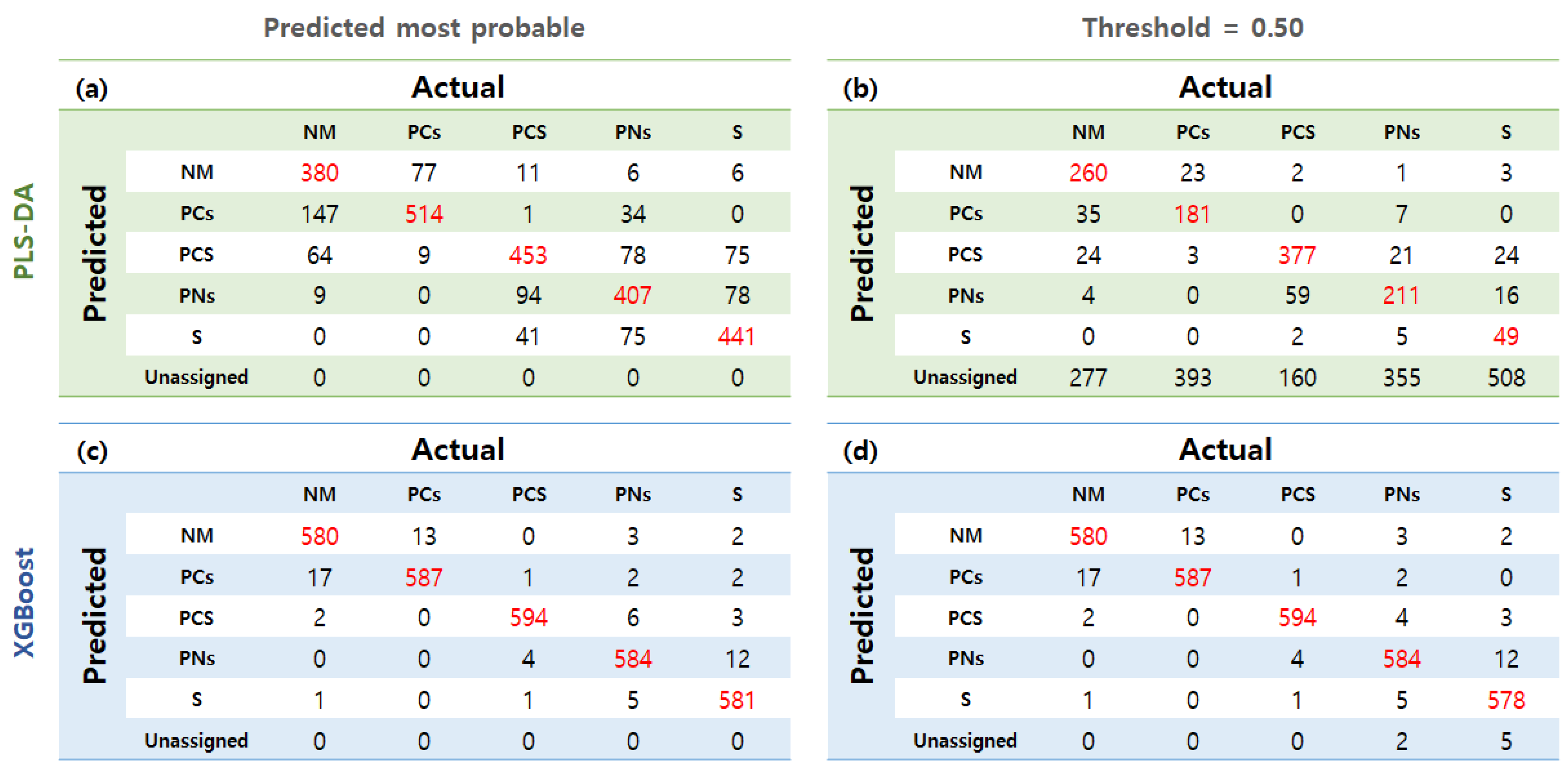
3.6. Classification Modeling with Full VNIR and SWIR Spectrum Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connor, A.M.; Luby, J.J.; Tong, C.B. Variation and heritability estimates for antioxidant activity, total phenolic content, and anthocyanin content in blueberry progenies. J. Am. Soc. Hortic. Sci. 2002, 127, 82–88. [Google Scholar] [CrossRef]
- Burdulis, D.; Sarkinas, A.; Jasutiene, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crops Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. The chemical and biological profiles of leaves from commercial blueberry varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Perez-Arcoiza, A.; Gomez-Jimenez, M.C.; Diaz-Espejo, A. Disentangling the link between leaf photosynthesis and turgor in fruit growth. Plant J. 2021, 107, 1788–1801. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S.; Deb, S.K.; Ritchie, G.L.; Wallace, R.W. Effect of deficit irrigation on physiology, plant growth, and fruit yield of cucumber cultivars. Plant Stress 2021, 1, 100004. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; CABI: Boston, MA, USA, 2018. [Google Scholar]
- Acharya, C.L.; Kapur, O.C. Using organic wastes as compost and mulch for potato (Solanum tuberosum) in low water-retaining hill soils of north-west India. Indian J. Agric. Sci. 2001, 71, 306–509. [Google Scholar]
- Pande, K.K.; Dimri, D.C.; Kamboj, P. Effect of various mulches on growth, yield and quality attributes of apple. Indian J. Hortic. 2005, 62, 145–147. [Google Scholar]
- Burkhard, N.; Lynch, D.; Percival, D.; Sharifi, M. Organic mulch impact on vegetation dynamics and productivity of highbush blueberry under organic production. HortScience 2009, 44, 688–696. [Google Scholar] [CrossRef]
- Goel, L.; Shankar, V.; Sharma, R. Effect of organic mulches on agronomic parameters–A case study of tomato crop (Lycopersicon esculentum Mill.). Int. J. Recycl. Org. Waste Agric. 2020, 9, 297–307. [Google Scholar]
- Lee, J.H.; Kim, K.S.; Shin, S.O.; Cho, J.H.; Chen, Y.J.; Kim, I.H. Effects of Dietary Pine Cone Meal on Egg Production, Egg Quality, Serum Cholesterol and Cholesterol Content and Fatty Acid Composition of Egg Yolk in Laying Hens. Korean J. Poult. 2007, 34, 223–229. (In Korean) [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.W.; Shin, S.O.; Cho, J.H.; Chen, Y.J.; Kim, I.H. Effects of Dietary Pine Cone Meal on Growth Performance, Blood Characteristics, Carcass Quality and Fecal Noxious Gases Compounds in Finishing Pigs. J. Anim. Sci. Technol. 2007, 49, 761–772. (In Korean) [Google Scholar]
