Improvement in Physiochemical Characteristics of ‘Prime Seedless’ Grapes by Basal Defoliation with Foliar-Sprayed Low-Biuret Urea and Cyanocobalamin under Mediterranean Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment
2.2. Vegetative Growth
2.3. Leaf Analysis
2.3.1. Chlorophyll (C55H72MgN4O5) Content
2.3.2. Proline (C5H9NO2) Content
2.3.3. Nutrient Contents
2.4. Berry Set and Total Yield
2.5. Fruit Physiochemical Characteristics
2.5.1. Cluster Parameters
2.5.2. Decay
2.5.3. Berry Parameters
2.6. Statistical Analysis
3. Results
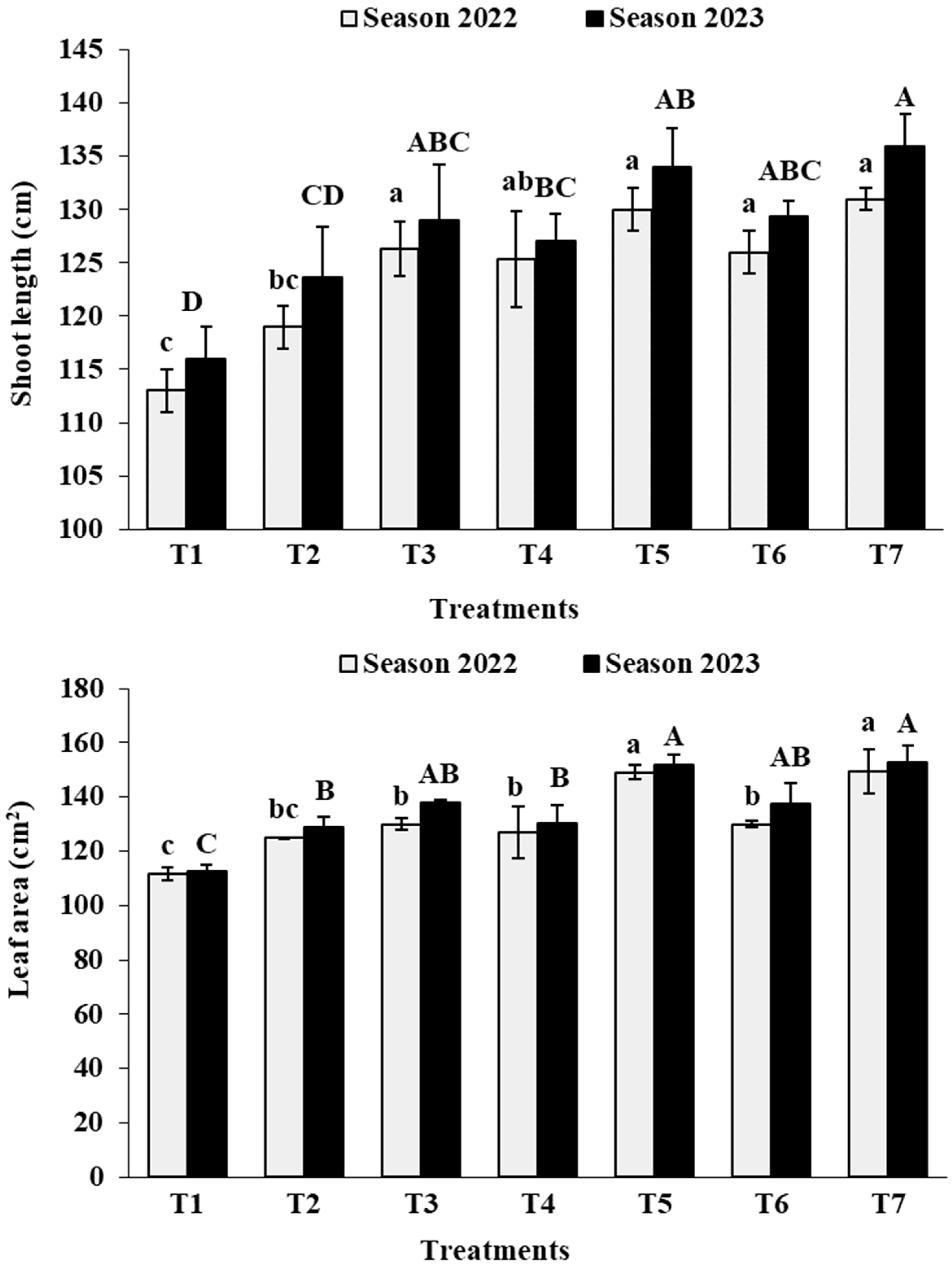
3.1. Vegetative Growth
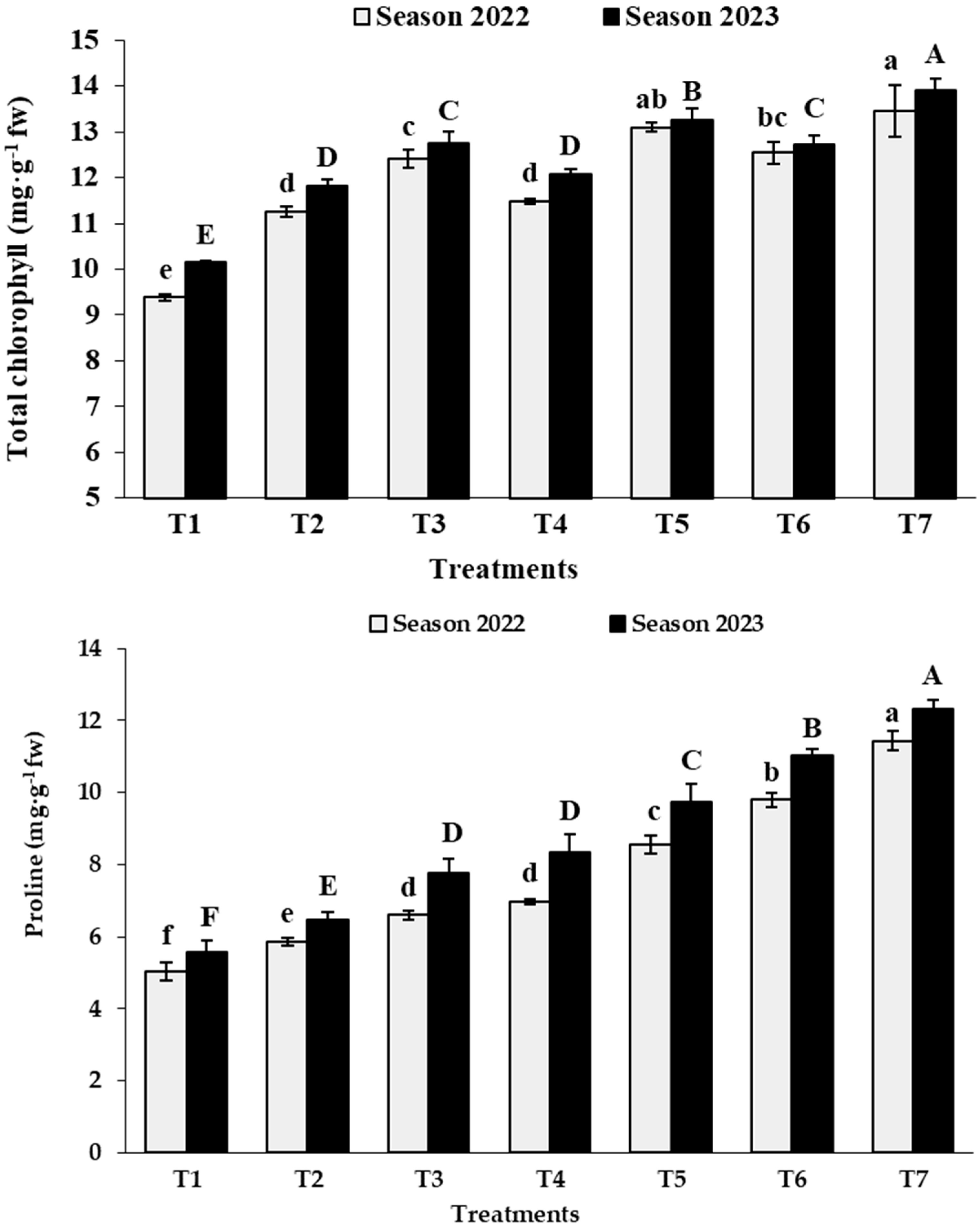
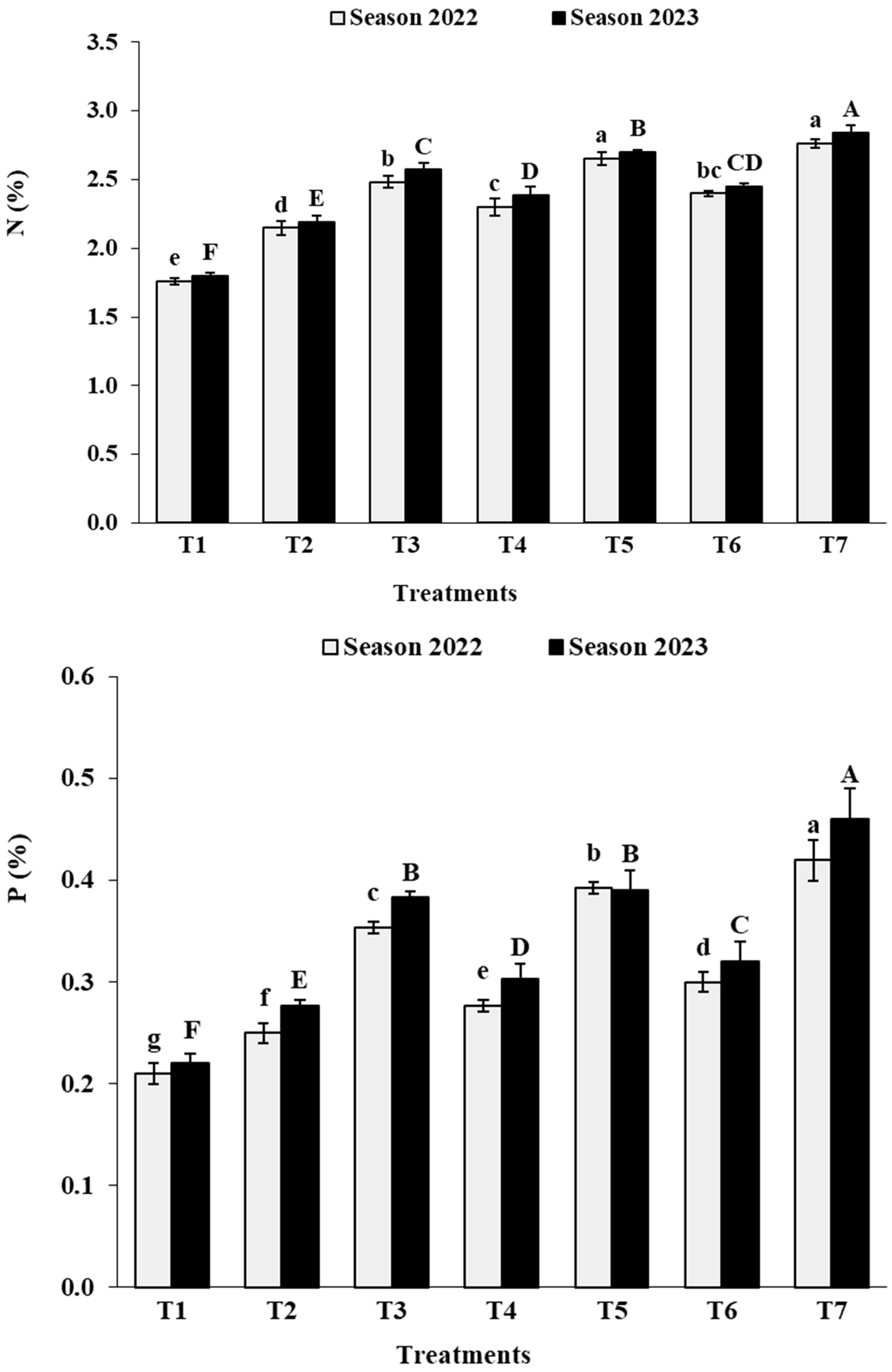
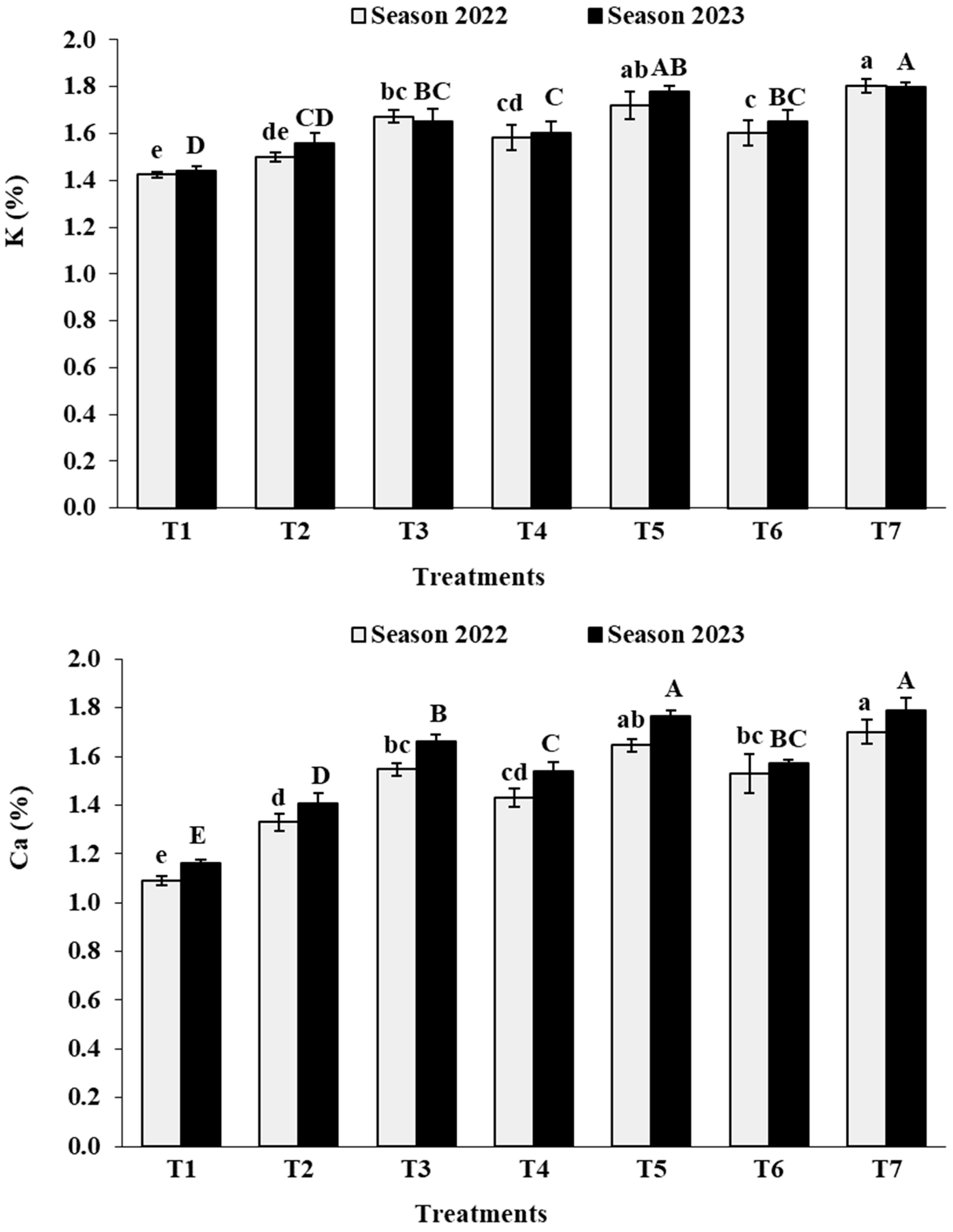
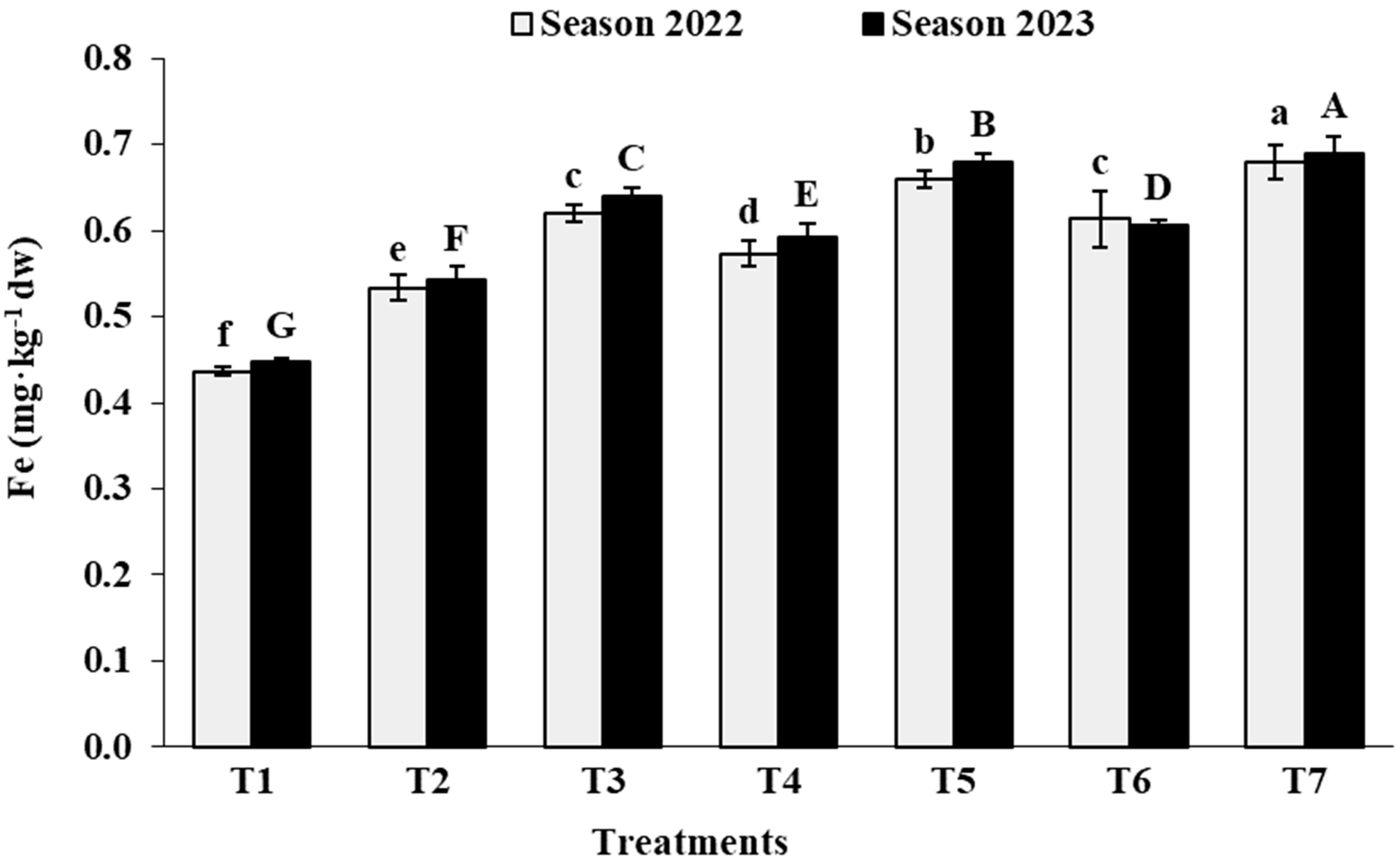
