Effects of Neonicotinoid Seed Treatments on Cotton Seedling Physiology, Nutrition, and Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Chemical Analysis
2.2.1. Chlorophylls
2.2.2. Nutrient Analysis
2.3. Statistical Analysis
3. Results
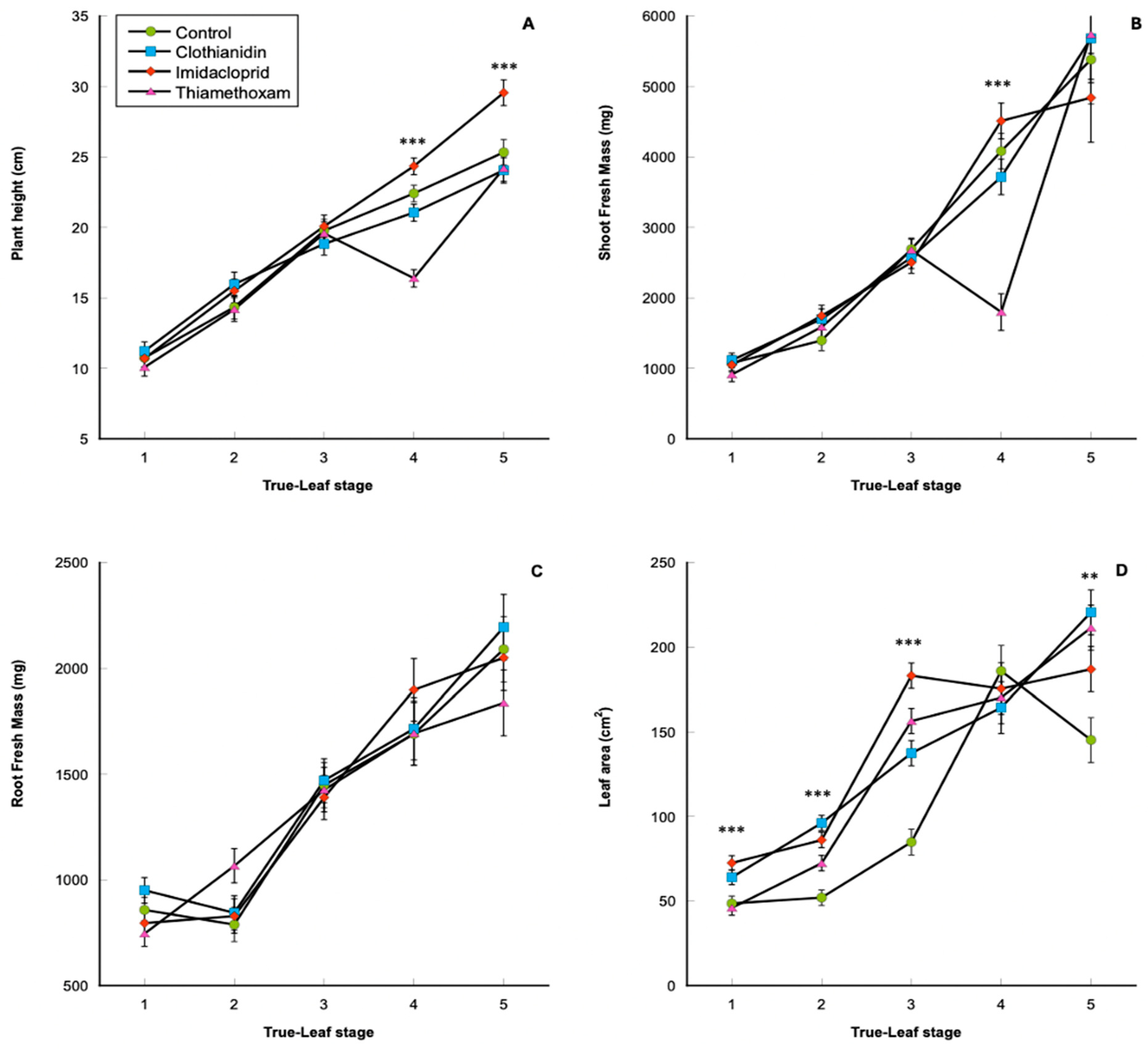
3.1. Plant Physiological Data
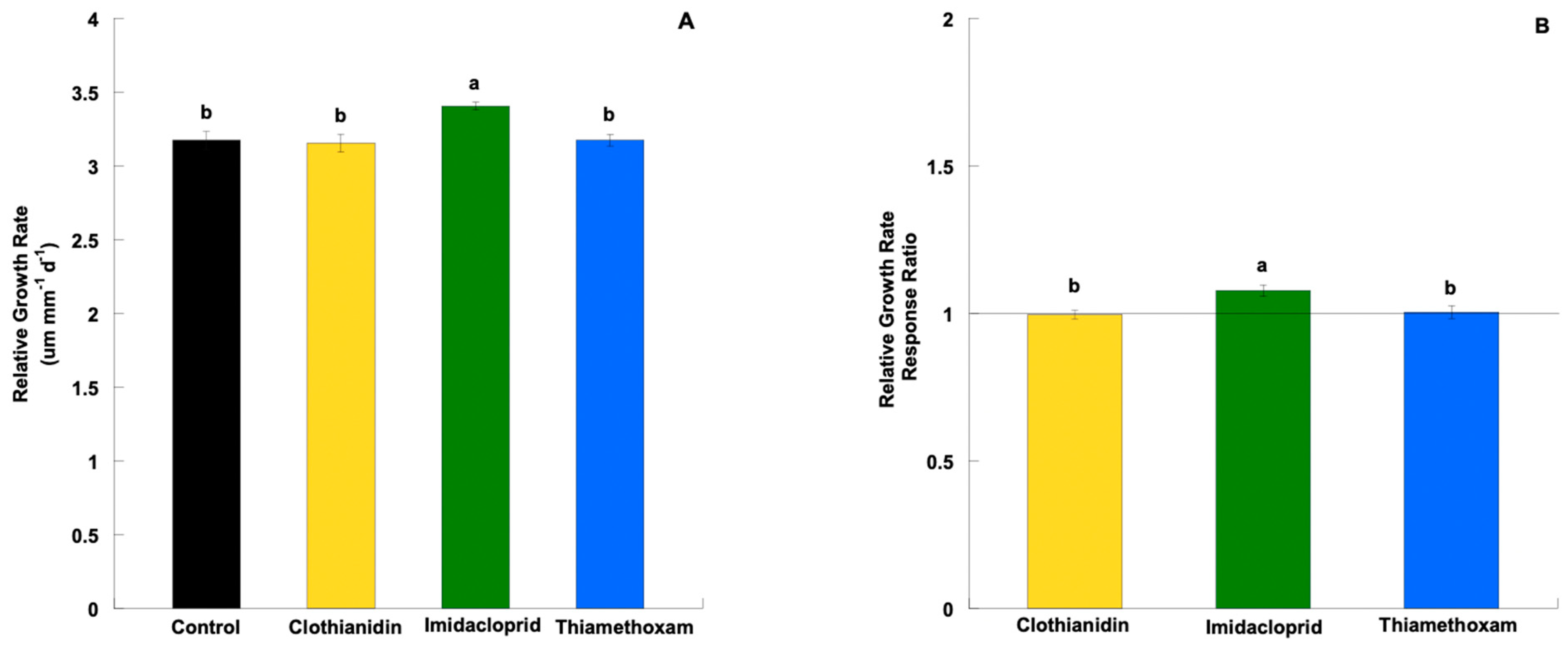
3.2. Relative Growth Rate and Response Ratio
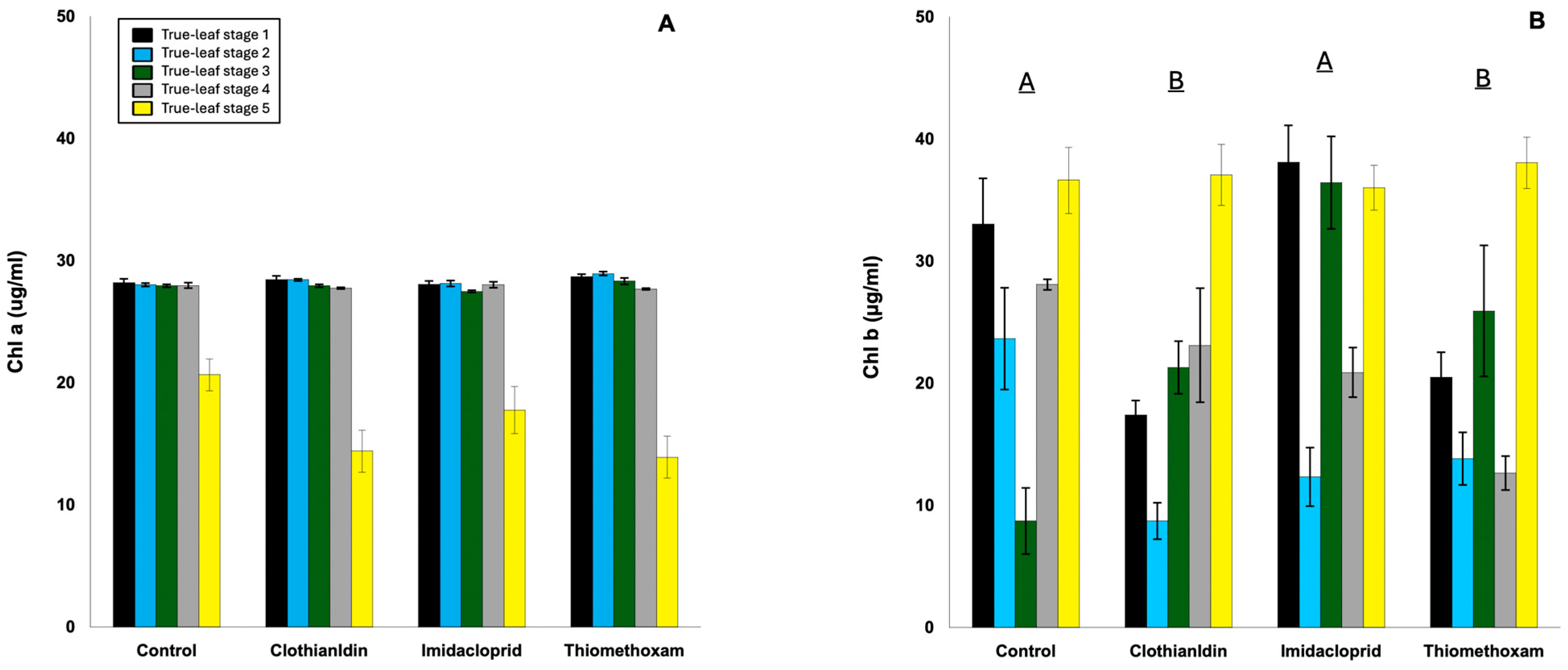
3.3. Chlorophyll Content in Cotton Seedlings
3.4. Nutrient Analysis
4. Discussion
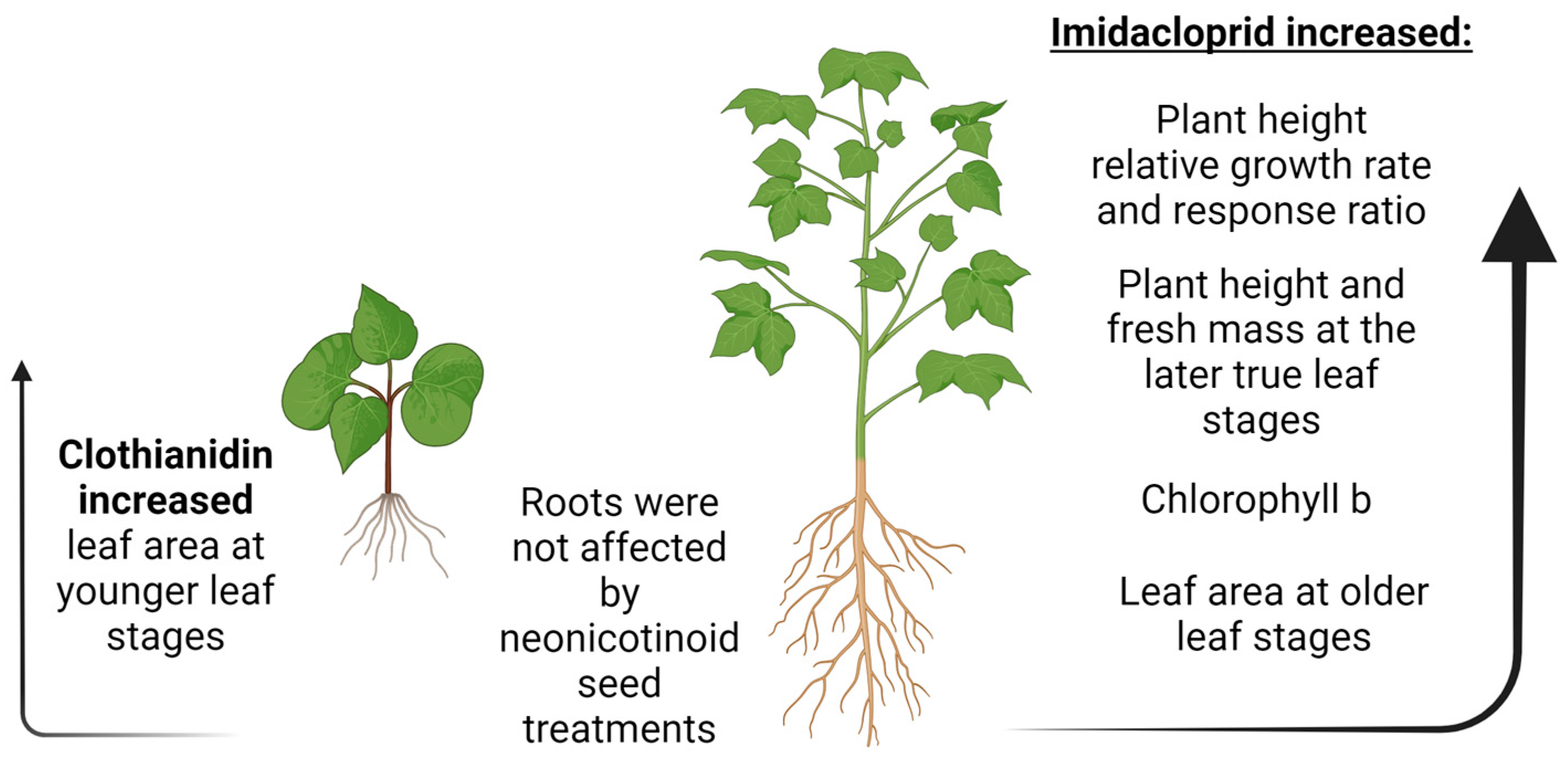
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hungerford, A.; Arita, S.; Johansson, R. Examining USDA 2019 Acreage and Yield Estimates. Farmdoc Dly. 2019, 9, 185. [Google Scholar]
- Deguine, J.P.; Ferron, P.; Russell, D. Sustainable pest management for cotton production. A review. Agron. Sustain. Dev. 2008, 28, 113–137. [Google Scholar] [CrossRef]
- Leigh, T.F.; Roach, S.H.; Watson, T.F.; King, E.; Phillips, J.; Coleman, R. Biology and ecology of important insect and mite pests of cotton. In Cotton Insect and Mites: Characterization and Management; The Cotton Foundation: Memphis, TN, USA, 1996; pp. 17–86. [Google Scholar]
- Vyavhare, S.S.; Reed, B. Control of cotton fleahopper in cotton using foliar applied insecticides, 2019. Arthropod Manag. Tests 2020, 45, tsaa090. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; Terry, L.I. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agric. For. Entomol. 2003, 5, 301–310. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Liu, T.X.; Adamczyk, J.J. Thrips (Thysanoptera: Thripidae) on cotton in the lower Rio Grande valley of Texas: Species composition, seasonal abundance, damage, and control. Southwest. Entomol. 2009, 34, 417–430. [Google Scholar] [CrossRef]
