Sensitivity of Lithuanian Zymoseptoria tritici to Quinone Outside Inhibitor and Succinate Dehydrogenase Inhibitor Fungicides
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaf Sampling and Isolation of Zymoseptoria tritici
2.2. Fungicide Sensitivity Testing
2.3. Fungicides
2.4. Statistical Analysis
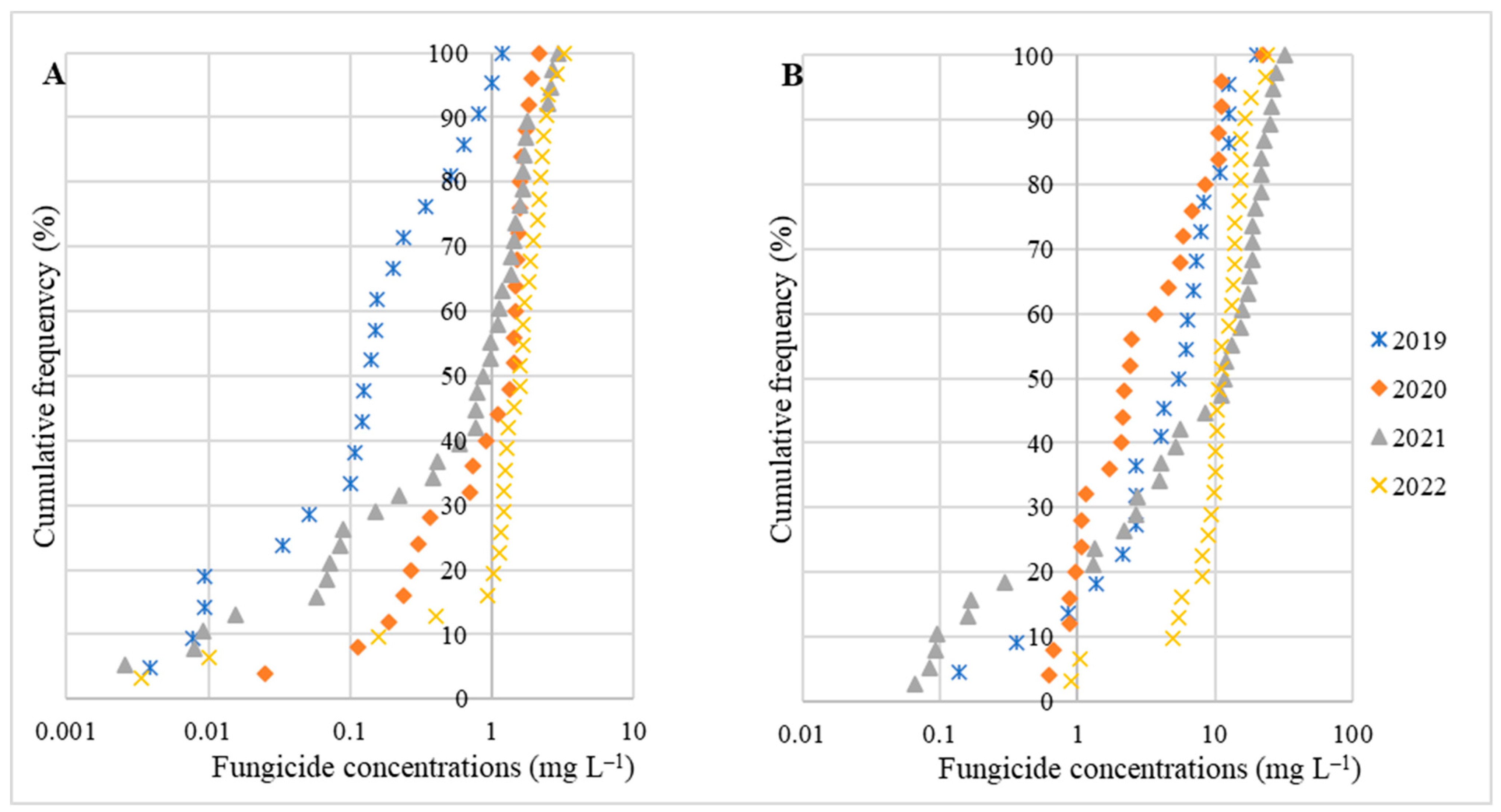
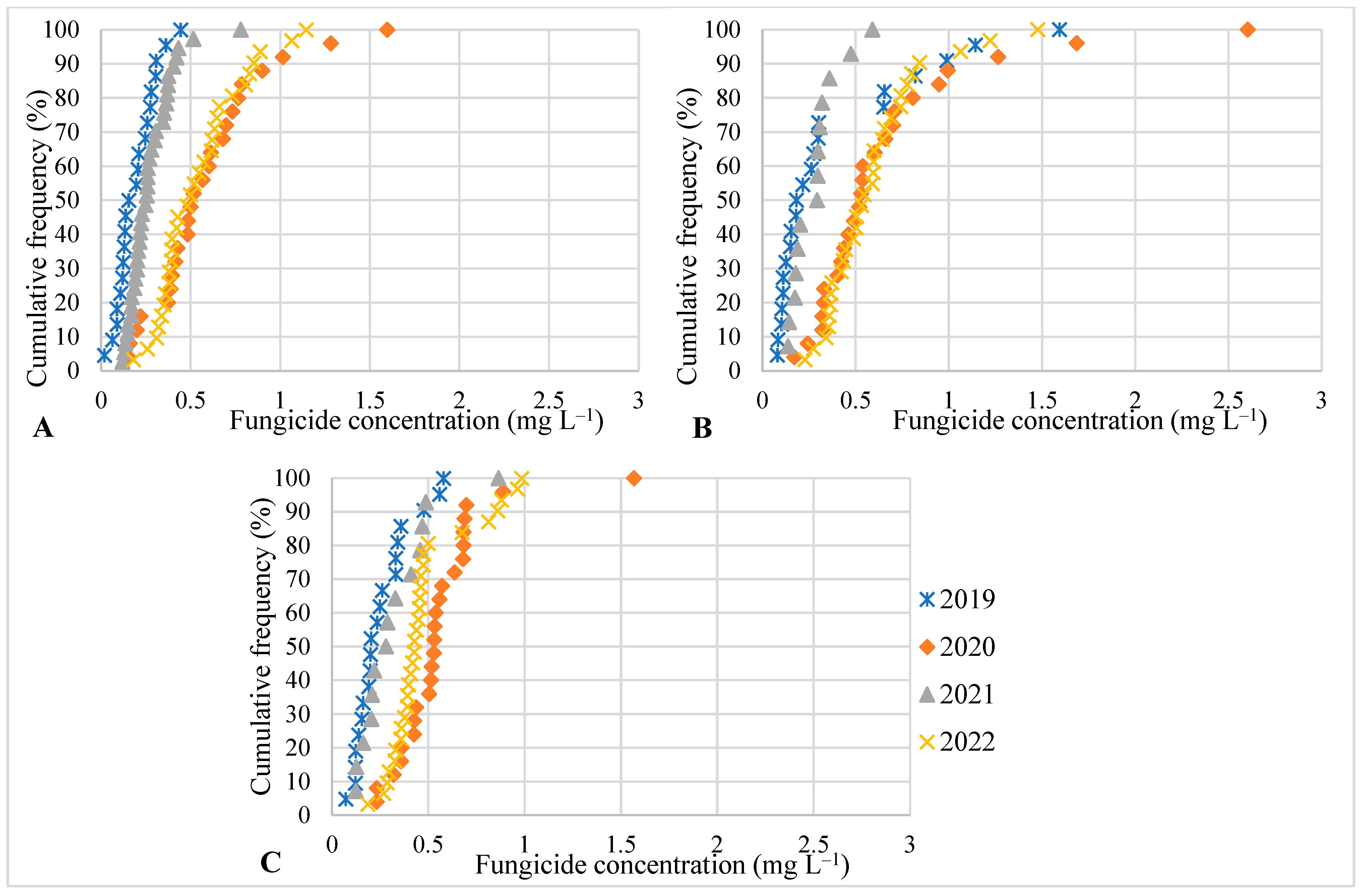
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quaedvlieg, W.; Kema, G.H.J.; Groenewald, J.Z.; Verkley, G.J.M.; Seifbarghi, S.; Razavi, M.; Mirzadi Gohari, A.; Mehrabi, R.; Crous, P.W. Zymoseptoria gen. nov.: A new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia Mol. Phylogeny Evol. Fungi 2011, 26, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.N.; Hovmøller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Goral, T.; et al. IPM Strategies and their dilemmas including an introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Hansen, J.G.; Semaskiene, R.; Korbas, M.; Danielewicz, J.; Glazek, M.; Maumene, C.; Rodemann, B.; Weigand, S.; et al. Four azoles’ profile in the control of septoria, yellow rust and brown rust in wheat across Europe. Crop Prot. 2018, 105, 16–27. [Google Scholar] [CrossRef]
- FRAC (Fungicide Resistance Action Committe). Available online: https://www.frac.info/ (accessed on 1 March 2024).
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar] [PubMed]
- Fouché, G.; Michel, T.; Lalève, A.; Wang, N.X.; Young, D.H.; Meunier, B.; Debieu, D.; Fillinger, S.; Walker, A.S. Directed evolution predicts cytochrome b G37V target site modification as probable adaptive mechanism towards the QiI fungicide fenpicoxamid in Zymoseptoria tritici. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Lucas, J.A.; Clark, W.S.; Burnett, F.J. QoI resistance development in populations of cereal pathogens in the UK. In Proceedings of the BCPC International Congress—Crop Science and Technology, Glasgow, UK, 10–12 November 2003; pp. 689–694. [Google Scholar]
- Torriani, S.F.F.; Brunner, P.C.; McDonald, B.A.; Sierotzki, H. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag. Sci. 2009, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Drabešová, J.; Ryšánek, P.; Brunner, P.; McDonald, B.A.; Croll, D. Population genetic structure of Mycosphaerella gramicola and Quinone Outside Inhibitor (QoI) resistance in the Czech Republic. Eur. J. Plant Pathol. 2013, 135, 211–224. [Google Scholar] [CrossRef][Green Version]