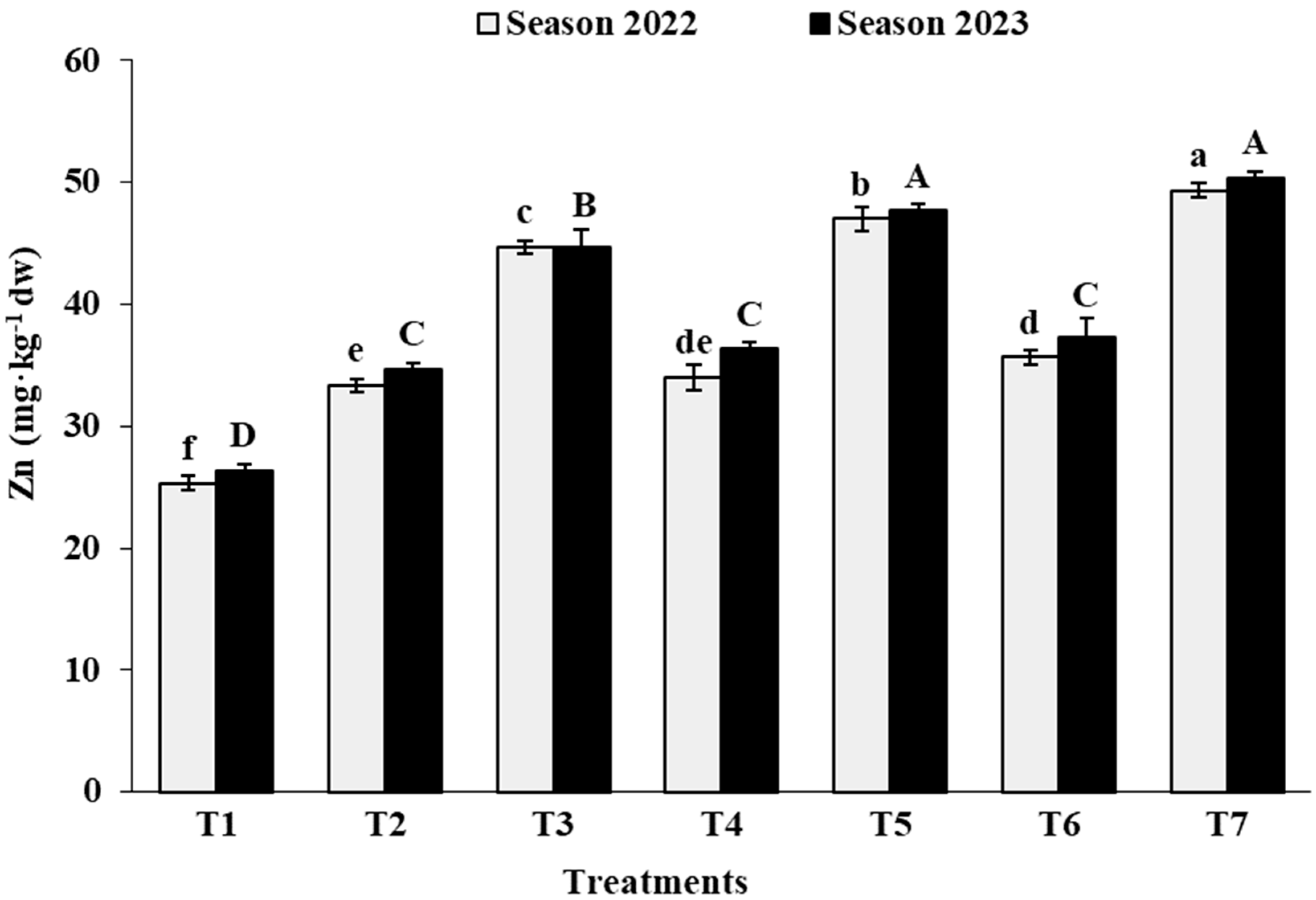
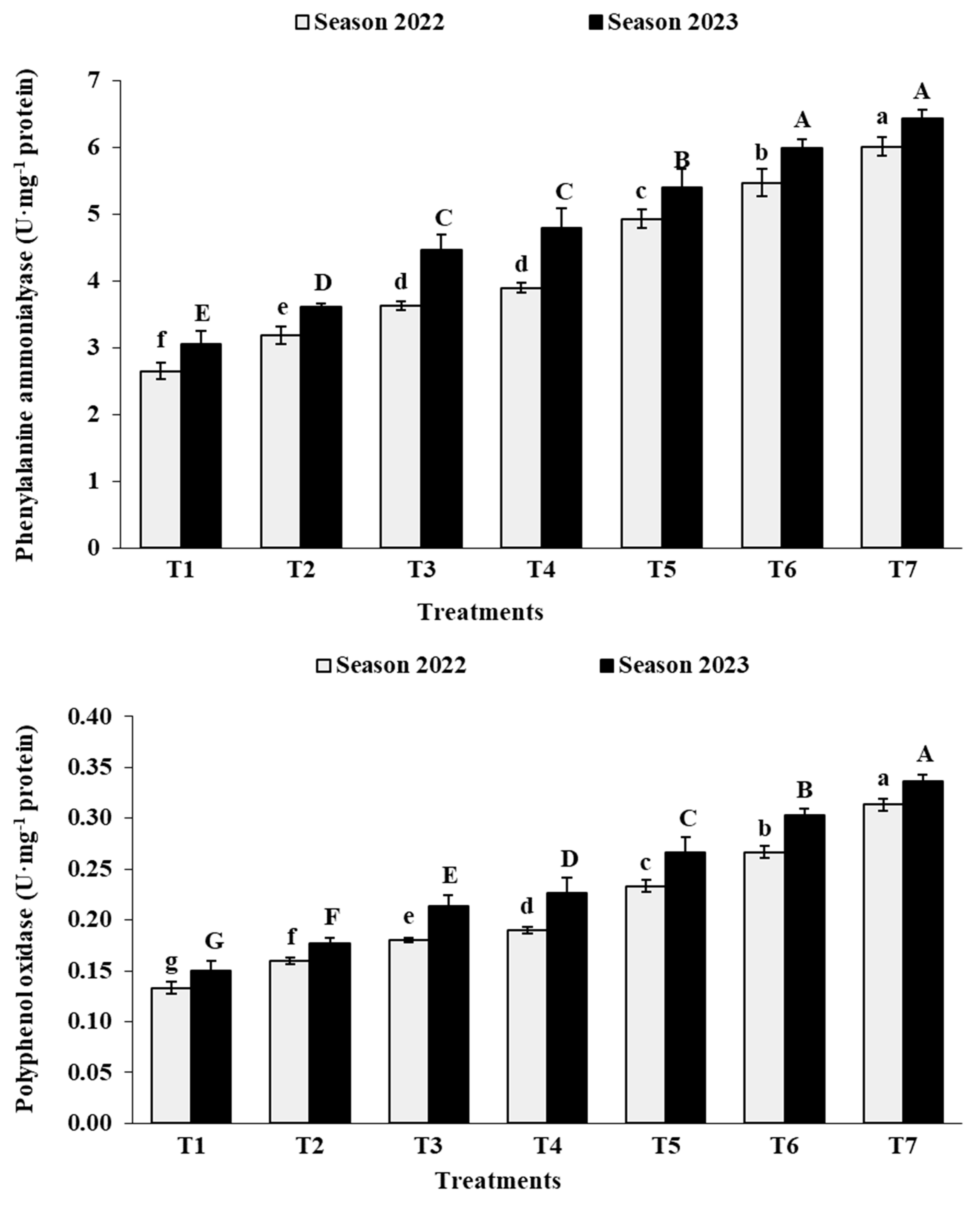
3.2. Leaf Analysis
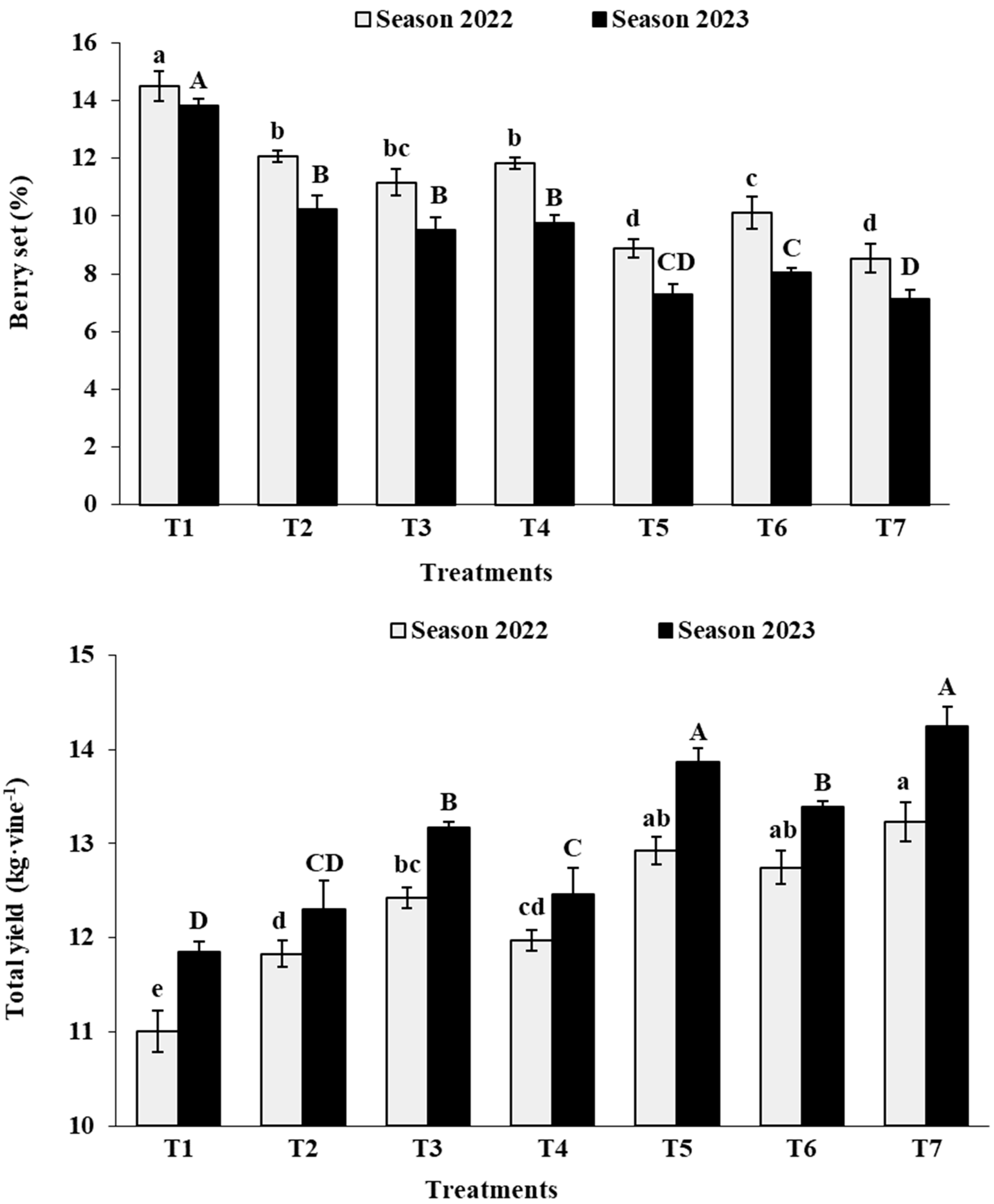
3.3. Fruit Set and Total Yield
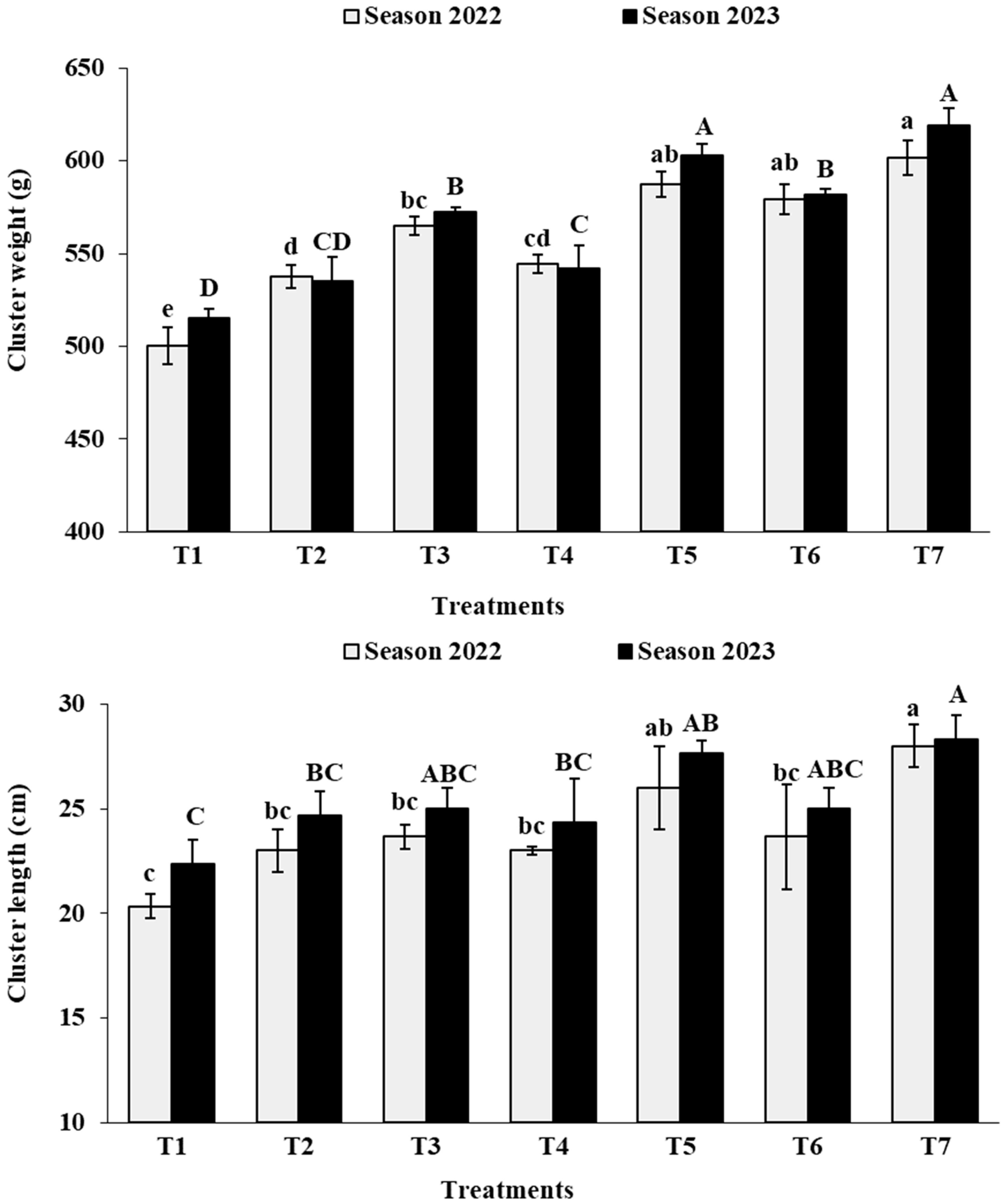
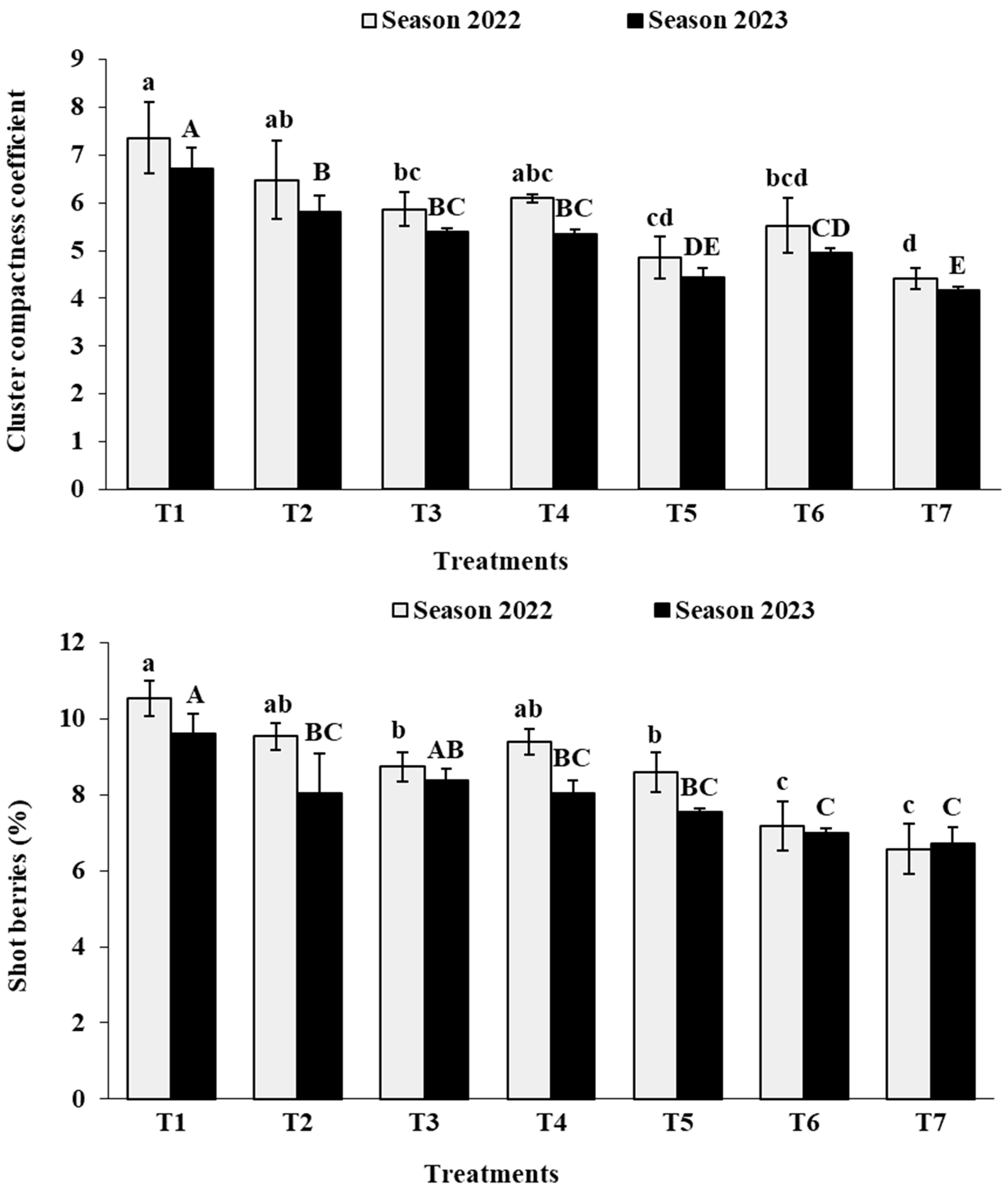
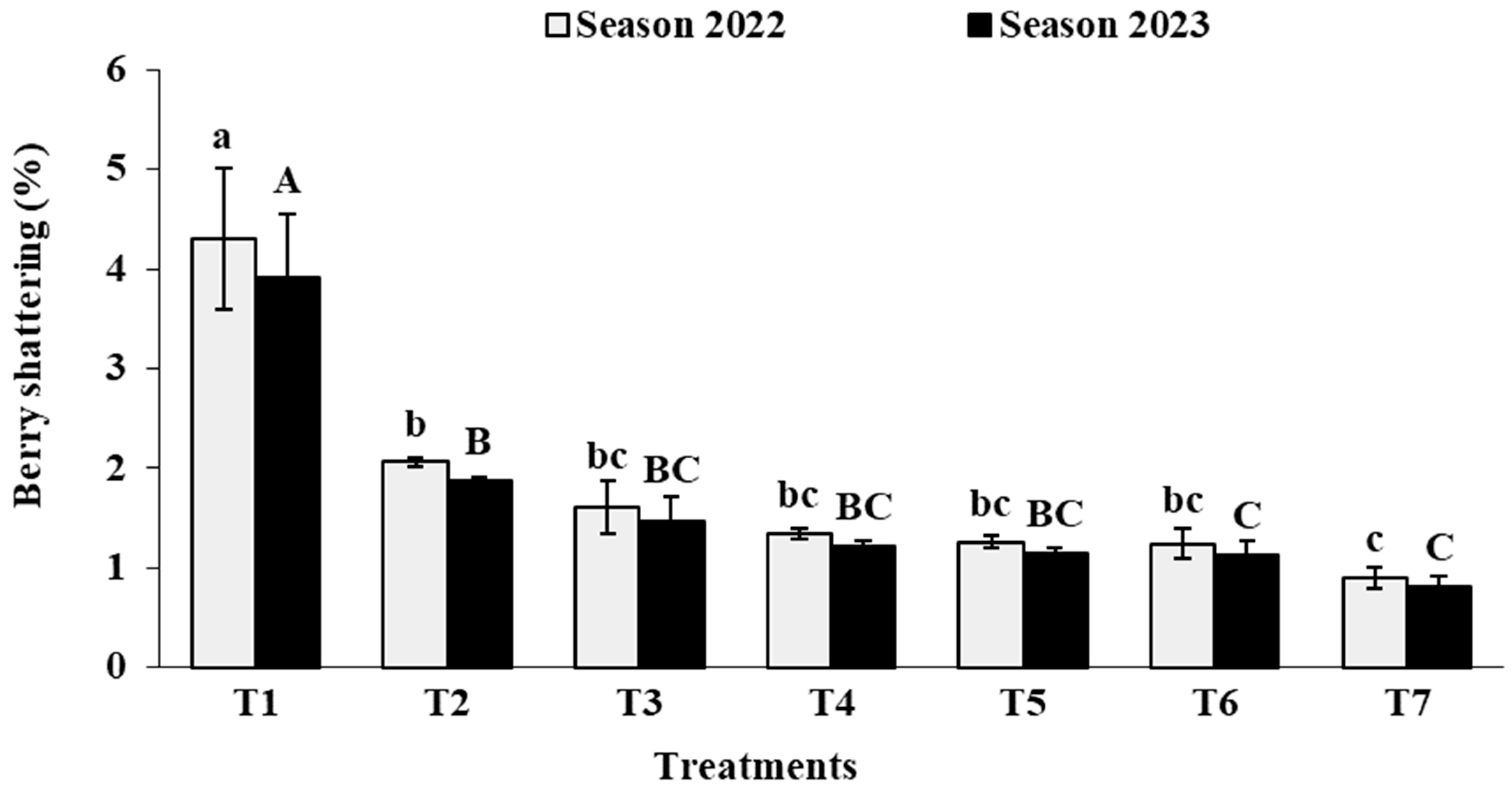
3.4. Cluster Physical Characteristics
3.5. Decay Percentage
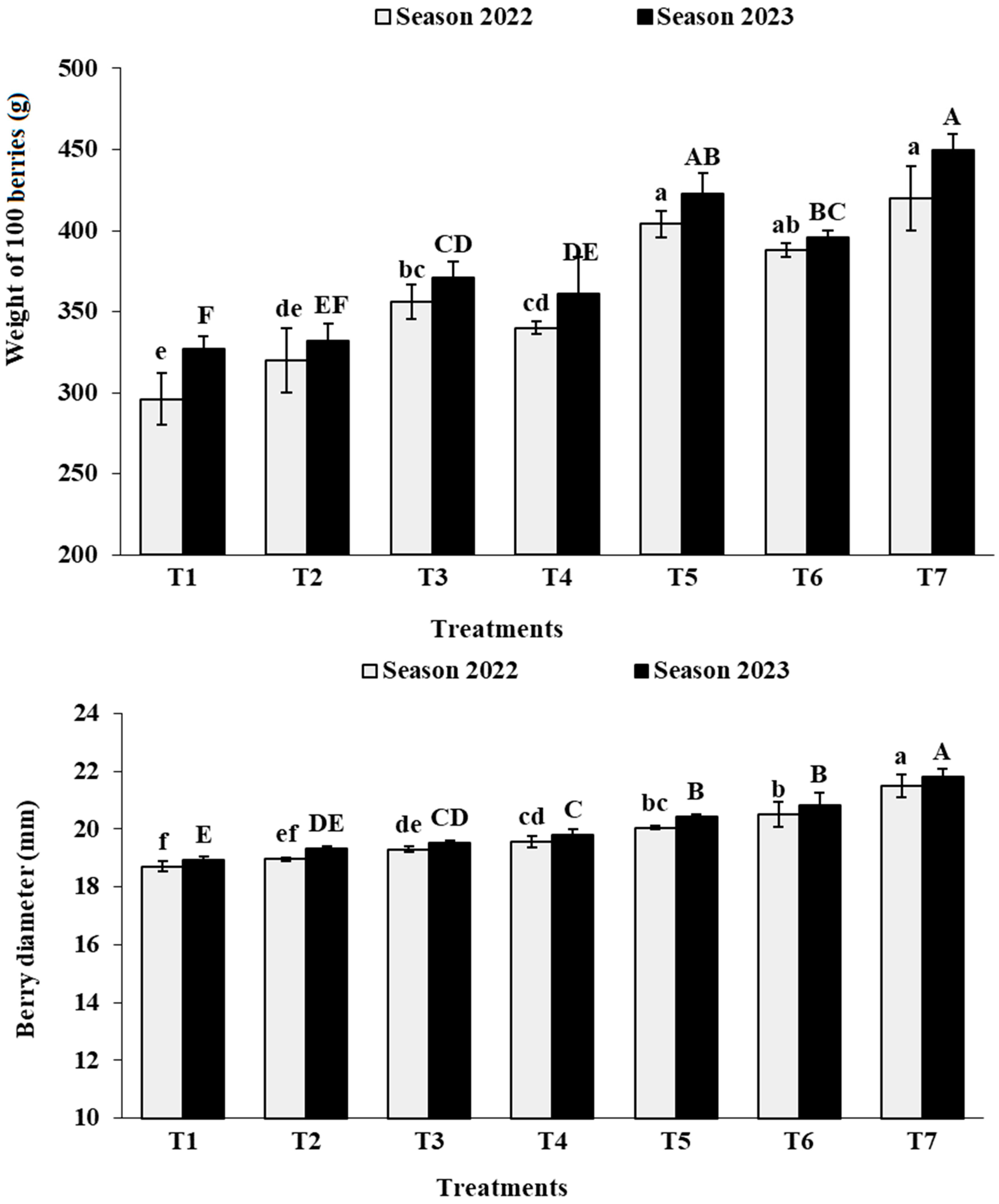
