The Seasonal Response of N2O Emissions to Increasing Precipitation and Nitrogen Deposition and Its Driving Factors in Temperate Semi-Arid Grassland
Abstract
1. Introduction
2. Materials and Methods
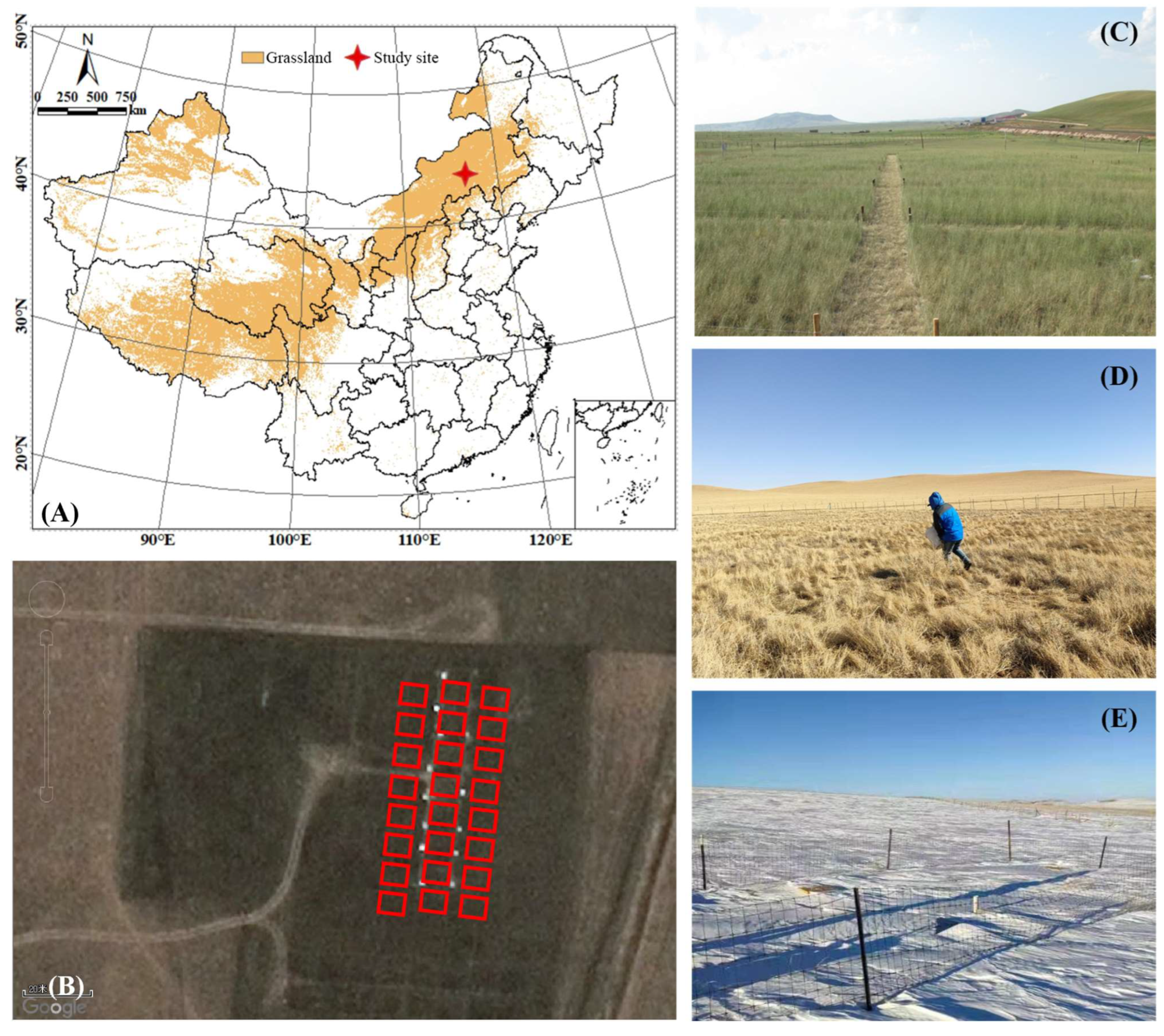
2.1. Study Site
2.2. Experimental Design
2.3. Sampling and Measurement
2.4. Statistical Analyses
3. Results
3.1. Meteorological Factors and Soil Properties
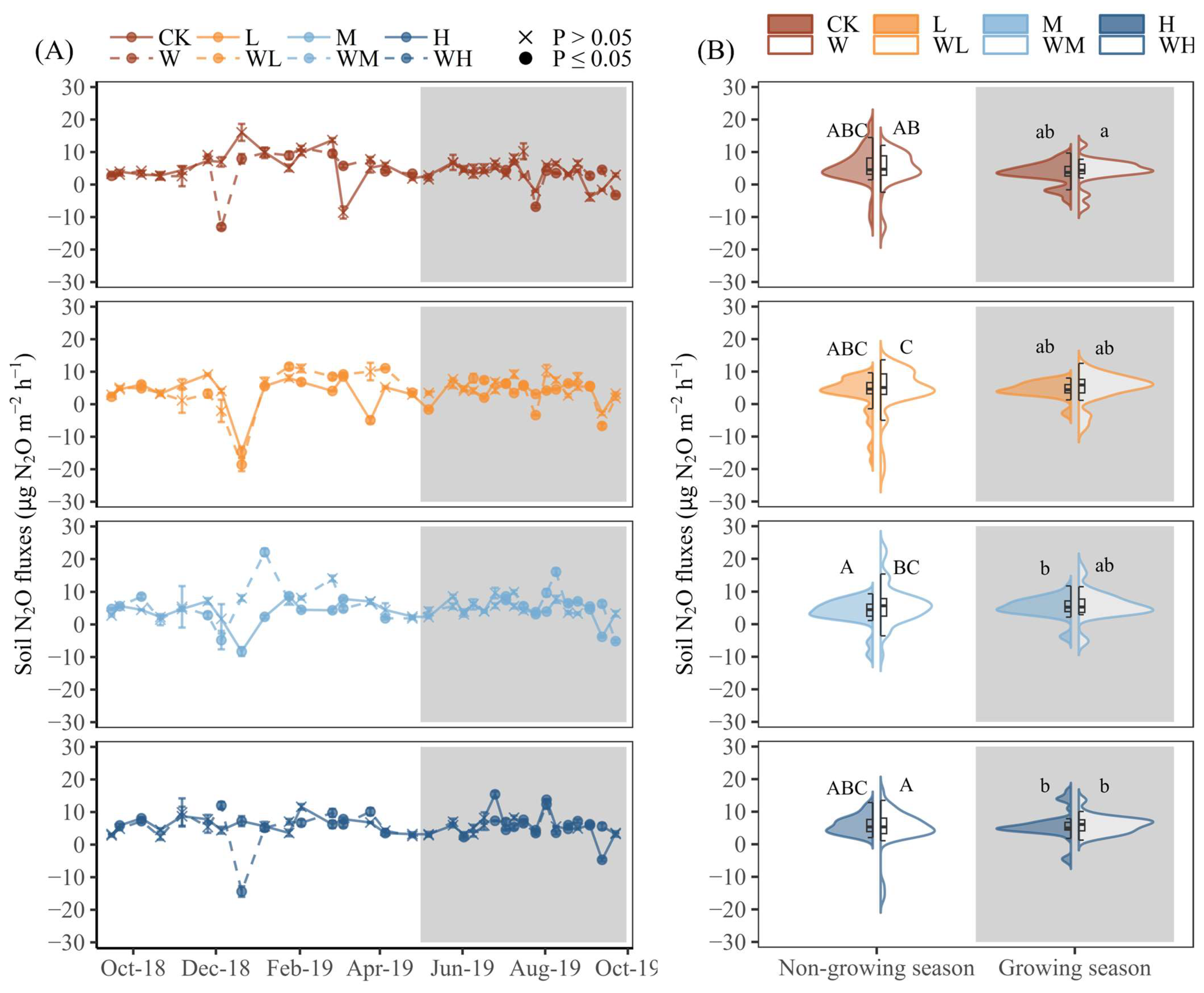
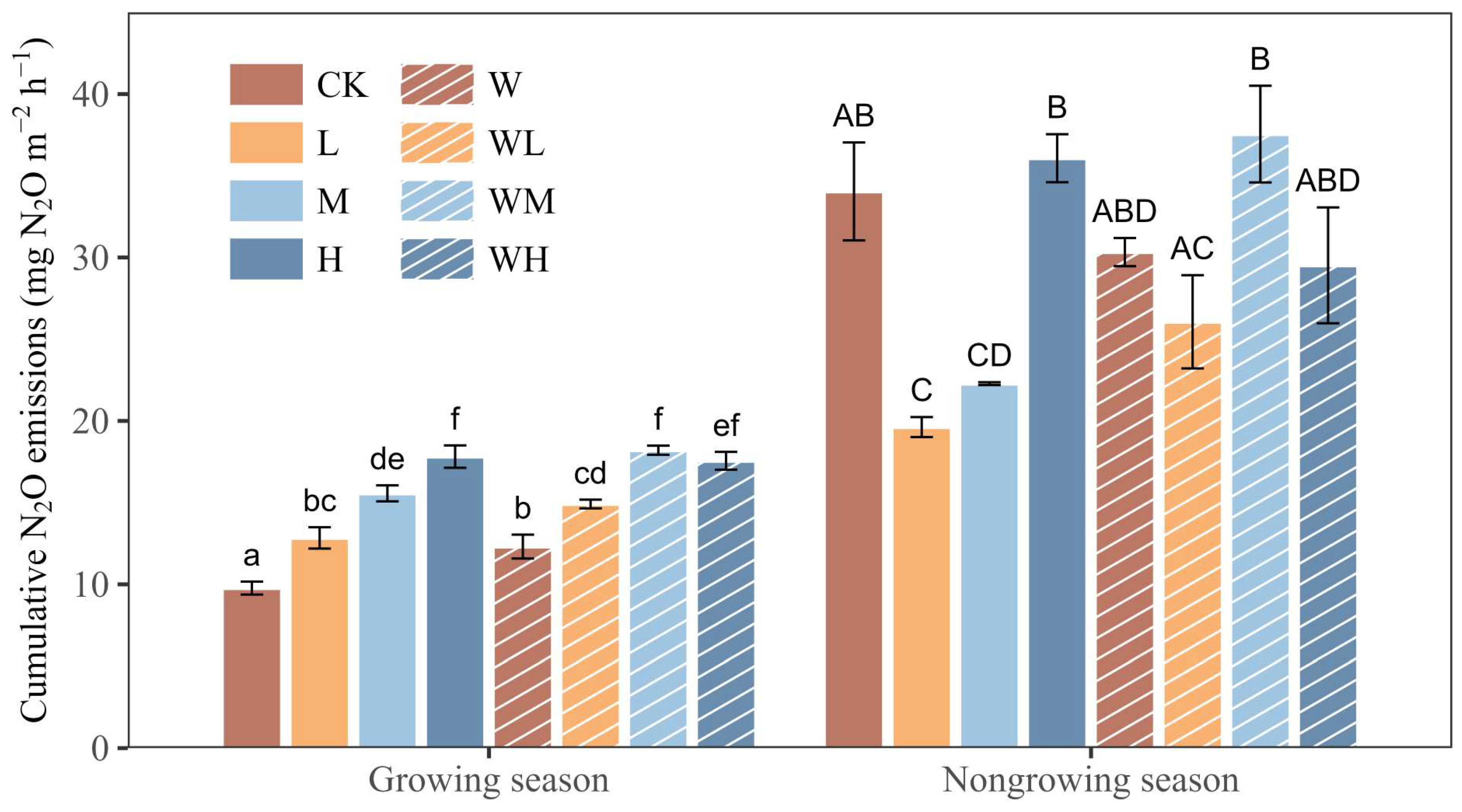
3.2. Effects of Water and N Addition on Soil N2O Emissions
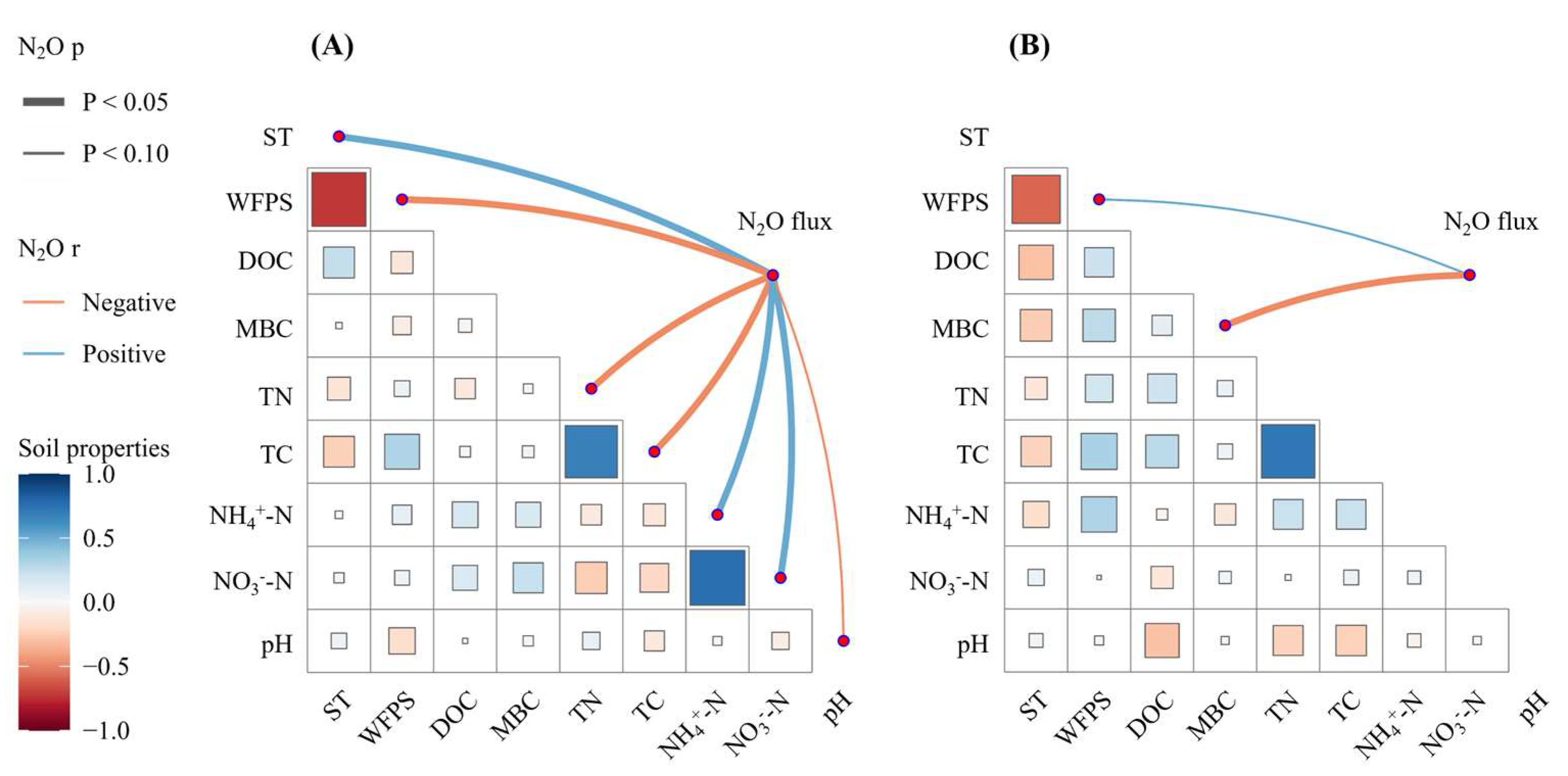
3.3. Controlling Factors on Soil N2O Emissions
4. Discussion
4.1. Seasonal Variation and Contribution of N2O
4.2. Effect of Water and N Addition on N2O Flux during the Growing Season
4.3. Effect of Water and N Addition on N2O Flux during the Non-Growing Season
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC (The Intergovernmental Panel on Climate Change). 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Working Group III contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- IPCC (The Intergovernmental Panel on Climate Change). Climate Change 2022: Mitigation of Climate Change; Working Group III contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Qiu, Q.; Wu, L.; Ouyang, Z.; Li, B.; Xu, Y.; Wu, S.; Gregorich, E.G. Effects of Plant-Derived Dissolved Organic Matter (DOM) on Soil CO2 and N2O Emissions and Soil Carbon and Nitrogen Sequestrations. Appl. Soil Ecol. 2015, 96, 122–130. