Use of Eucalyptus Charcoal Waste in the Formulation of Substrate for the Cultivation of Two Strains (LED 20/11 and LED 20/12) of Lentinula edodes
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, X.; Li, W.; Li, W.; Chen, W.; Zhang, Z.; Wu, D.; Yang, Y. Quality characteristics and non-volatile taste formation mechanism of Lentinula edodes during hot air drying. Food Chem. 2022, 393, 133378. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Gao, Q.; Rong, C.; Liu, Y.; Song, S.; Yu, Q.; Zhou, K.; Liao, Y. Comparative transcriptome analysis of abnormal cap and healthy fruiting bodies of the edible mushroom Lentinula edodes. Fungal Genet. Biol. 2021, 156, 103614. [Google Scholar] [CrossRef] [PubMed]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fang, X.; Wu, W.; Chen, H.; Mu, H.; Gao, H. Effects of high-temperature pre-drying on the quality of air-dried shiitake mushrooms (Lentinula edodes). Food Chem. 2019, 285, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, T.; Yang, Y.L.; Liu, H.G.; Wang, Y.Z. Arsenic concentrations and associated health risks in Laccaria mushrooms from Yunnan (SW China). Biol. Trace Elem. Res. 2015, 164, 261–266. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Andrade, M.C.N.; Sales-Campos, C.; Carvalho, C.S.M.; Aguiar, L.V.B.; Minhoni, M.T.A. Uso de resíduos madeireiros da Amazônia brasileira no cultivo in vitro de Lentinus strigosus. Rev. Ambiência 2013, 9, 189–196. [Google Scholar] [CrossRef]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci. Hortic. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, S.Y.; Han, J.G.; Kong, W.S.; Jung, M.Y.; Kim, S.H. Fatty acids and stable isotope ratios in shiitake mushrooms (Lentinula edodes) indicate the origin of the cultivation substrate used: A preliminary case study in Korea. Foods 2020, 9, 1210. [Google Scholar] [CrossRef]