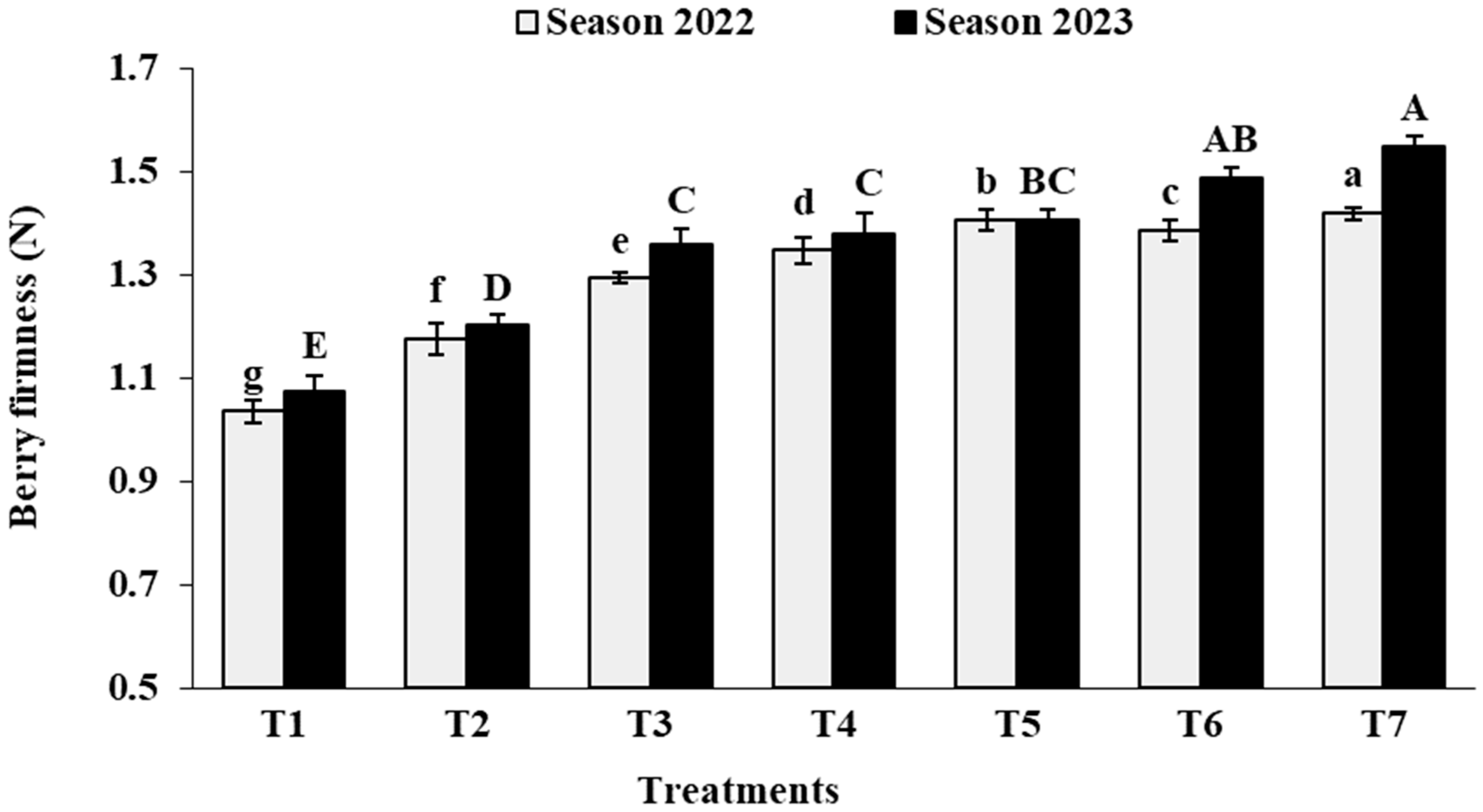
3.6. Berry Physical Characteristics
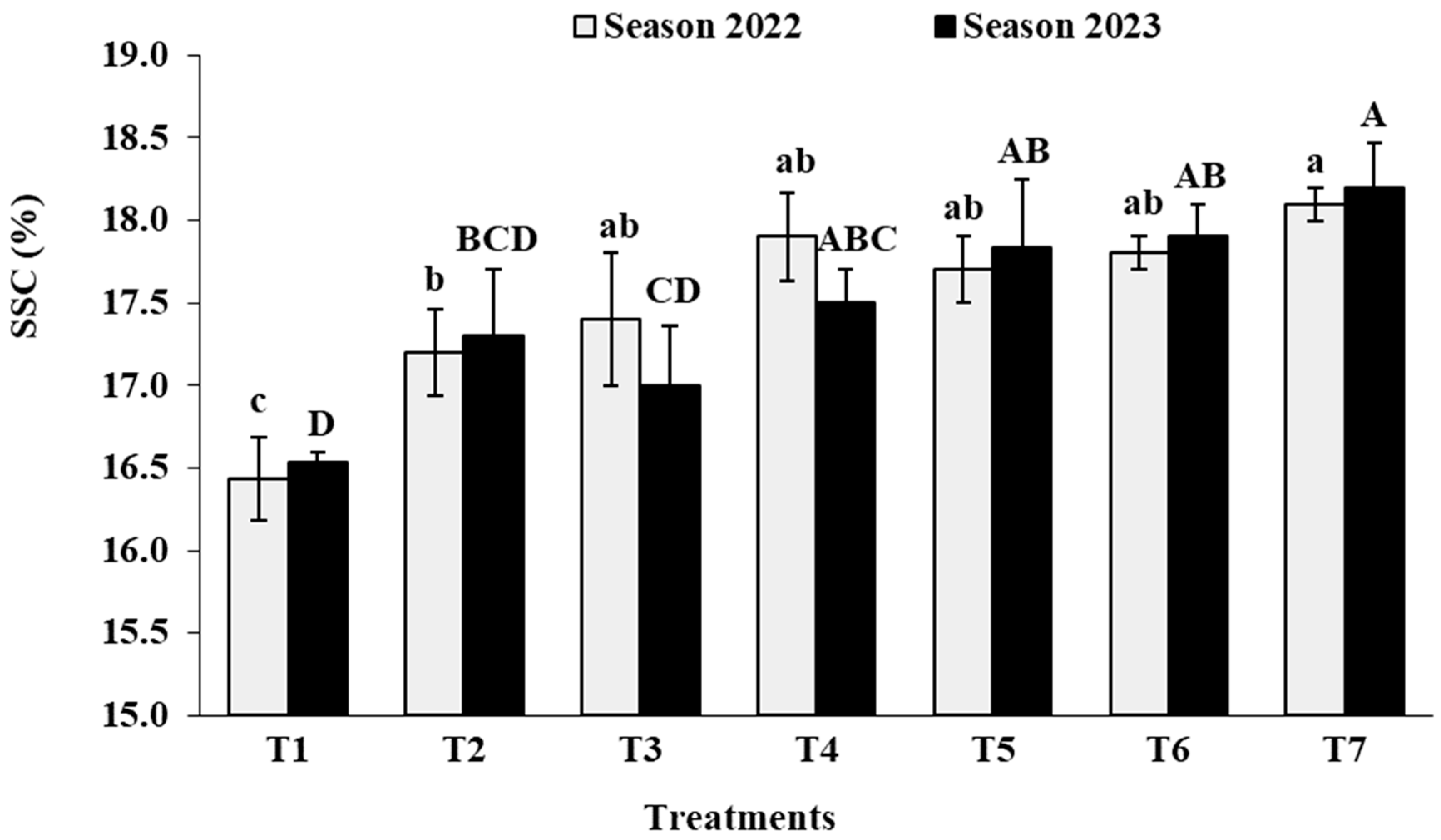
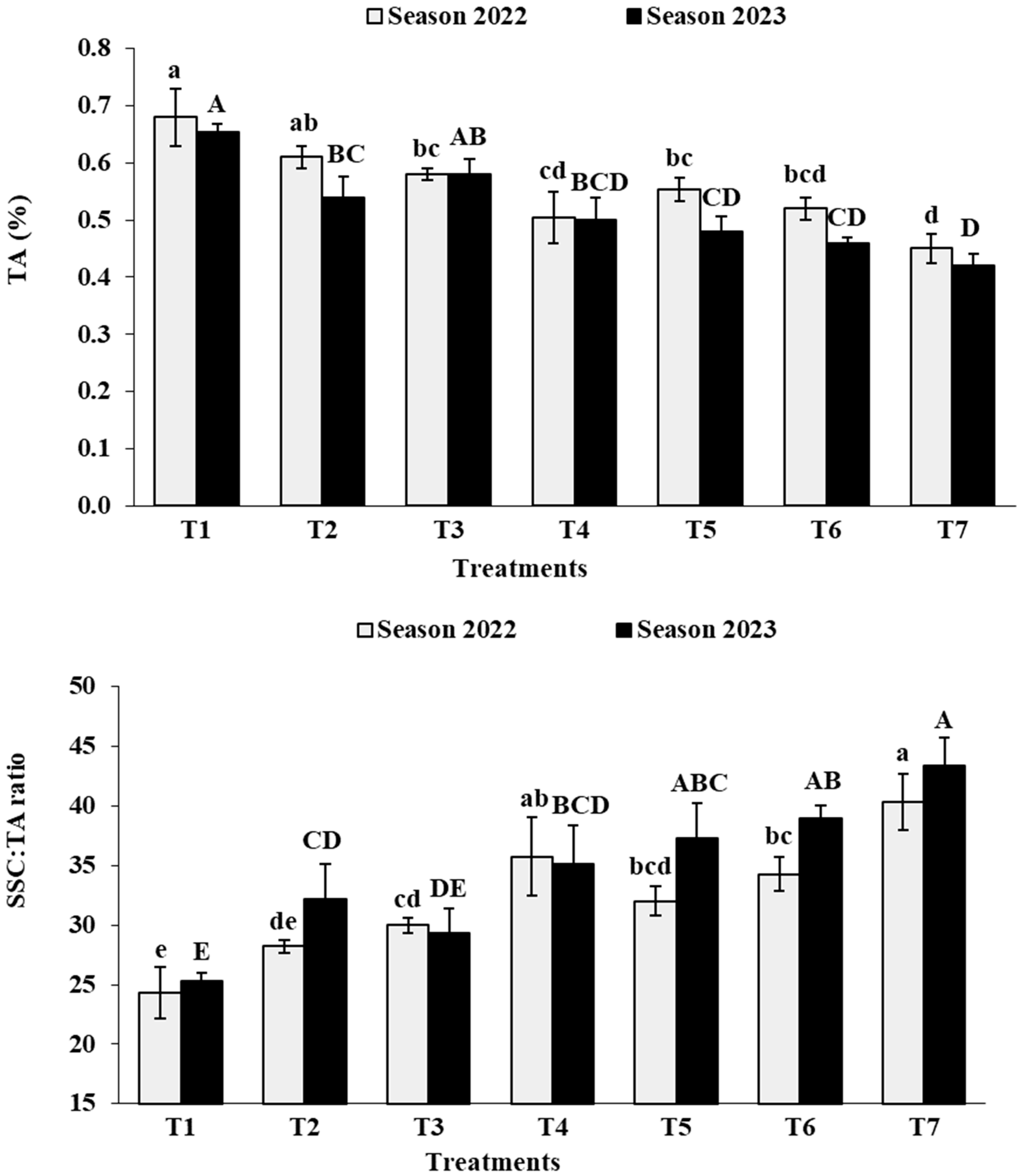
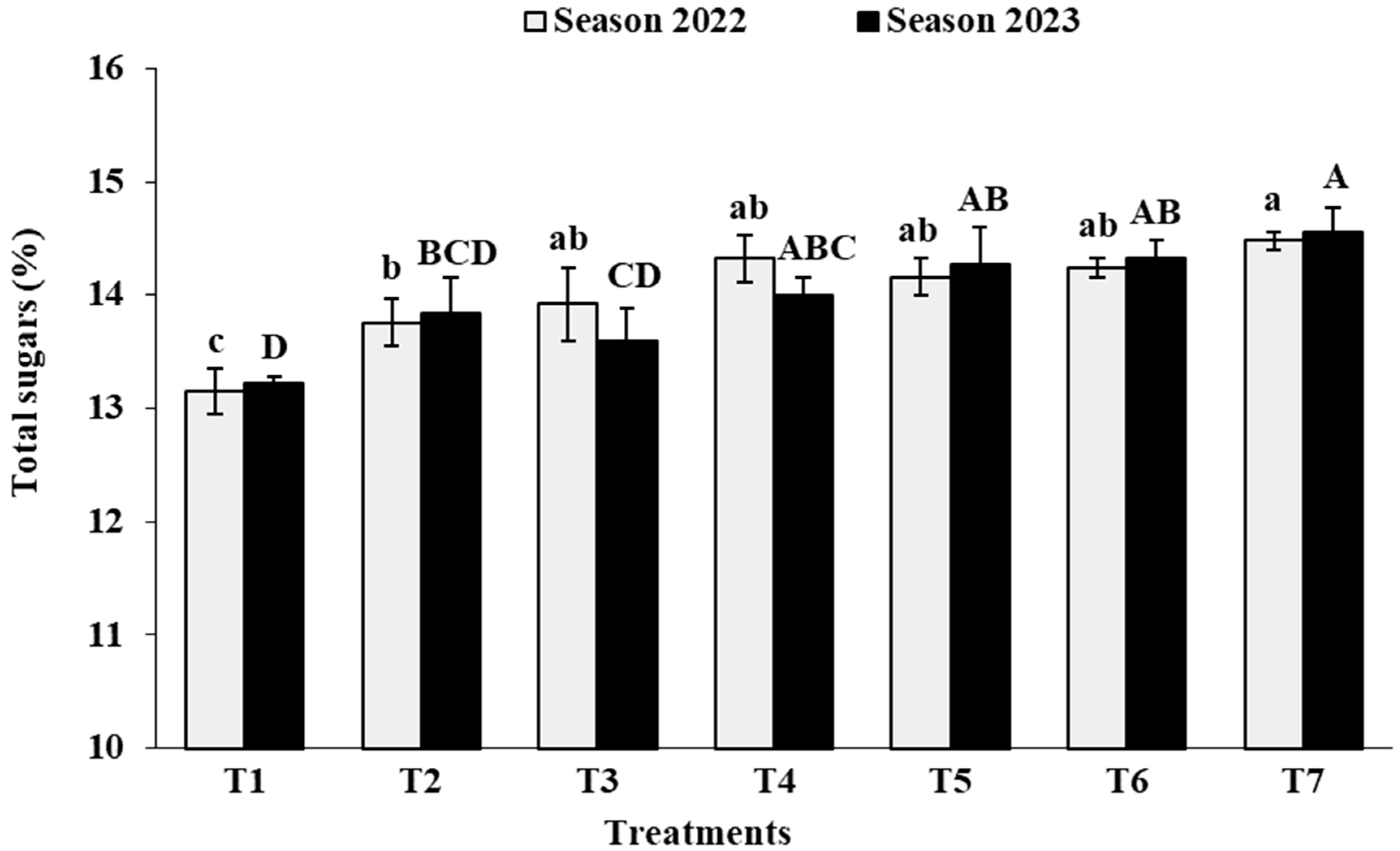
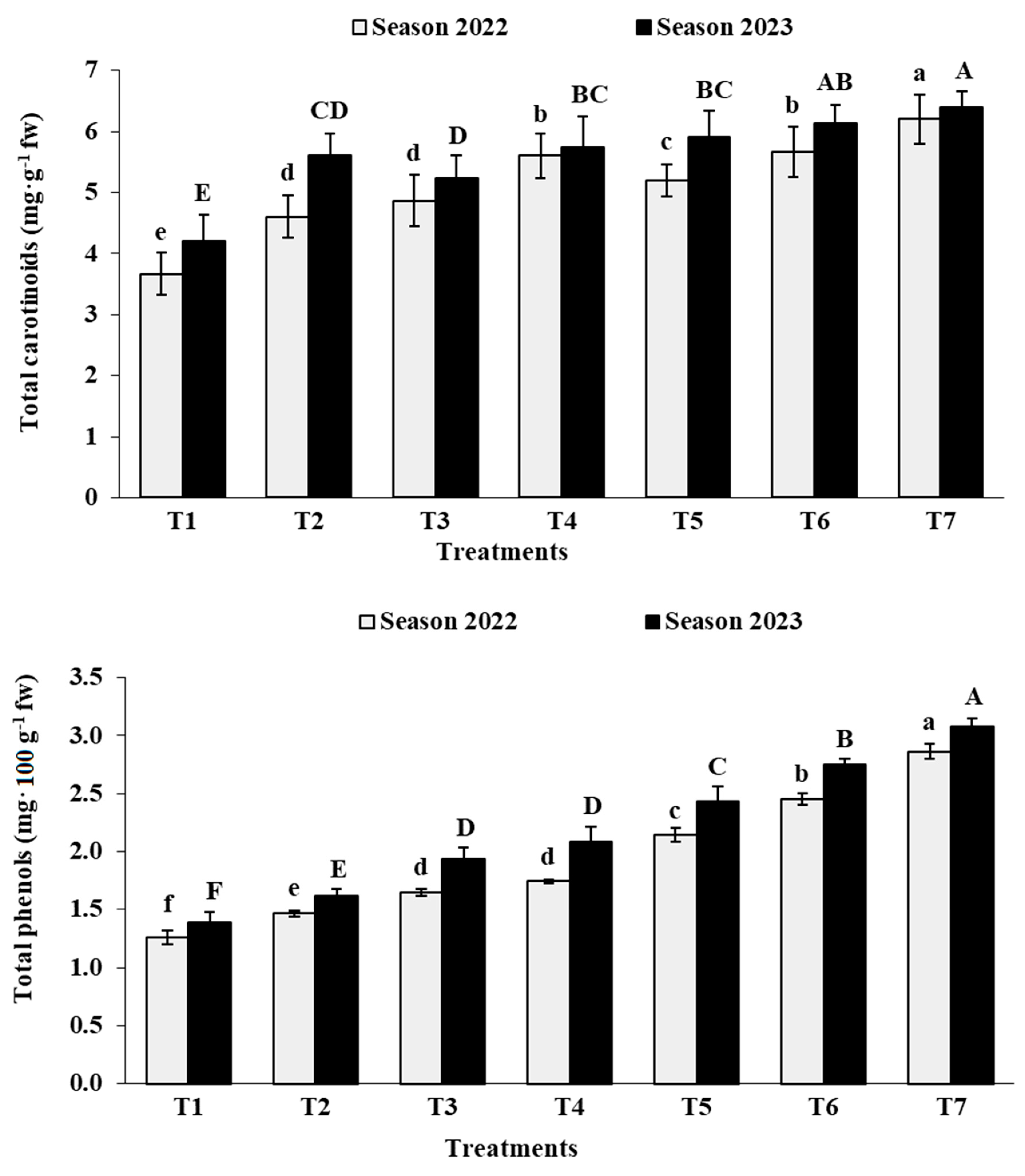
3.7. Berry Chemical Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vivier, M.A.; Pretorius, I.S. Genetically tailored grapevines for the wine industry. Trends Biotechnol. 2002, 20, 72–478. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Grapes Facts and Figures; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 March 2024).
- Omar, S.; Akingbe, O. Overview of Egypt’s Table Grape Sector; Foreign Agricultural Service, Report # EG2020–0055; United States Department of Agriculture (USDA): Washington, DC, USA, 2020.
