Differences in Soil Microflora between the Two Chinese Geographical Indication Products of “Tricholoma matsutake Shangri-la” and “T. matsutake Nanhua”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples
2.2. Soil Physical and Chemical Property Testing
2.3. Microbial Diversity Sequencing
2.4. Data Statistics and Analysis
3. Results
3.1. Comparison of Soil Physical and Chemical Properties
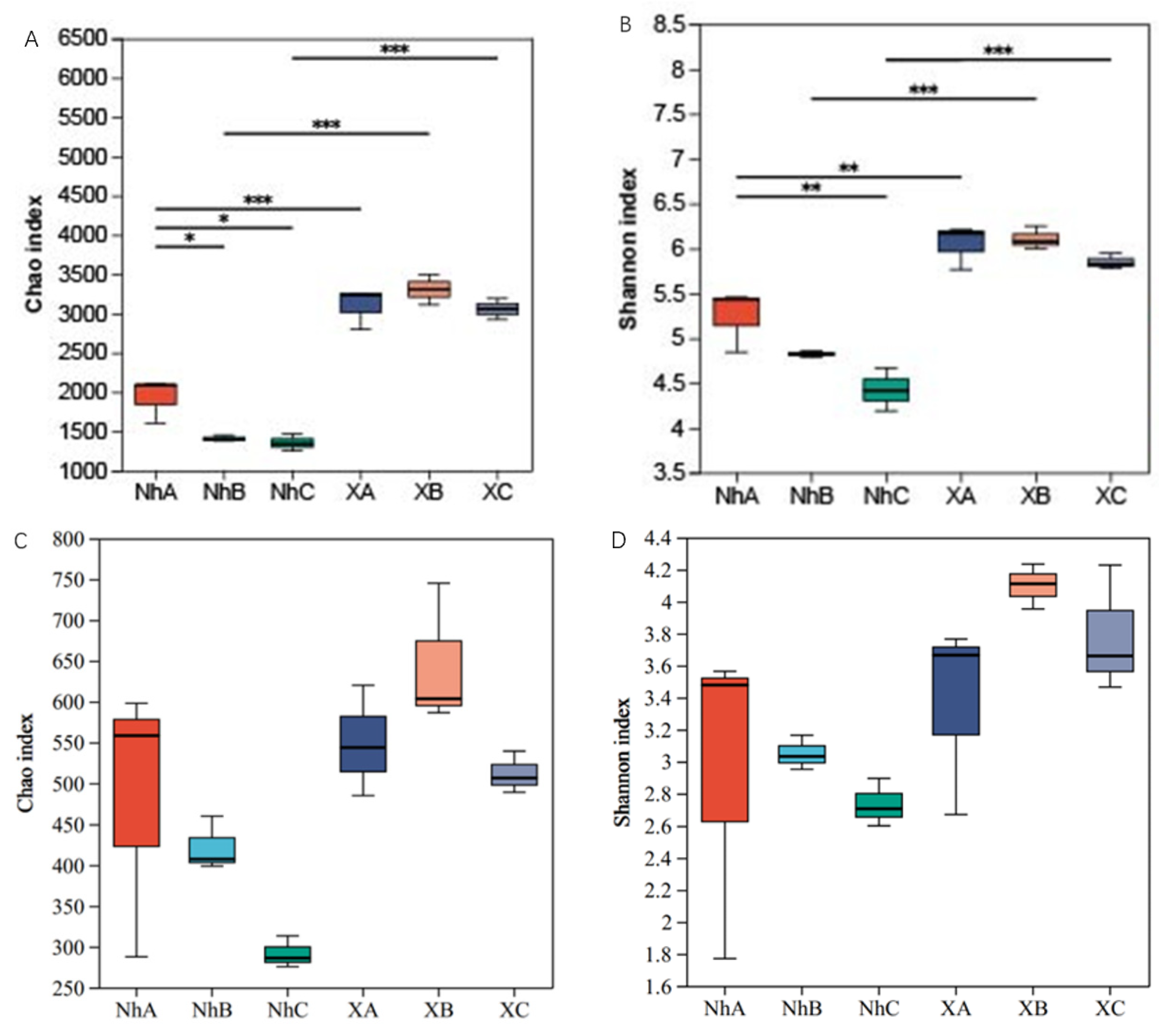
3.2. Analysis of Soil Microbial Diversity
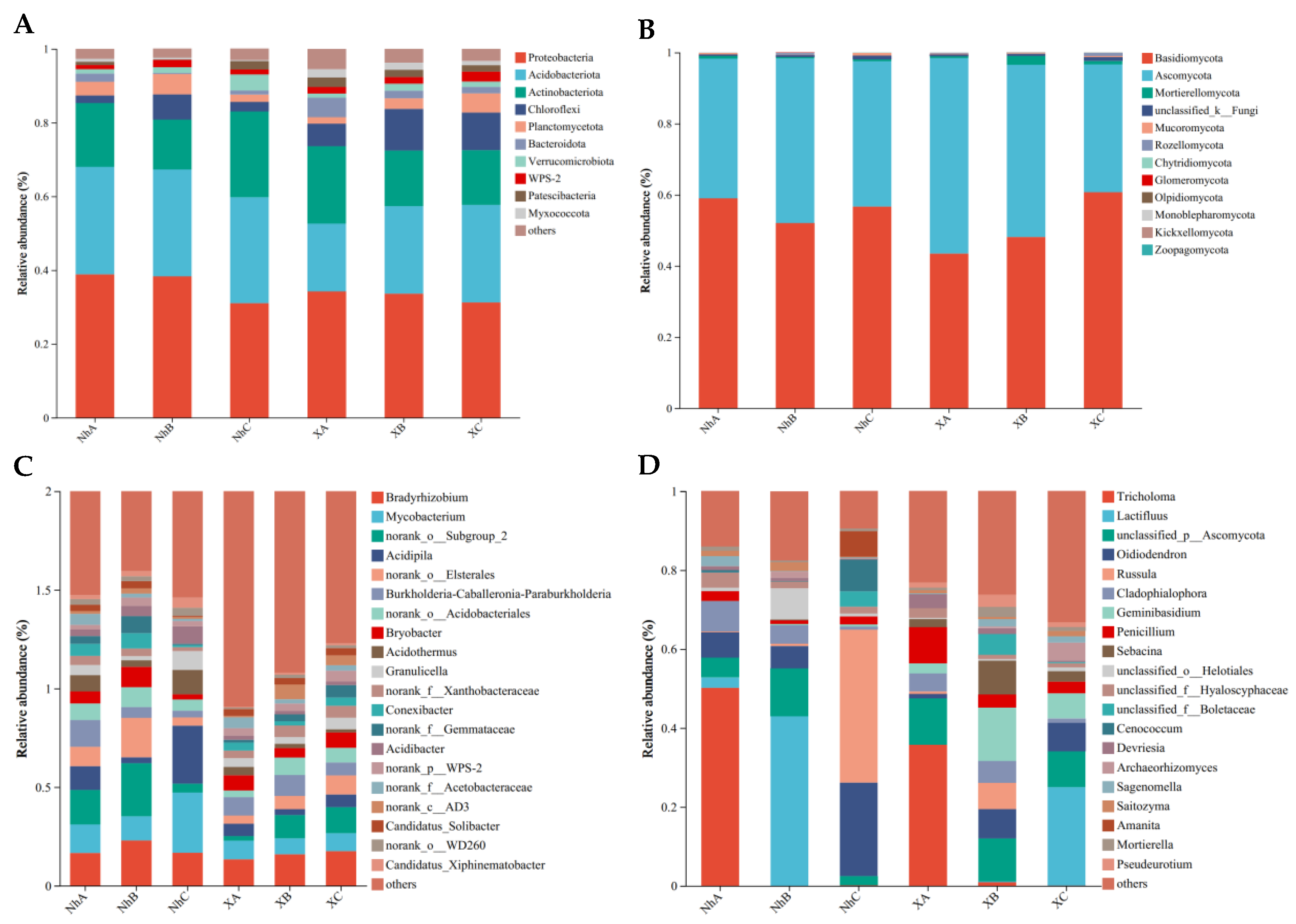
3.3. Soil Microbial Community Structure
3.4. Functional Predictive Analysis
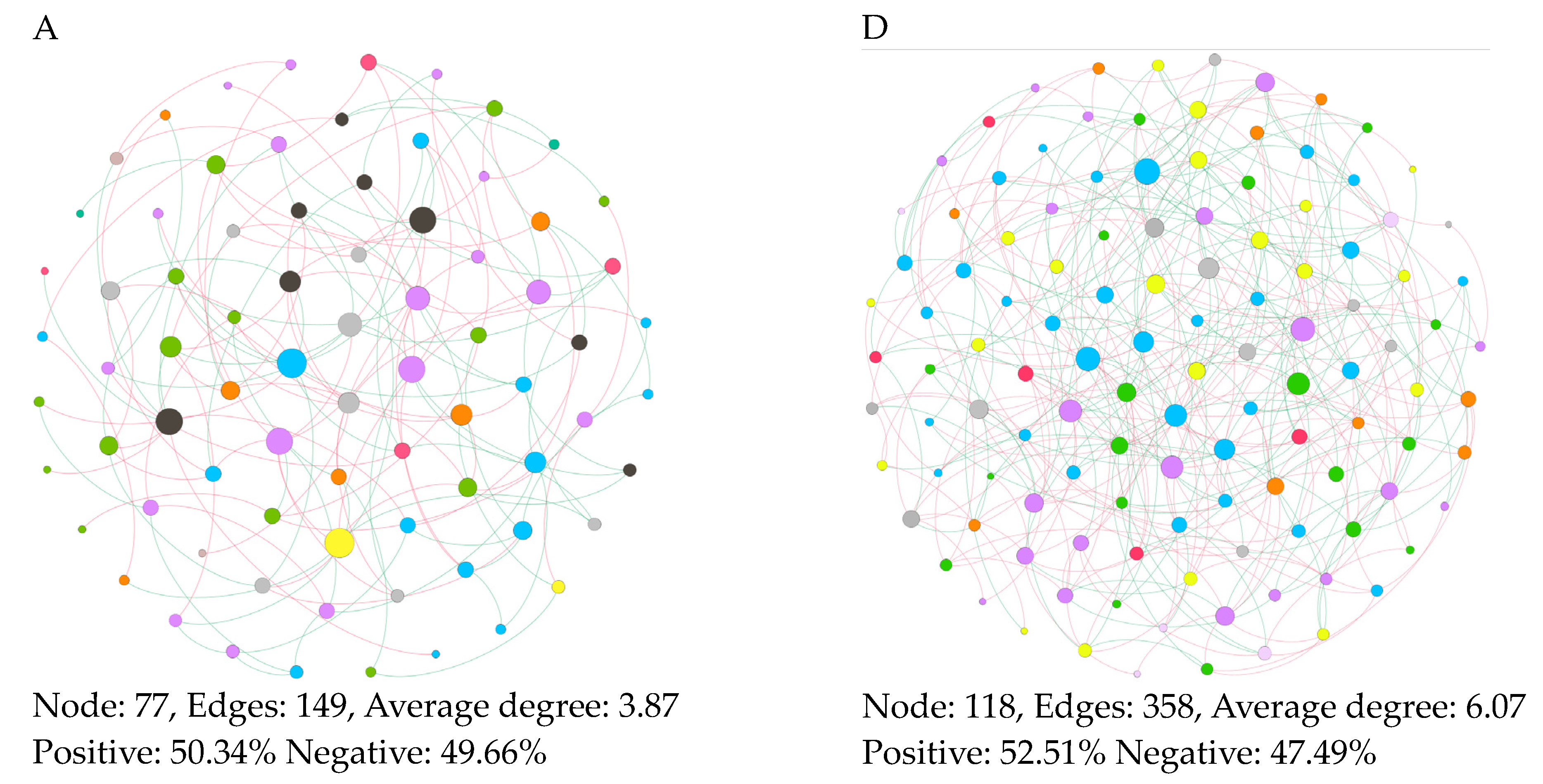
3.5. Features of Co-Occurrence Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamanaka, T.; Ota, Y.; Konno, M.; Kawai, M.; Ohta, A.; Neda, H.; Terashima, Y.; Yamada, A. The host ranges of conifer-associated Tricholoma matsutake, Fagaceae-associated T. bakamatsutake and T. fulvocastaneum are wider in vitro than in nature. Mycologia 2014, 106, 397–406. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Li, Y.; Wang, K.; Yang, R.; Yao, Y. Status of global macrofungal conservation basedon red lists and suggestions for a comprehensive strategy in China. Acta Edulis Fungi 2021, 28, 108–114. (In Chinese) [Google Scholar]
- Yao, Y.J.; Wei, J.; Zhuang, W.; Cai, L.; Liu, D.; Li, J.; Wei, T.; Li, Y.; Wang, K.; Wu, H. Development of red list assessment of macrofungi in China. Biodivers. Sci. 2020, 28, 8–14. (In Chinese) [Google Scholar]
- Wang, Y.; Xue, W. Effect of different temperature and humidity on preservation of Tricholoma matsutake. Sci. Technol. Food Ind. 2012, 33, 366–389. [Google Scholar]
- Yang, X.; He, J.; Li, C.; Ma, J.; Yang, Y.; Xu, J. Matsutake Trade in Yunnan Province, China: An Overview. Econ. Bot. 2008, 62, 269–277. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Huang, W.; Xiong, C.; Yang, Z.; Zheng, L. The nutrition and quality of Tricholoma matsutake in seven different areas of sichuan. Food Ferment. Ind. 2014, 40, 97–100. [Google Scholar]