- Moonmoon, M.; Shelly, N.J.; Khan, M.A.; Uddin, M.N.; Hossain, K.; Tania, M.; Ahmed, S. Effects of different levels of wheat bran, rice bran and maize powder supplementation with saw dust on the production of shiitake mushroom (Lentinus edodes (Berk.) Singer). Saudi J. Biol. Sci. 2011, 18, 323–328. [Google Scholar] [CrossRef]
- Balan, V.; Zhu, W.; Krishnamoorthy, H.; Benhaddou, D.; Mowrer, J.; Husain, H.; Eskandari, A. Challenges and opportunities in producing high-quality edible mushrooms from lignocellulosic biomass in a small scale. Appl. Microbiol. Biotechnol. 2022, 106, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, M.; Murray, D.J.; Ward, F. Influence of substrate formulation and autoclave treatment on Lentinula edodes production. Mushroom Sci. 2000, 15, 803–810. [Google Scholar]
- Morais, M.H.; Ramos, A.C.; Matos, N.; Oliveira, E.J.S. Production of shiitake mushroom (Lentinus edodes) on lignocellulosic residues. Food Sci. Technol. Int. 2000, 6, 123–128. [Google Scholar] [CrossRef]
- Curvetto, N.; Figlas, D.; Delmastro, S. Sunflower seed hulls as substrate for the cultivation of shiitake mushrooms. Horttech 2002, 12, 652–655. [Google Scholar] [CrossRef]
- Philippoussis, A.N.; Diamantopoulou, P.A.; Zervakis, G.I. Correlation of the properties of several lignocellulosic substrates to the crop performance of the shiitake mushroom Lentinula edodes. World J. Microbiol. Biotechnol. 2003, 19, 551–557. [Google Scholar] [CrossRef]
- Gaitán-Hernández, R.; López-Peña, D.; Esqueda, M.; Gutiérrez, A. Review of bioactive molecules production, biomass, and basidiomata of shiitake culinary-medicinal mushrooms, Lentinus edodes (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 841–850. [Google Scholar] [CrossRef]
- Kannan, C. Effect of spawn density and bed substrates on the sporophore yield of Lentinus edodes. Plant Arch. 2019, 19, 365–368. [Google Scholar]