- North, J.H.; Gore, J.; Catchot, A.L.; Stewart, S.D.; Lorenz, G.M.; Musser, F.R.; Cook, D.R.; Kerns, D.L.; Dodds, D.M.; Giles, K. Value of neonicotinoid insecticide seed treatments in mid-south cotton (Gossypium hirsutum [Malvales: Malvaceae]) Production Systems. J. Econ. Entomol. 2018, 111, 10–15. [Google Scholar] [CrossRef]
- Jones, A.G.; Mason, C.J.; Felton, G.W.; Hoover, K. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci. Rep. 2019, 9, 2792. [Google Scholar] [CrossRef]
- Macedo, W.R.; Castro, P.R.D.C. Thiamethoxam: Molecule moderator of growth, metabolism and production of spring wheat. Pestic. Biochem. Phys. 2011, 100, 299–304. [Google Scholar] [CrossRef]
- Castro, P.R.D.C.; Serciloto, C.M.; Pereira, M.A.; Rodrigues, J.L.M.; Rossi, G. Agroquímicos de Controle Hormonal, Fosfitos e Potencial de Aplicação dos Aminoácidos na Agricultura Tropical; Série Produtor Rural; Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo: Piracicaba, Brazil, 2019; 83p. [Google Scholar]
- Cataneo, A.C.; Ferreira, L.C.; Carvalho, J.C.; Andréo-Souza, Y.; Corniani, N.; Mischan, M.M.; Nunes, J.C. Improved germination of soybean seed treated with thiamethoxam under drought conditions. Seed Sci. Technol. 2010, 38, 248–251. [Google Scholar] [CrossRef]
- Pynenburg, G.M.; Sikkema, P.H.; Gillard, C.L. Agronomic and economic assessment of intensive pest management of dry bean (Phaseolus vulgaris). J. Crop Prot. 2011, 30, 340–348. [Google Scholar] [CrossRef]