- Lee, J.H.; Yang, H.Y.; Lee, H.S.; Hong, S.K. Chemical composition and antimicrobial activity of essential oil from cones of Pinus koraiensis. J. Microbiol. Biotechnol. 2008, 18, 497–502. [Google Scholar]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of copper from aqueous solution by chemically activated pine cone: A kinetic study. J. Chem. Eng. 2011, 175, 260–270. [Google Scholar] [CrossRef]
- Bello, A.; Manyala, N.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Renewable pine cone biomass derived carbon materials for supercapacitor application. RSC Adv. 2016, 6, 1800–1809. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Mac Regenstein, J.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef] [PubMed]
- Larco, H.; Strik, B.C.; Bryla, D.R.; Sullivan, D.M. Mulch and fertilizer management practices for organic production of highbush blueberry. I: Plant growth and allocation of biomass during establishment. HortScience 2013, 48, 1250–1261. [Google Scholar] [CrossRef]
- Han, G.D.; Jung, D.H.; Heo, S.; Chung, Y.S. SPAD value difference between blueberry cultivar ‘STAR’ by planted ground and pot. Phyton 2022, 91, 2583. [Google Scholar]
- Retamal-Salgado, J.; Loor, B.; Hirzel, J.; López, M.D.; Undurraga, P.; Zapata, N.; Vergara-Retamales, R.; Olivares-Soto, H. Chlorophyll fluorescence and fruit quality response of blueberry to different mulches. Agronomy 2022, 12, 1702. [Google Scholar] [CrossRef]
- Calderón-Orellana, A.; Hermosilla, N.; Bastías, R.M. Impact of the Covering Material on Drought Tolerance Responses and Soil Water Content in Two Cultivars of Young Blueberry Plants under Protected Cultivation. Water 2023, 15, 2326. [Google Scholar] [CrossRef]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Henriques, F.S. Leaf chlorophyll fluorescence: Background and fundamentals for plant biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]
- Mayanja, I.K.; Diepenbrock, C.H.; Vadez, V.; Lei, T.; Bailey, B.N. Practical considerations and limitations of using leaf and canopy temperature measurements as a stomatal conductance proxy: Sensitivity across environmental conditions, scale, and sample size. Plant Phenomics 2024, in press. [CrossRef]
- Amodio, M.L.; Chaudhry, M.M.A.; Colelli, G. Spectral and hyperspectral technologies as an additional tool to increase information on quality and origin of horticultural crops. Agronomy 2019, 10, 7. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, P.C.; Petropoulos, G.P.; Pavlides, A.; Srivastava, P.K.; Koutsias, N.; Deng, K.A.K.; Bao, Y. Hyperspectral remote sensing in precision agriculture: Present status, challenges, and future trends. In Hyperspectral Remote Sensing, 1st ed.; Pandey, P.C., Srivastava, P.K., Balzter, H., Bhattacharya, B., Petropoulos, G.P., Eds.; Elsevier: Cambridge, MA, USA, 2020; Volume Earth Observation, pp. 121–146. [Google Scholar]
- Fiorani, F.; Schurr, U. Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef]