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Xu, Q.; Cao, G.; Guo, X.; Zhou, H.; Du, Y. Global Analysis of Nitrification Inhibitors on Grasslands Nitrous Oxide Emission Rates. Biochem. Syst. Ecol. 2021, 97, 104289. [Google Scholar] [CrossRef]
- Liu, X.; Qi, Y.; Dong, Y.; Peng, Q.; He, Y.; Sun, L.; Jia, J.; Cao, C. Response of Soil N2O Emissions to Precipitation Pulses under Different Nitrogen Availabilities in a Semiarid Temperate Steppe of Inner Mongolia, China. J. Arid Land 2014, 6, 410–422. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; Wolf, B.; Yao, Z.; Liu, C.; Butterbach-Bahl, K.; Brüggemann, N. Long-Term Grazing Effects on Soil-Atmosphere Exchanges of CO2, CH4 and N2O at Different Grasslands in Inner Mongolia: A Soil Core Study. Ecol. Indic. 2019, 105, 316–328. [Google Scholar] [CrossRef]
- Du, Y.; Ke, X.; Li, J.; Wang, Y.; Cao, G.; Guo, X.; Chen, K. Nitrogen Deposition Increases Global Grassland N2O Emission Rates Steeply: A Meta-Analysis. Catena 2021, 199, 105105. [Google Scholar] [CrossRef]