- Macedo, W.R.; Araújo, D.K.; Castro, P.R.D.C. Unravelling the physiologic and metabolic action of thiamethoxam on rice plants. Pestic. Biochem. Phys. 2013, 107, 244–249. [Google Scholar] [CrossRef]
- Endres, L.; Oliveira, N.G.; Ferreira, V.M.; Silva, J.V.; Barbosa, G.V.S.; Maia, J.S.O. Morphological and physiological response of sugarcane under abiotic stress to neonicotinoid insecticides. Theor. Exp. Plant Physiol. 2016, 28, 347–355. [Google Scholar] [CrossRef]
- Bredeson, M.M.; Lundgren, J.G. Field and forage crops, thiamethoxam seed treatments have no impact on pest numbers or yield in cultivated sunflowers. J. Econ. Entomol. 2015, 108, 2665–2671. [Google Scholar] [CrossRef]
- Preetha, G.; Stanley, J. Influence of neonicotinoid insecticides on the plant growth attributes of cotton and okra. J. Plant Nutr. 2012, 35, 1234–1245. [Google Scholar] [CrossRef]
- Patil, B.C.; Patil, S.B.; Vdikeri, S.S.; Khadi, B.M. Effect of imidacloprid seed treatment on growth, yield, seedling vigor and biophysical parameters in cotton (Gossypium spp.) genotypes. In Proceedings of the World Cotton Research Conference–III, Cape Town, South Africa, 9–13 March 2003. [Google Scholar]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. Physiologic response of cotton to the insecticide imidacloprid under high-temperature stress. J. Plant Growth Regul. 2008, 27, 77–82. [Google Scholar] [CrossRef]
- Lauxen, L.R.; Almeida, A.D.S.; Deuner, C.; Meneghello, G.E.; Villela, F.A. Physiological response of cotton seeds treated with thiamethoxam under heat stress. J. Seed Sci. 2016, 38, 140–147. [Google Scholar] [CrossRef]