- Hagerty, C.H.; Anderson, N.P.; Mundt, C.C. Temporal dynamics and spatial variation of Zymoseptoria tritici azoxystrobin and propiconazole fungicide resistance: A hierarchical survey of commercial winter wheat fields in the Willamette Valley of Oregon. Phytopathology 2017, 107, 345–352. [Google Scholar] [CrossRef]
- Mäe, A.; Fillinger, S.; Sooväli, P.; Heick, T.M. Fungicide sensitivity shifting of Zymoseptoria tritici in the Finnish-Baltic region and a novel insertion in the MFS1 promoter. Front. Plant Sci. 2020, 11, 519898. [Google Scholar] [CrossRef]
- Lavrukaitė, K.; Heick, T.M.; Ramanauskienė, J.; Armonienė, R.; Ronis, A. Fungicide sensitivity levels in the Lithuanian Zymoseptoria tritici population in 2021. Front. Plant Sci. 2023, 13, 1075038. [Google Scholar] [CrossRef] [PubMed]
- Sierotzki, H.; Scalliet, G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Glättli, A.; Grote, T.; Stammler, G. SDH-inhibitors: History, biological performance and molecular mode of action. In Modern Fungicides and Antifungal Compounds; Dehne, H.W., Deising, H.B., Gisi, U., Kuck, K.H., Russell, P.E., Lyr, H., Eds.; DPG: Braunschweig, Germany, 2011; Volume VI, pp. 159–170. [Google Scholar]
- Rehfus, A.; Strobel, D.; Bryson, R.; Stammler, G. Mutations in sdh genes in field isolates of Zymoseptoria tritici and impact on the sensitivity to various succinate dehydrogenase inhibitors. Plant Pathol. 2018, 67, 175–180. [Google Scholar] [CrossRef]
- Vestergård, N.F.; Jørgensen, L.N.; Hellin, P.; Heick, T.M. Fungicide spraying intensity in the field drives the selection of amino acid alteration conferring resistance in Zymoseptoria tritici. Eur. J. Plant Pathol. 2023, 166, 385–401. [Google Scholar] [CrossRef]
- Dooley, H.; Shaw, M.W.; Mehenni-Ciz, J.; Spink, J.; Kildea, S. Detection of Zymoseptoria tritici SDHI-insensitive field isolates carrying the SdhC-H152R and SdhD-R47W substitutions. Pest Manag. Sci. 2016, 72, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Hellin, P.; Duvivier, M.; Heick, T.M.; Fraaije, B.A.; Bataille, C.; Clinckemaillie, A.; Legrève, A.; Jørgensen, L.N.; Andersson, B.; Samils, B.; et