- You, L.; Ros, G.H.; Chen, Y.; Yang, X.; Cui, Z.; Liu, X.; Jiang, R.; Zhang, F.; de Vries, W. Global Meta-Analysis of Terrestrial Nitrous Oxide Emissions and Associated Functional Genes under Nitrogen Addition. Soil Biol. Biochem. 2022, 165, 108523. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, Y.; Peng, Q.; Li, Z.; He, Y.; Yan, Z.; Qin, S. Effects of Nitrogen and Water Addition on N2O Emissions in Temperate Grasslands, Northern China. Appl. Soil Ecol. 2022, 177, 104548. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Mueller, N.; Bernoux, M.; Kasimir, Ä.; van der Weerden, T.J.; Ogle, S.M. Improved Accuracy and Reduced Uncertainty in Greenhouse Gas Inventories by Refining the IPCC Emission Factor for Direct N2O Emissions from Nitrogen Inputs to Managed Soils. Glob. Chang. Biol. 2021, 27, 6536–6550. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, S.X.; Ma, B.; Bork, E.W. Watering Increased DOC Concentration but Decreased N2O Emission from a Mixed Grassland Soil under Different Defoliation Regimes. Biol. Fertil. Soils 2016, 52, 987–996. [Google Scholar] [CrossRef]
- Long, X.-E.; Shen, J.-P.; Wang, J.-T.; Zhang, L.-M.; Di, H.; He, J.-Z. Contrasting Response of Two Grassland Soils to N Addition and Moisture Levels: N2O Emission and Functional Gene Abundance. J. Soils Sediments 2017, 17, 384–392. [Google Scholar] [CrossRef]