- Sabbe, W.E.; Zelinski, L.J. Plant analysis as an aid in fertilizing cotton. In Soil Testing and Plant Analysis; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2018; pp. 469–493. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S.; Ahmed, B.; Syed, A.; Bahkali, A.H. Physiological disruption, structural deformation and low grain yield induced by neonicotinoid insecticides in chickpea: A long term phytotoxicity investigation. Chemosphere 2021, 262, 128388. [Google Scholar] [CrossRef]
- Macedo, W.R.; Fernandes, G.M.; Possenti, R.A.; Lambais, G.R.; Castro, P.R.D.C. Responses in root growth, nitrogen metabolism and nutritional quality in Brachiaria with the use of thiamethoxam. Acta Physiol. Plant. 2013, 35, 205–211. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Marthews, T.R.; Vogt, D.R.; Purves, D.; Rees, M.; Hector, A.; Turnbull, L.A. How to fit nonlinear plant growth models and calculate growth rates: An update for ecologists. Methods Ecol. Evol. 2012, 3, 245–256. [Google Scholar] [CrossRef]
- Mahmood, T.; Iqbal, M.S.; Li, H.; Nazir, M.F.; Khalid, S.; Sarfraz, Z.; Hu, D.; Baojun, C.; Geng, X.; Tajo, S.M.; et al. Differential seedling growth and tolerance indices reflect drought tolerance in cotton. BMC Plant Biol. 2022, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.N.; Fipke, G.M.; Minussi, W.J.E.; Márchese, J.A. ImageJ software as an alternative method for estimating leaf area in oats. Acta Agron. 2020, 69, 162–169. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll Fluorescence Signatures of Leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 1987, 131, 101–110. [Google Scholar] [CrossRef]
