Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment Layout
2.2. Plant Growth and Biomass
2.3. Enzymatic Antioxidants
2.4. Photosynthetic Pigments
2.5. Total Soluble Sugars
2.6. Total Soluble Proteins
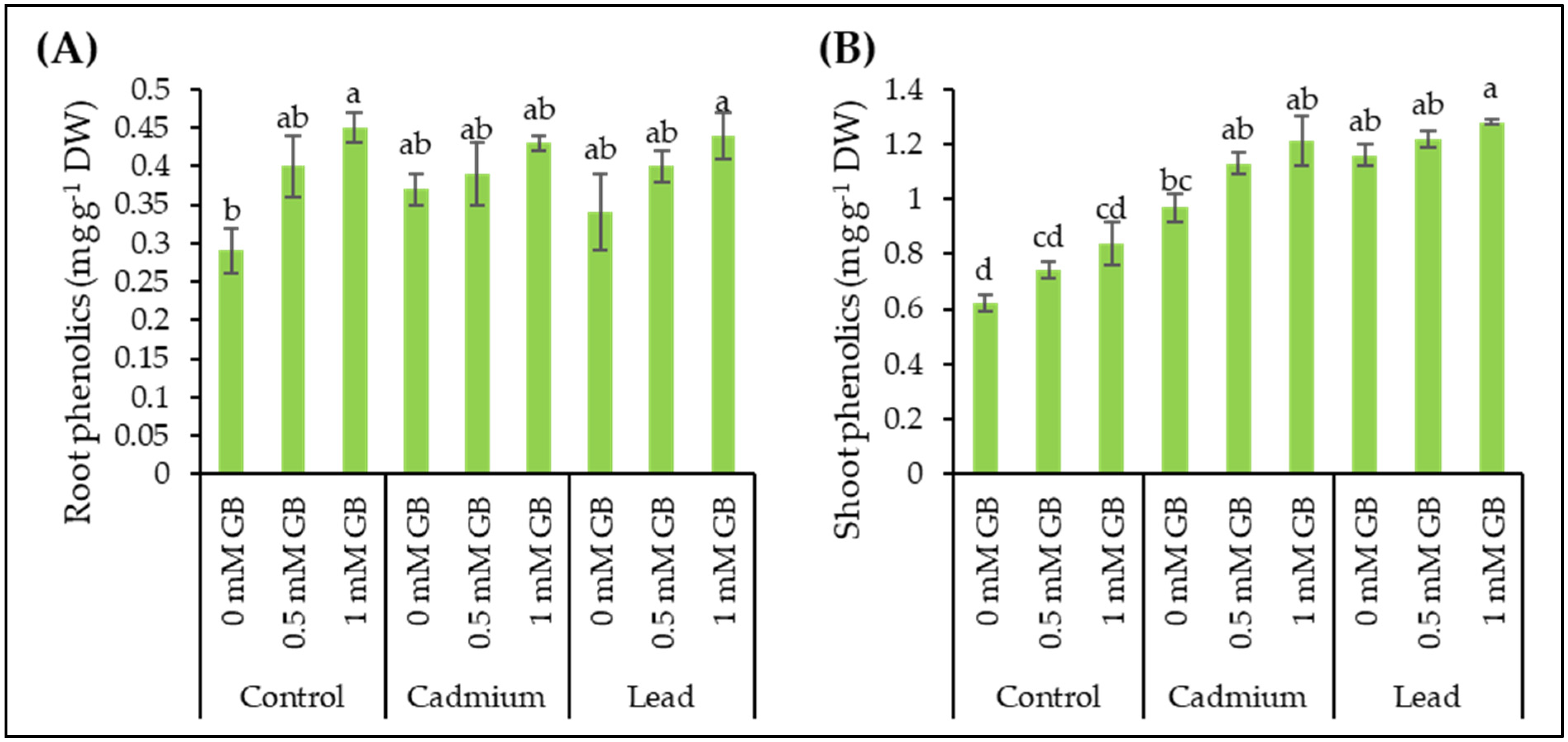
2.7. Phenolic Compounds
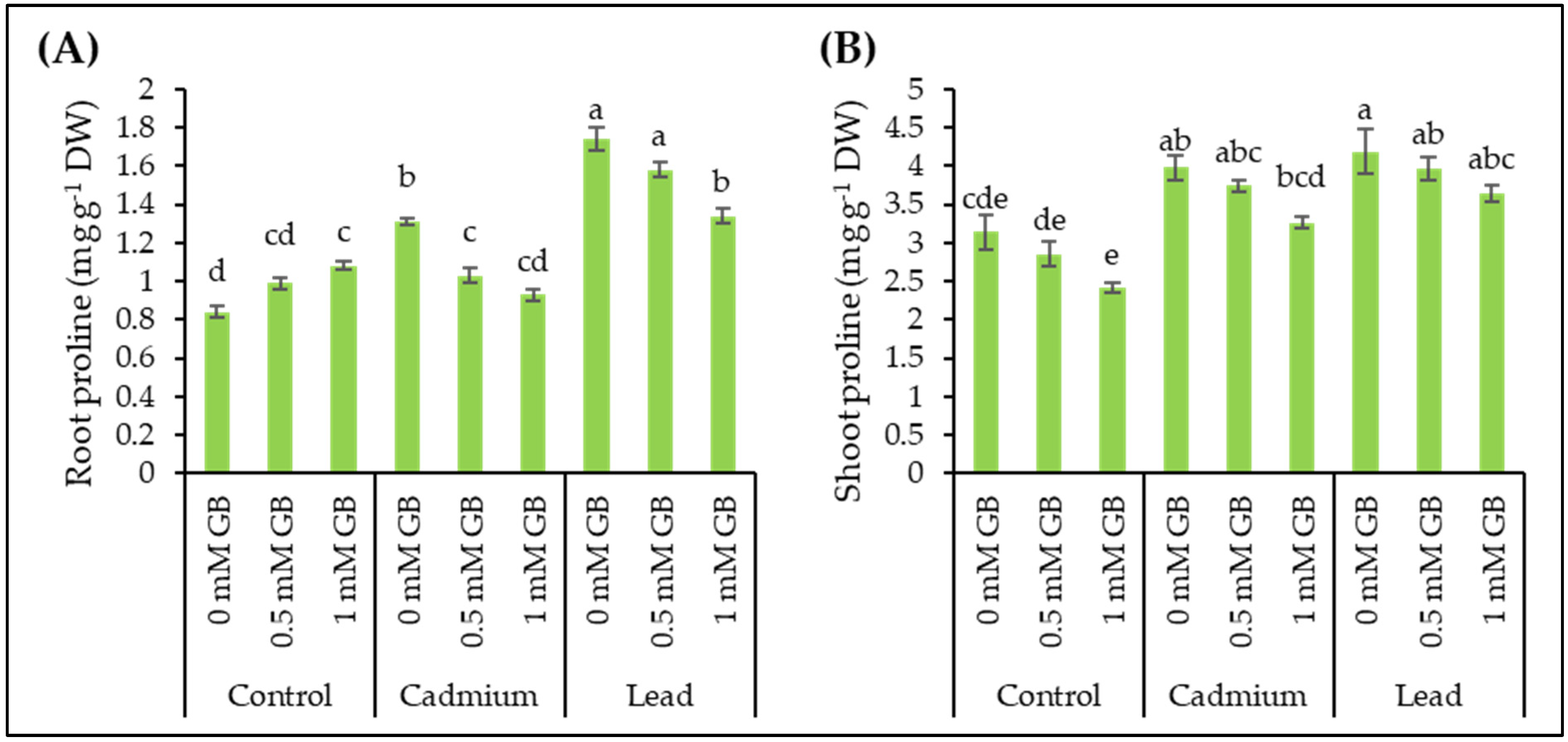
2.8. Free Proline
2.9. Statistical Analysis
3. Results
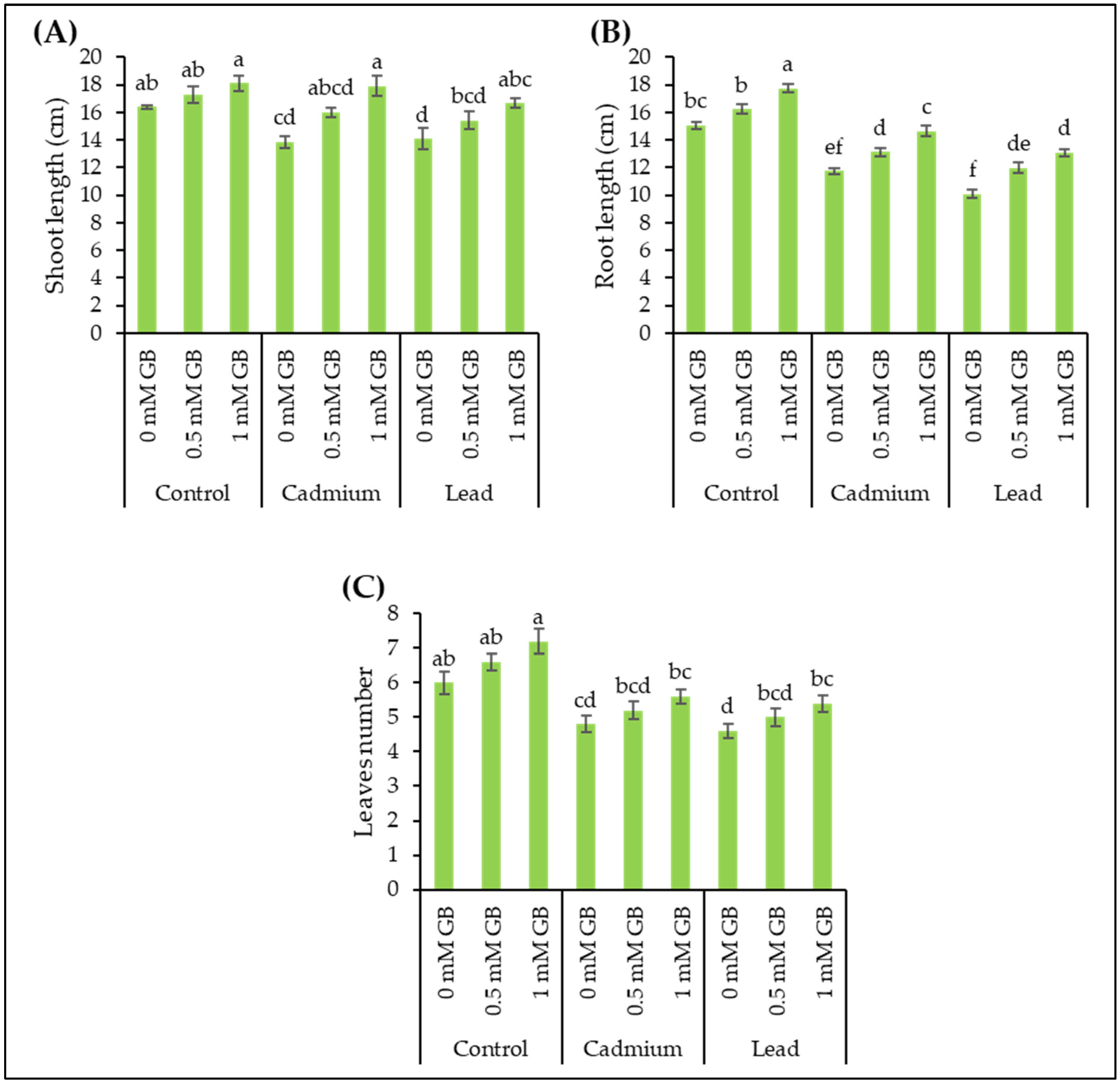
3.1. Morphological Plant Growth
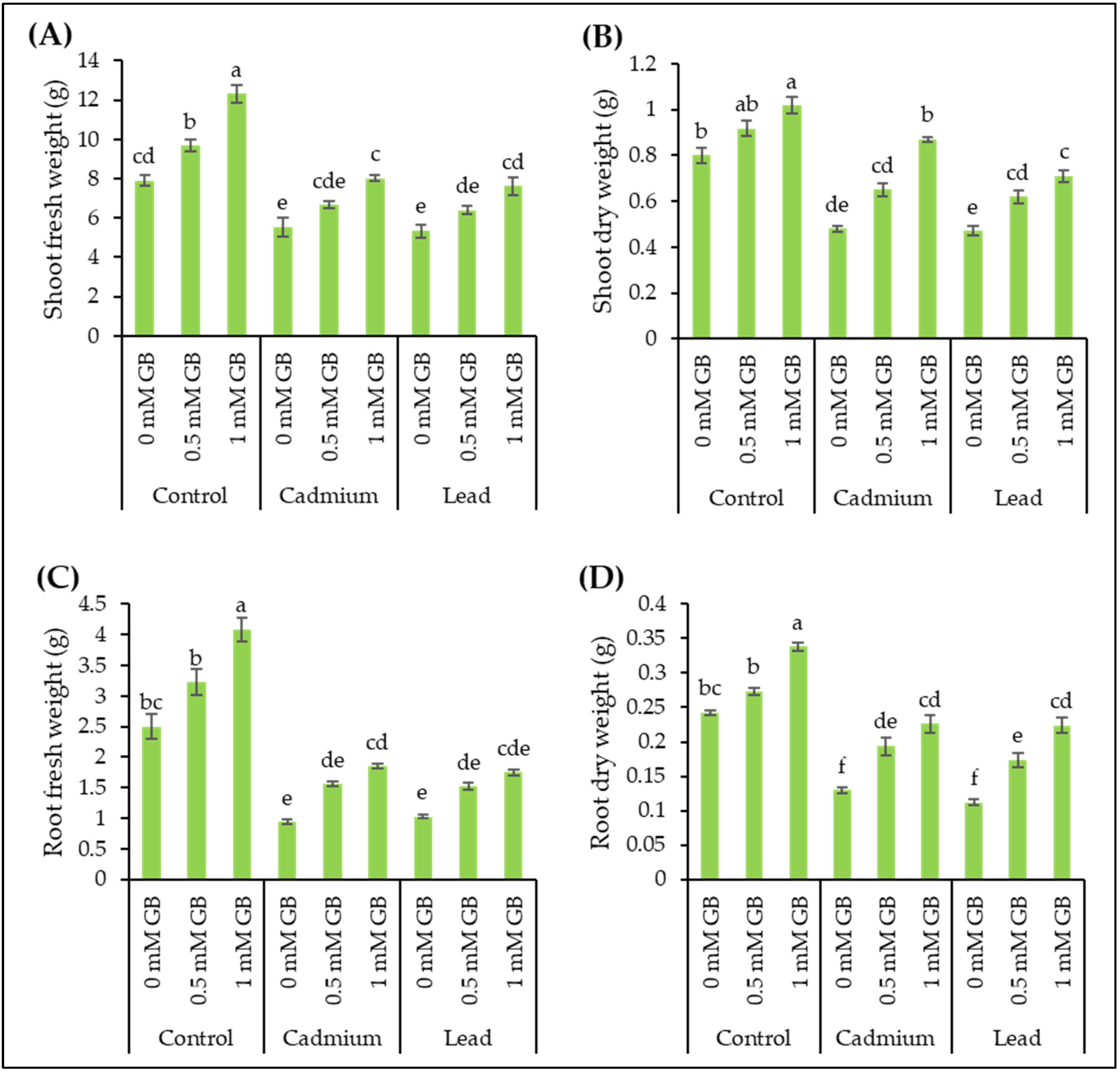
3.2. Plant Biomass
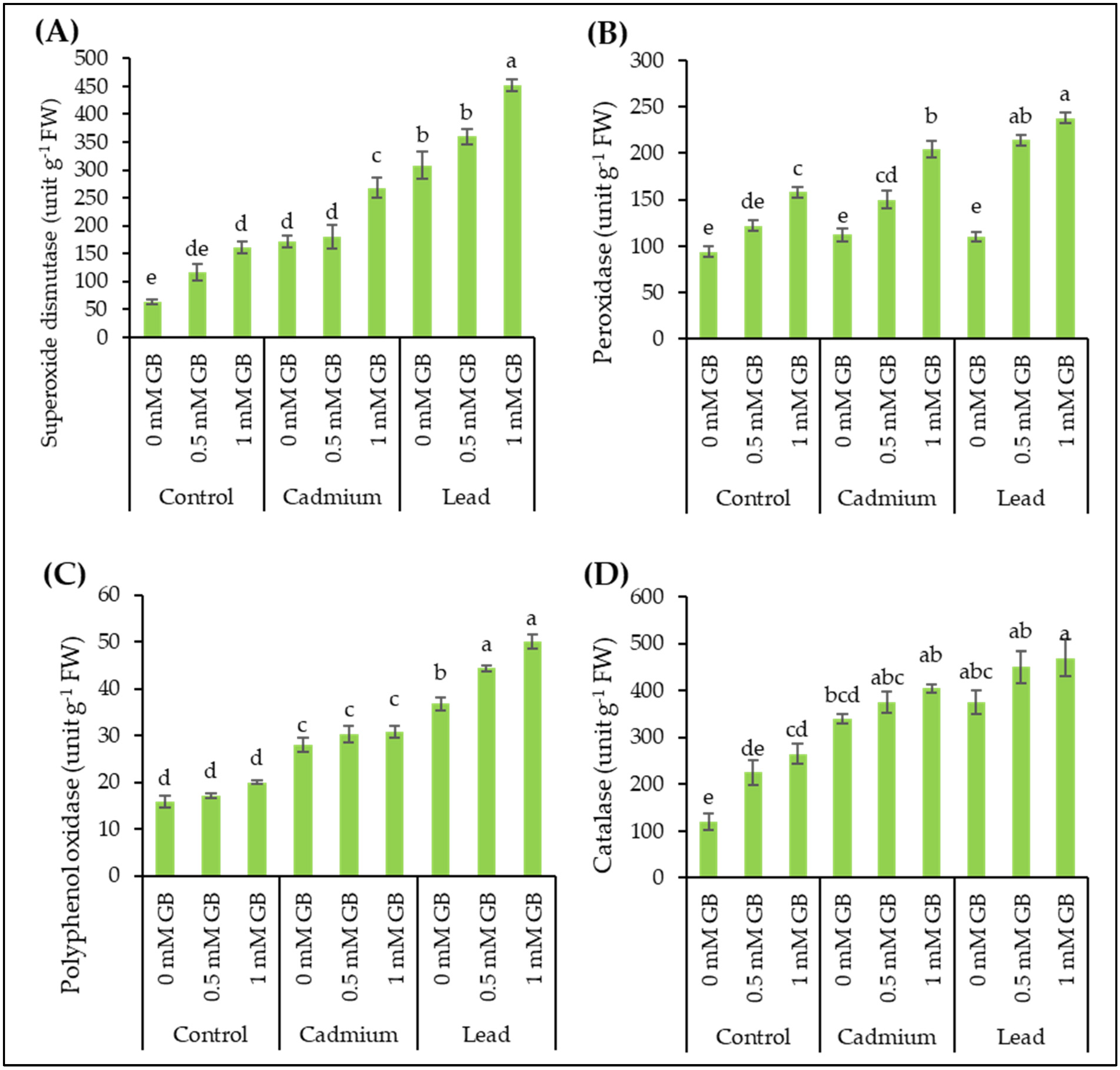
3.3. Antioxidant Enzymes
3.4. Leaf Pigments
3.5. Soluble Sugars
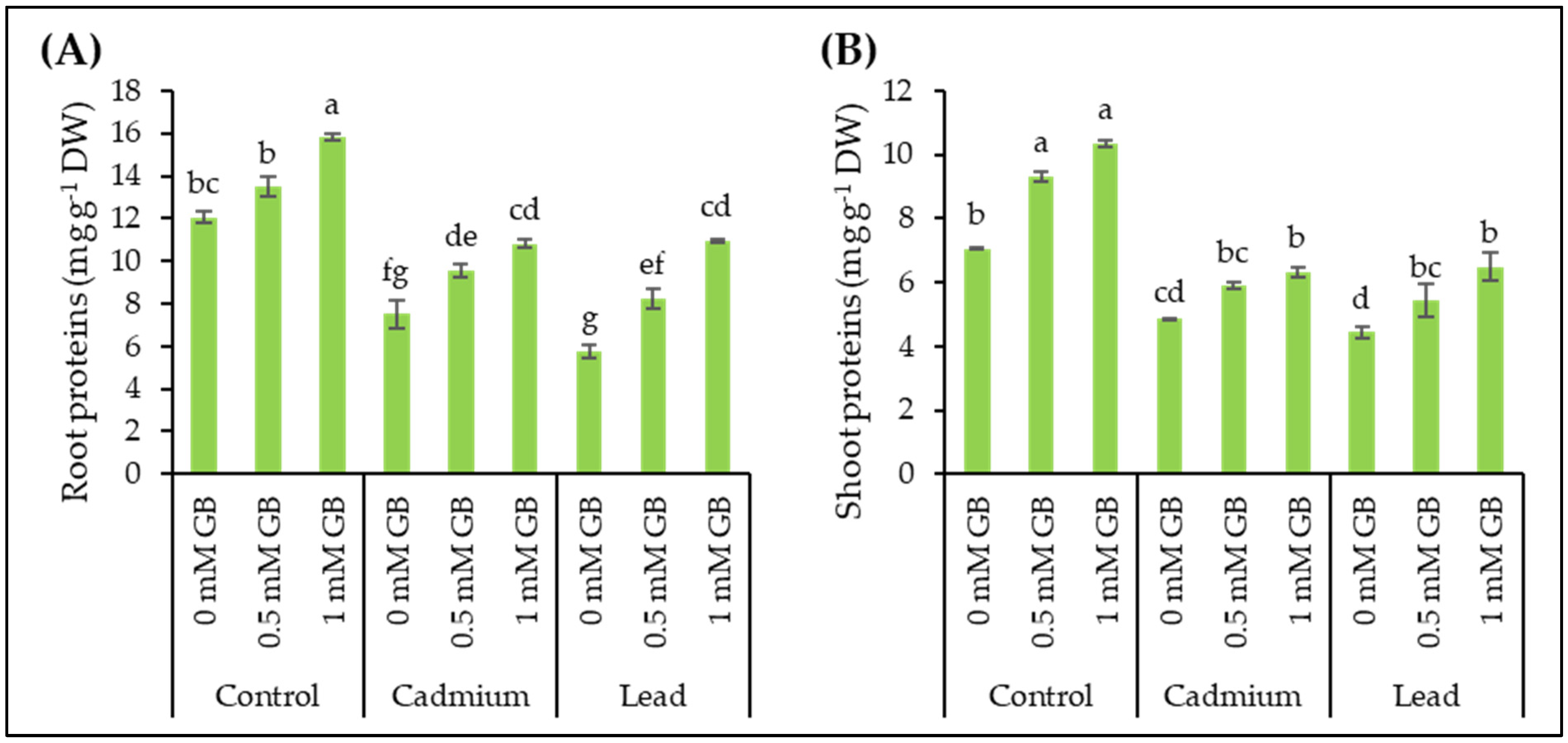
3.6. Soluble Proteins
3.7. Phenolic Compounds
3.8. Free Proline
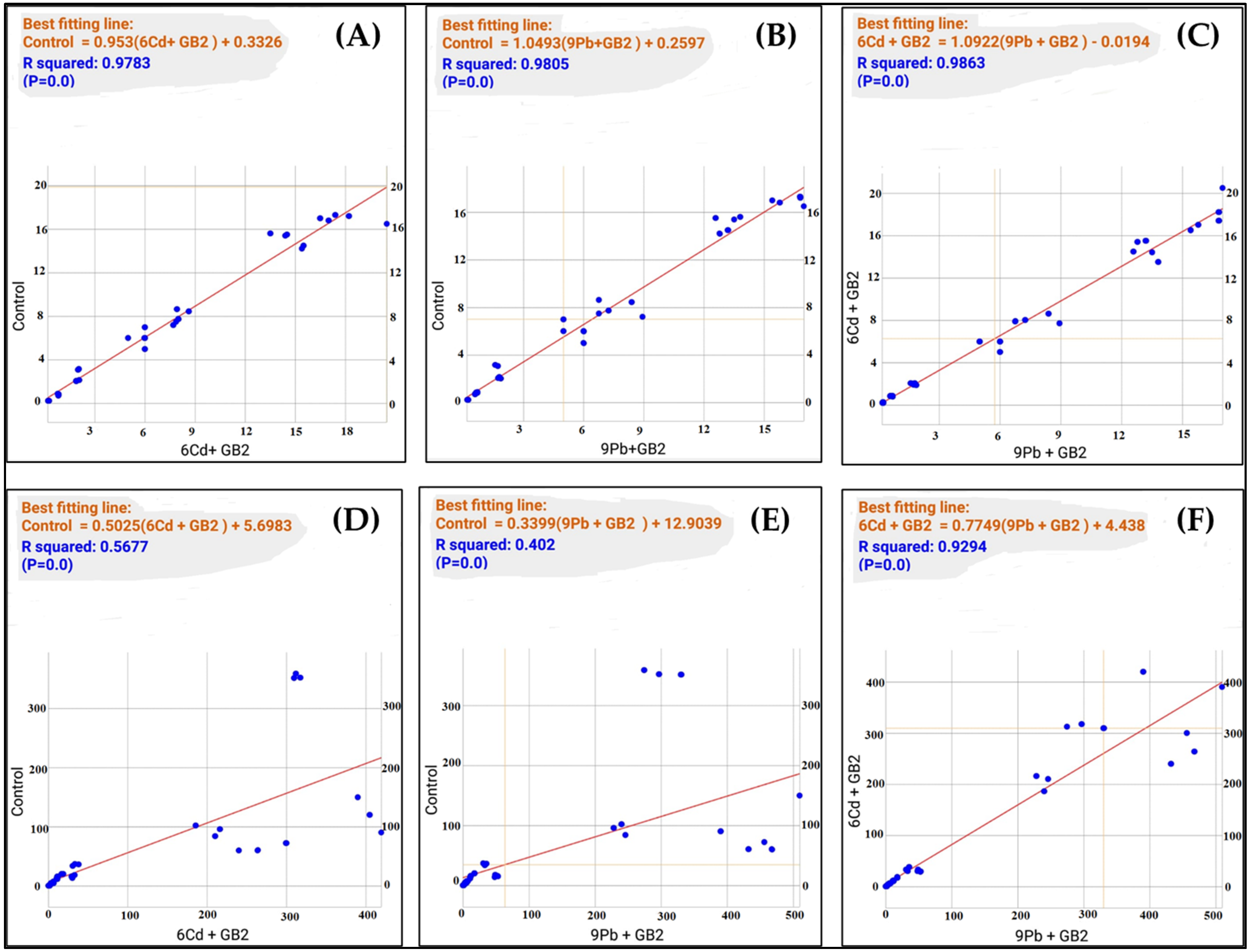
3.9. Correlation Analysis
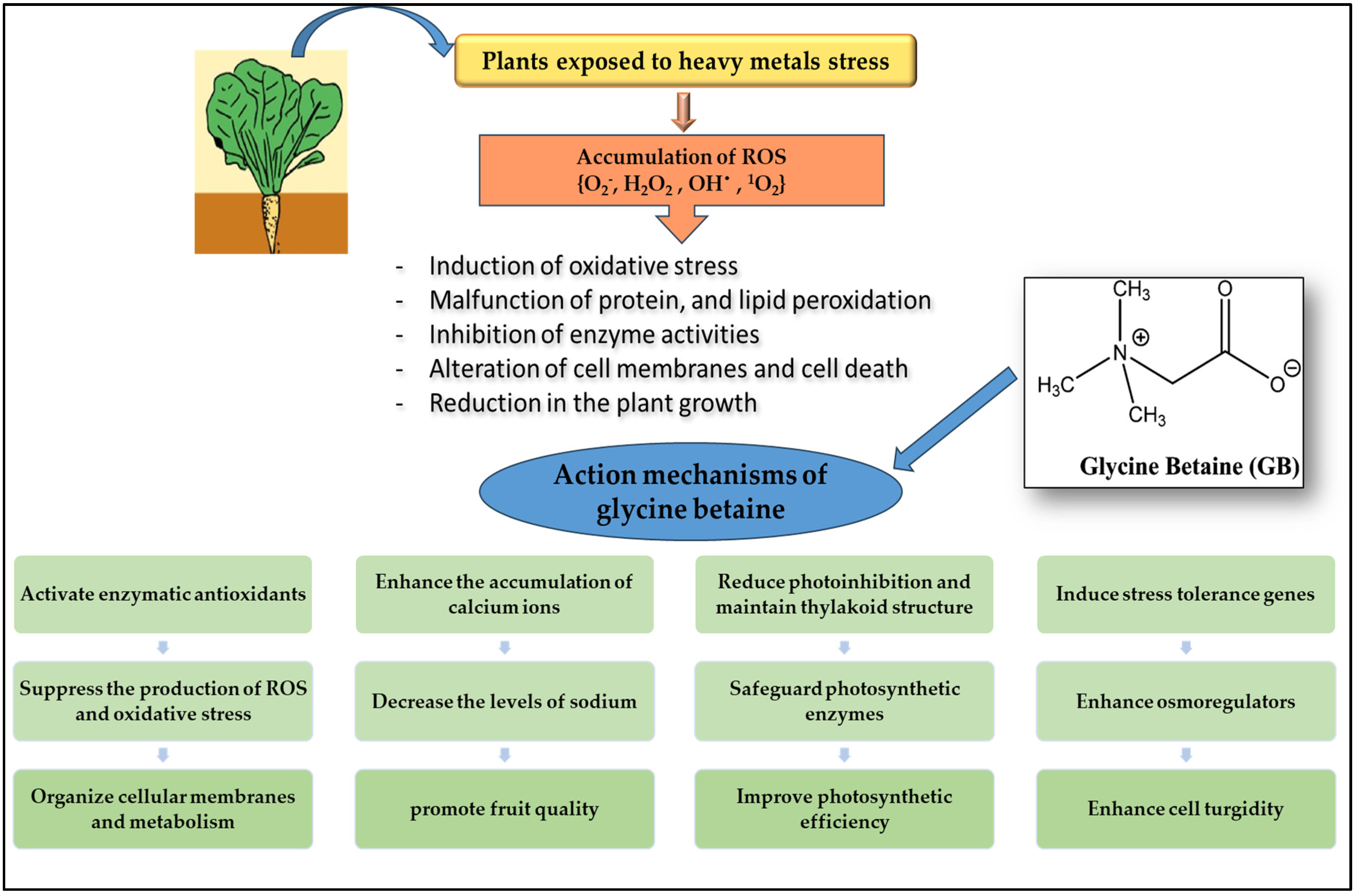
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2020, 39, 641–655. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Yu, J.; Gao, Y.; Liu, X.; Wang, X.; Wu, X. Effects of Exogenous Glycinebetaine on Cadmium-Induced Changes in Photosynthetic Performance, Antioxidative Metabolism and ATPase in Cucumber Seedlings. Plant Soil Environ. 2022, 68, 401–409. [Google Scholar] [CrossRef]
- Hawkes, S.J. What Is a “Heavy Metal”? J. Chem. Educ. 1997, 74, 1374. [Google Scholar] [CrossRef]
- Ewais, E.A.; Ismail, M.A.; Amin, M.A.; Badawy, A.A. Efficiency of Salicylic Acid and Glycine on Sugar Beet Plants Grown under Heavy Metals Pollution. Egypt. J. Biotechnol. 2015, 48, 112–126. [Google Scholar]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed]
- Chenery, S.R.; Izquierdo, M.; Marzouk, E.; Klinck, B.; Palumbo-Roe, B.; Tye, A.M. Soil-Plant Interactions and the Uptake of Pb at Abandoned Mining Sites in the Rookhope Catchment of the N. Pennines, UK—A Pb Isotope Study. Sci. Total Environ. 2012, 433, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, A.; Seth, C.S. Does Jasmonic Acid Regulate Photosynthesis, Clastogenecity, and Phytochelatins in Brassica juncea L. in Response to Pb-Subcellular Distribution? Chemosphere 2020, 243, 125361. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy Metal Stress and Crop Productivity. In Crop Production and Global Environmental Issues; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Bali, S.; Jamwal, V.L.; Kaur, P.; Kohli, S.K.