The Influence of Different, Long-Term Fertilizations on the Chemical and Spectroscopic Properties of Soil Organic Matter
Abstract
:1. Introduction
2. Material and Methods
2.1. Long-Term Field Experiment
2.2. Soil Characteristics
2.3. The Content of C and N
2.4. Fractional Analysis of SOM
2.5. Isolation of HUM
2.6. TC-GC/MS Analysis of Bulk Soil and HUM
2.7. UV-Vis Analysis of HUM
2.8. Fluorescence Spectroscopy of HUM
2.9. Graphical and Statistical Methods
3. Results and Discussion
3.1. The Content of Carbon and Nitrogen and Sorption Properties
3.2. Fractional Composition of SOM
3.3. TC-GC/MS Analysis of Bulk Soil and HUM
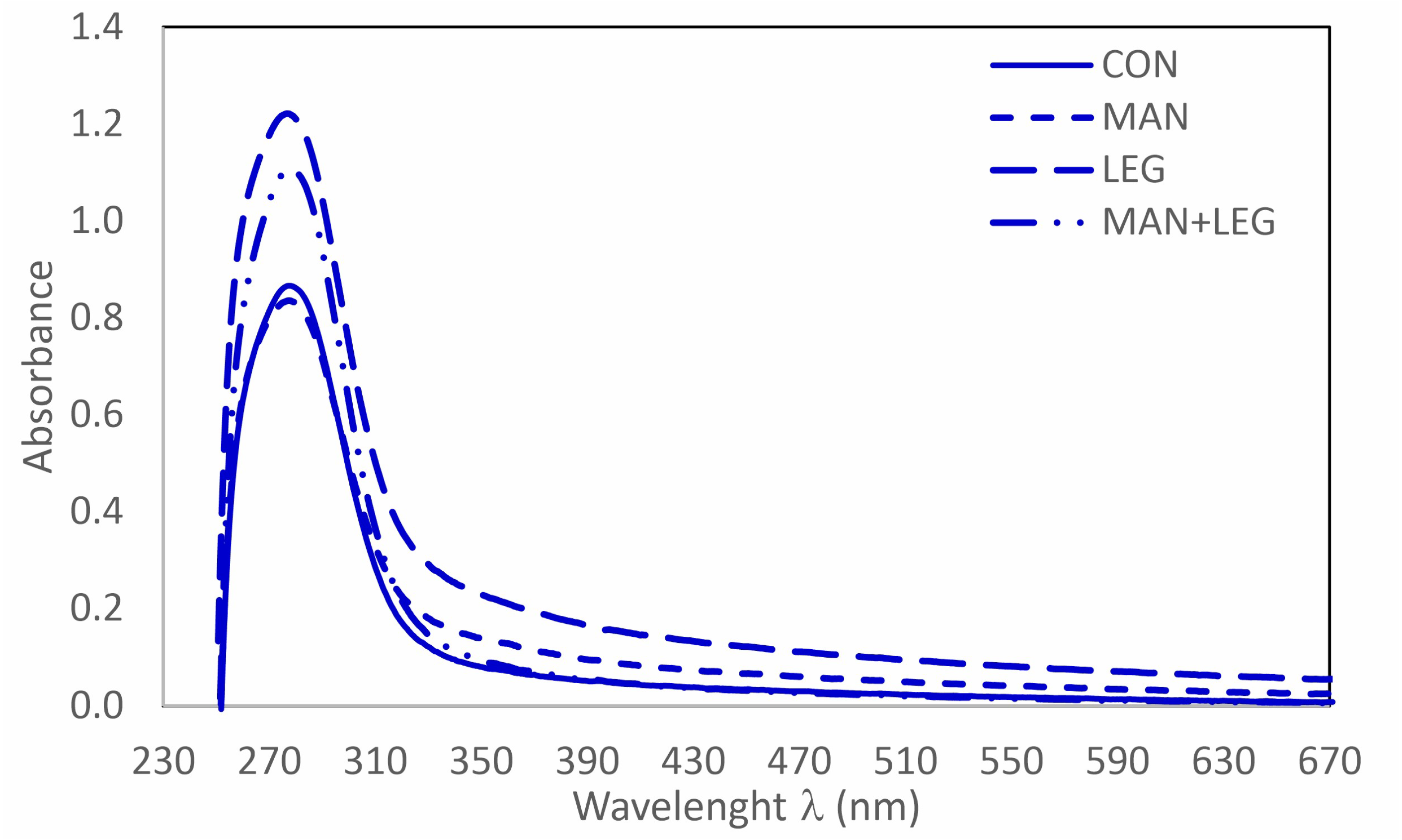
3.4. UV-Vis Analysis of HUM
3.5. Fluorescence Analysis of HUM
3.5.1. Synchronous Scan Fluorescence Spectra (SSF)
3.5.2. EEM Spectra
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papadis, E.; Tsatsaronis, G. Challenges in the decarbonization of the energy sector. Energy 2020, 205, 118025. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, D.S.; Bradbury, N.J.; Coleman, K. How the Rothamsted Classical Experiments have been used to develop and test models for the turnover of carbon and nitrogen in soil. In Long-Term Experiments in Agricultural and Ecological Sciences; Johnston, A.E., Leigh, R.A., Eds.; CABI International: Wallingford, UK, 1994; pp. 117–138. [Google Scholar]