al. Spatio-temporal distribution of DMI and SDHI fungicide resistance of Zymoseptoria tritici throughout Europe based on frequencies of key target-site alterations. Pest Manag. Sci. 2021, 77, 5576–5588. [Google Scholar] [CrossRef] [PubMed]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Temporal Changes in Sensitivity of Zymoseptoria tritici Field Populations to Different Fungicidal Modes of Action. Agriculture 2021, 11, 269. [Google Scholar] [CrossRef]
- Yamashita, M.; Fraaije, B.A. Non-target site SDHI resistance is present as standing genetic variation in field populations of Zymoseptoria tritici. Pest Manag. Sci. 2018, 74, 672–681. [Google Scholar] [CrossRef]
- Heick, T.M.; Justesen, A.F.; Jørgensen, L.N. Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic-Baltic region—A status. Eur. J. Plant Pathol. 2017, 149, 669–682. [Google Scholar] [CrossRef]
- Blake, J.J.; Gosling, P.; Fraaije, B.A.; Burnett, F.J.; Knight, S.M.; Kildea, S.; Paveley, N.D. Changes in field dose-response curves for demethylation inhibitor (DMI) and quinone outside inhibitor (QoI) fungicides against Zymoseptoria tritici, related to laboratory sensitivity phenotyping and genotyping assays. Pest Manag. Sci. 2018, 74, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Bryson, R.J.; Stammler, G.; Hu, A.; Strobel, D.; Meyer, L.; Moronval, M.H. Mefentrifluconazole—The first Isopropanol-azole fungicide for the control of Zymoseptoria tritici including field isolates with known complex CYP51 haplotypes. In Proceedings of the 12e Conference International Sur les Mal. des Plantes, Vegephyl, Tours, France, 11–12 December 2018; pp. 222–231. [Google Scholar]
- Owen, W.J.; Yao, C.; Myung, K.; Kemmitt, G.; Leader, A.; Meyer, K.G.; Bowling, A.J.; Slanec, T.; Kramer, V.J. Biological characterization of fenpicoxamid, a new fungicide with utility in cereals and other crops. Pest Manag. Sci. 2017, 73, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System COM (2020) 381 Final; European Commission: Maastricht, The Netherlands, 2020. [Google Scholar]
- Barrès, B.; Corio-Costet, M.-F.; Debieu, D.; Délye, C.; Fillinger, S.; Grosman, J.; Micoud, A.; Siedwart, M.; Walker, A.-S. Trends and Challenges in Pesticide Resistance Detection. Trends Plant Sci. 2016, 21, 834–853. [Google Scholar] [CrossRef]