- Chen, F.; Martín, C.; Lestander, T.A.; Grimm, A.; Xiong, S. Shiitake cultivation as biological preprocessing of lignocellulosic feedstocks–Substrate changes in crystallinity, syringyl/guaiacyl lignin and degradation-derived by-products. Bioresour. Technol. 2022, 344, 126256. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Zhang, L.; Li, Q.; Song, C.; Shang, X.; Bao, D.; Tan, Q.; Lv, B. Corncob as a Substrate for the Cultivation of Lentinula edodes. Waste Biomass Valori 2022, 13, 929–939. [Google Scholar] [CrossRef]
- Viotto, R.S.; Maia, A.A.D.; Yamaji, F.M.; Morais, L.C. Thermogravimetric investigation of spent shiitake substrate to solid biofuel. Can. J. Chem. Eng. 2018, 96, 845–854. [Google Scholar] [CrossRef]
- Brandão, V.D.S.; Matos, A.T.D.; Fontes, M.P.; Martinez, M.A. Retenção de poluentes em filtros orgânicos operando com águas residuárias da suinocultura. Rev. Bras. Eng. Agric. Ambient. 2003, 7, 329–334. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Ataíde, C.H. Valorization of Eucalyptus urograndis wood via carbonization: Product yields and characterization. Energy 2019, 172, 509–516. [Google Scholar] [CrossRef]
- Dias Júnior, A.F.; Suuchi, M.A.; Sant’Anna Neto, A.; da Silva, J.G.M.; da Teixeira, Á.M.; de Souza, N.D.; Protásio, T.P.; Brito, J.O. Blends of charcoal fines and wood improve the combustibility and quality of the solid biofuels. Bioenergy Res. 2021, 14, 344–354. [Google Scholar] [CrossRef]
- MacPhee, J.A.; Gransden, J.F.; Giroux, L.; Price, J.T. Possible CO2 mitigation via addition of charcoal to coking coal blends. Fuel Process Technol. 2009, 90, 16–20. [Google Scholar] [CrossRef]
- Noh, J.H.; Ko, H.G.; Park, H.S.; Koo, C.D. Selection of parental strain on the sawdust cultivation and mycelial growth and cultural characteristics of Lentinula edodes hybrid strains. J. Mushrooms 2015, 13, 41–49. [Google Scholar] [CrossRef]
