Quantitative Analysis of Atrazine Impact on UAV-Derived Multispectral Indices and Correlated Plant Pigment Alterations: A Heatmap Approach
Abstract
:1. Introduction
2. Materials and Methods
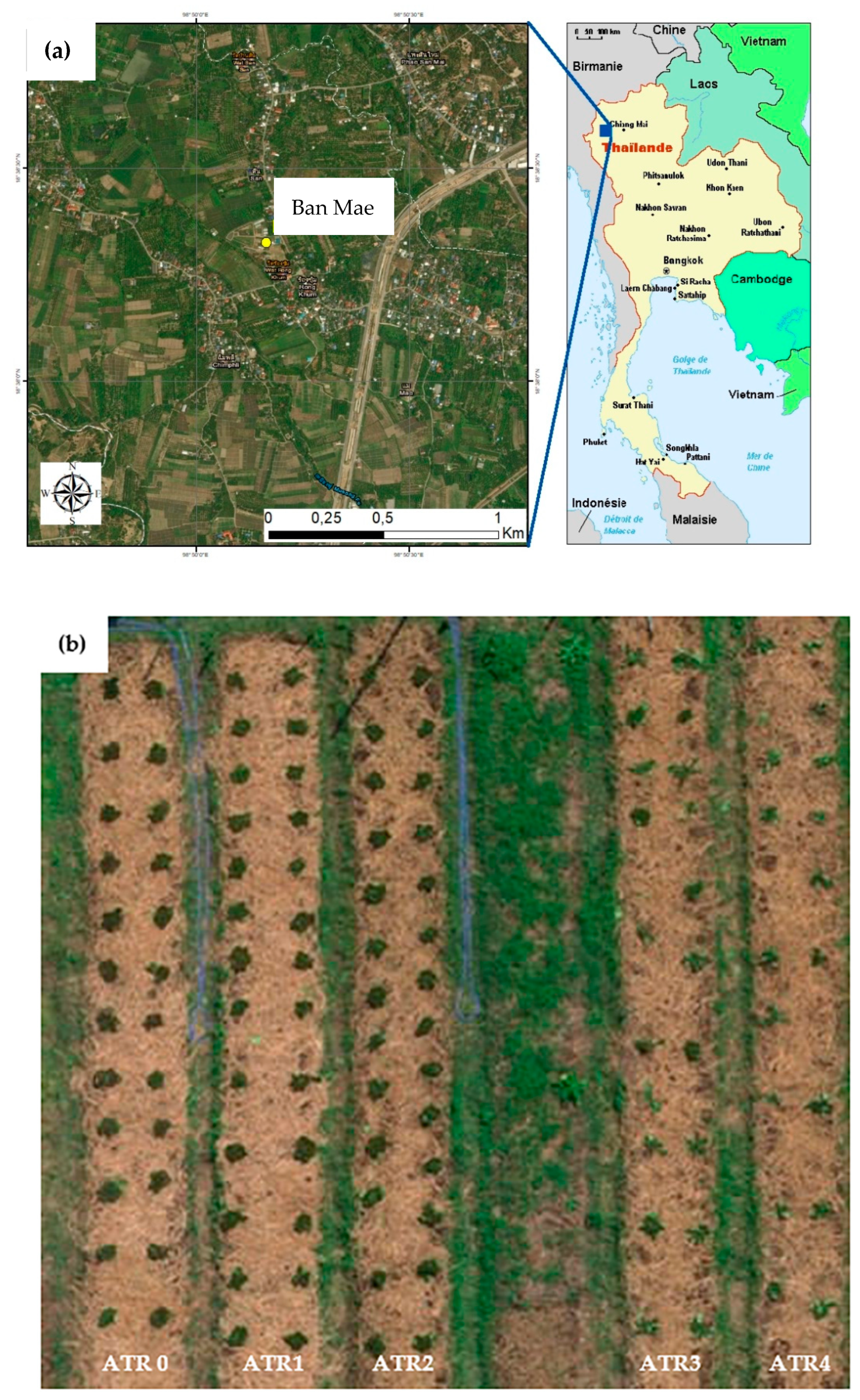
2.1. Field Study Area and Soil Characteristics
2.2. Field Experiment and Sample Collection
2.3. Quantification of Atrazine Uptake
2.3.1. Soil and Plant Extraction
2.3.2. LC-MS/MS Analysis
2.4. Quantification of Pigments
2.4.1. Chlorophyll and Carotenoids
2.4.2. Anthocyanin
2.5. Acquision of Spectral Data in the Field Using UAVs and Image Processing
2.6. Data Analysis
3. Results
3.1. Atrazine Uptake in Red Cos Lettuce
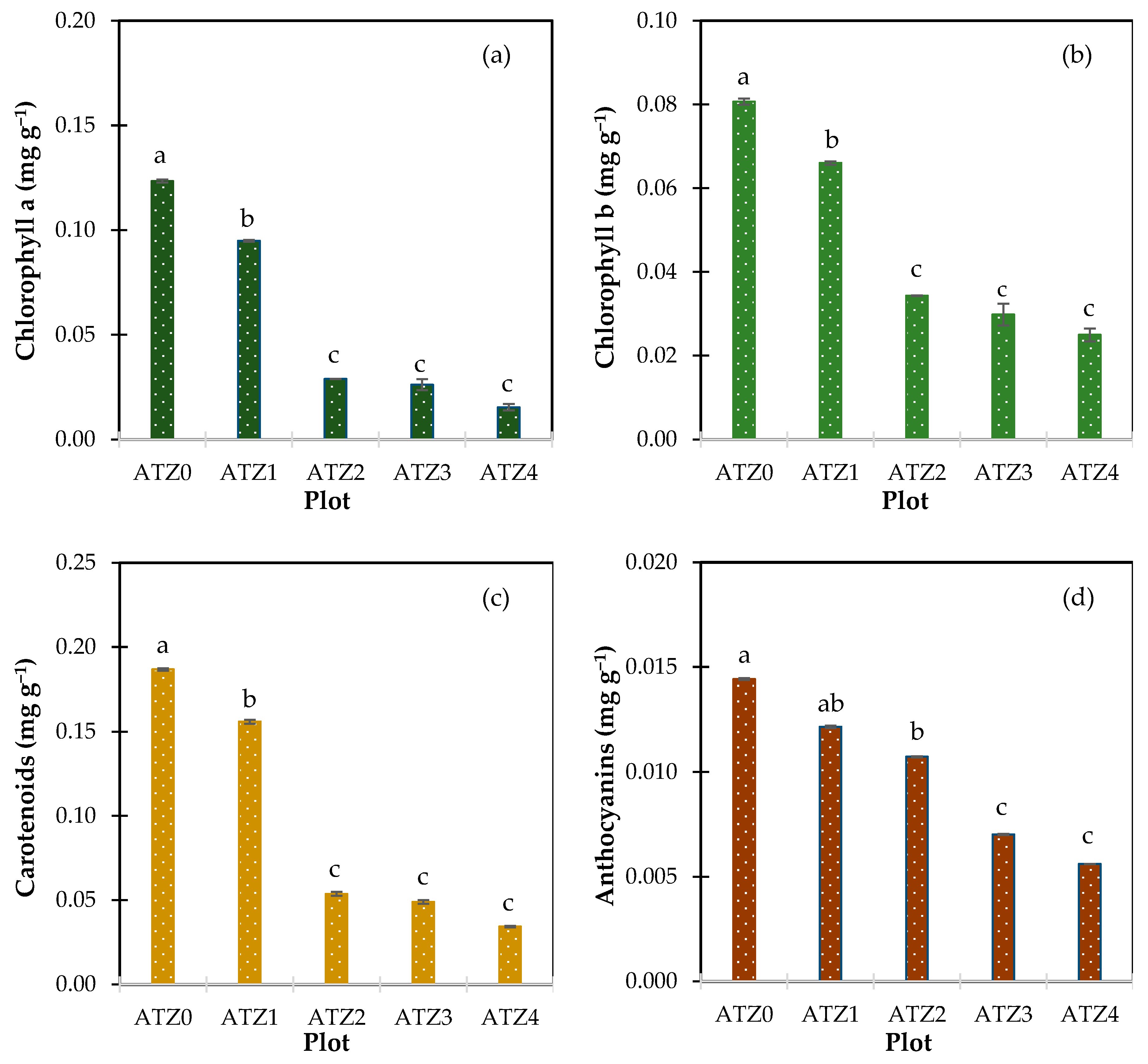
3.2. Atrazine’s Impact on Leaf Pigment Levels
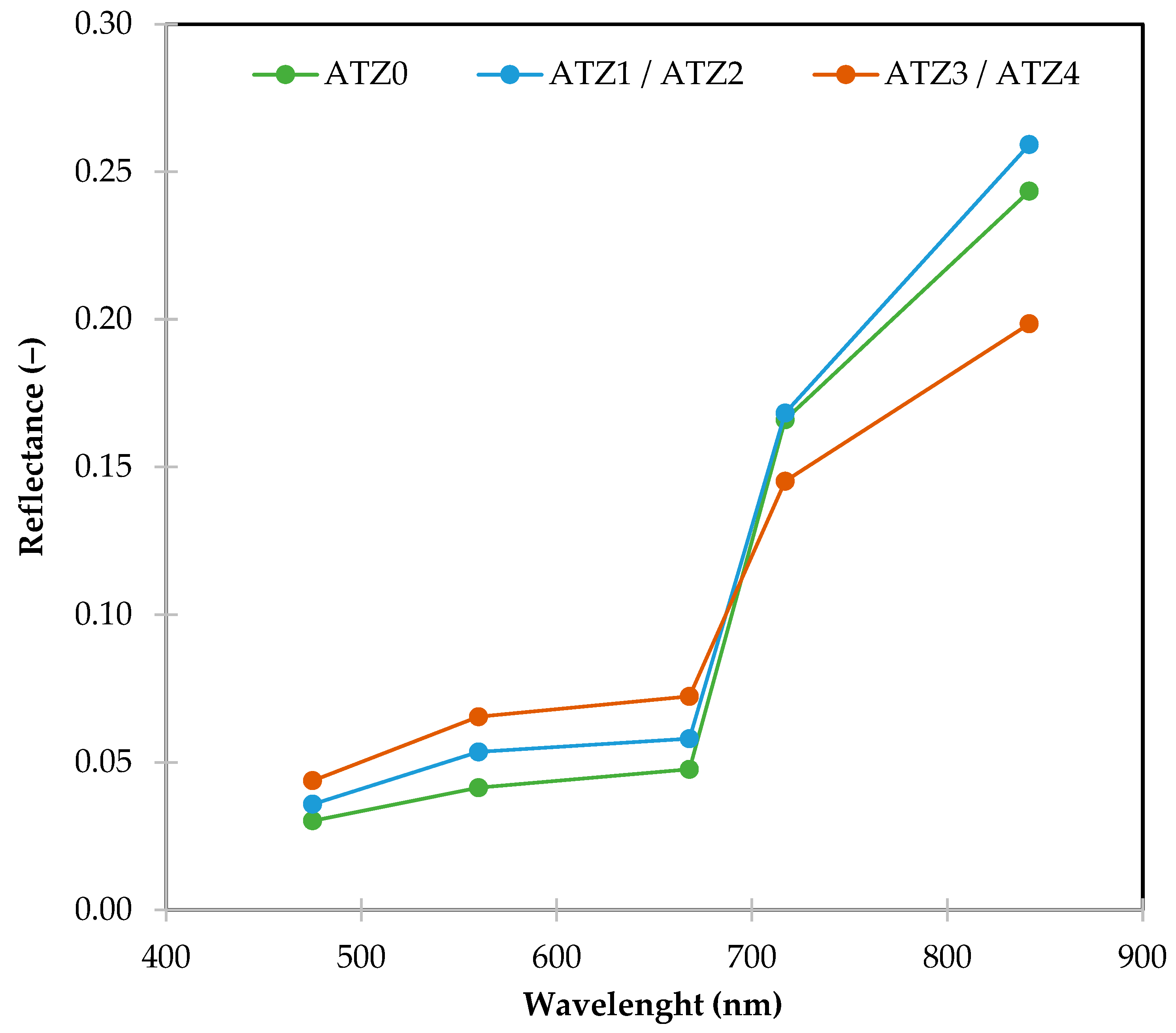
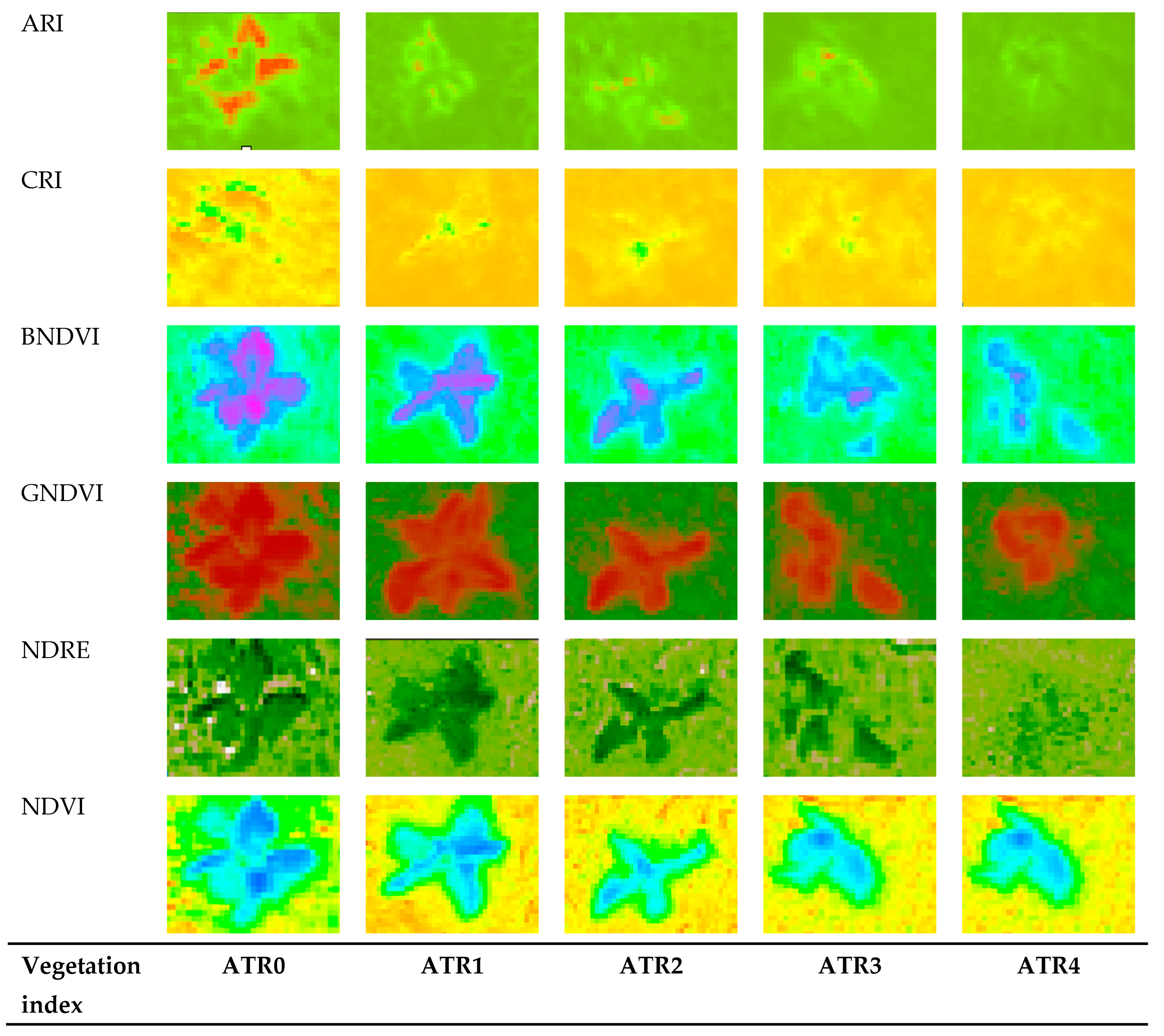
3.3. Insights from Heatmap Analysis
4. Discussion
4.1. Atrazine Uptake Dynamics and Pigment Changes in Red Cos Lettuce under Soil Application
4.2. Capabilities of Heatmap Analysis in Predicting Pigment Changes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Procedure for Heatmap Generation