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ahmad, P. Role of P-Type ATPase Metal Transporters and Plant Immunity Induced by Jasmonic Acid against Lead (Pb) Toxicity in Tomato. Ecotoxicol. Environ. Saf. 2019, 174, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Pignata, M.L.; Lascano, H.R.; Rodriguez, J.H. Assessment of Lead Tolerance on Glycine max (L.) Merr. at Early Growth Stages. Environ. Sci. Pollut. Res. 2021, 28, 22843–22852. [Google Scholar] [CrossRef] [PubMed]
- Bharwana, S.A.; Ali, S.; Farooq, M.A.; Iqbal, N.; Hameed, A.; Abbas, F.; Ahmad, M.S.A. Glycine Betaine-Induced Lead Toxicity Tolerance Related to Elevated Photosynthesis, Antioxidant Enzymes Suppressed Lead Uptake and Oxidative Stress in Cotton. Turk. J. Botany 2014, 38, 281–292. [Google Scholar] [CrossRef]
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide Stimulates Root Protection, and Leaf Antioxidant Enzymes in Lead Stressed Rice Plants: Central Roles to Minimize Pb Content and Oxidative Stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A Critical Review on Speciation, Mobilization and Toxicity of Lead in Soil-Microbe-Plant System and Bioremediation Strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Drought Induces Oxidative Stress and Enhances the Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, S.; Kumar, V.; Singh, P.; Khyalia, P.; Verma, S.; Saini, S.; Sharma, A. Foliar Application of Glycine Betaine to Ameliorate Lead Toxicity in Barley Plants by Modulating Antioxidant Enzyme Activity and Biochemical Parameters. Environ. Res. Commun. 2023, 5, 075002. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, Y.; Han, C.; Zhu, W.; Gu, j.; He, J. Application of Exogenous Glycinebetaine Alleviates Lead Toxicity in Pakchoi (Brassica chinensis L.) by Promoting Antioxidant Enzymes and Suppressing Pb Accumulation. Environ. Sci. Pollut. Res. 2022, 29, 25568–25580. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Meunier, J.-D.; Miche, H.; Keller, C. Effect of Silicon on Reducing Cadmium Toxicity in Durum Wheat (Triticum turgidum L. Cv. Claudio W.) Grown in a Soil with Aged Contamination. J. Hazard. Mater. 2012, 209, 326–334. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of Inorganic Amendments for in Situ Stabilization of Cadmium in Contaminated Soils and Its Phyto-Availability to Wheat and Rice under Rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-Induced Oxidative Damage in Mustard [Brassica juncea (L.) Czern. & Coss.] Plants Can Be Alleviated by Salicylic Acid. S. Afr. J. Bot. 2011, 77, 36–44. [Google Scholar]