- Horváth, L.; Grosz, B.; Machon, A.; Tuba, Z.; Nagy, Z.; Czóbel, S.Z.; Balogh, J.; Péli, E.; Fóti, S.Z.; Weidinger, T.; et al. Estimation of Nitrous Oxide Emission from Hungarian Semi-Arid Sandy and Loess Grasslands; Effect of Soil Parameters, Grazing, Irrigation and Use of Fertilizer. Agric. Ecosyst. Environ. 2010, 139, 255–263. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Baggs, E.M.; Clough, T.J.; Fuchs, K.; Petersen, S.O. Mitigation of Nitrous Oxide Emissions in the Context of Nitrogen Loss Reduction from Agroecosystems: Managing Hot Spots and Hot Moments. Curr. Opin. Environ. Sustain. 2020, 47, 46–53. [Google Scholar] [CrossRef]
- Yue, P.; Zuo, X.; Li, K.; Li, X.; Wang, S.; Misselbrook, T. No Significant Change Noted in Annual Nitrous Oxide Flux under Precipitation Changes in a Temperate Desert Steppe. Land Degrad. Dev. 2022, 33, 94–103. [Google Scholar] [CrossRef]
- Liu, W.; Lü, X.-T.; Yang, Y.; Hou, L.; Shao, C.; Yuan, W.; Pan, Q.; Li, L. Hot Moments of N2O Emission Under Water and Nitrogen Management in Three Types of Steppe. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG006877. [Google Scholar] [CrossRef]
- Li, K.; Gong, Y.; Song, W.; Lv, J.; Chang, Y.; Hu, Y.; Tian, C.; Christie, P.; Liu, X. No Significant Nitrous Oxide Emissions during Spring Thaw under Grazing and Nitrogen Addition in an Alpine Grassland. Glob. Chang. Biol. 2012, 18, 2546–2554. [Google Scholar] [CrossRef]