- Govender, M.; Govender, P.J.; Weiersbye, I.M.; Witkowski, E.T.F.; Ahmed, F. Review of commonly used remote sensing and ground-based technologies to measure plant water stress. Water SA 2009, 35, 741–752. [Google Scholar] [CrossRef]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Pettorelli, N. The Normalized Difference Vegetation Index, 1st ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Tan, C.W.; Wang, D.L.; Zhou, J.; Du, Y.; Luo, M.; Zhang, Y.J.; Guo, W.S. Assessment of Fv/Fm absorbed by wheat canopies employing in-situ hyperspectral vegetation indexes. Sci. Rep. 2018, 8, 9525. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Marín, D.; Paz, P.; Guerrero, J.E.; Garrido-Varo, A.; Sánchez, M.T. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J. Food Eng. 2010, 99, 294–302. [Google Scholar] [CrossRef]
- Sánchez, M.T.; de la Haba, M.J.; Benítez-López, M.; Fernández-Novales, J.; Garrido-Varo, A.; Pérez-Marín, D. Non-destructive characterization and quality control of intact strawberries based on NIR spectral data. J. Food Eng. 2012, 110, 102–108. [Google Scholar] [CrossRef]
- Furbank, R.T.; Silva-Perez, V.; Evans, J.R.; Condon, A.G.; Estavillo, G.M.; He, W.; Newman, S.; Poiré, R.; Hall, A.; He, Z. Wheat physiology predictor: Predicting physiological traits in wheat from hyperspectral reflectance measurements using deep learning. Plant Methods 2021, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Ramraj, S.; Uzir, N.; Sunil, R.; Banerjee, S. Experimenting XGBoost algorithm for prediction and classification of different datasets. IET Control Theory Appl. 2016, 9, 651–662. [Google Scholar]
- Azim, M.A.; Islam, M.K.; Rahman, M.M.; Jahan, F. An effective feature extraction method for rice leaf disease classification. Telkomnika 2021, 19, 463–470. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, Y.; Wei, L.; Yang, X.; Huang, C. Study on the Optimization of Hyperspectral Characteristic Bands Combined with Monitoring and Visualization of Pepper Leaf SPAD Value. Sensors 2021, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.; Kim, S.H.; Maeng, W.Y.; Lee, I.E.; Chang, K.W.; Lee, J.J. Effects of soil acidity and organic matter by application of organic materials and soil mulching with pine needles for soil surface management in blueberry eco-friendly farming. Korean J. Soil Sci. 2013, 46, 556–562. (In Korean) [Google Scholar] [CrossRef]
- Tyurin, I.V. A new modification of the volumetric method of determining soil organic matter by means of chromic acid. Pochvovedenie 1931, 26, 36–47. [Google Scholar]
- Cox, M. The Lancaster soil test method as an alternative to the Mehlich 3 soil test method. Soil Sci. 2001, 166, 484–489. [Google Scholar] [CrossRef]