- Ahmed, O.A.; Abd El-Aziz, M.H. Description and evaluation of some newly introduced grape cultivars under Egyptian conditions. J. Agric. Chem. Biotechnol. Mansoura Univ. 2021, 12, 127–136. [Google Scholar]
- Van der Merwe, G.G. Guidelines for the Preparation of Table Grapes for Export 2014/2015; South African Table Grape Industry: Paarl, South Africa, 2014. [Google Scholar]
- El-Halaby, E.H.S.; El-Salhy, A.M.; Al-Wasfy, M.M.; Ibrahim, R.A. Effect of GA3, urea and yeast spraying on fruiting of Flame Seedless grapevines under sandy soil conditions. Assiut J. Agric. Sci. 2015, 46, 95–106. [Google Scholar]
- Intrieri, C.; Filippetti, I.; Allegro, G.; Centinari, M.; Poni, S. Early defoliation (hand vs mechanical) for improved crop control and grape composition in Sangiovese (Vitis vinifera L.). Aust. J. Grape Wine Res. 2008, 14, 25–32. [Google Scholar] [CrossRef]
- Sabbatini, P.; Howell, G.S. Effects of early defoliation on yield, fruit composition, and harvest season cluster rot complex of grapevines. HortScience 2010, 45, 1804–1808. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.T.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar] [CrossRef]
- Austin, C.N.; Wilcox, W.F. Effects of fruit-zone leaf removal, training systems, and irrigation on the development of grapevine powdery mildew. Am. J. Enol. Vitic. 2011, 62, 193–198. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.T. Grapevine quality: A multiple-choice issue. Sci. Hortic. 2017, 234, 445–462. [Google Scholar] [CrossRef]
- Tardaguila, J.; de Toda, F.M.; Poni, S.; Diago, M.P. Impact of early leaf removal on yield and fruit and wine composition of Vitis vinifera L. Graciano and Carignan. Am. J. Enol. Vit. 2010, 61, 372–381. [Google Scholar] [CrossRef]
- Vander, W.J.; Medina-Meza, I.G.; Frioni, T.; Sivilotti, P.; Falchi, R.; Sabbatini, P. Enhancement of fruit technological maturity and alteration of the flavonoid metabolomic profile in Merlot (Vitis vinifera L.) by early mechanical leaf removal. J. Agric. Food Chem. 2018, 66, 9839–9849. [Google Scholar] [CrossRef]
- Acimovic, D.; Tozzini, L.; Green, A.; Sivilotti, P.; Sabbatini, P. Identification of a defoliation severity threshold for changing fruit set, bunch morphology and fruit composition in Pinot noir. Aust. J. Grape Wine Res. 2016, 22, 399–408. [Google Scholar] [CrossRef]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Bourdin, G.; Spring, J.-L. Mechanisation of pre-flowering leaf removal under the temperate climate conditions of Switzerland. This Article Is Published in Cooperation With the 22nd GiESCO International Meeting, Hosted by Cornell University in Ithaca, NY, July 17–21, 2023. OENO One 2023, 57, 291–302. [Google Scholar] [CrossRef]
- Abd El-Khalek, A.; El-Kenawy, M.A.; Belal, B.E.; Hassan, I.F.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Basal defoliation, salicylic acid and cyanocobalamin to ameliorate the physiological and biochemical characteristics of flood-irrigated ‘Crimson Seedless’ grapevines in a semi-arid Mediterranean climate. Folia Hortic. 2023, 35, 307–332. [Google Scholar] [CrossRef]
- Sabbatini, P.P. Leaf Removal: A Tool to Improve Crop Control and Fruit Quality in Vinifera Grapes; Michigan Grape & Wine Industry Council Research Report; Michigan State Universty Extension: East Lansing, MI, USA, 2015. [Google Scholar]
- Intrieri, C.; Filippetti, I.; Allegro, G.; Valentini, G.; Pastore, C.; Colucci, E. The effectiveness of basal shoot mechanical leaf removal at the onset of bloom to control crop on cv. Sangiovese (V. vinifera L.): Report on a three-year trial. S. Afr. J. Enol. Viticul. 2016, 37, 193–198. [Google Scholar] [CrossRef][Green Version]
- Frioni, T.; Shijian, Z.; Alberto, P.; Paolo, S.; Rachele, F.; Paolo, S. Leaf removal and cluster thinning efficiencies are highly modulated by environmental conditions in cool climate viticulture. Am. J. Enol. Vitic. 2017, 68, 325–335. [Google Scholar] [CrossRef]
- Ameer, M.S.; Doaa, M.H. Manual defoliation treatments affected yield and cluster quality of grapevines cv. Crimson Seedless. J. Plant Prod. 2020, 11, 377–381. [Google Scholar]
- Vander, W.J.; Gottschalk, C.; Steven, R.S.; Nasrollahiazar, E.; Poni, S.; Sabbatini, P. Impact of pre-bloom leaf removal and wine grape quality production parameters: A systematic review and meta-analysis. Front. Plant Sci. 2012, 11, 621585. [Google Scholar] [CrossRef]
- Sivilotti, P.; Herrera, J.C.; Lisjak, K.; Basaesnik, H.; Ssabbatini, P.; Peterlunger, E.; Castellarin, S.D. Impact of leaf removal, applied before and after flowering, on anthocyanin, tannin, and methoxypyrazine concentrations in ‘Merlot’ (Vitis vinifera L.) grapes and wines. J. Agric. Food Chem. 2016, 64, 4487–4496. [Google Scholar] [CrossRef]
- Petrie, P.R.; Trought, M.C.T.; Howell, G.S.; Buchan, G.D. The effect of leaf removal and canopy height on whole-vine gas exchange and fruit development of Vitis vinifera L. ‘Sauvignon blanc’. Funct. Plant Biol. 2003, 30, 711–717. [Google Scholar] [CrossRef]
- Mosetti, D.; Herrera, J.C.; Sabbatini, P.; Green, A.; Alberti, G.; Peterlunger, E.; Lisjak, K.; Castellarin, S.D. Impact of leaf removal after berry set on fruit composition and bunch rot ‘Sauvignon blanc’. Vitis 2016, 55, 57–64. [Google Scholar]
- Verdenal, T.; Spangenberg, J.E.; Zufferey, V.; Lorenzini, F.; DienesNagy, A.; Gindro, K.; Spring, J.L.; Viret, O. Leaf-to-fruit ratio affects the impact of foliar-applied nitrogen on N accumulation in the grape must. OENO One 2016, 50, 23–33. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Lovatt, C.J. Properly timing foliar-applied fertilizers increases efficacy: A review and update on timing foliar nutrient applications to citrus and avocado. HortTechnology 2013, 23, 536–541. [Google Scholar] [CrossRef]
- Gutierrez-Gamboa, G.; Diez-Zamudio, F.; Stefanello, L.O.; Tassinari, A.; Brunetto, G. Application of foliar urea to grapevines: Productivity and flavour components of grapes. Aust. J. Grape Wine Res. 2022, 28, 27–40. [Google Scholar] [CrossRef]
- El-Salhy, A.M.; Ebtsam, M.F.; Eman, A.A.; Mona, M.D. Effect of GA3 and some plant extracts spraying on fruiting of Early Sweet Seedless grapevine. Int. J. Agric. Sci. 2019, 1, 54–63. [Google Scholar]
- Fawzi, M.I.F.; Laila, F.H.; Shahin, M.F.M.; Merwad, M.A.; Genaidy, E.A.A. Influence of spraying urea, boron and active dry yeast on growth, yield, leaf chemical composition and fruit quality of Superior grapevines growth in sandy soil conditions. Middle East J. Appl. Sci. 2014, 4, 740–747. [Google Scholar]
- Radwan, E.M.A.; Khodair, O.A.; Silem, A.A.E.M. Effect of some compounds spraying on fruiting of Superior Seedless grapevines under Assiut conditions. J. Plant Prod. 2019, 10, 59–64. [Google Scholar] [CrossRef]
- Alvarez, P.E.P.; Garde-Cerdán, T.; García-Escudero, E.; Martínez-Vidaurre, J.M. Effect of two doses of urea foliar application on leaves and grape nitrogen composition during two vintages. J. Sci. Food Agric. 2016, 97, 2524–2532. [Google Scholar] [CrossRef]
- Mikkelsen, R.L. Biuret in Urea Fertilizers. Better Crops 2007, 91, 6–7. [Google Scholar] [CrossRef]
- Guirguis, N.S.; Gumana, A.H.; Stino, R.G.; Merhreki, A.M. Effect of carbonate, urea and cyanamide on thinning and apical blooms under arid conditions in Egypt. Hort. Abst. 1996, 66, 92. [Google Scholar]
- Asensi-Fabado, M.A.; Munne’-Bosch, S. Vitamins in plants: Occurrence, biosynthesis, and antioxidant function. Trend Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Roje, S. Vitamin B biosynthesis in plants. Phytochemistry 2007, 68, 1904–1921. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.D.; Nemoto-Smith, E.; Deery, E.; Baker, J.A.; Schroeder, S.; Brown, D.G.; Tullet, J.M.A.; Howard, M.J.; Brown, I.R.; Smith, A.G.; et al. Construction of Fluorescent Analogs to Follow the Uptake and Distribution of Cobalamin (Vitamin B 12) in Bacteria, Worms, and Plants. Cell Chem. Biol. 2018, 25, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.C. Megaloblastic anemias. In Hematology: Basic Principles and Practice; Hoffman, R., Benz, E.J., Jr., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elseiver Inc.: Amesterdam, The Netherlands, 2018; pp. 514–545. [Google Scholar]
- Smith, A.G.; Croft, M.T.; Moulin, M.; Webb, M.E. Plants need their vitamins too. Curr. Opin. Plant Biol. 2007, 10, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Rivera, J.; Zapata, C.; Norambuena, J.; Sandoval, A.; Chavez, R.; Orellana, O.; Levican, G. Cobalamin protection against oxidative stress in the acidophilic iron-oxidizing bacterium Leptospirillum group II CF-1. Front. Microbiol. 2016, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Vitamin B-12. Adv. Nutrit. 2012, 3, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Zeh, M.; Leggewie, G.; Hoefgen, R.; Hesse, H. Cloning and characterization of a cDNA encoding a cobalamin-independent methionine synthase from potato (Solanum tuberosum L.). Plant Mol. Biol. 2002, 48, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, M.; Atta, M.; Mulliez, E. S-adenosylmethionine: Nothing goes to waste. Trend. Biochem. Sci. 2004, 29, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.C.; Vanhille, R.P.; Harrison, S.T.L. Reactive oxygen species generated in the presence of fine pyrite particles and its implication in thermophilic mineral bioleaching. Appl. Microbiol. Biotechnol. 2013, 97, 2735–2742. [Google Scholar] [CrossRef]
- Vasques, L.; Parra, A.; Quesille-Villalobos, A.M.; Galvez, G.; Navarette, P.; Latorre, M.; Toro, M.; Gonzalez, M.; Reyes-Jara, A. Cobalamin cbip mutant shows decreased tolerance to low temperature and copper stress in Listeria monocytogenes. Biol. Res. 2022, 55, 9. [Google Scholar]
- Lo’ay, A.A. Cyanocobalamin control fruit ripening of persimmon fruits. J. Plant Prod. 2010, 1, 1653–1663. [Google Scholar] [CrossRef]
- Lo’ay, A.A. Biological indicators to minimize berry shatter during handling of ‘Thompson seedless’ grapevines. World Appl. Sci. J. 2011, 12, 1107–1113. [Google Scholar]
- Samaan, L.G.; El-Dengawy, F.F.; Lo’ay, A.A.; El-Fayoumy, H.M. Exogenous spray of mango (Mangifera indica L.) trees with antioxidant solutions in relation to changes in fruit quality and storability at harvest and during cold storage. J. Plant Prod. 2011, 2, 617–639. [Google Scholar] [CrossRef]
- Samaan, L.G.; Iraqi, M.A.; Lo’ay, A.A.; Serag, T.A. Treatments to increase storability and marketability of guava (Psidium guajava L.) fruits. J. Plant Prod. Mansoura Univ. 2012, 3, 857–876. [Google Scholar] [CrossRef][Green Version]
- El-Baz, E.; Lo’ay, A.A.; Ibrahium, E.G.I.; El-Deeb, M.R.I. Effect of cobalt and some vitamins as foliar application treatment and productivity and quality of Williams banana cultivar. J. Plant Prod. 2016, 7, 777–786. [Google Scholar] [CrossRef]
- Abd El-Bary, A.A. Effect of spraying GA3 and cyanocobalamin (Vit. B12) on fruit set, yield, and fruit quality of Le-Conte Pear Trees. J. Plant Prod. 2017, 8, 555–558. [Google Scholar] [CrossRef]
- Lo’ay, A.A. Improvement berry color skin profile by exogenous cyanocobalamin treatment of ‘Crimson seedless’ grapevines. Egypt. J. Basic Appl. Sci. 2017, 4, 231–235. [Google Scholar] [CrossRef]
- Dokoozlian, N.; Luvisi, D.; Moriyama, M.; Schrader, P. Cultural practices improve color, size of ‘Crimson Seedless’. Hilgardia 1995, 49, 36–40. [Google Scholar] [CrossRef]
- Tardaguila, J.; Petrie, P.R.; Poni, S.; Diago, M.P.; Martinez de Toda, F. Effects of mechanical thinning on yield and fruit composition of Tempranillo and Grenache grapes trained to a vertical shoot-positioned canopy. Am. J. Enol. Vitic. 2008, 59, 412–417. [Google Scholar] [CrossRef]
- Lurie, S.; Lichter, A.; Kaplunov, T.; Zutahy, Y.; Oren-shamie, M.; Ovadia, R. Improvement of ‘Crimson Seedless’ grape color by abscisic acid treatment. Acta Hortic. 2010, 880, 183–189. [Google Scholar] [CrossRef]
- Gambacorta, G.; Antonacci, D.; La Gatta, M.; Faccia, M.; La Gatta, B.; Pati, S.; Coletta, A.; La Notte, E. Phenolic composition of Aglianico and Nero di Troia grapes and wines as affected by cover cropping and irrigation. Ital. J. Food Sci. 2011, 23, 381–394. [Google Scholar]
- Baiano, A.; De Gianni, A.; Previtali, M.A.; Del Nobile, M.A.; Novello, V.; De Palma, L. Effects of defoliation on quality attributes of Nero di Troia (Vitis vinifera L.) grape and wine. Food Res. Int. 2015, 75, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, L.; Alba, V.; Di Gennaro, D.; Amendolagine, A.M.; Gentilesco, G.; Masi, G. Grape and wine quality of Vitis vinifera ‘Nero di Troia’ in response to moderate deficit irrigation. Acta Hortic. 2017, 1150, 485–492. [Google Scholar] [CrossRef]
- Khan, A.S.; Malik, A.U.; Perez, M.A.; Saleem, B.A.; Rajwana, I.A.; Shaheen, T.; Anwar, R. Foliar application of low-biuret urea and fruit canopy position in the tree influence the leaf nitrogen status and physico-chemical characteristics of Kinnow mandarin (Citrus reticulata Blanco). Pak. J. Bot. 2009, 41, 73–85. [Google Scholar]
- Worldweatheronline. El-Sadat, Menoufia, Egypt Historical Weather. Available online: https://www.worldweatheronline.com/sadat-city-weather-averages/al-buhayrah/eg.aspx (accessed on 19 March 2024).