- 1.
- Data Preparation:
- Load the dataset from the Excel file, including variables like atrazine concentration, plant pigments, and multispectral indices;
- Perform necessary preprocessing steps, such as handling missing values and confirming appropriate data types for correlation analysis.
- 2.
- Correlation Analysis:
- Calculate Pearson correlation coefficients between all pairs of variables;
- Organize correlation coefficients into a matrix format, with rows and columns corresponding to variables.
- 3.
- Heatmap Visualization:
- Use a heatmap to visualize the correlation matrix, with color intensity indicating the strength and direction of correlations;
- Include clear labels for variables along each axis;
- Apply a diverging colormap to distinguish positive from negative correlations, and highlight significant correlations as needed.
References
- Giannini-Kurina, F.; Borello, J.; Cañas, I.; Hang, S.; Balzarini, M. Mapping atrazine persistence in soils of central Argentina using INLA. Soil Tillage Res. 2022, 219, 105320. [Google Scholar] [CrossRef]
- Huang, M.Y.; Zhao, Q.; Duan, R.Y.; Liu, Y.; Wan, Y.Y. The effect of atrazine on intestinal histology, microbial community and short chain fatty acids in Pelophylax nigromaculatus tadpoles. Environ. Pollut. 2021, 288, 117702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.; Ma, F.; Bai, S.; Yang, J.; Qi, S. Pseudomonas sp, ZXY-1 a newly isolated and highly efficient atrazine-degrading bacterium and optimization of biodegradation using response surface methodology. J. Environ. Sci. 2017, 54, 52–159. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Zhu, Y. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Szewczyk, R.; Różalska, S.; Mironenka, J.; Bernat, P. Atrazine biodegradation by mycoinsecticide Metarhizium robertsii: Insights into its amino acids and lipids profile. J. Environ. Manag. 2020, 262, 110304. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Fang, W.; Chen, L.; Zhang, P.; Zhang, G.; Zhang, H.; Liang, J.; Wang, Q.; Ma, W. Toxicological effects, environmental behaviors and remediation technologies of herbicide atrazine in soil and sediment: A comprehensive review. Chemosphere 2022, 307 Pt 3, 136006. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Jafari, S.; Moeini, Z.; Jaskulak, M.; Keshtgar, L.; Badeenezhad, A.; Azhdarpoor, A.; Rostami, M.; Zorena, K.; Dehghani, M. Current methods and technologies for degradation of atrazine in contaminated soil and water: A review. Environ. Technol. Innov. 2021, 24, 102019. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Wang, Y.; Zhou, X.; Wang, S.; Wang, Z. Meta-analysis and experimental validation identified atrazine as a toxicant in the male reproductive system. Environ. Sci. Pollut. Res. 2021, 28, 37482–37497. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Department of Agriculture. 2022. Available online: https://www.doa.go.th/ard/wp-content/uploads/2023/02/%E0%B8%AA%E0%B8%A3%E0%B8%B8%E0%B8%9B%E0%B8%82%E0%B9%89%E0%B8%AD%E0%B8%A1%E0%B8%B9%E0%B8%A5%E0%B8%AA%E0%B9%88%E0%B8%87%E0%B8%AD%E0%B8%AD%E0%B8%81%E0%B8%9B%E0%B8%B5-2565-%E0%B8%A3%E0%B8%B2%E0%B8%A2%E0%B8%A5%E0%B8%B0%E0%B9%80%E0%B8%AD%E0%B8%B5%E0%B8%A2%E0%B8%94.pdf (accessed on 10 February 2024).