- Cobos, J.D.V.; Viejó, F.G.; Cobo, J.C.; Sulca, R.L.; Verdugo, D.N.; Moncayo, M.F.G.; Endara, A.G. Chemical and productivity characterization of parental and hybrid strains of Lentinula edodes cultivated in different agricultural residues. Emir. J. Food Agric. 2021, 33, 260–265. [Google Scholar] [CrossRef]
- Zied, D.C.; Penachio, S.M.; Dias, E.S.; Minhoni, M.T.A.; Ferraz, R.A.; Vieites, R.L. Influence of productivity and processing method on physicochemical characteristics of white button mushrooms in Brazil. J. Sci. Food Agric. 2014, 94, 2850–2855. [Google Scholar] [CrossRef]
- Sheikha, A.F.; Hu, D.M. How to trace the geographic origin of mushrooms? Trends Food Sci. Technol. 2018, 78, 292–303. [Google Scholar] [CrossRef]
- Rao, N.K.; Reddy, L.J.; Bramel, P.J. Potential of wild species for genetic enhancement of some semi-arid food crops. Genet. Resour. Crop Evol. 2003, 50, 707–721. [Google Scholar]
- Lee, S.J.; Kim, H.H.; Kim, S.H.; Kim, S.H.; Sung, N.J. The effect of different culture conditions of liquid spawn on the quality characteristics of shiitake mushroom (Lentinula edodes). J. Mushrooms 2019, 17, 99–106. [Google Scholar]
- Xiong, S.; Martín, C.; Eilertsen, L.; Wei, M.; Myronycheva, O.; Larsson, S.H.; Lestander, T.A.; Atterhem, L.; Jönsson, L.J. Energy-efficient substrate pasteurisation for combined production of shiitake mushroom (Lentinula edodes) and bioethanol. Bioresour. Technol. 2019, 274, 65–72. [Google Scholar] [CrossRef]
- Sousa, M.A.C.; Costa, L.M.A.S.; Pereira, T.S.; Zied, D.C.; Rinker, D.L.; Dias, E.S. Enzyme activity and biochemical changes during production of Lentinula edodes (Berk.) Pegler. Food Sci. Technol. 2019, 39, 774–780. [Google Scholar] [CrossRef]
- Roz, A.L.; Ricardo, J.F.C.; Nakashima, G.T.; Santos, L.R.O.; Yamaji, F.M. Maximização do teor de carbono fixo em biocarvão aplicado ao sequestro de carbono. Rev. Bras. Eng. Agrícola Ambient. 2015, 19, 810–814. [Google Scholar] [CrossRef]
