Exogenous Gibberellic Acid Ameliorates Chilling Injury in Peach (Prunus persica L.) by Improving the Antioxidant System
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material and Post-Harvest Treatments
- (1)
- Control group (CK): These peaches were immersed in sterile, deionized water.
- (2)
- GA3 group: These peaches were immersed in 0.1 mmol/L of GA3.
2.2. Measurement of IB Index
2.3. Measurement of Firmness
2.4. Measurements of Ethylene Production and Respiration Rate
2.5. Measurements of H2O2 and MDA Content
2.6. Measurements of SOD and CAT Activity
2.7. Extraction of Total RNA and RNA-Seq
2.8. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.9. Statistical Analysis
3. Results
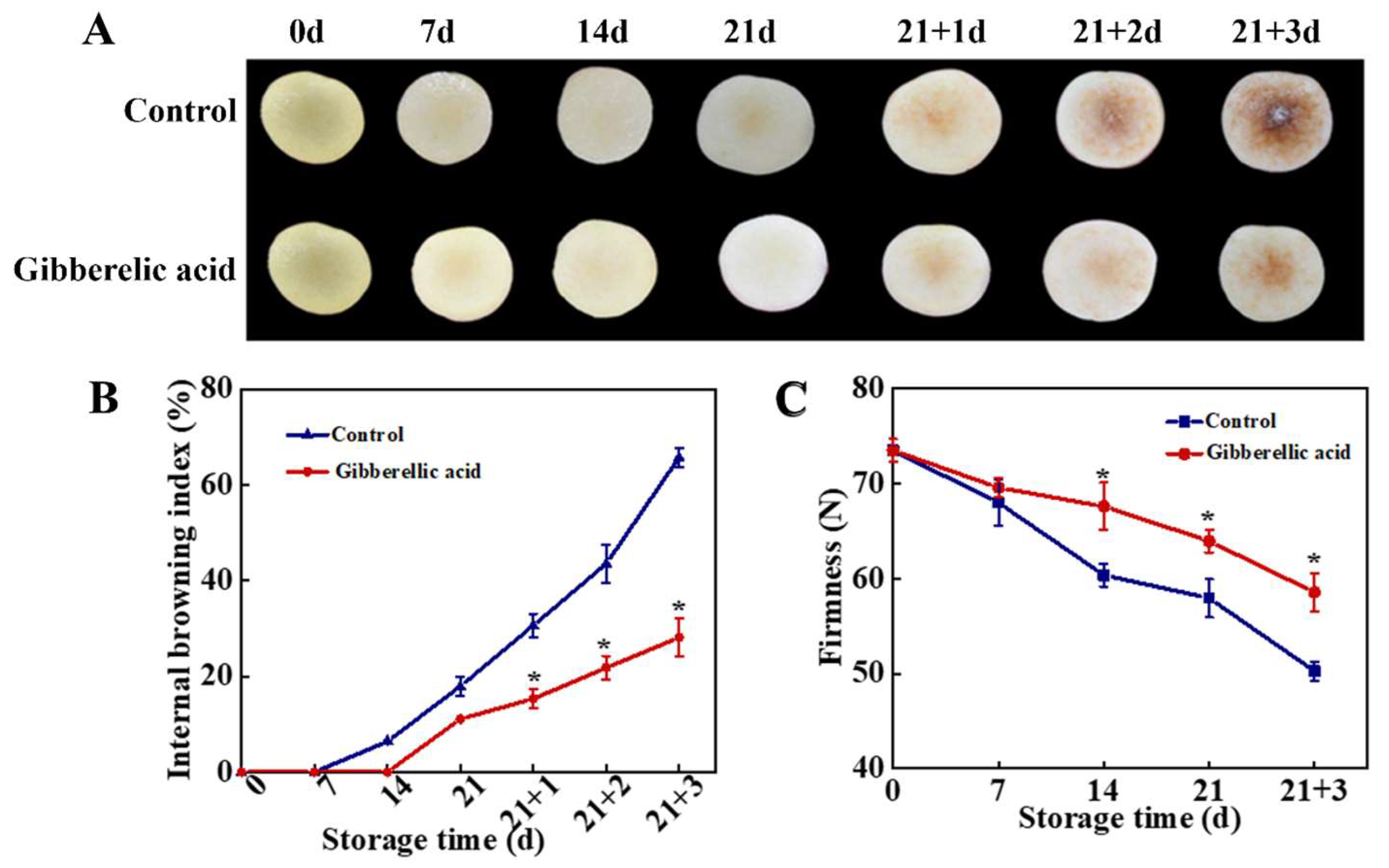
3.1. Effect of GA3 Treatment on IB Index and Fruit Firmness
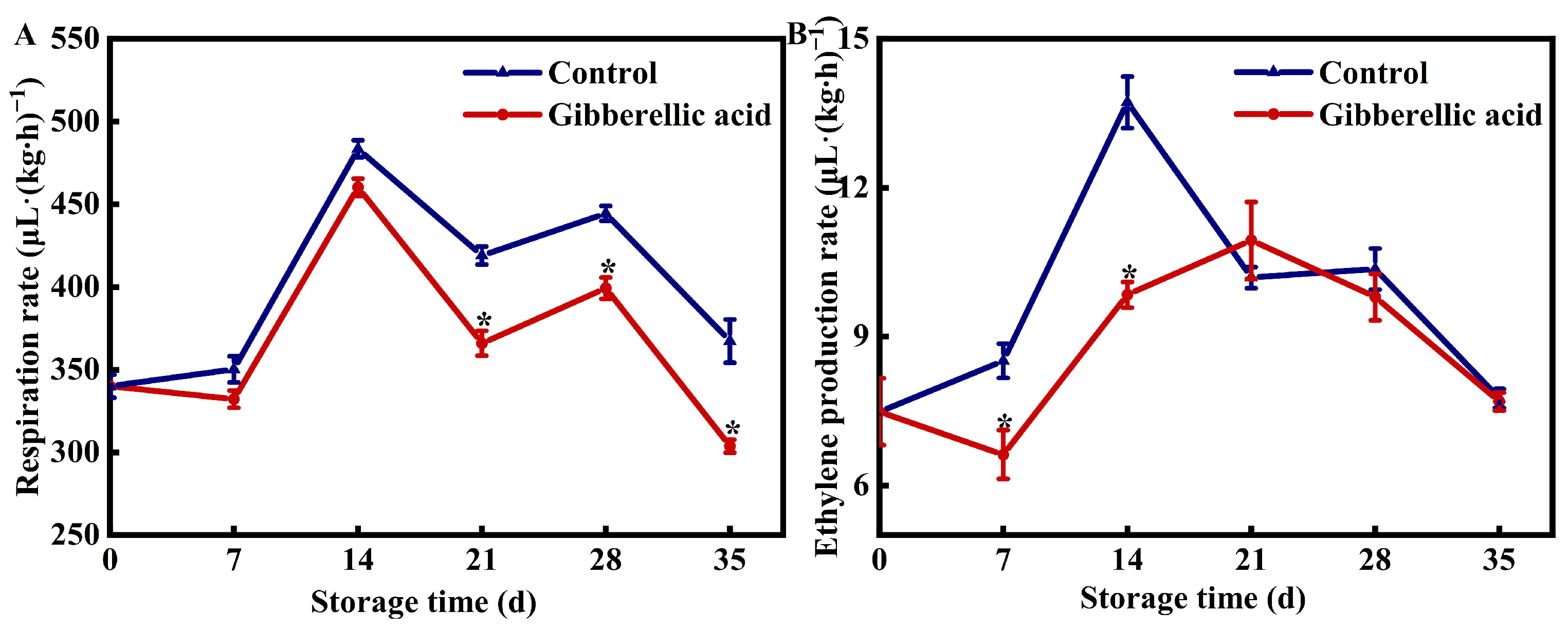
3.2. Effect of GA3 on the Respiration and Ethylene Production Rate of Peach Fruit
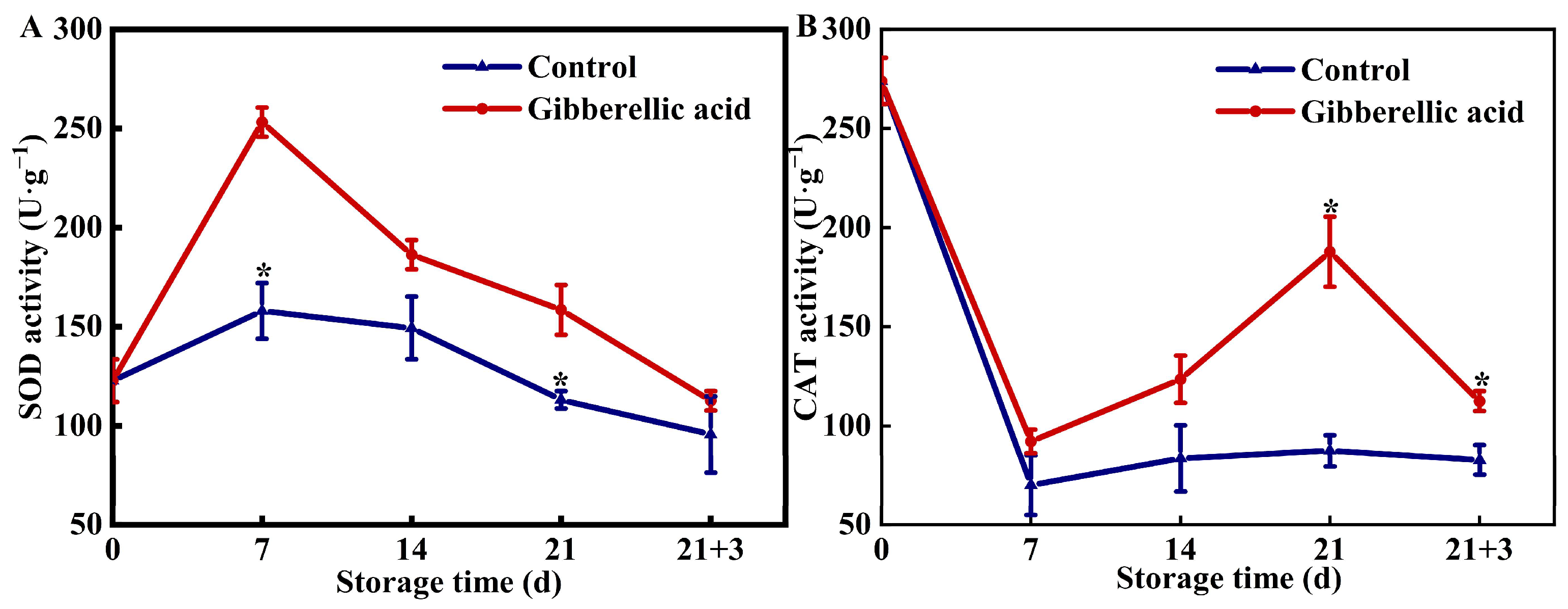
3.3. Effects of Exogenous GA3 on Enzymes Related to Antioxidant Activity
3.4. Effects of Exogenous GA3 on MDA and H2O2 Contents
3.5. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Bal, E. Effect of melatonin treatments on biochemical quality and postharvest life of nectarines. J. Food Meas. Charact. 2020, 15, 288–295. [Google Scholar] [CrossRef]
- Jing, G.; Zhou, J.; Zhu, S. Effects of nitric oxide on mitochondrial oxidative defence in postharvest peach fruits. J. Sci. Food Agric. 2016, 96, 1997–2003. [Google Scholar] [CrossRef]
- Nian, Y.; Wang, N.; Li, R.; Shao, Y.; Li, W. Cold shock treatment alleviates chilling injury in papaya fruit during storage by improving antioxidant capacity and related gene expression. Sci. Hortic. 2022, 294, 110784. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and drought priming induce tolerance to subsequent heat and drought stress by regulating leaf photosynthesis, root morphology, and antioxidant defense in maize seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Che, Y.; Yao, T.; Wang, H.; Wang, Z.; Zhang, H.; Sun, G.; Zhang, H. Potassium ion regulates hormone, Ca(2+) and H(2)O(2) signal transduction and antioxidant activities to improve salt stress resistance in tobacco. Plant Physiol. Biochem. 2022, 186, 40–51. [Google Scholar] [CrossRef]