- Almberg, K.S.; Turyk, M.E.; Jones, R.M.; Rankin, K.; Freels, S.; Stayner, L.T. Atrazine contamination of drinking water and adverse birth outcomes in community water systems with elevated Atrazine in Ohio, 2006–2008. Int. J. Environ. Res. Public Health 2018, 15, 1889. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Z.; Hou, A.; Wang, X.; Zhou, Z.; Qin, B.; Cao, B.; Zhang, Y. Impact of atrazine on soil microbial properties: A meta-analysis. Environ. Pollut. 2023, 323, 121337. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.J.; Doucette, W.J.; Moore, M.T. Atrazine uptake, translocation, bioaccumulation and biodegradation in cattail (Typha latifolia) as a function of exposure time. Chemosphere 2022, 287 Pt 1, 132104. [Google Scholar] [CrossRef]
- Urseler, N.; Bachetti, R.; Morgante, V.; Agostini, E.; Morgante, C. Atrazine behavior in an agricultural soil: Adsorption–desorption, leaching, and bioaugmentation with Arthrobacter sp. strain AAC22. J. Soils Sediments 2022, 22, 93–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Sun, Y.; Chang, C.; Yu, J.; Zhang, W. Fusion of multispectral aerial imagery and vegetation indices for machine learning-based ground classification. Remote Sens. 2021, 13, 1411. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, Z.; Zhang, J.; Shuai, G. Highly efficient paddy classification using UAV-based orthorectified image. In Proceedings of the IGARSS 2017—2017 IEEE International Geoscience and Remote Sensing Symposium IEEE, Fort Worth, TX, USA, 23–28 July 2017. [Google Scholar]
- Pölönen, I.; Saari, H.; Kaivosoja, J.; Honkavaara, E.; Pesonen, L. Hyperspectral imaging-based biomass and nitrogen content estimations from light-weight UAV. In Proceedings of the SPIE—The International Society for Optical Engineering 8887, Dresden; SPIE: Florence, Italy, 2013. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Maimaitiyiming, M.; Hartling, S.; Peterson, K.T.; Maw, M.J.W.; Shakoor, N.; Mockler, T.; Fritschi, F.B. Vegetation Index Weighted Canopy Volume Model (CVMVI) for soybean biomass estimation from Unmanned Aerial System-based RGB imagery. ISPRS J. Photogramm. Remote Sens. 2019, 151, 27–41. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Gilliot, J.M.; Michelin, J.; Hadjard, D.; Houot, S. An accurate method for predicting spatial variability of maize yield from UAV-based plant height estimation: A tool for monitoring agronomic field experiments. Precis. Agric. 2020, 22, 897–921. [Google Scholar] [CrossRef]
- Romero, M.; Luo, Y.; Su, B.; Fuentes, S. Vineyard water status estimation using multispectral imagery from an UAV platform and machine learning algorithms for irrigation scheduling management. Comput. Electron. Agric. 2018, 147, 109–117. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-based chlorophyll content estimation by evaluating vegetation index responses under different crop coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- He, Y.; Peng, J.; Liu, F.; Zhang, C.U.; Kong, W. Critical review of fast detection of crop nutrient and physiological information with spectral and imaging technology. Trans. Chin. Soc. Agric. Eng. 2015, 31, 174–189. [Google Scholar] [CrossRef]
- Abdulridha, J.; Min, A.; Rouse, M.N.; Kianian, S.; Isler, V.; Yang, C. Evaluation of stem rust disease in wheat fields by drone hyperspectral imaging. Sensors 2023, 23, 4154. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. (Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Yu, K.; Lenz-Wiedemann, V.; Chen, X.; Bareth, G. Estimating leaf chlorophyll of barley at different growth stages using spectral indices to reduce soil background and canopy structure effects. ISPRS J. Photogramm. Remote Sens. 2014, 97, 58–77. [Google Scholar] [CrossRef]
- Kopačková-Strnadová, V.; Koucká, L.; Jelének, J.; Lhotáková, Z.; Oulehle, F. Canopy top, height and photosynthetic pigment estimation using parrot sequoia multispectral imagery and the unmanned aerial vehicle (UAV). Remote Sens. 2021, 13, 705. [Google Scholar] [CrossRef]