- Sykes, E.M.; Sackett, K.E.; Severns, P.M.; Mundt, C.C. Sensitivity variation and cross-resistance of Zymoseptoria tritici to azole fungicides in North America. Eur. J. Plant Pathol. 2018, 151, 269–274. [Google Scholar] [CrossRef]
- Heick, T.M.; Matzen, N.; Jørgensen, N.L. Reduced field efficacy and sensitivity of demethylation inhibitors in the Danish and Swedish Zymoseptoria tritici populations. Eur. J. Plant Pathol. 2020, 157, 625–636. [Google Scholar] [CrossRef]
- Ronis, A.; Jørgensen, L.N.; Semaškienė, R.; Gaurilčikienė, I.; Ramanauskienė, J. Sensitivity of Mycosphaerella graminicola isolates to demethylation-inhibiting (DMI) fungicides. Zemdirbyste-Agriculture 2014, 101, 177–184. [Google Scholar] [CrossRef][Green Version]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Estep, L.K.; Zala, M.; Anderson, N.P.; Sackett, K.E.; Flowers, M.D.; McDonald, B.A.; Mundt, C. First report of resistance to QoI fungicides in North American populations of Zymoseptoria tritici, causal agent of Septoria tritici blotch of wheat. Plant Dis. 2013, 97, 1511. [Google Scholar] [CrossRef] [PubMed]
- Taher, K.; Graf, S.; Fakhfakh, M.M.; Salah, H.B.; Yahyaoui, A.; Rezgui, S.; Nasraoui, B.; Stammler, G. Sensitivity of Zymoseptoria tritici isolates from Tunisia to pyraclostrobin, fluxapyroxad, epoxiconazole, metconazole, prochloraz and tebuconazole. J. Phytopathol. 2014, 162, 442–448. [Google Scholar] [CrossRef]
- Stewart, T.M.; Perry, A.J.; Evans, M.J. Resistance of Zymoseptoria tritici to azoxystrobin and epoxiconazole in the lower North Island of New Zeland. N. Z. Plant Protect. 2014, 67, 304–313. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Kiguchi, S.; Suemoto, H.; Iwahashi, F. Antifungal activity of metyltetraprole against the existing QoI-resistant isolates of various plant pathogenic fungi: Metyltetraprole against QoI-R isolates. Pest Manag. Sci. 2020, 76, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Bayon, C.; Atkins, S.; Cools, H.J.; Lucas, J.A.; Fraaije, M.W. Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control septoria leaf blotch in wheat. Mol. Plant Pathol. 2012, 13, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Scalliet, G.; Bowler, J.; Luksch, T.; Kirchhofer-Allan, L.; Steinhauer, D.; Ward, K.; Niklaus, M.; Verras, A.; Csukai, M.; Daina, A.; et al. Mutagenesis and functional studies with succinate dehydrogenase inhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS ONE 2012, 7, e35429. [Google Scholar] [CrossRef] [PubMed]