- National Institute of Agricultural Science and Technology. Methods of Soil and Plant Analysis; Rural Development Administration (NIAST): Suwon, Republic of Korea, 2000; pp. 103–146. (In Korean) [Google Scholar]
- Mänd, P.; Hallik, L.; Peñuelas, J.; Nilson, T.; Duce, P.; Emmett, B.A.; Beier, C.; Estiarte, M.; Garadnai, J.; Kalapos, T.; et al. Responses of the reflectance indices PRI and NDVI to experimental warming and drought in European shrublands along a north–south climatic gradient. Remote Sens. Environ. 2010, 114, 626–636. [Google Scholar] [CrossRef]
- Daughtry, C.S.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey Iii, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Rock, B.N. Detection of changes in leaf water content using near-and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Cho, M.; Sobhan, I.; Skidmore, A.; de Leeuw, J. Discriminating species using hyperspectral indices at leaf and canopy scales. In Proceedings of the XXI Congress: Silk Road for Information from Imagery, Beijing, China, 3–11 July 2008; pp. 369–376. [Google Scholar]
- Haubrock, S.N.; Chabrillat, S.; Lemmnitz, C.; Kaufmann, H. Surface soil moisture quantification models from reflectance data under field conditions. Int. J. Remote Sens. 2008, 29, 3–29. [Google Scholar] [CrossRef]
- Albert, T.; Karp, K.; Starast, M.; Paal, T. The effect of mulching and pruning on the vegetative growth and yield of the half-high blueberry. Agron. Res. 2010, 8, 759–769. [Google Scholar]
- Peterson, L.A.; Stang, E.J.; Dana, M.N. Blueberry Response to NH4-N and NO3-N. J. Am. Soc. Hortic. Sci. 1988, 113, 9–12. [Google Scholar] [CrossRef]
- Muñoz-Vega, P.; Paillán, H.; Serri, H.; Donnay, D.; Sanhueza, C.; Merino, E.; Hirzel, J. Effects of organic fertilizers on the vegetative, nutritional, and productive parameters of blueberries ‘Corona’, ‘Legacy’, and ‘Liberty’. Chil. J. Agric. Res. 2016, 76, 201–212. [Google Scholar] [CrossRef]
- Iqbal, M.; Bakesh, P.; Kumar, R.; Wali, V.K.; Bhushan, B. Influence of Mulching on Fruit Quality of Aonla (Emblica officinalis Gaertn.) Cv. Na-7. Ecol. Environ. Conserv. 2015, 21, 263–268. [Google Scholar]
- Karp, K.; Noormets, M.; Starast, M.; Paal, T. The influence of mulching on nutrition and yield of Northblue blueberry. In Proceedings of the VIII International Symposium on Vaccinium Culture, Sevilla, Spain; Oeiras, Portugal, 3–8 May 2004; pp. 301–306. [Google Scholar]
- Akhtar, K.; Wang, W.; Khan, A.; Ren, G.; Afridi, M.Z.; Feng, Y.; Yang, G. Wheat straw mulching offset soil moisture deficient for improving physiological and growth performance of summer sown soybean. Agric. Water Manag. 2019, 211, 16–25. [Google Scholar] [CrossRef]
- Dvořák, P.; Martin, K.R.Á.L. Effects of organic mulching on soil water potential and SPAD values as factors on yield of potatoes (Solanum tuberosum L.). J. Agric. Sci. 2019, 25, 147–154. [Google Scholar] [CrossRef]