- Johnston, A.E.; Poultion, P.R. The importance of long-term experiments in agriculture: Their management to ensure continued crop production and soil fertility; the Rothamsted experience. Eur. J. Soil Sci. 2018, 69, 113–125. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.J. (Ed.) Rothamsted Long-Term Experiments: Guide to the Classical and Other Long-Term Experiments, Datasets and Sample Archive. Rothamsted Research. 2018. Available online: http://www.era.rothamsted.ac.uk/eradoc/book/248 (accessed on 20 December 2023).
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-X.; Wei, Y.-X.; Li, R.-C.; Chen, Z.; Wang, H.-D.; Virk, A.L.; Lal, R.; Zhao, X.; Zhang, H.-L. Improving soil aggregates stability and soil organic carbon sequestration by no-till and legume-based crop rotations in the North China Plain. Sci. Total Environ. 2022, 847, 157518. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.M.; Katayama, A. Humin as an External Electron Mediator for Microbial Pentachlorophenol Dechlorination: Exploration of Redox Active Structures Influenced by Isolation Methods. Int. J. Environ. Res. Public Health 2018, 15, 2753. [Google Scholar] [CrossRef] [PubMed]
- Spaccini, R.; Piccolo, A.; Conte, P.; Haberhauer, G.; Gerzabek, M.H. Increased soil organic carbon sequestration through hydrophobic protection by humic substances. Soil Biol. Biochem. 2002, 34, 1839–1851. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 18, 176. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Chapter One—Vindication of humic substances as a key component of organic matter in soil and water. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–37. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- IHSS. 2010. Available online: https://humic-substances.org/what-are-humic-substances-2/ (accessed on 10 December 2023).