- Joseph, J.L.S. Biochar for Environmental Management, 1st ed.; Earthscan: London, UK; Sterling, VA, USA, 2009; 404p. [Google Scholar]
- Hilscher, A.; Heister, K.; Siewert, C.; Knicker, H. Mineralisation and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Org. Geochem. 2009, 40, 332–342. [Google Scholar] [CrossRef]
- Madari, B.E.; Costa, A.R.; Castro, L.M.; Santos, J.L.S.; Benites, V.D.M.; Rocha, A.D.O.; Machado, P.D.A. Carvão vegetal como condicionador de solo para arroz de terras altas (cultivar Primavera): Um estudo prospectivo; Embrapa Arroz e Feijão. Comunicado Técnico, 125; Embrapa Arroz e Feijão: Santo Antônio de Goiás, Brazil, 2006; pp. 1–4. [Google Scholar]
- Rodrigues, A.E.; Royse, D.J. Yield, size and bacterial blotch resistance of Pleurotus eryngii grown on cottonseed hulls/oak sawdust supplemented with manganese, copper and whole ground soybean. Bioresour. Technol. 2007, 98, 1898–1906. [Google Scholar] [CrossRef]
- Zied, D.C.; Caitano, C.E.C.; Pardo-Gimenez, A.; Dias, E.S.; Zeraik, M.L.; Pardo, J.E. Using of appropriated strains in the practice of compost supplementation for Agaricus subrufescens production. Front. Sustain. Food Syst. 2018, 2, 26. [Google Scholar] [CrossRef]
- Kerketta, A.; Shukla, C.S.; Singh, H.K. Evaluation of different casing materials for growth and yield of button Musrhroom (Agaricus bisporus (L.) Sing.). J. Pharmacogn. Phytochem. 2019, 8, 207–209. [Google Scholar]
- Rather, M.A.; Ahmad, I.; Shah, A.; Hajam, Y.A.; Amin, A.; Khursheed, S.; Ahmad, I.; Rasool, S. Exploring opportunities of Artificial Intelligence in aquaculture to meet increasing food demand. Food Chem. X 2024, 22, 101309. [Google Scholar] [CrossRef]