- Liu, G.; Wang, H.; Zhou, B.; Guo, X.; Hu, X. Compositional analysis and nutritional studies of Tricholoma matsutake collected from Southwest China. J. Med. Plants Res. 2010, 4, 1222–1227. [Google Scholar]
- Su, K.; Wang, Z.; Duan, F.; Liu, Q.; Liu, Z. Preliminary study on the cultivation pine-mycorrhizas of Tricholoma matsutake. Edible Fungi 2002, 6, 35–36. (In Chinese) [Google Scholar]
- Su, K. Investigation on the ecological environment of Tricholoma matsutake in Chuxiong and Zhongdian areas of Yunnan. Edible Fungi China 2002, 3, 19–20. (In Chinese) [Google Scholar]
- Cao, Z.; Yao, Y. Morphological and Biogeographic study on Tricholoma matsutake-complex. Mycosystema 2004, 23, 43–55. (In Chinese) [Google Scholar]
- Oh, S.Y.; Park, M.S.; Lim, Y.W. The Influence of Microfungi on the Mycelial Growth of Ectomycorrhizal Fungus Tricholoma matsutake. Microorganisms 2019, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, F.; Chen, Y.; Gong, M. Studies on culture conditions of Tricholoma matsutake. For. Res. 2000, 13, 410–415. [Google Scholar]
- Brown, M.; Timothy, M.; Li, H.L.; Karunarathna, S.C. Applied mycology can contribute to sustainable rural livelihoods: Building upon China’s matsutake management initiatives. Environ. Manag. 2008, 61, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Yamada, A.; Maruyama, T.; Endo, N.; Yamamoto, K.; Ohira, T.; Shimokawa, T. Root endophyte interaction between ectomycorrhizal basidiomycete Tricholoma matsutake and arbuscular mycorrhizal tree Cedrela odorata, allowing in vitro synthesis of rhizospheric “shiro”. Mycorrhiza 2013, 23, 235–242. [Google Scholar] [CrossRef]