- Wilde, S.A.; Corey, R.B.; Lyer, J.G.; Voight, G.K. Soil and Plant Analysis for Tree Culture, 3rd ed.; Oxford and IBH. Publishing Co.: New Delhi, India, 1985; pp. 93–106. [Google Scholar]
- Chapman, H.D.; Pratt, F.P. Methods of Analysis for Soils, Plants and Waters, 1st ed.; University of California, Division of Agricultural Sciences: Davis, CA, USA, 1961; p. 309. [Google Scholar]
- Ali, E.M.; Shabaan-Dessouki, S.A.; Soliman, A.R.I.; El Shenawy, S. Characterization of chemical water quality in the Nile River. Egypt. Int. J. Pure App. Biosci. 2014, 2, 35–53. [Google Scholar]
- Abuzaid, A.S. Evaluating surface water quality for irrigation in Dakahlia governorate using water quality index and GIS. J. Soil Sci. Agric. Eng. 2018, 9, 481–490. [Google Scholar]
- El Sayed, S.M.; Hegab, M.H.; Mola, H.R.A.; Ahmed, N.M.; Goher, M.E. An integrated water quality assessment of Damiatta and Rosetta branches (Nile River, Egypt) using chemical and biological indices. Environ. Monit. Assess. 2020, 192, 228. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elwafa, T.S.A. Effect of different levels of pruning on growth, yield and fruit quality of Prime Seedless grapevines under Gable supporting system. J. Plant Prod. Mansours Univ. 2021, 12, 1279–1283. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wolf, B.A. Comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Evenhuis, B.; Dewaard, P.W. Nitrogen Determination; Department of Agriculture Research, Royal Tropical Institute: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Jones, J.B.; Wolf, B.; Mills, H.A. Plant analysis handbook. In A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, GA, USA, 1991; p. 212. [Google Scholar]
- Tendon, H.L.S. Analysis of Soils, Plants, Waters and Fertilizers; Fertilizer Development and Consultation Organization: New Delhi, India, 2005. [Google Scholar]
- Chang, K.L.; Bray, R.H. Determination of calcium and magnesium in soil and plant material. Soil Sci. 1951, 72, 449–458. [Google Scholar] [CrossRef]
- Rashid, A. Mapping Zinc Fertility of Soil Using Indicator Plant and Soil Analysis. Ph.D. Thesis, University of Hawaii, Manoa, HI, USA, 1986. [Google Scholar]
- Mohamed, A.K.A.; El-Sese, A.M. Effect of some chemical compounds and growth regulators on regularity of bud break, flowering, and fruiting of Red Roomy grapevine (Vitis vinifera L.). Assiut J. Agric. Sci. 2004, 35, 165–181. [Google Scholar]
- Guelfat-Reich, S.; Safran, B. Indices of maturity for table grapes as determined by variety. Am. J. Enol. Viticult. 1971, 22, 13–18. [Google Scholar] [CrossRef]
- Mohamed, A.K.A.; Mohamed, F.E.; Gouda, A.M.; Ibrahim, R.A.; Madkor, Y.M.A. Improve the yield and quality of red roomy and Thompson seedless grape cultivars. Assiut J. Agric. Sci. 2017, 48, 38–58. [Google Scholar]
- Khalil, A.; Nazir, N.; Din, S.; Sharma, M.K.; Kumar, A. Response of Budload and Fertilizer on Berry Shape, Quality and Shot Berry Disorder in Grapes cv. Sahebi. Biol. Forum–Int. J. 2022, 14, 12–16. [Google Scholar]
- Fawzi, M.I.F.; Hagagg, L.F.; Shahin, M.F.M.; El-Hady, E.S. Effect of hand thinning, girdling and boron spraying application on, vegetative growth, fruit quality and quantity of Thompson seedless grapevines. Mid. East J. Agric. Res. 2019, 8, 506–513. [Google Scholar]
- Hegazi, A.H.; Samara, N.R.; Bndok, S.A.; Enas, A.S. Effect of ethephon, acetic and citric acid on berry quality and storage ability of Flame Seedless grapes. J. Plant Prod. Mansoura Univ. 2014, 5, 1795–1806. [Google Scholar] [CrossRef]
- Junior, O.J.C.; Youssef, K.; Koyama, R.; Ahmed, S.; Dominguez, A.R.; Mühlbeier, D.T.; Roberto, S.R. Control of Gray Mold on Clamshell-Packaged ‘Benitaka’ Table Grapes Using Sulphur Dioxide Pads and Perforated Liners. Pathogens 2019, 8, 271. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruit (Fragaria × anannasa Cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- El-Abbasy, U.K.; Abdel-Hameed, M.A.; Hatterman-Valenti, H.M.; El-Shereif, A.R.; Abd El-Khalek, A.F. Effectiveness of Oregano and Thyme Essential Oils as Alternatives for Sulfur Dioxide in Controlling Decay and Gray Mold and Maintaining Quality of ‘Flame Seedless’ Table Grape (Vitis vinifera L.) during Cold Storage. Agronomy 2023, 13, 3075. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R. Applications of salt solutions before and after harvest affect the quality and incidence of postharvest gray mold of ‘Italia’ table grapes. Postharvest Biol. Technol. 2014, 87, 95–102. [Google Scholar] [CrossRef]
- Watkins, C.; Harman, J. Use of penetrometer to measure flesh firmness of fruit. Orchard. New Zealand 1981, 54, 14–16. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemist International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colourimetric methods of determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mackinny, G. Absorption of light by chlorophyll soluation. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Khan, N.U.; Vaidyanathan, C.S. A new simple spectrophotometric assay of phenylalanine ammonia-lyase. Curr. Sci. 1986, 55, 391–393. [Google Scholar]
- Jiang, Y.M.; Zhang, Z.Q.; Joyce, D.C.; Ketsa, S. Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biol. Technol. 2002, 26, 241–252. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1990. [Google Scholar]
- Diago, M.P.; Ayestarán, B.; Guadalupe, Z.; Poni, S.; Tardáguila, J. Impact of prebloom and fruit-set basal leaf removal on the flavonol and anthocyanin composition of Tempranillo grapes. Am. J. Enol. Viticult. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Beslic, Z.; Todic, S.; Matijasevic, S. Effect of Timing of Basal Leaf Removal on Yield Components and Grape Quality of Grapevine cvs Cabernet Sauvignon and Prokupac (Vitis vinifera L.). Bulg. J. Agric. Sci. 2013, 19, 96–102. [Google Scholar]
- Palliotti, A.; Gatti, M.; Poni, S. Early Leaf Removal to Improve Vineyard Efficiency: Gas Exchange, Source-to-Sink Balance, and Reserve Storage Responses. Am. J. Enol. Viticult. 2011, 62, 219–228. [Google Scholar] [CrossRef]
- Kemp, B.; Harrison, R.; Creasy, G.L. The effect of timing of mechanical leaf removal on Pinot noir berry and wine composition. In Proceedings of the 7th International Cool Climate Symposium, Seattle, WA, USA, 1 August 2017; pp. 50–58, 98. [Google Scholar]
- Ghada, S. Effect of Vegetative Shoot Thinning on Growth, Yield and Bunch Quality of Black Monukka and Red Globe Grape Cultivars. Egypt. J. Hortic. 2015, 41, 299–311. [Google Scholar]
- Sun, Q.; Sacks, G.L.; Lerch, S.D.; VandenHeuvel, J.E. Impact of shoot and cluster thinning on yield, fruit composition, and wine quality of Corot noir. Am. J. Enol. Viticult. 2012, 63, 49–56. [Google Scholar] [CrossRef]
- Oliveira, C.; Silva-Ferreira, A.C.; Costa, P.; Guerra, J.; Guedes, D.E.; Pinho, P. Effect of some viticultural parameters on the grape carotenoid profile. J. Agric. Food Chem. 2004, 52, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Razek, E.; Treutter, D.; Saleh, M.M.S.; El-Shammaa, M.; Amira, A.F.; Abdel-Hamid, N.; Abou-Rawash, M. Effect of defoliation and fruit thinning on fruit quality of ‘Crimson Seedless’ Grape. Res. J. Agric. Biol. Sci. 2010, 6, 289–295. [Google Scholar]
- Poni, S.; Bernizzoni, F.; Civardi, S.; Libelli, N. Effects of pre-bloom leaf removal on growth of berry tissues and must composition in two red Vitis vinifera L. cultivars. Am. J. Enol. Viticult. 2009, 15, 185–193. [Google Scholar]
- Grierson, W. Maturity and grade standards. In Fresh Citrus Fruits; Wardowski, W.F., Miller, W.M., Hall, D.J., Grierson, W., Eds.; Florida Science Source, Inc.: Longboat Key, FL, USA, 2006; pp. 23–48. [Google Scholar]
- Diego, S.I.; Elena, L.; Javier, R. Early defoliation reduces cluster compactness and improves grape composition in Mando, an autochthonous cultivar of Vitis vinifera from southeastern Spain. Sci. Hortic. 2014, 167, 71–75. [Google Scholar]
- Brunetto, G.; Ceretta, C.A.; Melo, G.W.; Girotto, E.; Ferreira, P.A. Application of nitrogen sources on grapevines and effect on yield and must composition. Rev. Bras. Frutic. 2013, 35, 1042–1051. [Google Scholar] [CrossRef]
- Nijjar, G.S. Nutrition of Fruit Trees; Kilyany Publishers: New Delhi, India, 1985; pp. 206–234. [Google Scholar]
- Mohsen, F.S.; Ali, A.A. Foliar spray of Gibberellin (GA3) and Urea to improve growth, yield, bunch and berry quality of Red Globe grapevine. Curr. Sci. Int. 2019, 8, 193–202. [Google Scholar]
- El-Salhy, A.M.; Ahmed, A.K.I.; Masoud, A.A.B.; Abozeed, A.A. Effect of berry thinning, CPPU spraying and pinching on cluster and berry quality of two grapevine cultivars’. Assiut J. Agric. Sci. 2009, 40, 92–107. [Google Scholar] [CrossRef]
- Krook, J.; Van’t Slot, K.A.E.; Vreugdenhil, D.; Dijkema, C.; Van der Plas, L.H.W. The triose-hexose phosphate cycle and the sucrose cycle in carrot (Daucus carota L.) cell suspensions are controlled by respiration and PPi: Fructose-6- phosphate Phosphotransferase. J. Plant Physiol. 2000, 156, 595–604. [Google Scholar] [CrossRef]
- El-Baz, E.; El Eraky, M.A.; Lo’ay, A.A.; El-Deeb, M.R.I. Vitamins application and persimmon (diospyros kaki L. Cv ‘Costata’), fruit quality. J. Plant Prod. 2011, 2, 367–375. [Google Scholar] [CrossRef][Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments—A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Shahbaz, M.; Ashraf, M.; Akram, N.A. Alleviation of drought- induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot. 2009, 41, 621–632. [Google Scholar]