- Kiiker, R.; Juurik, M.; Heick, T.M.; Mäe, A. Changes in DMI, SDHI, and QoI fungicide sensitivity in the Estonian Zymoseptoria tritici population between 2019 and 2020. Microorganisms 2021, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.; Walker, A.S. Multiple mechanisms account for resistance to sterol 14alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2011, 67, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Omrane, S.; Audéon, C.; Ignace, A.; Duplaix, C.; Aouini, L.; Kema, G.; Walker, A.S.; Fillinger, S. Plasticity of the MFS1 promoter leads to multidrug resistance in the wheat pathogen Zymoseptoria tritici. mSphere 2017, 2, e00393-17. [Google Scholar] [CrossRef]
- Patry-Leclaire, S.; Neau, E.; Pitarch, A.; Walker, A.S.; Fillinger, S. Plasticity of the MFS1 promotor is not the only driver of Multidrug resistance in Zymoseptoria tritici. bioRxiv 2023. [Google Scholar] [CrossRef]
- Glaab, A.; Weilacher, X.; Hoffmeister, M.; Strobel, D.; Stammler, G. Occurrence and distribution of CYP51 haplotypes of Zymoseptoria tritici in recent years in Europe. J. Plant Dis. Prot. 2024, 2024, 1–8. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Heick, T.M.; O’Driscoll, A.; Clark, B.; Waite, K.; Blake, J.; Glazek, M.; Maumene, C.; Couleaud, G.; et al. Shifting sensitivity of septoria tritici blotch compromises field performance and yield of main fungicides in Europe. Front. Plant Sci. 2022, 13, 1060428. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.; Salat, M.; Frey, R.; Mosbach, A.; Luksch, T.; Balmer, D.; Hansesn, R.; Widdison, S.; Logan, G.; Dietrich, R.A.; et al. A dispensable paralog of succinate dehydrogenase subunit C mediates standing resistance towards a subclass of SDHI fungicides in Zymoseptoria tritici. PLoS Pathog. 2019, 15, e1007780. [Google Scholar] [CrossRef] [PubMed]