- Gerke, J. Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef]
- Janzen, H. The Future of Humic Substances Research: Preface to a Debate. J. Environ. Qual. 2019, 48, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; De Nobili, M.; Chen, Y.; McKnight, D.M.; Wells, M.J.M.; Weber, J. Using Humic Fractions to Understand Natural Organic Matter Processes in Soil and Water: Selected Studies and Applications. J. Environ. Qual. 2019, 48, 1633–1643. [Google Scholar] [CrossRef]
- Schnitzer, M.; Monreal, C.M. Chapter Three—Quo Vadis Soil Organic Matter Research? A Biological Link to the Chemistry of Humification. Adv. Agron. 2011, 113, 143–217. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.; Paustin, K. Stabilization mechanisms of soil organic matter: Implications for C saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Zaccone, C.; Plaza, C.; Ciavatta, C.; Miano, T.M.; Shotyk, W. Advances in the determination of humification degree in peat since Achard (1786): Applications in geochemical and paleoenvironmental studies. Earth-Sci. Rev. 2018, 185, 163–178. [Google Scholar] [CrossRef]
- Cong, R.H.; Xu, M.G.; Wang, X.B.; Zhang, W.J.; Yang, X.Y.; Huang, S.M.; Wang, B.R. An analysis of soil carbon dynamics in long-term soil fertility trials in China. Nutr. Cycl. Agroecosyst. 2012, 93, 201–213. [Google Scholar] [CrossRef]
- Goyal, S.; Mishra, M.M.; Hooda, I.S.; Singh, R. Organic matter-microbial biomass relationships in field experiments under tropical conditions: Effects of inorganic fertilization and organic amendments. Soil Biol. Biochem. 1992, 24, 1081–1084. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Wu, X.; Han, F.; Zhang, T. Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil Tillage Res. 2010, 106, 268–274. [Google Scholar] [CrossRef]
- Huang, Q.-H.; Li, D.-M.; Liu, K.-L.; Yu, X.-C.; Ye, H.-C.; Hu, H.-W.; Xu, X.-L.; Wang, S.-L.; Zhou, L.-L.; Duan, Y.-L.; et al. Effects of Long-Term Organic Amendments on Soil Organic Carbon in a Paddy Field: A Case Study on Red Soil. J. Integr. Agric. 2014, 13, 570–576. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.-P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, M.; Wang, B.; Wang, X. Soil organic carbon, total nitrogen and grain yields under long-term fertilizations in the upland red soil of southern China. Nutr. Cycl. Agroecosyst. 2009, 84, 59–69. [Google Scholar] [CrossRef]
- Huang, Q.R.; Hu, F.; Huang, S.; Li, H.X.; Yuan, Y.H.; Pan, G.X.; Zhang, W.J. Effect of long-term fertilization on organic carbon and nitrogen in a subtropical paddy soil. Pedosphere 2009, 19, 727–734. [Google Scholar] [CrossRef]
- Huang, S.; Peng, X.; Huang, Q.; Zhang, W. Soil aggregation and organic carbon fractions affected by long-term fertilization in a red soil of subtropical China. Geoderma 2010, 154, 364–369. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Changes in soil biological and biochemical characteristics in a long-term field trial on a sub-tropical inceptisol. Soil Biol. Biochem. 2006, 38, 1577–1582. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat-maize cropping system in northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Mi, W.; Sun, Y.; Gao, Q.; Liu, M.; Wu, L. Changes in humus carbon fractions in paddy soil given different organic amendments and mineral fertilizers. Soil Tillage Res. 2019, 195, 104421. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; An, T.; Wei, D.; Chi, F.; Zhou, B. Effects of long-term fertilization on soil humic acid composition and structure in Black Soil. PLoS ONE 2017, 12, e0186918. [Google Scholar] [CrossRef] [PubMed]