- Molla, S.M.H.; Rastegar, S.; Omran, V.G.; Khademi, O. Ameliorative effect of melatonin against storage chilling injury in pomegranate husk and arils through promoting the antioxidant system. Sci. Hortic. 2022, 295, 110889. [Google Scholar] [CrossRef]
- Xiong, S.; Zhou, F.; Jiang, A.; Yang, L.; Hu, W. Ethanol vapor ameliorates chilling injury and maintains postharvest quality by increasing antioxidant capacity of hardy kiwifruit (Actinidia arguta). Sci. Hortic. 2024, 327, 112796. [Google Scholar] [CrossRef]
- Kantakhoo, J.; Ose, K.; Imahori, Y. Effects of hot water treatment to alleviate chilling injury and enhance phenolic metabolism in eggplant fruit during low temperature storage. Sci. Hortic. 2022, 304, 111325. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; López-Climent, M.; Gómez-Cadenas, A.; Zacarías, L. Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biol. Technol. 2016, 111, 214–223. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Dai, X.; Yu, M.; Yu, Z. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Fang, Y.; Yan, Z.; Shi, J.; Gao, L.; Wang, Q.; Jiang, A. Effect of folic acid on the postharvest physiology of broccoli during storage. Food Chem. 2021, 339, 127981. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.N.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Erogul, D.; Sen, F. The effect of preharvest gibberellic acid applications on fruit quality of ‘Angelino’ plums during storage. Sci. Hortic. 2016, 202, 111–116. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, Y.; Zhao, J.; Nie, Y.; Zhang, Y.; Sheng, J.; Tang, X. Effects of Postharvest Gibberellic Acid Treatment on Chilling Tolerance in Cold-Stored Tomato (Solanum lycopersicum L.) Fruit. Food Bioprocess Technol. 2016, 9, 1202–1209. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, Y. Guanosine 3′,5′-cyclic monophosphate mediates gibberellic acid-induced chilling tolerance and defense response in postharvest peach fruit. Postharvest Biol. Technol. 2019, 155, 80–85. [Google Scholar] [CrossRef]