- Ali, Q.; Ashraf, M.; Shahbaz, M.; Humera, H. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 2008, 40, 211–219. [Google Scholar]
- Rajkumar, M. Influence of potassium on growth parameters and yield of grapes cv. Muscat. J. Emerg. Technol. Innov. Res. 2018, 5, 294–302. [Google Scholar]
- Cyr, D.R.; Bewley, D. Seasonal variation in nitrogen storage reserves in the roots of leafy spurge (Euphorbia esula) and responses to decapitation and defoliation. Physiol. Plant. 1990, 78, 361–366. [Google Scholar] [CrossRef]
- Prins, A.H.; Verkaar, H.J. Defoliation: Do physiological and morphological responses lead to (over) compensation? In Pests and Pathoges: Plant Responses to Foliar Attack; Ayres, P.G., Ed.; Environmental Plant Biology Series; BIOS Sci. Pub.: Devon, UK, 1992; Chapter 2; pp. 13–31. [Google Scholar]
- Caspari, H.W.; Lang, A. Carbohydrate supply limits fruit set in commercial Sauvignon blanc grapevines. In Proceedings of the 4th International Symposium on Cool Climate Enology and Viticulture, Rochester, NY, USA, 16–20 July 1996; pp. II-9–II-13. [Google Scholar]
- Caspari, H.W.; Lang, A.; Alspach, P. Effects of girdling and leaf removal on fruit set and vegetative growth in grape. Am. J. Enol. Viticult. 1998, 49, 359–366. [Google Scholar] [CrossRef]
- Yorgos, K.; Georgiadou, A.; Tikos, P.; Kallithraka, S.; Koundouras, S. Effects of Severity of Post-flowering Leaf Removal on Berry Growth and Composition of Three Red Vitis vinifera L. Cultivars Grown under Semiarid Conditions. J. Agric. Food Chem. 2012, 60, 6000–6010. [Google Scholar]
- Sternad, L.M.; Sivilotti, P.; Butinar, L.; Laganis, J.; Vrhovsek, U. Pre-flowering leaf removal alters grape microbial population and offers good potential for a more sustainable and cost-effective management of a Pinot noir vineyard. Aust. J. Grape Wine Res. 2015, 21, 439–450. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees-Struct. Funct. 2006, 20, 348–355. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhong, X.M.; Fan, Y.; Wang, H.C.; Li, J.G.; Huang, X.M. Burst of reactive oxygen species in pedicel-mediated fruit abscission after carbohydrate supply was cut off in longan (Dimocarpus longan). Front. Plant Sci. 2015, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Munemura, I.; Tomita, R.; Kobayashi, K. Reactive oxygen species in leaf abscission signaling. Plant Signal. Behav. 2008, 3, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, B.K. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Hajian, G.; Ghasemnezhad, M.; Ghazvini, R.F.; Khaledian, M.R. Effects of regulated deficit irrigation on vegetative growth, fruit yield and quality of Japanese plum (Prunus salicina Lindell’Methly’). Agric. Conspec. Sci. 2020, 85, 61–70. [Google Scholar]
- Boud, A. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 9, 335. [Google Scholar] [CrossRef]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef]
- Manoj, B.S.; Gupta, M.; Sachin, G. Enhanced lignin and quinone accumulation undervarying degree of drought stress influenced by chitosan. Pharma Innov. J. 2021, 10, 1030–1033. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]



| Month | Year | Temperature (°C) | Humidity (%) | Rainfall (mm·Month−1) | Wind Speed (km·h−1) | Cloud (%) | Sun (h·Month−1) | UV Index |
|---|---|---|---|---|---|---|---|---|
| September | 2021 | 34.2 | 53 | 0.0 | 12.1 | 5 | 379 | 7 |
| 2022 | 31.5 | 51 | 0.0 | 13.2 | 5 | 378 | 7 | |
| October | 2021 | 31.5 | 55 | 0.8 | 11.0 | 10 | 389 | 6 |
| 2022 | 33.0 | 59 | 0.0 | 11.8 | 11 | 389 | 5 | |
| November | 2021 | 27.8 | 62 | 2.6 | 11.1 | 8 | 378 | 6 |
| 2022 | 29.1 | 57 | 1.8 | 10.3 | 27 | 359 | 6 | |
| December | 2021 | 19.7 | 66 | 4.2 | 13.1 | 22 | 380 | 5 |
| 2022 | 21.5 | 65 | 5.3 | 10.2 | 19 | 370 | 4 | |
| January | 2022 | 17.0 | 54 | 4.1 | 13.9 | 31 | 369 | 4 |
| 2023 | 21.5 | 59 | 3.0 | 13.0 | 19 | 385 | 5 | |
| February | 2022 | 18.2 | 57 | 10.0 | 12.2 | 31 | 334 | 4 |
| 2023 | 21.7 | 60 | 4.6 | 12.5 | 27 | 324 | 4 | |
| March | 2022 | 20.5 | 58 | 2.3 | 14.6 | 23 | 371 | 7 |
| 2023 | 23.3 | 62 | 6.7 | 14.2 | 14 | 394 | 5 | |
| April | 2022 | 23.9 | 51 | 0.8 | 13.3 | 15 | 382 | 7 |
| 2023 | 29.2 | 51 | 3.8 | 15.3 | 9 | 386 | 8 | |
| May | 2022 | 29.0 | 38 | 0.0 | 14.2 | 10 | 393 | 7 |
| 2023 | 39.9 | 37 | 0.0 | 13.3 | 3 | 398 | 8 | |
| June | 2022 | 32.6 | 44 | 0.0 | 13.5 | 7 | 386 | 8 |
| 2023 | 39.3 | 41 | 0.0 | 12.9 | 1 | 386 | 9 | |
| July | 2022 | 36.7 | 46 | 0.0 | 12.5 | 8 | 398 | 8 |
| 2023 | 39.7 | 41 | 0.0 | 13.2 | 2 | 398 | 9 | |
| August | 2022 | 36.1 | 47 | 0.0 | 12.8 | 4 | 398 | 8 |
| 2023 | 36.0 | 43 | 0.0 | 11.8 | 2 | 398 | 8 |
| Parameter | Soil Depth (cm) | Water | |||
|---|---|---|---|---|---|
| 0–30 | 30–60 | 60–90 | |||
| Clay (%) | 4.29 | 4.29 | 4.29 | Transparency (cm) | 132.5 |
| Silt (%) | 3.36 | 3.36 | 3.36 | Permeability index (%) | 55.64 |
| Sand (%) | 92.31 | 92.31 | 92.31 | Water quality index | 21.54 |
| Texture | Sandy | Sandy | Sandy | pH | 7.33 |
| Field capacity (%) | 13.77 | 13.71 | 13.71 | Total dissolved salts (mg·L−1) | 204.9 |
| Permanent wilting point (%) | 6.65 | 6.62 | 6.62 | E.C. (μmhos·cm−1) | 558.8 |
| pH (1:2.5 extract) | 8.08 | 8.05 | 8.01 | O2 (%) | 95.80 |
| Organic material (%) | 2.10 | 0.55 | 0.35 | CaCO3 (mg·L−1) | 100.6 |
| E.C. (dS·m−1) [1:5 extract] | 2.03 | 2.01 | 2.01 | HCO3− (mg·L−1) | 159.5 |
| CaCO3 (%) | 1.83 | 1.41 | 1.88 | CO32− (mg·L−1) | 7.00 |
| HCO3− (meq·100 g−1) | 0.30 | 0.37 | 0.40 | SO42− (mg·L−1) | 10.13 |
| CO32− (meq·100 g−1) | 0.00 | 0.00 | 0.00 | SiO2 (mg·L−1) | 1.21 |
| SO42− (meq·100 g−1) | 3.17 | 4.04 | 4.13 | Cl− (mg·L−1) | 0.4.0 |
| Cl− (meq·100 g−1) | 0.96 | 0.98 | 1.08 | Na+ (mg·L−1) | 29.20 |
| Na+ (meq·100 g−1) | 0.48 | 0.66 | 1.42 | Ca2+ (mg·L−1) | 6.00 |
| Ca2+ (meq·100 g−1) | 0.80 | 0.20 | 1.25 | Mg2+ (mg·L−1) | 0.70 |
| Mg2+ (meq·100 g−1) | 0.33 | 0.97 | 1.16 | N (mg·L−1) | 1.56 |
| N (mg·kg−1) | 3.00 | 2.00 | 1.00 | P (mg·L−1) | 0.094 |
| P (mg·kg−1) | 1.00 | 2.00 | 1.00 | K (mg·L−1) | 8.81 |
| K (mg·kg−1) | 27.0 | 24.0 | 23.0 | Fe (mg·L−1) | 0.23 |
| Fe (mg·kg−1) | 1.48 | 1.21 | 111 | Mn (mg·L−1) | 0.005 |
| Mn (mg·kg−1) | 1.10 | 150 | 1.21 | Zn (mg·L−1) | 0.60 |
| Zn (mg·kg−1) | 0.18 | 0.11 | 0.11 | Cu (mg·L−1) | 0.018 |
| Cu (mg·kg−1) | 4.24 | 2.10 | 0.75 | Co (mg·L−1) | 1.56 |
| Pb (mg·L−1) | 0.77 | ||||
| B (mg·L−1) | 0.03 | ||||
| Mo (mg·L−1) | 0.009 | ||||
| Al (mg·L−1) | 0.03 | ||||
| Ni (mg·L−1) | 0.014 | ||||
| Se (mg·L−1) | 0.021 | ||||
| As (mg·L−1) | 0.044 | ||||
| V (mg·L−1) | 0.014 | ||||
| Month | Dripper Discharge Amount (L·h−1) | Number of Drippers per Vine | Irrigation Period (h·Day−1) | Daily Water Quantity (L·Vine−1) | Monthly Water Quantity (L·Vine−1) |
|---|---|---|---|---|---|
| September | 4 | 4 | 1 h, 45 m | 28.00 | 840.00 |
| October | 4 | 4 | 0 h, 45 m | 12.00 | 372.00 |
| November | 4 | 4 | 0 h, 30 m | 7.98 | 239.40 |
| December | 4 | 4 | 0 h, 08 m | 2.13 | 66.03 |
| January | 4 | 4 | 0 h, 09 m | 2.40 | 74.40 |
| February | 4 | 4 | 0 h, 10 m | 2.68 | 75.04 |
| March | 4 | 4 | 1 h, 00 m | 16.00 | 496.00 |
| April | 4 | 4 | 1 h, 29 m | 23.73 | 711.90 |
| May | 4 | 4 | 2 h, 00 m | 32.00 | 992.00 |
| June | 4 | 4 | 2 h, 11 m | 34.93 | 1047.90 |
| July | 4 | 4 | 2 h, 13 m | 35.47 | 1099.57 |
| August | 4 | 4 | 2 h, 15 m | 36.00 | 1116.00 |
| Annual water quantity (m3·vine−1) = 7.13 m3 | |||||
| Annual water quantity (m3·hectare−1) = 11,883.73 m3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Khalek, A.F.; Mazrou, Y.S.A.; Hatterman-Valenti, H.M.; Awadeen, A.A.; El-Mogy, S.M.M.; El-Kenawy, M.A.; Belal, B.E.A.; Mohamed, M.A.; Hassan, I.F.; El-Wakeel, H.F.; et al. Improvement in Physiochemical Characteristics of ‘Prime Seedless’ Grapes by Basal Defoliation with Foliar-Sprayed Low-Biuret Urea and Cyanocobalamin under Mediterranean Climate. Agronomy 2024, 14, 815. https://doi.org/10.3390/agronomy14040815
Abd El-Khalek AF, Mazrou YSA, Hatterman-Valenti HM, Awadeen AA, El-Mogy SMM, El-Kenawy MA, Belal BEA, Mohamed MA, Hassan IF, El-Wakeel HF, et al. Improvement in Physiochemical Characteristics of ‘Prime Seedless’ Grapes by Basal Defoliation with Foliar-Sprayed Low-Biuret Urea and Cyanocobalamin under Mediterranean Climate. Agronomy. 2024; 14(4):815. https://doi.org/10.3390/agronomy14040815
Chicago/Turabian StyleAbd El-Khalek, Ahmed F., Yasser S. A. Mazrou, Harlene M. Hatterman-Valenti, Ashraf A. Awadeen, Shimaa M. M. El-Mogy, Mosaad A. El-Kenawy, Bassam E. A. Belal, Mahmoud A. Mohamed, Islam F. Hassan, Hassan F. El-Wakeel, and et al. 2024. "Improvement in Physiochemical Characteristics of ‘Prime Seedless’ Grapes by Basal Defoliation with Foliar-Sprayed Low-Biuret Urea and Cyanocobalamin under Mediterranean Climate" Agronomy 14, no. 4: 815. https://doi.org/10.3390/agronomy14040815
APA StyleAbd El-Khalek, A. F., Mazrou, Y. S. A., Hatterman-Valenti, H. M., Awadeen, A. A., El-Mogy, S. M. M., El-Kenawy, M. A., Belal, B. E. A., Mohamed, M. A., Hassan, I. F., El-Wakeel, H. F., Makhlouf, A. H., Omar, A. E.-D. K., & Alam-Eldein, S. M. (2024). Improvement in Physiochemical Characteristics of ‘Prime Seedless’ Grapes by Basal Defoliation with Foliar-Sprayed Low-Biuret Urea and Cyanocobalamin under Mediterranean Climate. Agronomy, 14(4), 815. https://doi.org/10.3390/agronomy14040815