- Percival, G.C.; Keary, I.P.; Noviss, K. The potential of a chlorophyll content SPAD meter to quantify nutrient stress in foliar tissue of sycamore (Acer pseudoplatanus), English oak (Quercus robur), and European beech (Fagus sylvatica). Arboric. Urban For. 2008, 34, 89–100. [Google Scholar] [CrossRef]
- Kumagai, E.; Araki, A.; Kubota, F. Correlation of chlorophyll meter readings with gas exchange and chlorophyll fluorescence in flag leaves of rice (Oryza sativa L.) plants. Plant Prod. Sci. 2009, 12, 50–53. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Wei, J.; Jiang, J.; Li, Y.; Chen, J.; Yu, H. Growth, fruit yield, photosynthetic characteristics, and leaf microelement concentration of two blueberry cultivars under different long-term soil pH treatments. Agronomy 2019, 9, 357. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zeng, Q.; Wei, J.; Yu, H. The effect of soil pH on plant growth, leaf chlorophyll fluorescence and mineral element content of two blueberries. In Proceedings of the XI International Vaccinium Symposium, Orlando, FL, USA, 10–14 April 2016; pp. 269–276. [Google Scholar]
- Farzi, R.; Gholami, M.; Baninasab, B.; Gheysari, M. Evaluation of different mulch materials for reducing soil surface evaporation in semi-arid region. Soil Use Manag. 2017, 33, 120–128. [Google Scholar] [CrossRef]
- Rho, H.; Yu, D.J.; Kim, S.J.; Lee, H.J. Limitation factors for photosynthesis in ‘Bluecrop’ highbush blueberry (Vaccinium corymbosum) leaves in response to moderate water stress. J. Plant Biol. 2012, 55, 450–457. [Google Scholar] [CrossRef]
- Oliveira, R.M.D.; Cunha, F.F.D.; Silva, G.H.D.; Andrade, L.M.; Morais, C.V.D.; Ferreira, P.M.O.; Raimundi, F.P.G.; Freitas, A.R.J.; Souza, C.M.D.; Oliveira, R.A.D. Evapotranspiration and crop coefficients of Italian zucchini cultivated with recycled paper as mulch. PLoS ONE 2020, 15, e0232554. [Google Scholar] [CrossRef] [PubMed]
- Kovar, M.; Brestic, M.; Sytar, O.; Barek, V.; Hauptvogel, P.; Zivcak, M. Evaluation of hyperspectral reflectance parameters to assess the leaf water content in soybean. Water 2019, 11, 443. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Yang, Y.; Hu, G.; Ge, D.; Liu, H.; Cao, H. A cloud computing-based approach using the visible near-infrared spectrum to classify greenhouse tomato plants under water stress. Comput. Electron. Agric. 2021, 181, 105966. [Google Scholar]
- Bruno, T.J.; Svoronos, P.D. CRC Handbook of Fundamental Spectroscopic Correlation Charts, 1st ed.; CRC Press: Florida, FL, USA, 2005. [Google Scholar]
- Liu, C.; Zhang, G.; Zhu, H.; Zhu, Y. Effects of irrigation and nitrogen-sulfur combined application on grain yield and water use efficiency in winter wheat. J. Henan Agric. Sci. 2015, 44, 13–18. [Google Scholar]