- Sedberry, J.E. Plant-Tissue Analysis as a Diagnostic Aid in Crop Production. LSU Agricultural Experiment Station Reports, 783. 1987. Available online: https://repository.lsu.edu/agexp/867 (accessed on 7 February 2024).
- Ford, K.A.; Casida, J.E.; Chandran, D.; Gulevich, A.G.; Okrent, R.A.; Durkin, K.A.; Sarpong, R.; Bunnelle, E.M.; Wildermuth, M.C. Neonicotinoid insecticides induce salicylate-associated plant defense responses. Proc. Natl. Acad. Sci. USA 2010, 107, 17527–17532. [Google Scholar] [CrossRef] [PubMed]
- Sirchio, K.; Sutton, A. Syngenta professional products focuses chemical technology on new applications to enhance the quality of life. Chimia 2007, 61, 17. [Google Scholar] [CrossRef]
- Alford, A.; Krupke, C. Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS ONE 2017, 12, e0173836. [Google Scholar]
- Godói, C.T.D.; Campos, S.O.; Monteiro, S.H.; Ronchi, C.P.; Silva, A.A.; Guedes, R.N.C. Thiamethoxam in soybean seed treatment: Plant bioactivation and hormesis, besides whitefly control? Sci. Total Environ. 2023, 857, 159443. [Google Scholar] [CrossRef] [PubMed]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objectives, and adaptive system for expressing rice development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef]
- Pereira, J.M.; Fernandes, P.M.; Veloso, V.R.S. Physiological effect of the insecticide thiamethoxam on sugarcane. Arq. Inst. Biol. 2015, 77, 159–164. [Google Scholar] [CrossRef]