- Choppala, G.; Saifullah; Bolan, N.; Bibi, S.; Iqbal, M.; Rengel, Z.; Kunhikrishnan, A.; Ashwath, N.; Ok, Y.S. Cellular Mechanisms in Higher Plants Governing Tolerance to Cadmium Toxicity. Crit. Rev. Plant Sci. 2014, 33, 374–391. [Google Scholar] [CrossRef]
- Zhang, G.; Ba, Q.; Chen, S.; Liu, F.; Li, G. Exogenous Application of Glycine Betaine Alleviates Cadmium Toxicity in Super Black Waxy Maize by Improving Photosynthesis, the Antioxidant System and Glutathione-Ascorbic Acid Cycle Metabolites. Cereal Res. Commun. 2020, 48, 449–458. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus Amendment Decreased Cadmium (Cd) Uptake and Ameliorates Chlorophyll Contents, Gas Exchange Attributes, Antioxidants, and Mineral Nutrients in Wheat (Triticum aestivum L.) under Cd Stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Arshad, A.; Iqbal, M.; Hussain, I. Interactive Effects of Chitosan and Cadmium on Growth, Secondary Metabolism, Oxidative Defense, and Element Uptake in Pea (Pisum sativum L.). Arab. J. Geosci. 2020, 13, 847. [Google Scholar] [CrossRef]
- Aamer, M.; Muhammad, U.H.; Li, Z.; Abid, A.; Su, Q.; Liu, Y.; Adnan, R.; Muhammad, A.U.K.; Tahir, A.K.; Huang, G. Foliar Application of Glycinebetaine (GB) Alleviates the Cadmium (Cd) Toxicity in Spinach through Reducing Cd Uptake and Improving the Activity of Anti-Oxidant System. Appl. Ecol. Environ. Res. 2018, 16, 7575–7583. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Banerjee, A.; Bhuyan, M.H.M.B.; Roychoudhury, A.; Al Mahmud, J.; Fujita, M. Targeting Glycinebetaine for Abiotic Stress Tolerance in Crop Plants: Physiological Mechanism, Molecular Interaction and Signaling. Phyton 2019, 88, 185. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Jain, P.; Pandey, B.; Singh, P.; Singh, R.; Singh, S.P.; Sonkar, S.; Gupta, R.; Rathore, S.S.; Singh, A.K. Plant Performance and Defensive Role of Glycine Betaine under Environmental Stress. In Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management; Springer: Cham, Switzerland, 2021; pp. 225–248. [Google Scholar]