- Virnodkar, S.S.; Pachghare, V.K.; Patil, V.C.; Jha, S.K. Remote sensing and machine learning for crop water stress determination in various crops: A critical review. Precis. Agric. 2020, 21, 1121–1155. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, K.; Jin, X.; Ji, J.; Zhu, X. Identification of Blueberry Fruit Maturity Using Hyperspectral Images Technology. J. Agric. Sci. Technol. 2020, 22, 1008-0864. [Google Scholar]
- Huang, Y.; Wang, D.; Liu, Y.; Zhou, H.; Sun, Y. Measurement of early disease blueberries based on vis/nir hyperspectral imaging system. Sensors 2020, 20, 5783. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, J.; Kim, J.; Jeong, S.; Kim, M.; Park, S.; Moon, K.D. Hyperspectral imaging technigue for monitoring moisture content of blueberry during the drying process. Food Sci. Preserv. 2021, 28, 445–455. [Google Scholar] [CrossRef]
- Zheng, Z.; An, Z.; Liu, X.; Chen, J.; Wang, Y. Finite element analysis and near-infrared hyperspectral reflectance imaging for the determination of blueberry bruise grading. Foods 2022, 11, 1899. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.B.; McGonigle, A.J.; Willmott, J.R. Hyperspectral imaging in environmental monitoring: A review of recent developments and technological advances in compact field deployable systems. Sensors 2019, 19, 3071. [Google Scholar] [CrossRef] [PubMed]
- Damour, G.; Simonneau, T.; Cochard, H.; Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010, 33, 1419–1438. [Google Scholar] [CrossRef] [PubMed]

| Data Set (Data Format) | Parameters and Wavelengths | References |
|---|---|---|
| Leaf growth and physiology data (200 measured data × 5 treatments × 10 parameters) | 1. LW; 2. LL; 3. LW/LL; 4. SPAD; 5. Fo; 6. Fm; 7. Fv/Fm; 8. ABS/RC; 9. DIo/RC; 10. gs | |
| VI data (2000 measured data × 5 treatments × 8 VIs) | 1. Normalized difference vegetation index (NDVI): (R800 − R670)/(R800 + R670) | [45] |
| 2. Modified chlorophyll absorption ratio index (MCARI): [(R700 − R670) − 0.2 × (R700 − R550)] × (R700/R670) | [46] | |
| 3. Soil-adjusted vegetation index (SAVI): (1 + 0.16) × (R800 − R670)/(R800 + R670 + 0.16) | [47] | |
| 4. Photochemical reflectance index (PRI): (R570 − R531)/(R531 + R570) | [48] | |
| 5. Moisture stress index (MSI): R1600/R820 | [49] | |
| 6. Normalized difference water index (NDWI): (R860 − R1240)/(R860 + R1240) | [50] | |
| 7. Disease water stress index (DWSI): (R802 + R547)/(R1657 + R682) | [51] | |
| 8. Normalized soil moisture index (NSMI): (R1800 − R2119)/(R1800 + R2119) | [52] | |
| Full VNIR spectra (2000 measured reflectance (%) data × 5 treatments × 466 wavelengths) | R485–950 nm (units of 1 nm) | |
| Full SWIR spectra (2000 measured reflectance (%) data × 5 treatments × 378 wavelengths) | R1101–2300 nm (Units of 3 nm) |