- Kumada, K. Chemistry of Soil Organic Matter; Elsevier: Amsterdam, The Netherlands, 1987; p. 241. [Google Scholar]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, V.; Miano, T. Fluorescence properties of humic acid interaction products with s-triazine and bipyridilium herbicides and their Cu complexes: A multivariate approach. J. Soils Sediments 2018, 18, 1347–1354. [Google Scholar] [CrossRef]
- Řezáčová, V.; Gryndler, M. Fluorescence Spectroscopy: A tool to characterize humic substances in soil colonized by microorganisms? Folia Microbiol. 2006, 51, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Senesi, N.; Loffredo, E.; D’Orazio, V.; Brunetti, G.; Miano, T.M.; La Cava, P. Adsorption of pesticides by humic acids from organic amendments and soils. In Humic Substances and Chemical Contaminants; Clapp, C.E., Hayes, M.H.B., Senesi, N., Bloom, P.R., Jardine, P.M., Eds.; SSSA: Madison, WI, USA, 2001; pp. 129–154. [Google Scholar] [CrossRef]
- Ferreira, F.P.; Buurman, P.; Macias-Vazquez, I.F.; Otero, X.L.; Boluda, R. Pyrolysis-Gas Chromatography/Mass Spectrometry of Soil Organic Matter Extracted from a Brazilian Mangrove and Spanish Salt Marshes. Soil Sci. Soc. Am. J. 2013, 73, 841–851. [Google Scholar] [CrossRef]
- Zhou, P.; Pana, G.X.; Spaccini, R.; Picco, A. Molecular changes in particulate organic matter (POM) in a typical Chinese paddy soil under different long-term fertilizer treatments. Eur. J. Soil Sci. 2010, 61, 231–242. [Google Scholar] [CrossRef]
- Mercik, S. Long-term agricultural experiments in Eastern Europe! Long-term continuous experiments in Poland, Bulgaria, Czech Republic and Slovakia. In Long-Term Experiments in Agricultural and Ecological Sciences; Johnston, A.E., Leigh, R.A., Eds.; CABI International: Wallingford, UK, 1994; pp. 211–219. [Google Scholar]
- Mercik, S.; Stępien, W. The most important soil properties and yields of plants in 80 years of static fertilizing experiments in Skierniewice. Fragm. Agron. 2005, 22, 189–201. [Google Scholar]
- WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; IUSS Working Group, International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Volume 4, ISBN 9798986245119. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Mehlich, A. Mehlich 3 soil test extractant. A modification o the Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006; p. 995. [Google Scholar]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis: Part 3; Sparks, E., Ed.; SSSA Book Series; SSSA: Madison, WI, USA, 1996; Volume 5, pp. 1011–1069. [Google Scholar]