- Goglio, P.; Ponsioen, T.; Carrasco, J.; Milenkovi, I.; Kiwala, L.; Van Mierlo, K.; Helmes, R.; Tei, F.; Oosterkamp, E.; Pérez, M. An environmental assessment of Agaricus bisporus ((JE Lange) Imbach) mushroom production systems across Europe. Eur. J. Agron. 2024, 155, 127108. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lehmann, J.; Solomon, D.; Sohi, S.; Thies, J.E.; Skjemstad, J.O.; Luizão, F.J.; Engelhard, M.H.; Neves, E.G.; Wirick, S. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta 2008, 72, 6069–6078. [Google Scholar] [CrossRef]
- Fuss, S.; Jones, C.D.; Kraxner, F.; Peters, G.P.; Smith, P.; Tavoni, M.; Vuuren, D.P.; Canadell, J.G.; Jackson, R.B.; Milne, J.; et al. Research priorities for negative emissions. Environ. Res. Lett. 2016, 11, 115007. [Google Scholar] [CrossRef]
- Whitman, T.; Hanley, K.; Enders, A.; Lehmann, J. Predicting pyrogenic organic matter mineralization from its initial properties and implications for carbon management. Org. Geochem. 2013, 64, 76–83. [Google Scholar] [CrossRef]
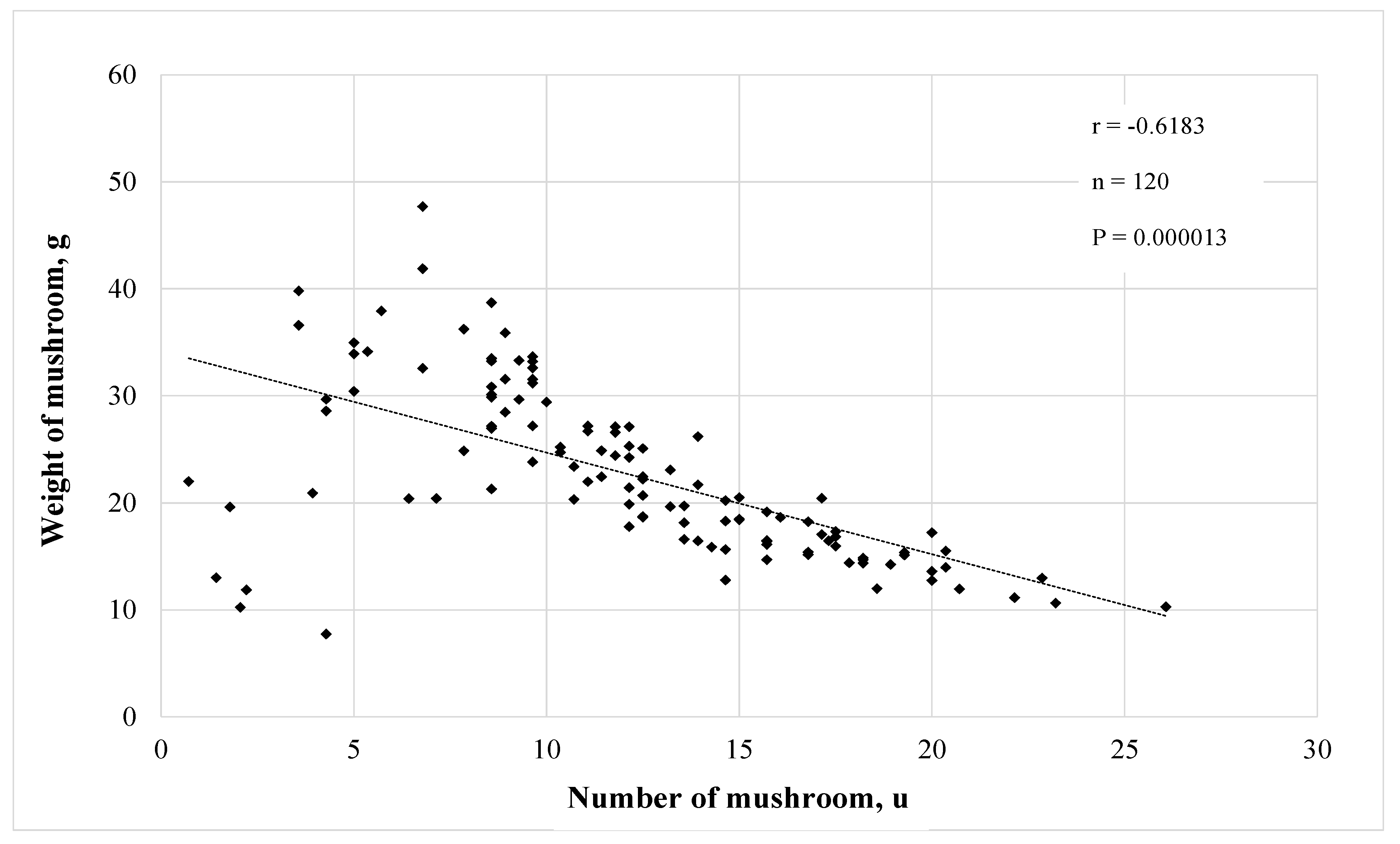
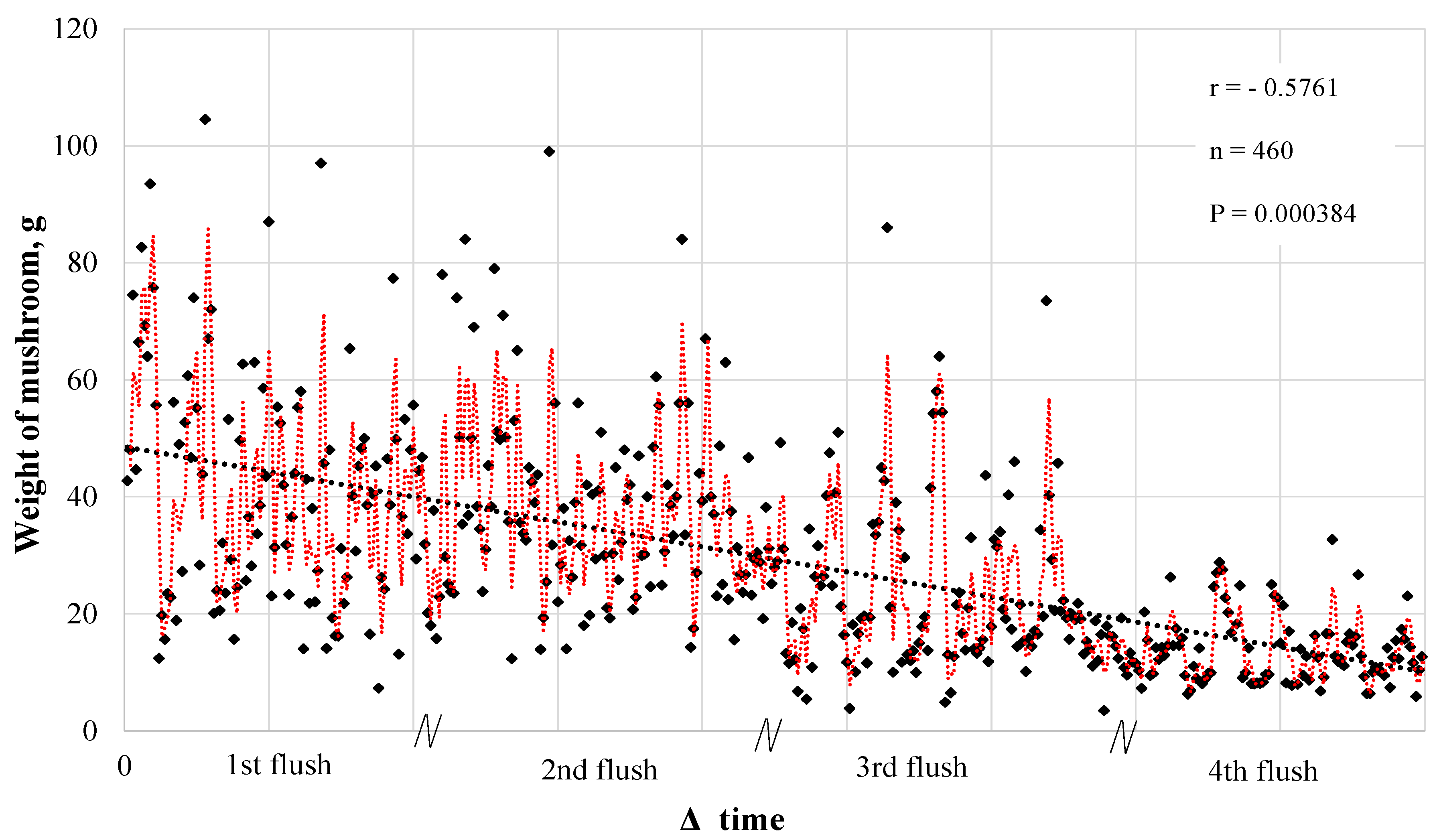
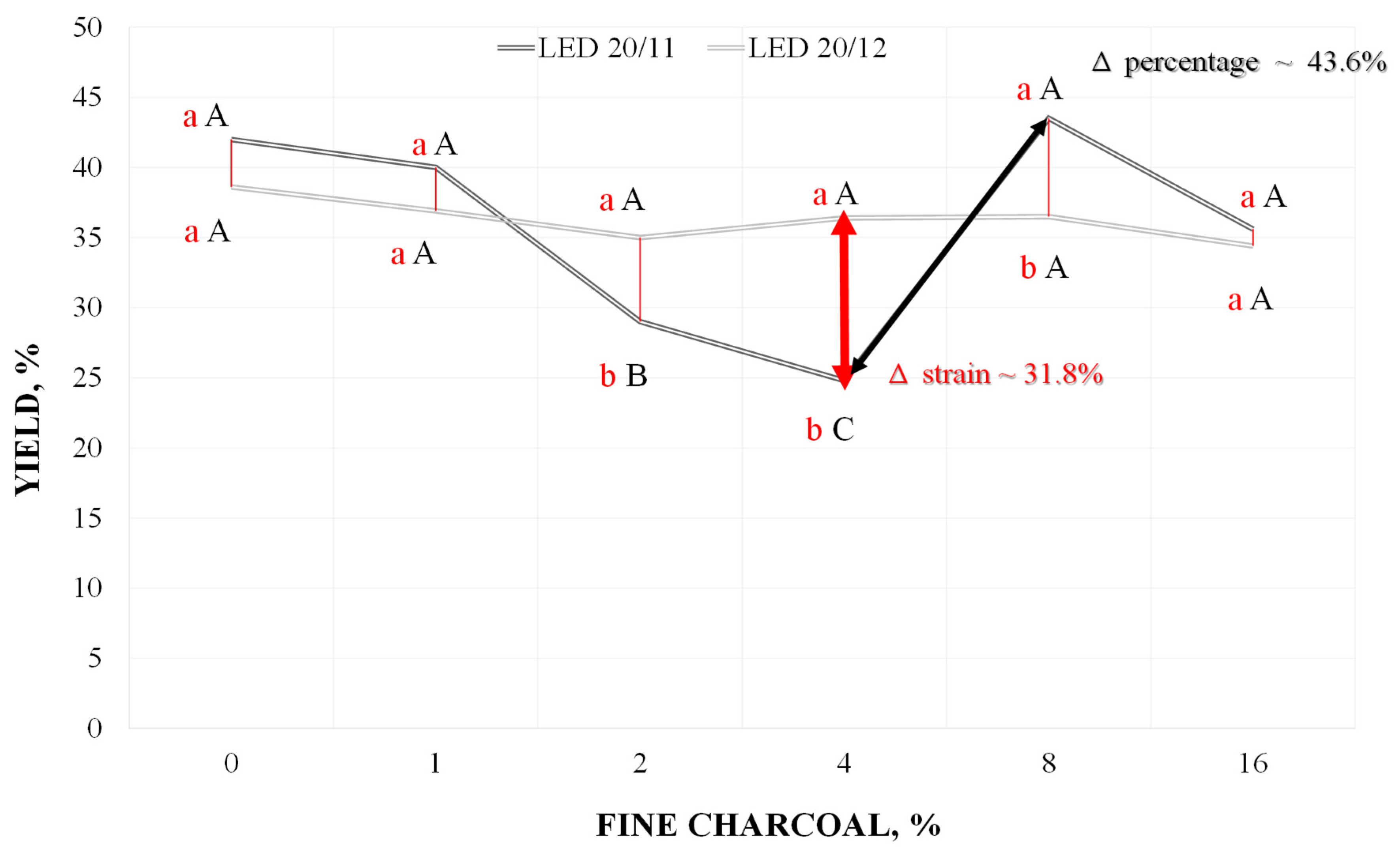
| Substrate | N | P2O5 | K | Ca | Mg | S | O.M. | O.C. | Na | Cu | Fe | Mn | Zn | C/N Ratio | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | mg/kg | ||||||||||||||
| 0% | 0.89 | 1.03 | 0.39 | 0.80 | 0.34 | 0.04 | 87.43 | 49.44 | 766.21 | 5.89 | 402.75 | 117.88 | 39.29 | 55/1 | 5.52 |
| 1% | 0.75 | 1.06 | 0.36 | 0.81 | 0.32 | 0.04 | 89.17 | 50.00 | 774.00 | 3.96 | 356.68 | 122.86 | 33.69 | 66/1 | 5.42 |
| 2% | 0.84 | 1.23 | 0.38 | 0.84 | 0.35 | 0.04 | 85.55 | 48.33 | 797.33 | 3.93 | 424.80 | 129.80 | 37.37 | 57/1 | 5.42 |
| 4% | 1.05 | 1.19 | 0.38 | 0.80 | 0.34 | 0.04 | 87.59 | 49.44 | 810.97 | 5.91 | 429.10 | 135.82 | 37.40 | 47/1 | 5.44 |
| 8% | 0.79 | 1.07 | 0.37 | 0.83 | 0.35 | 0.05 | 85.90 | 48.33 | 884.36 | 3.95 | 404.80 | 138.22 | 33.57 | 61/1 | 5.41 |
| 16% | 1.30 | 1.04 | 0.38 | 1.38 | 0.38 | 0.05 | 85.07 | 47.78 | 1081.33 | 1.98 | 401.60 | 168.16 | 35.61 | 36/1 | 5.35 |
| Sawdust * | 0.23 | 0.007 | 0.97 | 1.36 | 0.057 | - | - | 55.18 | 670 | 1.82 | - | - | 14.62 | 238/1 | 5.40 |