- Mäkelä, P.; Munns, R.; Colmer, T.D.; Condon, A.G.; Peltonen-Sainio, P. Effect of Foliar Applications of Glycinebetaine on Stomatal Conductance, Abscisic Acid and Solute Concentrations in Leaves of Salt-or Drought-Stressed Tomato. Funct. Plant Biol. 1998, 25, 655–663. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary Ammonium and Tertiary Sulfonium Compounds in Higher Plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Sorwong, A.; Sakhonwasee, S. Foliar Application of Glycine Betaine Mitigates the Effect of Heat Stress in Three Marigold (Tagetes erecta) Cultivars. Hortic. J. 2015, 84, 161–171. [Google Scholar] [CrossRef]
- Jabeen, N.; Abbas, Z.; Iqbal, M.; Rizwan, M.; Jabbar, A.; Farid, M.; Ali, S.; Ibrahim, M.; Abbas, F. Glycinebetaine Mediates Chromium Tolerance in Mung Bean through Lowering of Cr Uptake and Improved Antioxidant System. Arch. Agron. Soil Sci. 2016, 62, 648–662. [Google Scholar] [CrossRef]
- Chen, W.P.; Li, P.H.; Chen, T.H.H. Glycinebetaine Increases Chilling Tolerance and Reduces Chilling-Induced Lipid Peroxidation in Zea mays L. Plant Cell Environ. 2000, 23, 609–618. [Google Scholar] [CrossRef]
- He, X.; Richmond, M.E.A.; Williams, D.V.; Zheng, W.; Wu, F. Exogenous Glycinebetaine Reduces Cadmium Uptake and Mitigates Cadmium Toxicity in Two Tobacco Genotypes Differing in Cadmium Tolerance. Int. J. Mol. Sci. 2019, 20, 1612. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Iqbal, M.; Ashraf, M.A.; Hussain, I.; Shafiq, F.; Yousaf, A.; Zaheer, A. Glycine Betaine Counteracts the Inhibitory Effects of Waterlogging on Growth, Photosynthetic Pigments, Oxidative Defence System, Nutrient Composition, and Fruit Quality in Tomato. J. Hortic. Sci. Biotechnol. 2018, 93, 385–391. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and Abiotic Stress Tolerance in Plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- EL-Sharnoby, H.M.; Badr, E.A.; Abo Elenen, F.F. Influence of Foliar Application of Algae Extract and Nitrogen Fertilization on Yield and Quality of Sugar Beet Grown in Reclaimed Sandy Soil. SVU-Int. J. Agric. Sci. 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Islam, M.J.; Uddin, M.J.; Hossain, M.A.; Henry, R.; Begum, K.; Sohel, A.T.; Mou, M.A.; Ahn, J.; Cheong, E.J.; Lim, Y.S. Exogenous Putrescine Attenuates the Negative Impact of Drought Stress by Modulating Physio-Biochemical Traits and Gene Expression in Sugar Beet (Beta vulgaris L.). PLoS ONE 2022, 17, e0262099. [Google Scholar] [CrossRef] [PubMed]