- Weber, J.; Jamroz, E.; Kocowicz, A.; Dębicka, M.; Bekier, J.; Ćwieląg-Piasecka, I.; Ukalska-Jaruga, A.; Mielnik, L.; Bejger, R.; Jerzykiewicz, M. Optimized isolation method of humin fraction from mineral soil material. Environ. Geochem. Health 2022, 44, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- van Eekeren, N.; de Boer, H.; Bloem, J.; Schouten, T.; Rutgers, M.; de Goede, R.; Brussaard, L. Soil biological quality of grassland fertilized with adjusted cattle manure slurries in comparison with organic and inorganic fertilizers. Biol. Fertil. Soils 2009, 45, 595–608. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Brummelhol, A.; Kuka, K. The Effects of Manure Application and Herbivore Excreta on Plant and Soil Properties of Temperate Grasslands—A Review. Agronomy 2023, 13, 3010. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.-F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Veloso, M.G.; Cecagno, D.; Bayer, C. Legume cover crops under no-tillage favor organomineral association in microaggregates and soil C accumulation. Soil Tillage Res. 2019, 190, 139–146. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Zhang, D.; Li, L.; Sheng, L. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
- Mercik, S.; Stepien, W.; Figat, E. Dynamika zmian zawartości węgla i azotu w glebie oraz losy N z nawozów mineralnych i organicznych w statycznych doświadczeniach nawozowych. Zesz. Probl. Postępów Nauk. Rol. 1995, 421, 277–283. [Google Scholar]
- Mercik, S.; Rumpel, J.; Stepień, W. Zawartość oraz dynamika rozkładu organicznych związków węgla i azotu w zależności od wieloletniego nawożenia mineralnego i organicznego. Zesz. Probl. Postępów Nauk. Rol. 1999, 467, 9–167. [Google Scholar]
- Sharaf, A.; Wu, J.; Fan, W.; Hu, J.; Oppoku-Kwanowaa, Y.; El-Rahim, M.A.; Moussa, A.A. Changes in Soil Humic Acid Composition after Nine Years of Repeated Application of Organic Wastes in Black Soil: A Study Using Solid-State FT-IR and (13C-NMR). Anal. Pol. J. Environ. Stud. 2021, 30, 5211–5223. [Google Scholar] [CrossRef]
- Schmidt, L.; Warnstorff, K.; Dörfel, H.; Leinweber, P.; Lange, H.; Merbach, W. The influence of fertilization and rotation on soil organic matter and plant yields in the long-term Eternal Rye trial in Halle (Saale), Germany. J. Plant Nutr. Soil Sci. 2000, 163, 639–648. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Eckhardt, K.-U.; Schlichting, A.; Hofmann, D.; Schulten, H.-R. Analytical pyrolysis and soft-ionization mass spectrometry. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2009; pp. 539–588. [Google Scholar]
- Jandl, G.; Leinweber, P.; Schulten, H.-R.; Eusterhues, K. The concentrations of fatty acids in organo-mineral particle-size fractions of a Chernozem. Eur. J. Soil Sci. 2004, 55, 459–469. [Google Scholar] [CrossRef]
- Schulten, H.R.; Leinweber, P. New insights in organic mineral particles: Composition, properties and models of molecular structure. Biol. Fertil. Soils 2000, 30, 399–432. [Google Scholar] [CrossRef]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- Enev, V.; Doskočil, L.; Kubíková, L.; Klučáková, M. The medium-term effect of natural compost on the spectroscopic properties of humic acids of Czech soils. J. Agric. Sci. 2018, 156, 877–887. [Google Scholar] [CrossRef]
- Fuentes, M.; Gonzalez-Gaitano, G.; Garcia-Mina, J.M. The usefulness of UV-visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org. Geochem. 2006, 37, 1949–1959. [Google Scholar] [CrossRef]
- Senesi, N.; d’Orazio, V. Fluorescence spectroscopy. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Academic Press: Amsterdam, The Netherlands, 2005; pp. 35–52. [Google Scholar]
- Hur, J.; Lee, D.-H.; Shin, H.-S. Comparison of the structural, spectroscopic, and phenanthrene binding characteristics of humic acids from soils and lake sediments. Org. Geochem. 2009, 40, 1091–1099. [Google Scholar] [CrossRef]