| Fine charcoal * | 0.21 | 0.025 | 2.32 | 1.83 | 0.067 | - | - | 55.18 | 7568 | 1.94 | - | - | 8.38 | 262/1 | 7.40 |
| Strain * | 1st Flush | 2nd Flush | 3rd Flush | 4th Flush |
|---|---|---|---|---|
| Number of Mushrooms, u | ||||
| LED 20/11 | 5.5 ± 8.0 B | 2.9 ± 3.70 C | 5.1 ± 2.95 b B | 8.8 ± 2.79 b A |
| LED 20/12 | 7.7 ± 4.11 C | 3.3 ± 2.62 D | 11.0 ± 2.49 a B | 14.7 ± 3.41 a A |
| Weight of mushroom, g | ||||
| LED 20/11 | 44.9 ± 13.95 A | 34.9 ± 21.31 B | 31.9 ± 14.37 a B | 15.9 ± 6.75 a C |
| LED 20/12 | 36.4 ± 43.46 A | 35.6 ± 16.07 A | 16.5 ± 7.58 b B | 9.9 ± 4.68 b B |
| Yield, % | ||||
| LED 20/11 | 12.5 ± 3.91 A | 5.2 ± 2.11 C | 8.2 ± 3.79 B | 7.0 ± 5.17 B |
| LED 20/12 | 14.1 ± 10.91 A | 5.9 ± 2.94 C | 9.1 ± 6.92 B | 7.3 ± 8.43 C |
| Dose, % * | 1st Flush | 2nd Flush | 3rd Flush | 4th Flush |
|---|---|---|---|---|
| Number of Mushroom, u | ||||
| 0 | 6.9 ± 11.24 B | 3.1 ± 2.88 B | 9.1 ± 10.54 a A | 11.7 ± 6.03 A |
| 1 | 6.8 ± 5.37 B | 2.8 ± 1.66 C | 7.1 ± 3.92 a B | 11.4 ± 5.42 A |
| 2 | 4.8 ± 4.74 B | 2.9 ± 1.31 B | 6.5 ± 7.10 b A | 12.0 ± 9.65 A |
| 4 | 4.9 ± 7.32 B | 2.8 ± 1.59 B | 4.5 ± 4.37 b B | 12.5 ± 5.71 A |
| 8 | 6.4 ± 14.07 B | 4.0 ± 2.27 B | 7.8 ± 4.30 a B | 11.5 ± 9.36 A |
| 16 | 6.3 ± 6.86 B | 3.3 ± 4.49 C | 7.9 ± 3.11 a B | 10.9 ± 6.65 A |
| Weight of mushroom, g | ||||
| 0 | 48.1 ± 21.77 A | 37.0 ± 24.0 b A | 23.0 ± 10.48 B | 12.8 ± 4.15 a B |
| 1 | 44.8 ± 19.36 A | 50.1 ± 17.85 a A | 24.0 ± 10.96 B | 13.5 ± 3.05 a B |
| 2 | 38.6 ± 19.98 A | 28.3 ± 19.49 b B | 26.9 ± 13.32 B | 11.3 ± 8.89 b C |
| 4 | 32.9 ± 24.84 A | 25.2 ± 11.16 b A | 24.0 ± 20.39 A | 8.3 ± 7.95 b B |
| 8 | 47.9 ± 16.01 A | 37.8 ± 17.79 b A | 22.7 ± 12.57 B | 14.7 ± 8.55 a B |
| 16 | 31.8 ± 15.14 A | 33.4 ± 18.04 b A | 24.6 ± 17.43 A | 13.1 ± 4.67 a B |
| Yield, % | ||||
| 0 | 16.5 ± 4.93 a A | 5.7 ± 4.37 a C | 10.5 ± 2.59 a B | 7.5 ± 2.71 C |