- Murata, H.; Yamada, A.; Yokota, S.; Maruyama, T.; Endo, N.; Yamamoto, K.; Ohira, T.; Neda, H. Root endophyte symbiosis in vitro between the ectomycorrhizal basidiomycete Tricholoma matsutake and the arbuscular mycorrhizal plant Prunus speciosa. Mycorrhiza 2014, 24, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Yamada, A.; Maruyama, T.; Neda, H. Ectomycorrhizas in vitro between Tricholoma matsutake, a basidiomycete that associates with Pinaceae, and Betula platyphylla var. japonica, an early-successional birch species, in cool-temperate forests. Mycorrhiza 2015, 25, 237–241. [Google Scholar] [PubMed]
- Yamada, A. Cultivation studies of edible ectomycorrhizal mushrooms: Successful establishment of ectomycorrhizal associations in vitro and efficient production of fruiting bodies. Mycoscience 2022, 63, 235–246. [Google Scholar] [PubMed]
- Lee, W.H.; Han, S.K.; Kim, B.S.; Shrestha, B.; Lee, S.Y.; Ko, C.S.; Sung, G.H.; Sung, J.M. Proliferation of Tricholoma matsutake mycelial mats in pine forest using mass liquid inoculum. Mycobiology 2007, 35, 54–61. [Google Scholar] [CrossRef]
- Eto, S.; Taniguchi, M. Development of the spawns of Tricholoma matsutake using fungicides for the inoculation in forests. J. Mushroom Sci. Soc. Jpn. 2000, 8, 197–202. [Google Scholar]
- Yamada, A.; Maeda, K.; Kobayashi, H.; Murata, H. Ectomycorrhizal symbiosis in vitro between Tricholoma matsutake and Pinus densiflora seedlings that resembles naturally occurring ‘shiro’. Mycorrhiza 2006, 16, 111–116. [Google Scholar] [CrossRef]
- Vaario, L.M.; Fritze, H.; Spetz, P.; Heinonsalo, J.; Hanajik, P.; Pennanen, T. Tricholoma matsutake dominates diverse microbial communities in different forest soils. Appl. Environ. Microbiol. 2011, 77, 8523–8531. [Google Scholar] [CrossRef]
- Lian, C.; Narimatsu, M.; Nara, K.; Hogetsu, T. Tricholoma matsutake in a natural Pinus densiflora forest: Correspondence between above- and below-ground genets, association with multiple host trees and alteration of existing ectomycorrhizal communities. New Phytol. 2006, 171, 825–836. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Yang, S.; Yang, X.; Shi, L. Fungal Interactions Matter: Tricholoma matsutake Domination Affect Fungal Diversity and Function in Mountain Forest Soils. Biology 2021, 10, 1051. [Google Scholar] [CrossRef]