- Miano, T.M.; Senesi, N. Synchronous excitation fluorescence spectroscopy applied to soil humic substances chemistry. Sci. Total Environ. 1992, 117–118, 41–51. [Google Scholar] [CrossRef]
- Mobed, J.J.; Hemmingsen, S.L.; Autry, J.L.; McGown, L.B. Fluorescence characterization of IHSS humic substances: Total luminescence spectra with absorbance correction. Environ. Sci. Technol. 1996, 30, 3061–3065. [Google Scholar] [CrossRef]
- Kwiatkowska-Malina, J. Analysis of humic substances structure in soils after brown coal application with use of 3-D fluorescence spectroscopy. Inż. I Ochr. Sr. 2011, 14, 197–208, (In Polish with English Summary). [Google Scholar]
- Coble, P.G.; Del Castillo, C.E.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep-Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Baker, A.; Genty, D. Fluorescence wavelength and intensity variations of cave waters. J. Hydrol. 1999, 217, 19–34. [Google Scholar] [CrossRef]
- Reynolds, D.M. Rapid and direct determination of tryptophan in water using synchronous fluorescence, spectroscopy. Water Res. 2003, 37, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.M.; Schick, L.L.; Skorko, K.; Boss, E. Photodissolution of particulate organic matter from sediment. Limnol. Oceanogr. 2006, 51, 1064–1071. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinveg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the source of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Plaza, C.; Brunetti, G.; Senesi, N.; Polo, A. Fluorescence characterization of metal ion–humic acid interactions in soils amended with composted municipal solid wastes. Anal. Bioanal. Chem. 2006, 386, 2133–2140. [Google Scholar] [CrossRef]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Characterization, differentiation and classification of humic substances by fluorescence spectroscopy. Soil Sci. 1991, 152, 259–271. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, B.; Song, Y.; Qin, Y. Correlation between molecular absorption spectral slope ratios and fluorescence humification indices in characterizing CDOM. Aquat. Sci. 2011, 73, 103–112. [Google Scholar] [CrossRef]


| Treatment | pH (KCl) | CaCO3 g kg−1 | Mehlich 3 | Sand | Silt | Clay | ||
|---|---|---|---|---|---|---|---|---|
| P | K | Mg | ||||||
| (mg kg−1) | % | |||||||
| CON | 7.07 ± 0.01 a | 9.90 ± 2.4 a | 149 ± 4.2 a | 116 ± 3.9 a | 45 ± 9.6 a | 76.3 ± 1.2 a | 18.0 ± 1.0 a | 5.7 ± 0.6 a |
| MAN | 6.79 ± 0.43 a | 9.20 ± 1.2 a | 175 ± 6.8 b | 153 ± 9.3 b | 67 ± 2.6 b | 76.7 ± 0.6 a | 17.0 ± 0.0 a | 6.3 ± 0.6 ab |
| LEG | 6.95 ± 0.03 a | 8.47 ± 3.7 a | 157 ± 19.4 ab | 130 ± 4.5 a | 67 ± 7.4 b | 75.3 ± 2.1 a | 17.0 ± 1.0 a | 7.7 ± 1.2 b |
| MAN + LEG | 6.79 ± 0.03 a | 7.83 ± 1.2 a | 149 ± 8.6 a | 198 13.5 c | 35 ± 3.4 a | 75.7 ± 0.6 a | 18.0 ± 0.0 a | 6.3 ± 0.6 ab |
| Treatment | TOC | TN | TOC/TN | Ca++ | Mg++ | K+ | Na+ | H+ | CEC |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | cmol(+) kg−1 | ||||||||
| CON | 5.48 ± 1.0 a | 0.46 ± 0.1 a | 12.1 ± 1.0 a | 3.38 ± 0.09 a | 0.25 ± 0.05 a | 0.20 ± 0.01 a | 0.02 ± 0.00 a | 0.08 ± 0.0 ab | 3.92 ± 0.06 a |
| MAN | 8.12 ± 0.4 bc | 0.78 ± 0.1 b | 10.5 ± 1.5 a | 5.40 ± 0.77 b | 0.38 ± 0.01 b | 0.33 ± 0.03 c | 0.02 ± 0.01 a | 0.11 ± 0.01 c | 6.23 ± 0.79 c |