- Franzoni, G.; Spadafora, N.D.; Sirangelo, T.M.; Ferrante, A.; Rogers, H.J. Biochemical and molecular changes in peach fruit exposed to cold stress conditions. Mol. Hortic. 2023, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Maurya, V.K.; Ranjan, V.; Gothandam, K.M.; Pareek, S. Exogenous gibberellic acid treatment extends green chili shelf life and maintain quality under modified atmosphere packaging. Sci. Hortic. 2020, 269, 108934. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Shao, J.; Zhang, C.; Mei, L.; Wang, K.; Jin, P.; Zheng, Y. Hydrogen sulfide alleviates chilling injury in peach fruit by maintaining cell structure integrity via regulating endogenous H2S, antioxidant and cell wall metabolisms. Food Chem. 2022, 391, 133283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, C.; Brummell, D.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2021, 338, 128005. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Mao, L.; Min, T.; Zhao, Y.; Duan, Y.; Wang, H.; Lin, Q. Salicylic acid treatment inhibits ethylene synthesis and starch-sugar conversion to maintain apple fruit quality during shelf life. Sci. Hortic. 2023, 308, 111586. [Google Scholar] [CrossRef]
- Song, C.; Zhao, Y.; Li, A.; Qi, S.; Lin, Q.; Duan, Y. Postharvest nitric oxide treatment induced the alternative oxidase pathway to enhance antioxidant capacity and chilling tolerance in peach fruit. Plant Physiol. Biochem. 2021, 167, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 338, 12800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, J.; Brummell, D.A.; Song, C.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Abscisic acid alleviates chilling injury in cold-stored peach fruit by regulating the metabolism of sucrose. Sci. Hortic. 2022, 298, 111000. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, K.; Wu, C.; Zhao, Y.; Yin, X.; Zhang, B.; Grierson, D.; Chen, K.; Xu, C. Effect of Ethylene on Cell Wall and Lipid Metabolism during Alleviation of Postharvest Chilling Injury in Peach. Cells 2019, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Li, F.; Ali, M.; Zhang, X.; Liu, Y. Application of methyl jasmonate to control chilling tolerance of postharvest fruit and vegetables: A meta-analysis and eliciting metabolism review. Crit. Rev. Food Sci. Nutr. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Huang, T.; Liu, G.; Zhu, L.; Liu, J.; Xiang, Y.; Xu, X.; Zhang, Z. Mitigation of chilling injury in mango fruit by methyl jasmonate is associated with regulation of antioxidant capacity and energy homeostasis. Postharvest Biol. Technol. 2024, 211, 112801. [Google Scholar] [CrossRef]