- Agamy, R.; Hashem, M.; Alamri, S. Effect of Soil Amendment with Yeasts as Bio-Fertilizers on the Growth and Productivity of Sugar Beet. Afr. J. Agric. Res. 2013, 8, 46–56. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-Induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1974; Volume 1, p. 974. [Google Scholar]
- Matta, A. Accumulation of Phenols in Tomato Plants Infected by Different Forms of Fusarium oxysporum. Phytopathology 1969, 59, 512–513. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzimol. 1984, 105, 121–126. [Google Scholar]
- Vernon, L.P.; Seely, G.R. The Chlorophylls; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Umbreit, W.W.; Burris, R.H.; Stauffer, J.F. Manometric Techniques: A Manual Describing Methods Applicable to the Study of Tissue Metabolism; Burgess Publishing Company: Minneapolis, MN, USA, 1964. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dai, G.H.; Andary, C.; Cosson-Mondolot, L.; Boubals, D. Polyphenols and Resistance of Grapevines to Downy Mildew. In Proceedings of the International Symposium on Natural Phenols in Plant Resistance 381, Weihenstephan, Germany, 13–17 September 1993; pp. 763–766. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; The Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Xiao, W.; Cui, X. Salt-Tolerant and Plant-Growth-Promoting Bacteria Isolated from High-Yield Paddy Soil. Can. J. Microbiol. 2018, 64, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of Salt Tolerance and Interactive Impact of Azotobacter Chroococcum and/or Alcaligenes Faecalis Inoculation on Canola (Brassica napus L.) Plants Grown in Saline Soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.A.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of Seawater Stress Tolerance in Barley by the Endophytic Fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Kenawy, S.K.M.; Elkady, F.M.; Badawy, A.A. Grain-Priming with L-Arginine Improves the Growth Performance of Wheat (Triticum aestivum L.) Plants under Drought Stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A.H. Ameliorative Impact of an Extract of the Halophyte Arthrocnemum Macrostachyum on Growth and Biochemical Parameters of Soybean under Salinity Stress. J. Plant Growth Regul. 2021, 40, 1245–1256. [Google Scholar] [CrossRef]
- Bhatti, K.H.; Anwar, S.; Nawaz, K.; Hussain, K.; Siddiqi, E.H.; Usmansharif, R.; Talat, A.; Aneelakhalid, A. Effect of Exogenous Application of Glycinebetaine on Wheat (Triticum aestivum L.) under Heavy Metal Stres. Middle East J. Sci. Res. 2013, 14, 130–137. [Google Scholar] [CrossRef]