| LEG | 7.24 ± 0.5 b | 0.62 ± 0.2 ab | 12.5 ± 3.8 a | 3.60 ± 0.17 a | 0.42 ± 0.03 b | 0.24 ± 0.01 b | 0.01 ± 0.01 a | 0.07 ± 0.00 a | 4.35 ± 0.16 ab |
| MAN + LEG | 9.21 ± 1.2 c | 0.81 ± 0.1 b | 11.4 ± 0.8 a | 4.08 ± 0.45 a | 0.23 ± 0.03 a | 0.41 ± 0.03 d | 0.02 ± 0.00 a | 0.09 ± 0.02 bc | 4.83 ± 0.49 b |
| Treatment | FF | HA | FA | HUM | HA/FA | HUM |
|---|---|---|---|---|---|---|
| C % in TOC | g kg−1 | |||||
| CON | 15.3 ± 1.7 b | 53.4 ± 5.1 b | 7.0 ± 1.8 a | 24.3 ± 2.6 bc | 8.1 ± 2.5 b | 1.32 ± 0.19 a |
| MAN | 12.6 ± 0.3 a | 43.5 ± 2.2 a | 15.6 ± 2.9 b | 28.2 ± 1.1 c | 2.9 ± 0.6 a | 2.30 ± 0.21 b |
| LEG | 13.2 ± 0.2 ab | 56.6 ± 1.4 b | 10.1 ± 3.9 ab | 20.0 ± 2.6 ab | 6.1 ± 2.0 ab | 1.51 ± 0.25 a |
| MAN + LEG | 12.4 ± 1.7 a | 60.1 ± 5.0 b | 11.3 ± 5.2 ab | 16.2 ± 3.0 a | 6.4 ± 3.7 ab | 1.49 ± 0.30 a |
| Treatment | Bulk Soil | HUM | ||||
|---|---|---|---|---|---|---|
| Carbohydrates | Lignins | Lipids | Carbohydrates | Lignins | Lipids | |
| CON | 27.8 | 16.6 | 39.9 | 35.8 | 15.2 | 43.2 |
| MAN | 33.6 | 23.4 | 24.6 | 38.2 | 17.4 | 37.5 |
| LEG | 33.0 | 19.9 | 30.5 | 37.3 | 26.5 | 24.5 |
| MAN + LEG | 34.0 | 19.0 | 38.7 | 46.3 | 14.4 | 32.5 |
| Treatments | E280:E365 | E280:E665 | E465:E665 | ΔlogK | ε280 | ε665 |
|---|---|---|---|---|---|---|
| CON | 12.99 | 102.64 | 3.64 | 0.55 | 86.15 | 0.84 |
| MAN | 6.92 | 32.98 | 2.44 | 0.43 | 83.01 | 2.52 |
| LEG | 6.02 | 21.83 | 2.05 | 0.35 | 120.99 | 5.54 |
| MAN + LEG | 14.93 | 152.66 | 4.01 | 0.63 | 110.20 | 0.72 |
| Treatments | SSF Spectra | EEM Spectra | |||||
|---|---|---|---|---|---|---|---|
| %PLF | %FLF | %HLF | IFl325:IFl390 | HIX | f380/f430 | f450/f500 | |
| CON | 21.6 | 43.7 | 8.1 | 1.34 | 0.83 | 1.28 | 1.83 |
| MAN | 20.1 | 45.1 | 8.2 | 1.49 | 0.94 | 1.40 | 1.73 |
| LEG | 13.0 | 52.2 | 9.3 | 0.79 | 1.22 | 1.17 | 2.04 |
| MAN + LEG | 10.2 | 50.3 | 10.6 | 0.85 | 1.45 | 1.14 | 1.88 |
| Treatments | A | B | C | D | ||||
|---|---|---|---|---|---|---|---|---|
| λex/λem | IFl | λex/λem | IFl | λex/λem | IFL | λex/λem | IFl | |
| CON | 278/370 | 50.0 | 318/387 | 45.0 | 365/437 | 25.0 | --- | --- |
| MAN | 280/365 | 49.8 | 319/385 | 49.0 | 360/450 | 24.5 | --- | --- |
| LEG | 277/379 | 63.6 | 326/390 | 79.8 | 366/430 | 50.6 | 306/412 | 75.8 |
| MAN + LEG | 275/384 | 39.2 | 324/388 | 60.8 | 369/429 | 38.9 | 308/413 | 54.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, J.; Mielnik, L.; Leinweber, P.; Hewelke, E.; Kocowicz, A.; Jamroz, E.; Podlasiński, M. The Influence of Different, Long-Term Fertilizations on the Chemical and Spectroscopic Properties of Soil Organic Matter. Agronomy 2024, 14, 837. https://doi.org/10.3390/agronomy14040837
Weber J, Mielnik L, Leinweber P, Hewelke E, Kocowicz A, Jamroz E, Podlasiński M. The Influence of Different, Long-Term Fertilizations on the Chemical and Spectroscopic Properties of Soil Organic Matter. Agronomy. 2024; 14(4):837. https://doi.org/10.3390/agronomy14040837
Chicago/Turabian StyleWeber, Jerzy, Lilla Mielnik, Peter Leinweber, Edyta Hewelke, Andrzej Kocowicz, Elżbieta Jamroz, and Marek Podlasiński. 2024. "The Influence of Different, Long-Term Fertilizations on the Chemical and Spectroscopic Properties of Soil Organic Matter" Agronomy 14, no. 4: 837. https://doi.org/10.3390/agronomy14040837