- Simpson, C.R.; Nelson, S.D.; Melgar, J.C.; Jifon, J.; King, S.R.; Schuster, G.; Volder, A. Growth response of grafted and ungrafted citrus trees to saline irrigation. Sci. Hort. 2014, 169, 199–205. [Google Scholar] [CrossRef]
- Lima, A.F.; Aguirre, N.M.; Carvalho, G.A.; Grunseich, J.M.; Helms, A.M.; Peñaflor, M.F.G. Effects of neonicotinoid seed treatment on maize anti-herbivore defenses vary across plant genotypes. J. Pest Sci. 2024, 97, 199–212. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M. Leaf pigment content. Comp. Rem. Sens. 2017, 3, 117–142. [Google Scholar]
- Liu, T.; Luo, J.; Liu, S.; Li, T.; Li, H.; Zhang, L.; Zou, N. Clothianidin loaded TA/Fe (III) controlled-release granules: Improve pesticide bioavailability and alleviate oxidative stress. J. Hazard. Mater. 2021, 416, 125861. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bouwkamp, J.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Botany 1998, 49, 503–510. [Google Scholar]
- Ebel, R.C.; Wallace, B.; Elkins, C. Phytotoxicity of the systemic insecticide imidacloprid on tomato and cucumber in the greenhouse. Hort. Technol. 2000, 10, 144–147. [Google Scholar] [CrossRef]
- Mote, U.N.; Datkile, R.V.; Loage, G.R. Efficacy of imidacloprid as seed treatment against initial sucking pests of cotton. Pestology 1995, 19, 5–8. [Google Scholar]
| Seed Treatment | N (%) | P (%) | K (%) | Mg (%) | Ca(%) | S (%) | B (ppm) | Zn (ppm) | Mn (ppm) | Fe (ppm) | Cu (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2.48 | 0.42 | 2.66 | 0.64 | 2.79 | 1.05 | 50.50 | 30.67 | 97.50 | 58.83 | 3.83 |