- Oh, S.Y.; Fong, J.J.; Park, M.S.; Lim, Y.W. Distinctive feature of microbial communities and bacterial functional profiles in Tricholoma matsutake dominant soil. PLoS ONE 2016, 11, e0168573. [Google Scholar] [CrossRef]
- Vaario, L.M.; Yang, X.; Yamada, A. Biogeography of the Japanese gourmet fungus, Tricholoma matsutake: A review of the distribution and functional ecology of matsutake. Ecol. Stud. 2017, 230, 319–344. [Google Scholar]
- Kim, M.; Yoon, H.; You, Y.H.; Kim, Y.E.; Woo, J.R.; Seo, Y.; Lee, G.M.; Kim, Y.J.; Kong, W.S.; Kim, J.G. Metagenomic analysis of fungal communities inhabiting the fairy ring zone of Tricholoma matsutake. J. Microbiol. Biotechnol. 2013, 23, 1347–1356. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Bååth, E. Growth response of the bacterial community to pH in soils differing in pH. FEMS Microbiol. Ecol. 2010, 73, 149–156. [Google Scholar] [CrossRef]
- Murphy, D.V.; Cookson, W.R.; Braimbridge, M.; Marschner, P.; Davey L Jones, D.; Stockdale, E.; Abbott, L.K. Relationships between soil organic matter and the soil microbial biomass (size, functional diversity, and community structure) in crop and pasture systems in a semi-arid environment. Soil Res. 2011, 49, 582–594. [Google Scholar] [CrossRef]
- Ji, R.Q.; Xu, Y.; Si, Y.J.; Phukhamsakda, C.; Li, Y.; Meng, L.P.; Liu, S.Y.; Xie, M.L. Fungal-Bacterial Networks in the Habitat of SongRong (Tricholoma matsutake) and Driving Factors of Their Distribution Rules. J. Fungi 2022, 8, 575. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 1–495. [Google Scholar]
- Zeng, W.; Li, Z.; Jiang, T.; Cheng, D.; Yang, L.; Hang, T.; Duan, L.; Zhu, D.; Fang, Y.; Zhang, Y. Identification of Bacterial Communities and Tick-Borne Pathogens in Haemaphysalis spp. Collected from Shanghai, China. Trop. Med. Infect. Dis. 2022, 7, 413. [Google Scholar] [CrossRef]