- Cao, R.; Chen, Y.; Shen, M.; Chen, J.; Zhou, J.; Wang, C.; Yang, W. A simple method to improve the quality of NDVI time-series data by integrating spatiotemporal information with the Savitzky-Golay filter. Remote Sens. Environ. 2018, 217, 244–257. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Li, J.; Guo, X.; Wang, S.; Lu, J. Estimating biomass of winter oilseed rape using vegetation indices and texture metrics derived from UAV multispectral images. Comput. Electron. Agric. 2019, 166, 81–91. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y.; et al. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Pérez-Patricio, M.; Camas-Anzueto, J.L.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F.; Escobar-Gómez, E.; Voisin, Y.; Rios Rojas, C.; Grajales-Coutiño, R. Optical method for estimating the chlorophyll contents in plant leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.-T.; Mele, M.A.; Choi, I.-L.; Kang, H.-M. The effect of phosphorus and root zone temperature on anthocyanin of red romaine lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef]
- Elhadary, A.; Rabah, M.; Ghanim, E.; Mohie, R.; Taha, A. The influence of flight height and overlap on UAV imagery over featureless surfaces and constructing formulas predicting the geometrical accuracy. NRIAG J. Astron. Geophys. 2022, 11, 210–223. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 71, 38–45. [Google Scholar] [CrossRef]
- Banerjee, B.P.; Sharma, V.; Spangenberg, G.; Kant, S. Machine learning regression analysis for estimation of crop emergence using multispectral UAV imagery. Remote Sens. 2021, 13, 2918. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of water content in eucalyptus leaves. Aust. J. Bot. 1999, 47, 909–923. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Thompson, C.N.; Guo, W.; Sharma, B.; Ritchie, G.L. Using normalized difference red edge index to assess maturity in cotton. Crop Sci. 2019, 59, 2167–2177. [Google Scholar] [CrossRef]
- Carlson, T.N.; Ripley, D.A. On the relation between NDVI, fractional vegetation cover, and leaf area index. Remote Sens. Environ. 1997, 62, 241–252. [Google Scholar] [CrossRef]
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root uptake of pharmaceuticals and personal care product ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A novel biochar modified by chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, M.; Zhou, B.; Cui, L. Effects of biochar nanoparticles as a soil amendment on the structure and hydraulic characteristics of a sandy loam soil. Soil Use Manag. 2021, 38, 836–849. [Google Scholar] [CrossRef]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A. Relationship between lipophilicity and root uptake and translocation of non-ionised chemicals by barley. Pestic. Sci. 1982, 13, 495–504. [Google Scholar] [CrossRef]
- Roeth, F.W.; Lavy, T.L. Atrazine uptake by Sudangrass, sorghum, and corn. Weed Sci. 1971, 19, 93–97. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, R.; Singh, S.; Jagota, N.; Sharma, A. Chapter 4—Pesticide biology in plants: Plant uptake, translocation, and accumulation. In Pesticides in the Environment; Sharma, A., Kumar, V., Zheng, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 67–86. [Google Scholar] [CrossRef]
- Sandanayake, S.; Hettithanthri, O.; Buddhinie, P.K.C.; Vithanage, M. Plant uptake of pesticide residues from agricultural soils. In The Handbook of Environmental Chemistry; Rodríguez-Cruz, M.S., Sánchez-Martín, M.J., Eds.; Springer: Cham, Switerzerland, 2021; Volume 113. [Google Scholar] [CrossRef]
- Bai, X.; Sun, C.; Xie, J.; Song, H.; Zhu, Q.; Su, Y.; Qian, H.; Fu, Z. Effects of atrazine on photosynthesis and defense response and the underlying mechanisms in Phaeodactylum tricornutum. Environ. Sci. Pollut. Res. 2015, 22, 17499–17507. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Effects of atrazine and its two major derivatives on the photosynthetic physiology and carbon sequestration potential of a marine diatom. Ecotoxicol. Environ. Saf. 2020, 205, 111359. [Google Scholar] [CrossRef]
- Qian, H.F.; Sheng, G.D.; Liu, W.P.; Lu, Y.; Fu, Z.W. Inhibitory effects of atrazine on Chlorella vulgaris as assessed by real-time polymerase chain reaction. Environ. Toxicol. Chem. 2008, 27, 182–187. [Google Scholar] [CrossRef]
- Zhu, J.; PATRoldt, W.L.; Radwan, O.; Tranel, P.J.; Clough, S.J. Effects of photosystem-II interfering herbicides atrazine and bentazon on the soybean transcriptome. Plant Genome 2009, 2, 191–205. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Zhou, B.; Sapkota, S.; Wei, F.; Wang, Z. Atrazine and mesotrione-induced oxidative stress and impact on antioxidant enzymes and chlorophyll content in Bermudagrass. Planta Daninha 2018, 36, e018172227. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Trebst, A. Function of beta-carotene and tocopherol in photosystem II. J. Biosci. 2003, 58, 609–620. [Google Scholar] [CrossRef]