- Hagerty, C.H.; Klein, A.M.; Reardon, C.L.; Kroese, D.R.; Melle, C.J.; Graber, K.R.; Mundt, C.C. Baseline and temporal changes in sensitivity of Zymoseptoria tritici isolates to benzovindiflupyr in Oregon, U.S.A., and cross-sensitivity to other SDHI fungicides. Plant Dis. 2021, 105, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
| Fungicides | Years | |||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | |
| Azoxystrobin | 6.292 (0.136–20.250) 1 | 4.864 (0.616–22.540) | 9.787 (0.009–29.140) | 10.790 (0.905–23.925) |
| Pyraclostrobin | 0.282 (0.004–1.198) | 1.099 (0.025–2.128) | 1.175 0.007–3.197) | 1.563 (0.003–3.220) |
| Fluxapyroxad | 0.260 (0.072–0.579) | 0.564 (0.232–1.568) | 0.331 (0.124–0.864) | 0.483 (0.187–0.985) |
| Benzovindiflupyr | 0.194 (0.019–0.444) | 0.598 (0.153–1.597) | 0.277 (0.120–0.780) | 0.549 (0.180–1.144) |
| Bixafen | 0.379 (0.081–1.594) | 0.681 (0.171–2.602) | 0.283 (0.138–0.590) | 0.599 (0.229–1.478) |
| Azoxystrobin | Pyraclostrobin | Fluxapyroxad | Benzovindiflupyr | Bixafen | |
|---|---|---|---|---|---|
| Azoxystrobin | 1 | ||||
| Pyraclostrobin | 0.418 ** | 1 | |||
| Fluxapyroxad | 0.080 | 0.262 * | 1 | ||
| Benzovindiflupyr | −0.095 | 0.203 * | 0.550 ** | 1 | |
| Bixafen | −0.050 | 0.359 ** | 0.418 | 0.374 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrukaitė, K.; Almogdad, M.; Ramanauskienė, J.; Sabeckis, A. Sensitivity of Lithuanian Zymoseptoria tritici to Quinone Outside Inhibitor and Succinate Dehydrogenase Inhibitor Fungicides. Agronomy 2024, 14, 813. https://doi.org/10.3390/agronomy14040813
Lavrukaitė K, Almogdad M, Ramanauskienė J, Sabeckis A. Sensitivity of Lithuanian Zymoseptoria tritici to Quinone Outside Inhibitor and Succinate Dehydrogenase Inhibitor Fungicides. Agronomy. 2024; 14(4):813. https://doi.org/10.3390/agronomy14040813
Chicago/Turabian StyleLavrukaitė, Karolina, Mohammad Almogdad, Jūratė Ramanauskienė, and Aurimas Sabeckis. 2024. "Sensitivity of Lithuanian Zymoseptoria tritici to Quinone Outside Inhibitor and Succinate Dehydrogenase Inhibitor Fungicides" Agronomy 14, no. 4: 813. https://doi.org/10.3390/agronomy14040813
APA StyleLavrukaitė, K., Almogdad, M., Ramanauskienė, J., & Sabeckis, A. (2024). Sensitivity of Lithuanian Zymoseptoria tritici to Quinone Outside Inhibitor and Succinate Dehydrogenase Inhibitor Fungicides. Agronomy, 14(4), 813. https://doi.org/10.3390/agronomy14040813