- Wang, L.; Qi, Y.; Dong, Y.; Peng, Q.; Guo, S.; He, Y.; Li, Z. Effects and Mechanism of Freeze-Thawing Cycles on the Soil N2O Fluxes in the Temperate Semi-Arid Steppe. J. Environ. Sci. 2017, 56, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.G.; Romaniuk, R.I.; Cosentino, V.R.N.; Busto, M.; Gonzalez, F.A.; Taboada, M.A.; Alves, B.J.R.; Costantini, A.O. Winter Soil N2O Emissions from a Meat Production System under Direct Grazing of Argentine Pampa. Anim. Prod. Sci. 2021, 61, 156–162. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Wang, T. Freezing and Thawing Cycles Affect Nitrous Oxide Emissions in Rain-Fed Lucerne (Medicago Sativa) Grasslands of Different Ages. PeerJ 2021, 9, e12216. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lu, D.; Wang, G. Diurnal, Seasonal, and Inter-Annual Variations of N2O Fluxes from Native Semi-Arid Grassland Soils of Inner Mongolia. Soil Biol. Biochem. 2006, 38, 3474–3482. [Google Scholar] [CrossRef]
- Merbold, L.; Steinlin, C.; Hagedorn, F. Winter Greenhouse Gas Fluxes (CO2, CH4 and N2O) from a Subalpine Grassland. Biogeosciences 2013, 10, 3185–3203. [Google Scholar] [CrossRef]
- Fay, P.A.; Prober, S.M.; Harpole, W.S.; Knops, J.M.H.; Bakker, J.D.; Borer, E.T.; Lind, E.M.; MacDougall, A.S.; Seabloom, E.W.; Wragg, P.D.; et al. Grassland Productivity Limited by Multiple Nutrients. Nat. Plants 2015, 1, 15080. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, Q.; Liu, J.; Wang, M.; Zhou, X.; Li, M.; Wang, K.; Ding, J.; Peng, C. Spatial and Temporal Variations of N2O Emissions from Global Forest and Grassland Ecosystems. Agric. For. Meteorol. 2019, 266–267, 129–139. [Google Scholar] [CrossRef]
- Kong, L.; Song, J.; Ru, J.; Feng, J.; Hou, J.; Wang, X.; Zhang, Q.; Wang, H.; Yue, X.; Zhou, Z.; et al. Nitrogen Addition Does Not Alter Symmetric Responses of Soil Respiration to Changing Precipitation in a Semi-Arid Grassland. Sci. Total Environ. 2024, 921, 171170. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, Y.; Dong, Y.; Peng, Q.; Guo, S.; He, Y.; Li, Z. Precipitation and Nitrogen Deposition Alter Litter Decomposition Dynamics in Semiarid Temperate Steppe in Inner Mongolia, China. Rangel. Ecol. Manag. 2018, 71, 220–227. [Google Scholar] [CrossRef]
- Keller, M.; Reiners, W.A. Soil-Atmosphere Exchange of Nitrous Oxide, Nitric Oxide, and Methane under Secondary Succession of Pasture to Forest in the Atlantic Lowlands of Costa Rica. Glob. Biogeochem. Cycles 1994, 8, 399–409. [Google Scholar] [CrossRef]
- Qiu, J. A Global Synthesis of the Effects of Biological Invasions on Greenhouse Gas Emissions. Glob. Ecol. Biogeogr. 2015, 24, 1351–1362. [Google Scholar] [CrossRef]
- Brin, L.D.; Goyer, C.; Zebarth, B.J.; Burton, D.L.; Chantigny, M.H. Changes in Snow Cover Alter Nitrogen Cycling and Gaseous Emissions in Agricultural Soils. Agric. Ecosyst. Environ. 2018, 258, 91–103. [Google Scholar] [CrossRef]