- Yao, F.; Yang, S.; Wang, Z.; Wang, X.; Ye, J.; Wang, X.; DeBruyn, J.M.; Feng, X.; Jiang, Y.; Li, H. Microbial Taxa Distribution Is Associated with Ecological Trophic Cascades along an Elevation Gradient. Front. Microbiol. 2017, 8, 2071. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 2012, 8, E1002687. [Google Scholar] [CrossRef]
- Ye, X.; Li, Z.; Luo, X.; Wang, W.; Li, Y.; Li, R.; Zhang, B.; Qiao, Y.; Zhou, J.; Fan, J.; et al. A predatory myxobacterium controls cucumber Fusarium wilt by regulating the soil microbial community. Microbiome 2020, 8, 49. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal biogeography. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar]
- Amend, A.; Keeley, S.; Garbelotto, M. Forest age correlates with fine-scale spatial structure of matsutake mycorrhizas. Mycol. Res. 2009, 113, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y.; Akiyoshi, Y.; Hitoshi, F. Advances in the cultivation of the highly-prized ectomycorrhizal mushroom Tricholoma matsutake. Mycoscience 2020, 61, 49–57. [Google Scholar]
- Wang, Q.L.; Wang, R.L.; Zhang, L.P.; Han, Y.J.; Wang, M.T.; Chen, H.; Chen, J.; Guo, B. Climatic ecological suitability and potential distribution of Tricholoma matsutake in western Sichuan Plateau, China based on MaxEnt model. Ying Yong Sheng Tai Xue Bao 2021, 32, 2525–2533. [Google Scholar] [PubMed]
- Jeong, M.; Choi, D.H.; Cheon, W.J.; Kim, J.G. Pyrosequencing and Taxonomic Composition of the Fungal Community from Soil of Tricholoma matsutake in Gyeongju. J. Microbiol. Biotechnol. 2021, 31, 686–695. [Google Scholar] [CrossRef]