- Mohamed, A.A.A.; Dardiry, M.H.O.; Samad, A.; Abdelrady, E. Exposure to Lead (Pb) Induced Changes in the Metabolite Content, Antioxidant Activity and Growth of Jatropha curcas (L.). Trop. Plant Biol. 2020, 13, 150–161. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Abid, M.; Rizwan, M.; Ali, B.; Tanveer, A.; Ahmad, I.; Azam, M.; Ghani, M.A. Glycinebetaine Alleviates the Chromium Toxicity in Brassica oleracea L. by Suppressing Oxidative Stress and Modulating the Plant Morphology and Photosynthetic Attributes. Environ. Sci. Pollut. Res. 2020, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Sun, L.; Ma, C.; Li, L.; Li, G.; Hao, L. Reducing Basal Salicylic Acid Enhances Arabidopsis Tolerance to Lead or Cadmium. Plant Soil 2013, 372, 309–318. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Bharwana, S.A.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium Stress in Cotton Seedlings: Physiological, Photosynthesis and Oxidative Damages Alleviated by Glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Saidi, I.; Ayouni, M.; Dhieb, A.; Chtourou, Y.; Chaïbi, W.; Djebali, W. Oxidative Damages Induced by Short-Term Exposure to Cadmium in Bean Plants: Protective Role of Salicylic Acid. S Afr. J. Bot. 2013, 85, 32–38. [Google Scholar] [CrossRef]
- Hédiji, H.; Djebali, W.; Belkadhi, A.; Cabasson, C.; Moing, A.; Rolin, D.; Brouquisse, R.; Gallusci, P.; Chaïbi, W. Impact of Long-Term Cadmium Exposure on Mineral Content of Solanum lycopersicum Plants: Consequences on Fruit Production. S. Afr. J. Bot. 2015, 97, 176–181. [Google Scholar] [CrossRef]
- Dias, M.C.; Monteiro, C.; Moutinho-Pereira, J.; Correia, C.; Gonçalves, B.; Santos, C. Cadmium Toxicity Affects Photosynthesis and Plant Growth at Different Levels. Acta Physiol. Plant. 2013, 35, 1281–1289. [Google Scholar] [CrossRef]
- Cao, F.; Liu, L.; Ibrahim, W.; Cai, Y.; Wu, F. Alleviating Effects of Exogenous Glutathione, Glycinebetaine, Brassinosteroids and Salicylic Acid on Cadmium Toxicity in Rice Seedlings (Oryza sativa). Agrotechnology 2013, 2, 107–112. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Hussain, I.; Haider, M.Z.; Kanwal, U.; Iqbal, M. Exogenous Proline and Glycinebetaine Mitigate Cadmium Stress in Two Genetically Different Spring Wheat (Triticum aestivum L.) Cultivars. Rev. Bras. Bot. 2014, 37, 399–406. [Google Scholar] [CrossRef]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of Fungal-Fabricated Selenium Nanoparticles to Improve the Growth Performance of Helianthus annuus L. and Control of Cutworm Agrotis Ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Adrees, M.; Saeed, Z.; Ali, S.S.; Hafeez, M.; Ali, S.S.; Chatha, S.; Rizwan, M. Simultaneous Mitigation of Cadmium and Drought Stress in Wheat by Soil Application of Iron Nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.A.H.; Yousef, M.A.; Badawy, A.A.; Salem, S.S. Influence of Biosynthesized Magnesium Oxide Nanoparticles on Growth and Physiological Aspects of Cowpea (Vigna unguiculata L.) Plant, Cowpea Beetle, and Cytotoxicity. Biotechnol. J. 2023, 18, 2300301. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.A.; Kaviani, B.; Jahanshahi, H. Application of Exogenous Glycine Betaine on Some Growth Traits of Soybean (Glycine max L.) Cv. DPX in Drought Stress Conditions. Sci. Res. Essays 2012, 7, 432–436. [Google Scholar]
- Omer, A.M.; Osman, M.S.; Badawy, A.A. Inoculation with Azospirillum Brasilense and/or Pseudomonas Geniculata Reinforces Flax (Linum usitatissimum) Growth by Improving Physiological Activities under Saline Soil Conditions. Bot. Stud. 2022, 63, 15. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Jaleel, H.; Sadiq, Y.; Khan, M.M.A.; Shabbir, A. Response of Exogenous Salicylic Acid on Cadmium Induced Photosynthetic Damage, Antioxidant Metabolism and Essential Oil Production in Peppermint. Plant Growth Regul. 2018, 86, 273–286. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of Chromium Toxicity by Glycinebetaine Is Related to Elevated Antioxidant Enzymes and Suppressed Chromium Uptake and Oxidative Stress in Wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Abdel Latef, A.A.H. Influence of Glycine Betaine (Natural and Synthetic) on Growth, Metabolism and Yield Production of Drought-Stressed Maize (Zea mays L.) Plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Minh, L.T.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A.; et al. Involvement of Secondary Metabolites in Response to Drought Stress of Rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Abd El-sadek, M.E.; Kenawy, S.K.M.; Badawy, A.A. The Promotive Effect of Putrescine on Growth, Biochemical Constituents, and Yield of Wheat (Triticum aestivum L.) Plants under Water Stress. Agriculture 2023, 13, 587. [Google Scholar] [CrossRef]