| Actual Outcome | Types of Performance Evaluation | |||
|---|---|---|---|---|
| True | False | |||
| Predicted outcome | True | True positive (Tp) | False positive (Fp) | Precision = Tp/(Tp + Fp) Recall = Tp/(Tp + Fn) Accuracy = (Tp + Tn)/(Tp + Fp + Tn + Fn) False Positive Rate (FPR) = Fp/(Tn + Fp) F1 score = (2 × Precision × Recall)/(Precision + Recall) |
| False | False negative (Fn) | True negative (Tn) | ||
| pH | OM (%) | Available P2O5 (mg/kg) | TN (mg/g) | NH4+-N (mg/g) | NO3−-N (mg/g) | Exchangeable Cation (cmol/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | Na+ | Mg2+ | |||||||
| NM | 6.46 | 4.16 | 10.80 | 0.61 | 0.10 | 0.03 | 0.06 | 3.25 | 0.65 | 0.51 |
| PCs | 5.89 | 7.02 | 29.10 | 1.20 | 0.04 | 0.07 | 0.28 | 2.57 | 0.18 | 0.56 |
| PCS | 5.81 | 2.80 | 26.70 | 0.96 | 0.04 | 0.01 | 0.21 | 2.22 | 0.20 | 0.48 |
| PNs | 5.87 | 1.79 | 11.40 | 0.68 | 0.05 | 0.03 | 0.06 | 3.10 | 0.22 | 0.48 |
| S | 5.59 | 2.05 | 14.60 | 0.70 | 0.06 | 0.05 | 0.06 | 3.06 | 0.22 | 0.44 |
| LW (cm) | LL (cm) | LW/LL | SPAD | Fo/100 | Fm/100 | Fv/Fm | ABS/RC | DIo/RC | gs (mmol/m2/s) | |
|---|---|---|---|---|---|---|---|---|---|---|
| NM | 0.81 ± 0.22 d | 1.57 ± 0.42 c | 0.52 ± 0.09 a | 21.95 ± 6.16 bc | 48.13 ± 13.48 c | 195.18 ± 56.05 c | 0.75 ± 0.05 c | 1.81 ± 0.40 a | 0.47 ± 0.19 a | 148.89 ± 33.21 b |
| PCs | 1.23 ± 0.37 c | 2.67 ± 0.53 b | 0.46 ± 0.11 b | 24.94 ± 5.41 a | 49.25 ± 7.23 bc | 235.07 ± 36.54 b | 0.79 ± 0.03 a | 1.63 ± 0.21 c | 0.35 ± 0.09 bc | 195.09 ± 60.90 a |
| PCS | 1.52 ± 0.41 a | 2.97 ± 0.80 a | 0.52 ± 0.07 a | 23.09 ± 6.58 b | 49.07 ± 6.02 bc | 237.26 ± 30.62 b | 0.79 ± 0.02 a | 1.55 ± 0.15 d | 0.32 ± 0.06 c | 187.23 ± 54.93 a |
| PNs | 1.38 ± 0.39 b | 2.72 ± 0.76 b | 0.52 ± 0.11 a | 21.90 ± 4.38 bc | 50.98 ± 7.92 b | 230.17 ± 39.56 b | 0.78 ± 0.02 b | 1.69 ± 0.16 bc | 0.38 ± 0.06 b | 190.80 ± 56.11 a |
| S | 0.85 ± 0.32 d | 1.64 ± 0.59 c | 0.53 ± 0.12 a | 21.22 ± 4.79 c | 54.52 ± 6.84 a | 258.44 ± 31.26 a | 0.79 ± 0.02 a | 1.72 ± 0.20 b | 0.37 ± 0.07 b | 117.45 ± 26.72 c |
| NDVI | MCARI | SAVI | PRI × 10 | MSI | NDWI × 10 | DWSI | NSMI | |
|---|---|---|---|---|---|---|---|---|
| NM | 0.86 ± 0.05 c | 157.63 ± 36.24 a | 1.00 ± 0.06 c | 0.25 ± 0.31 c | 0.60 ± 0.09 b | 0.04 ± 0.66 b | 1.92 ± 0.27 b | 0.31 ± 0.03 d |
| PCs | 0.88 ± 0.05 a | 136.18 ± 53.38 c | 1.02 ± 0.05 a | 0.06 ± 0.28 d | 0.56 ± 0.09 c | 0.23 ± 0.59 a | 2.00 ± 0.29 a | 0.34 ± 0.03 a |
| PCS | 0.87 ± 0.06 b | 129.90 ± 46.08 d | 1.01 ± 0.07 b | −0.10 ± 0.30 e | 0.60 ± 0.11 b | 0.03 ± 0.76 b | 1.92 ± 0.31 b | 0.32 ± 0.02 c |
| PNs | 0.86 ± 0.05 c | 141.17 ± 47.38 b | 0.99 ± 0.06 c | 0.32 ± 0.28 b | 0.60 ± 0.10 b | −0.03 ± 0.68 c | 1.91 ± 0.27 b | 0.33 ± 0.03 b |
| S | 0.81 ± 0.07 d | 142.26 ± 43.80 b | 0.94 ± 0.08 d | 0.44 ± 0.34 a | 0.68 ± 0.11 a | −0.39 ± 0.66 d | 1.73 ± 0.26 c | 0.30 ± 0.03 e |