- Xu, Z.; Mahmood, K.; Rothstein, S.J. ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol. 2017, 58, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the Biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Pavlovic, D.; Nikolic, B.; Durovic, S.; Waisi, H.; Andelkovic, A.; Marisavljevic, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Phytomedicine 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Sandmann, G. Bleaching herbicides: Action mechanism in carotenoid biosynthesis, structural requirements and engineering of resistance. In Herbicide Classes in Development; Böger, P., Wakabayashi, K., Hirai, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Ridley, S.M. Carotenoids and herbicide action. In Carotenoid Chemistry and Biochemistry; Britton, G., Goodwin, T.W., Eds.; Elsevier: Pergamon, UK, 1982; pp. 353–369. [Google Scholar] [CrossRef]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Plant stress response and adaptation via anthocyanins: A review. Plant Stress 2023, 10, 100230. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Sharma, N.; Pandey, A.K.; Garg, M. Physiological and molecular response of colored wheat seedlings against phosphate deficiency is linked to accumulation of distinct anthocyanins. Plant Physiol. Biochem. 2022, 170, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jalil, S.; Cao, H.; Tsago, Y.; Sunusi, M.; Chen, Z.; Shi, C.; Jin, X. The purple leaf (pl6) mutation regulates leaf color by altering the anthocyanin and chlorophyll contents in rice. Plants 2020, 9, 1477. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef] [PubMed]
- Kuska, M.T.; Behmann, J.; Mahlein, A.K. Potential of hyperspectral imaging to detect and identify the impact of chemical welfare compounds on plant tissue. Pure Appl. Chem. 2018, 90, 1615–1624. [Google Scholar] [CrossRef]
- Slaton, M.R.; Hunt, E.R., Jr.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Hallik, L.; Kazantsev, T.; Kuusk, A.; Galmes, J.; Tomas, M.; Niinemets, U. Generality of relationships between leaf pigment contents and spectral vegetation indices in Mallorca (Spain). Reg. Environ. Change 2017, 17, 2097–2109. [Google Scholar] [CrossRef]
- Melkozernov, A.N. Excitation energy transfer in photosystem I from oxygenic organisms. Photosynth. Res. 2001, 70, 129–153. [Google Scholar] [CrossRef]
- Gibasiewicz, K.; Szrajner, A.; Ihalainen, J.A.; Germano, M.; Dekker, J.P.; van Grondelle, R. Characterization of low-energy chlorophylls in the PSI-LHCI supercomplex from Chlamydomonas reinhardtii. A site-selective fluorescence study. J. Phys. Chem. B 2005, 109, 21180–21186. [Google Scholar] [CrossRef]
- Oja, V.; Bichele, I.; Hüve, K.; Rasulov, B.; Laisk, A. Reductive titration of photosystem I and differential extinction coefficient of P700+ at 810–950 nm in leaves. Biochim. Biophys. Acta Bioenerg. 2004, 1658, 225–234. [Google Scholar] [CrossRef]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. The long-wavelength limit of plant photosynthesis. FEBS Lett. 2005, 579, 4017–4019. [Google Scholar] [CrossRef] [PubMed]
- Oja, V.; Eichelmann, H.; Peterson, R.B.; Rasulov, B.; Laisk, A. Deciphering the 820 nm signal: Redox state of donor side and quantum yield of photosystem I in leaves. Photosynth. Res. 2003, 78, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ihalainen, J.A.; Jensen, P.E.; Haldrup, A.; van Stokkum, I.H.M.; van Grondelle, R.; Scheller, H.V.; Dekker, J.P. Pigment organization and energy transfer dynamics in isolated, photosystem I (PSI) complexes from Arabidopsis thaliana depleted of the PSI-G, PSI-K, PSI-L, or PSI-N subunit. Biophys. J. 2002, 83, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Thapper, A.; Mamedov, F.; Mokvist, F.; Hammarström, L.; Styring, S. Defining the far-red limit of photosystem II in spinach. Plant Cell 2009, 21, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Parida, B.R. Environmental factors associated with seasonal variations of night-time plant canopy and soil respiration fluxes in deciduous conifer forest, Western Himalaya, India. Trees 2019, 33, 599–613. [Google Scholar] [CrossRef]
- Parida, B.R.; Kumari, A. Mapping and modeling mangrove biophysical and biochemical parameters using Sentinel-2A satellite data in Bhitarkanika National Park, Odisha. Model. Earth Syst. Environ. 2021, 7, 2463–2474. [Google Scholar] [CrossRef]
- Cheng, Q.; Wu, X. Correlation analysis of simulated MODIS vegetation indices and the red edge and rice agricultural parameter. In Remote Sensing for Agriculture, Ecosystems, and Hydrology IX; Neale, C.M.U., Owe, M., D’Urso, G., Eds.; SPIE: Florence, Italy, 2007; p. 67420U. [Google Scholar]