| Clothianidin | 2.55 | 0.42 | 2.27 | 0.62 | 2.94 | 1.02 | 52.00 | 30.50 | 105.17 | 57.00 | 3.67 |
| Imidacloprid | 2.29 | 0.43 | 2.44 | 0.59 | 2.60 | 1.05 | 46.33 | 32.83 | 100.50 | 53.00 | 3.50 |
| Thiamethoxam | 2.39 | 0.38 | 2.18 | 0.62 | 2.70 | 0.96 | 46.00 | 28.33 | 114.67 | 54.50 | 3.33 |
| DF | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| F ratio | 0.442 | 1.480 | 2.53 | 0.443 | 0.83 | 0.160 | 0.691 | 1.162 | 0.366 | 0.438 | 0.505 |
| p-value | 0.725 | 0.250 | 0.086 | 0.725 | 0.493 | 0.922 | 0.568 | 0.349 | 0.778 | 0.728 | 0.683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehrish, A.; Parajulee, M.; Vyavhare, S.; Coldren, C.; Laza, H.; Simpson, C.R. Effects of Neonicotinoid Seed Treatments on Cotton Seedling Physiology, Nutrition, and Growth. Agronomy 2024, 14, 799. https://doi.org/10.3390/agronomy14040799
Sehrish A, Parajulee M, Vyavhare S, Coldren C, Laza H, Simpson CR. Effects of Neonicotinoid Seed Treatments on Cotton Seedling Physiology, Nutrition, and Growth. Agronomy. 2024; 14(4):799. https://doi.org/10.3390/agronomy14040799
Chicago/Turabian StyleSehrish, Aqeela, Megha Parajulee, Suhas Vyavhare, Cade Coldren, Haydee Laza, and Catherine R. Simpson. 2024. "Effects of Neonicotinoid Seed Treatments on Cotton Seedling Physiology, Nutrition, and Growth" Agronomy 14, no. 4: 799. https://doi.org/10.3390/agronomy14040799
APA StyleSehrish, A., Parajulee, M., Vyavhare, S., Coldren, C., Laza, H., & Simpson, C. R. (2024). Effects of Neonicotinoid Seed Treatments on Cotton Seedling Physiology, Nutrition, and Growth. Agronomy, 14(4), 799. https://doi.org/10.3390/agronomy14040799