- Shallan, M.A.; Hassan, H.M.M.; Namich, A.A.M.; Ibrahim, A.A. Effect of Sodium Niroprusside, Putrescine and Glycine Betaine on Alleviation of Drought Stress in Cotton Plant. Am. J. Agric. Environ. Sci. 2012, 12, 1252–1265. [Google Scholar] [CrossRef]
- Raza, M.A.S.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous Application of Glycinebetaine and Potassium for Improving Water Relations and Grain Yield of Wheat under Drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Yao, W.Q.; Lei, Y.K.; Yang, P.; Li, Q.S.; Wang, L.L.; He, B.Y.; Xu, Z.M.; Zhou, C.; Ye, H.J. Exogenous Glycinebetaine Promotes Soil Cadmium Uptake by Edible Amaranth Grown during Subtropical Hot Season. Int. J. Environ. Res. Public Health 2018, 15, 1794. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of Glycine Betaine in the Thermotolerance of Plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]


| Morphological Parameters | |||
|---|---|---|---|
| Control | Cd+Gb2 | Pb+Gb2 | |
| Control | - | 0.989 | 0.990 |
| Cd+Gb2 | 0.989 | - | 0.993 |
| Pb+Gb2 | 0.990 | 0.993 | - |
| Biochemical Parameters | |||
| Control | Cd+Gb2 | Pb+Gb2 | |
| Control | - | 0.753 | 0.634 |
| Cd+Gb2 | 0.753 | - | 0.964 |
| Pb+Gb2 | 0.634 | 0.964 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawy, A.A.; Alamri, A.A.; Hussein, H.-A.A.; Salem, N.F.G.; Mashlawi, A.M.; Kenawy, S.K.M.; El-Shabasy, A. Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach. Agronomy 2024, 14, 797. https://doi.org/10.3390/agronomy14040797
Badawy AA, Alamri AA, Hussein H-AA, Salem NFG, Mashlawi AM, Kenawy SKM, El-Shabasy A. Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach. Agronomy. 2024; 14(4):797. https://doi.org/10.3390/agronomy14040797
Chicago/Turabian StyleBadawy, Ali A., Abdullah A. Alamri, Hebat-Allah A. Hussein, Noura F. G. Salem, Abadi M. Mashlawi, Sahar K. M. Kenawy, and A. El-Shabasy. 2024. "Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach" Agronomy 14, no. 4: 797. https://doi.org/10.3390/agronomy14040797
APA StyleBadawy, A. A., Alamri, A. A., Hussein, H.-A. A., Salem, N. F. G., Mashlawi, A. M., Kenawy, S. K. M., & El-Shabasy, A. (2024). Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach. Agronomy, 14(4), 797. https://doi.org/10.3390/agronomy14040797