- Choi, D.H.; Han, J.G.; Lee, K.H.; Ahn, G.-H. Promotion of Tricholoma matsutake mycelium growth by Penicillium citreonigrum. Mycobiology 2023, 51, 354–359. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Chen, C.; Li, S.; Huang, W.; Xiong, C.; Jin, X.; Zheng, L. Analysis of Bacterial Diversity and Communities Associated with Tricholoma matsutake Fruiting Bodies by Barcoded Pyrosequencing in Sichuan Province, Southwest China. J. Microbiol. Biotechnol. 2016, 26, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Oh, S.Y.; Yoo, S.; Park, M.S.; Fong, J.J.; Lim, Y.W. Successional Change of the Fungal Microbiome Pine Seedling Roots Inoculated with Tricholoma matsutake. Front. Microbiol. 2020, 11, 574146. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Cong, J.; Wang, M.; Zhao, M.; Lu, H.; Xie, C.; Yang, C.; Yuan, T.; Li, D.; et al. Variations of soil microbial community structures beneath broadleaved forest trees in temperate and subtropical climate zones. Front. Microbiol. 2017, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Jin, S.; Wu, J. Soil bacterial community is more sensitive than fungal community to canopy nitrogen deposition and understory removal in a Chinese fir plantation. Front. Microbiol. 2022, 13, 1015936. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, L.; Dai, S. Diversity of root-associated fungal endophytes in Rhododendron fortunei in subtropical forests of China. Mycorrhiza 2009, 19, 417–423. [Google Scholar] [CrossRef]
- Chipashvili, O.; Utter, D.R.; Bedree, J.K.; Ma, Y.; Schulte, F.; Mascarin, G.; Alayyoubi, Y.; Chouhan, D.; Hardt, M.; Bidlack, F.; et al. Episymbiotic Saccharibacteria suppresses gingival inflammation and bone loss in mice through host bacterial modulation. Cell Host Microbe 2021, 29, 1649–1662.e7. [Google Scholar] [CrossRef]



| Shangri-la City | Nanhua | |
|---|---|---|
| Shiro in the development stage of fruiting bodies | XA | NhA |
| Non-shiro | XB | NhB |
| Shiro in the hyphal incubation period | XC | NhC |
| Habitats | NhA | NhB | NhC | XA | XB | XC | |
|---|---|---|---|---|---|---|---|
| Soil Physicochemical Properties | |||||||