- Frampton, W.J.; Dash, J.; Watmough, G.; Milton, E.J. Evaluating the capabilities of Sentinel-2 for quantitative estimation of biophysical variables in vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 83–92. [Google Scholar] [CrossRef]
- da Silva, E.E.; Baio, F.H.R.; Teodoro, L.P.R.; da Silva Junior, C.A.; Borges, R.S.; Teodoro, P.E. UAV-multispectral and vegetation indices in soybean grain yield prediction based on in situ observation. Remote Sens. Appl. Soc. Environ. 2020, 18, 100318. [Google Scholar] [CrossRef]
- Silva Junior, C.A.; Nanni, M.R.; Shakir, M.; Teodoro, P.E.; de Oliveira-Júnior, J.F.; Cezar, E.; Shiratsuchi, L.S. Soybean varieties discrimination using non imaging hyperspectral sensor. Infrared Phys. Technol. 2018, 89, 338–350. [Google Scholar] [CrossRef]
- Duan, B.; Liu, Y.; Ging, Y.; Peng, Y.; Wu, X.; Zhu, R.; Fang, S. Remote estimation of rice LAI based on Fourier spectrum texture from UAV image. Plant Methods 2019, 15, 124. [Google Scholar] [CrossRef] [PubMed]



| Vegetation Indices | Synonym | Description | Equation | Reference |
|---|---|---|---|---|
| Anthocyanin Reflectance Index | ARI | Measures the levels of anthocyanin content, which can indicate stress responses or senescence in plants. | [36] | |
| Anthocyanin Reflectance Index 2 | ARI 2 | Measures the levels of anthocyanin content, which can indicate stress responses or senescence in plants. | [36] | |
| Carotenoid Reflectance Index | CRI | Measures the concentration of carotenoids, pigments that play a crucial role in plant health and stress tolerance. | [36] | |
| Blue Normalized Difference Vegetation Index | BNDVI | Assesses whether the target being observed contains live green vegetation, indicating biomass and health, but uses blue light for better detection of vegetation in areas with high soil background reflectance. | [37] | |
| Enhanced Vegetation Index | EVI | Optimized to enhance the vegetation signal with improved sensitivity in high biomass regions, and to facilitate better vegetation monitoring by de-coupling the canopy background signal and reducing atmospheric influences. | [38] | |
| Green Normalized Difference Vegetation Index | GNDVI | Utilizes green wavelengths and is sensitive to chlorophyll concentration, indicating plant health and photosynthetic capacity. | [39] | |
| Leaf Chlorophyll Index | LCI | Estimates the chlorophyll content of leaves, which is an indicator of plant health and nitrogen content. | [40] | |
| Modified Chlorophyll Absorption in Reflectance Index | MCARI | Designed to monitor subtle changes in chlorophyll content, making it useful for assessing vegetation health and stress. | [41] | |
| Normalized Difference Red Edge | NDRE | Measures the chlorophyll content in plants, especially useful in later stages of crop growth when the NDVI becomes less sensitive. | [42] | |
| Normalized Difference Vegetation Index | NDVI | Assesses whether the target being observed contains live green vegetation or not, indicating biomass and health. | [43] | |
| Soil Adjusted Vegetation Index | SAVI | Corrects the NDVI for the influence of soil brightness when vegetation cover is low. | [44] |
| Pigment | Equation | R2 |
|---|---|---|
| Chlorophyll a | [Chlorophyll a] = −0.0032 [ATR] + 0.1388 | 0.8713 |
| Chlorophyll b | [Chlorophyll b] = −0.0016 [ATR] + 0.0889 | 0.8817 |
| Carotenoids | [Carotenoids] = −0.0046 [ATR] + 0.2119 | 0.8457 |
| Anthocyanins | [Anthocyanins] = −0.0002 [ATR] + 0.0161 | 0.8833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonupara, T.; Udomkun, P.; Kajitvichyanukul, P. Quantitative Analysis of Atrazine Impact on UAV-Derived Multispectral Indices and Correlated Plant Pigment Alterations: A Heatmap Approach. Agronomy 2024, 14, 814. https://doi.org/10.3390/agronomy14040814
Boonupara T, Udomkun P, Kajitvichyanukul P. Quantitative Analysis of Atrazine Impact on UAV-Derived Multispectral Indices and Correlated Plant Pigment Alterations: A Heatmap Approach. Agronomy. 2024; 14(4):814. https://doi.org/10.3390/agronomy14040814
Chicago/Turabian StyleBoonupara, Thirasant, Patchimaporn Udomkun, and Puangrat Kajitvichyanukul. 2024. "Quantitative Analysis of Atrazine Impact on UAV-Derived Multispectral Indices and Correlated Plant Pigment Alterations: A Heatmap Approach" Agronomy 14, no. 4: 814. https://doi.org/10.3390/agronomy14040814