| PLS-DA (“VIP Scores > 1” Rank) | XGBoost (“Variable Scores” Rank) | |
|---|---|---|
| Leaf growth and physiological data | 1. LL; 2. LW; 3. gs | 1. gs; 2. SPAD; 3. ABS/RC; 4. Fm; 5. LW/LL; 6. Fo; 7. Fv/Fm; 8. LL; 9. DIo/RC; 10. LW |
| VI data | 1. NSMI; 2. PRI | 1. PRI; 2. MCARI; 3. NSMI; 4. NDVI; 5. NDWI; 6. MSI; 7. DWSI; 8. SAVI |
| Full VNIR spectra | 1. 675 nm; 2. 553 nm; 3. 527 nm; 4. 737 nm; 5. 783 nm | 1. 827 nm; 2. 690 nm; 3. 784 nm; 4. 514 nm; 5. 749 nm |
| Full SWIR spectra | 1. 1401 nm; 2. 1935 nm; 3. 1660 nm | 1. 1875 nm; 2. 2106 nm; 3. 1455 nm |
| Parameter | Model | NM | PCs | PCS | PNs | S | |
|---|---|---|---|---|---|---|---|
| Leaf growth and physiology | PLS-DA | P.M.P | 0.775 | 0.713 | 0.700 | 0.569 | 0.827 |
| Thres: 0.5 | 0.621 | 0.583 | 0.502 | 0.513 | 0.742 | ||
| XGBoost | P.M.P | 0.827 | 0.758 | 0.752 | 0.692 | 0.867 | |
| Thres: 0.5 | 0.831 | 0.750 | 0.733 | 0.704 | 0.844 | ||
| VIs | PLS-DA | P.M.P | 0.596 | 0.729 | 0.728 | 0.580 | 0.748 |
| Thres: 0.5 | 0.549 | 0.502 | 0.549 | 0.520 | 0.521 | ||
| XGBoost | P.M.P | 0.825 | 0.862 | 0.847 | 0.829 | 0.868 | |
| Thres: 0.5 | 0.803 | 0.855 | 0.837 | 0.810 | 0.860 | ||
| VNIR | PLS-DA | P.M.P | 0.796 | 0.890 | 0.830 | 0.801 | 0.843 |
| Thres: 0.5 | 0.711 | 0.642 | 0.799 | 0.659 | 0.539 | ||
| XGBoost | P.M.P | 0.980 | 0.985 | 0.993 | 0.983 | 0.983 | |
| Thres: 0.5 | 0.980 | 0.985 | 0.993 | 0.983 | 0.980 | ||
| SWIR | PLS-DA | P.M.P | 0.615 | 0.794 | 0.552 | 0.585 | 0.683 |
| Thres: 0.5 | 0.501 | 0.664 | 0.500 | 0.532 | 0.506 | ||
| XGBoost | P.M.P | 0.983 | 0.985 | 0.967 | 0.965 | 0.968 | |
| Thres: 0.5 | 0.984 | 0.984 | 0.965 | 0.962 | 0.968 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, U.; Jang, T.; Kim, D.; Cheong, E.J. Classification and Identification of Pinecone Mulching in Blueberry Cultivation Based on Crop Leaf Characteristics and Hyperspectral Data. Agronomy 2024, 14, 785. https://doi.org/10.3390/agronomy14040785
Jeong U, Jang T, Kim D, Cheong EJ. Classification and Identification of Pinecone Mulching in Blueberry Cultivation Based on Crop Leaf Characteristics and Hyperspectral Data. Agronomy. 2024; 14(4):785. https://doi.org/10.3390/agronomy14040785
Chicago/Turabian StyleJeong, Ukhan, Taeyoung Jang, Dohee Kim, and Eun Ju Cheong. 2024. "Classification and Identification of Pinecone Mulching in Blueberry Cultivation Based on Crop Leaf Characteristics and Hyperspectral Data" Agronomy 14, no. 4: 785. https://doi.org/10.3390/agronomy14040785
APA StyleJeong, U., Jang, T., Kim, D., & Cheong, E. J. (2024). Classification and Identification of Pinecone Mulching in Blueberry Cultivation Based on Crop Leaf Characteristics and Hyperspectral Data. Agronomy, 14(4), 785. https://doi.org/10.3390/agronomy14040785