- Yi, B.; Liu, Y.; Wu, Z.; Zheng, Y.; Chen, H.; Jin, P. Hydrogen sulfide alleviates chilling injury of zucchini fruit by regulating antioxidant capacity, endogenous hydrogen sulfide, proline, and polyamine metabolism. Postharvest Biol. Technol. 2024, 208, 112638. [Google Scholar] [CrossRef]
- Rastegar, S.; Hassanzadeh Khankahdani, H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Li, Q.Z.; Li, C.H.; Yu, X.C.; Shi, Q.H. Gibberellin A3 pretreatment increased antioxidative capacity of cucumber radicles and hypocotyls under suboptimal temperature. Afr. J. Agric. Res. 2011, 6, 4091–4098. [Google Scholar]
- Hu, S.; Ma, Y.; Xie, B.; Hou, Y.; Jia, Z.; Zhao, L.; Zheng, Y.; Jin, P. 24-Epibrassinolide improves chilling tolerance by regulating PpCBF5-mediated membrane lipid metabolism in peach fruit. Postharvest Biol. Technol. 2022, 186, 111844. [Google Scholar] [CrossRef]
- Huang, D.; Tian, W.; Feng, J.; Zhu, S. Interaction between nitric oxide and storage temperature on sphingolipid metabolism of postharvest peach fruit. Plant Physiol. Biochem. 2020, 151, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Dagar, A.; Weksler, A.; Friedman, H.; Lurie, S. Gibberellic acid (GA3) application at the end of pit ripening: Effect on ripening and storage of two harvests of ‘September Snow’ peach. Sci. Hortic. 2012, 140, 125–130. [Google Scholar] [CrossRef]
- Tian, S.-f.; Wang, Y.; Du, G.; Li, Y.-x. Changes in contents and antioxidant activity of phenolic compounds during gibberellin-induced development in Vitis vinifera L. ‘Muscat’. Acta Physiol. Plant. 2011, 33, 2467–2475. [Google Scholar] [CrossRef]
- Ahammed, G.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Liu, A.; Chen, S.; et al. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.A.; Chaves, A.R.; Añón, M.C. Effect of Gibberellic Acid on Ripening of Strawberry Fruits (Fragaria annanassa Duch.). J. Plant Growth Regul. 1994, 13, 87–91. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Moldovan, L.; Moldovan, N.I. Oxygen free radicals and redox biology of organelles. Histochem. Cell Biol. 2004, 122, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Geng, B.; Feng, J.; Zhu, S. Interactive effects of abscisic acid and nitric oxide on chilling resistance and active oxygen metabolism in peach fruit during cold storage. J. Sci. Food Agric. 2019, 99, 3367–3380. [Google Scholar] [CrossRef] [PubMed]