| pH | 4.47 | 4.37 | 4.41 | 5.30 | 5.53 | 5.47 | |
| OM (g/kg) | 104.83 | 93.62 | 106.44 | 38.11 | 20.72 | 40.24 | |
| N (g/kg) | 2.37 | 2.38 | 2.66 | 1.36 | 1.04 | 1.39 | |
| P (mg/kg) | 8.40 | 19.20 | 10.90 | 12.22 | 1.91 | 7.36 | |
| K (mg/kg) | 515.00 | 205.00 | 207.30 | 490.10 | 175.7 | 210.30 | |
| moisture (%) | 28.50 | 31.10 | 31.20 | 23.80 | 17.50 | 19.70 | |
| Zn (g/kg) | 2.41 | 1.84 | 2.09 | 1.60 | 1.21 | 1.27 | |
| Mn (g/kg) | 9.64 | 3.76 | 7.63 | 91.04 | 90.52 | 89.49 | |
| Cu (mg/kg) | 0.35 | 0.17 | 0.21 | 0.32 | 0.36 | 0.32 | |
| Mg (mg/kg) | 124 | 95 | 104 | 162 | 169 | 149 | |
| Ca (mg/kg) | 543.00 | 632.00 | 576.00 | 846.00 | 772.00 | 798.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Yu, P.; Yang, J.; Liu, J.; Zi, Z.; Li, D.; Liang, M.; Tian, G. Differences in Soil Microflora between the Two Chinese Geographical Indication Products of “Tricholoma matsutake Shangri-la” and “T. matsutake Nanhua”. Agronomy 2024, 14, 792. https://doi.org/10.3390/agronomy14040792
Yao C, Yu P, Yang J, Liu J, Zi Z, Li D, Liang M, Tian G. Differences in Soil Microflora between the Two Chinese Geographical Indication Products of “Tricholoma matsutake Shangri-la” and “T. matsutake Nanhua”. Agronomy. 2024; 14(4):792. https://doi.org/10.3390/agronomy14040792
Chicago/Turabian StyleYao, Chunxin, Ping Yu, Jisheng Yang, Jiaxun Liu, Zhengquan Zi, Defen Li, Mingtai Liang, and Guoting Tian. 2024. "Differences in Soil Microflora between the Two Chinese Geographical Indication Products of “Tricholoma matsutake Shangri-la” and “T. matsutake Nanhua”" Agronomy 14, no. 4: 792. https://doi.org/10.3390/agronomy14040792
APA StyleYao, C., Yu, P., Yang, J., Liu, J., Zi, Z., Li, D., Liang, M., & Tian, G. (2024). Differences in Soil Microflora between the Two Chinese Geographical Indication Products of “Tricholoma matsutake Shangri-la” and “T. matsutake Nanhua”. Agronomy, 14(4), 792. https://doi.org/10.3390/agronomy14040792