| 1 | 15.3 ± 6.07 a A | 7.0 ± 3.54 a B | 8.5 ± 2.59 a B | 7.7 ± 2.7 B |
| 2 | 9.4 ± 5.55 b A | 4.1 ± 2.13 b B | 8.7 ± 3.51 a A | 6.8 ± 3.33 A |
| 4 | 10.6 ± 5.95 b A | 3.6 ± 2.21 b C | 5.4 ± 2.55 b B | 5.2 ± 3.37 B |
| 8 | 12.1 ± 4.24 b A | 7.5 ± 2.09 a B | 9.0 ± 1.99 a B | 8.5 ± 2.90 B |
| 16 | 12.8 ± 6.43 b A | 5.6 ± 3.36 a C | 9.7 ± 1.85 a B | 7.2 ± 3.84 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zied, D.C.; Silva, B.D.; Caitano, C.E.C.; Vieira Junior, W.G.; da Silva Freitas, M.A.; Teixeira, P.A.G.; Pardo-Giménez, A. Use of Eucalyptus Charcoal Waste in the Formulation of Substrate for the Cultivation of Two Strains (LED 20/11 and LED 20/12) of Lentinula edodes. Agronomy 2024, 14, 811. https://doi.org/10.3390/agronomy14040811
Zied DC, Silva BD, Caitano CEC, Vieira Junior WG, da Silva Freitas MA, Teixeira PAG, Pardo-Giménez A. Use of Eucalyptus Charcoal Waste in the Formulation of Substrate for the Cultivation of Two Strains (LED 20/11 and LED 20/12) of Lentinula edodes. Agronomy. 2024; 14(4):811. https://doi.org/10.3390/agronomy14040811
Chicago/Turabian StyleZied, Diego Cunha, Bianca Domingues Silva, Cinthia Elen Cardoso Caitano, Wagner Gonçalves Vieira Junior, Marcos Antônio da Silva Freitas, Pedro Afonso Gomes Teixeira, and Arturo Pardo-Giménez. 2024. "Use of Eucalyptus Charcoal Waste in the Formulation of Substrate for the Cultivation of Two Strains (LED 20/11 and LED 20/12) of Lentinula edodes" Agronomy 14, no. 4: 811. https://doi.org/10.3390/agronomy14040811
APA StyleZied, D. C., Silva, B. D., Caitano, C. E. C., Vieira Junior, W. G., da Silva Freitas, M. A., Teixeira, P. A. G., & Pardo-Giménez, A. (2024). Use of Eucalyptus Charcoal Waste in the Formulation of Substrate for the Cultivation of Two Strains (LED 20/11 and LED 20/12) of Lentinula edodes. Agronomy, 14(4), 811. https://doi.org/10.3390/agronomy14040811