- Al-Taey, D.K. The role of GA and organic matter to reduce the salinity effect on growth and leaves contents of elements and antioxidant in pepper. Plant Archives. 2018, 18, 479–488. [Google Scholar]
- Ma, Y.; Hu, S.; Chen, G.; Zheng, Y.; Jin, P. Cold shock treatment alleviates chilling injury in peach fruit by regulating antioxidant capacity and membrane lipid metabolism. Food Qual. Saf. 2022, 6, fyab026. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hou, Y.; Zhu, X.; Zheng, Y.; Jin, P. Physiological and metabolomic analyses of hot water treatment on amino acids and phenolic metabolisms in peach cold tolerance. Postharvest Biol. Technol. 2021, 179, 111593. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Rang, X.; Han, H.; Pei, Z.; Zhao, J.; Zhu, Z.; Li, J.; Zhang, P.; Zhao, Y.; Duan, Y. Exogenous Gibberellic Acid Ameliorates Chilling Injury in Peach (Prunus persica L.) by Improving the Antioxidant System. Agronomy 2024, 14, 816. https://doi.org/10.3390/agronomy14040816
Sun H, Rang X, Han H, Pei Z, Zhao J, Zhu Z, Li J, Zhang P, Zhao Y, Duan Y. Exogenous Gibberellic Acid Ameliorates Chilling Injury in Peach (Prunus persica L.) by Improving the Antioxidant System. Agronomy. 2024; 14(4):816. https://doi.org/10.3390/agronomy14040816
Chicago/Turabian StyleSun, Haixin, Xuena Rang, Haonan Han, Zhenhao Pei, Jingyi Zhao, Zhifeng Zhu, Jiangkuo Li, Peng Zhang, Yaoyao Zhao, and Yuquan Duan. 2024. "Exogenous Gibberellic Acid Ameliorates Chilling Injury in Peach (Prunus persica L.) by Improving the Antioxidant System" Agronomy 14, no. 4: 816. https://doi.org/10.3390/agronomy14040816
APA StyleSun, H., Rang, X., Han, H., Pei, Z., Zhao, J., Zhu, Z., Li, J., Zhang, P., Zhao, Y., & Duan, Y. (2024). Exogenous Gibberellic Acid Ameliorates Chilling Injury in Peach (Prunus persica L.) by Improving the Antioxidant System. Agronomy, 14(4), 816. https://doi.org/10.3390/agronomy14040816