- Li, J.; Yao, N.; Li, X.; Zhao, Y.; Zhang, A.; Lan, Z.-L.; Fan, T. Dynamics of CO2 and N2O in Seasonal Frozen Soil Profiles for a Typical Steppe in Inner Mongolia. Environ. Sci. 2018, 39, 2330–2338. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Pan, B.; Zheng, X.; Yu, J.; Ding, H.; Zhang, Y. Pathways of Soil N2O Uptake, Consumption, and Its Driving Factors: A Review. Environ. Sci. Pollut. Res. 2022, 29, 30850–30864. [Google Scholar] [CrossRef]
- Frasier, R.; Ullah, S.; Moore, T.R. Nitrous Oxide Consumption Potentials of Well-Drained Forest Soils in Southern Québec, Canada. Geomicrobiol. J. 2010, 27, 53–60. [Google Scholar] [CrossRef]
- Hu, J.; Inglett, K.S.; Clark, M.W.; Inglett, P.W.; Reddy, K.R. Nitrous Oxide Production and Consumption by Denitrification in a Grassland: Effects of Grazing and Hydrology. Sci. Total Environ. 2015, 532, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Stuchiner, E.R.; von Fischer, J.C. Using Isotope Pool Dilution to Understand How Organic Carbon Additions Affect N2O Consumption in Diverse Soils. Glob. Chang. Biol. 2022, 28, 4163–4179. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mania, D.; Mousavi, S.A.; Lycus, P.; Arntzen, M.Ø.; Woliy, K.; Lindström, K.; Shapleigh, J.P.; Bakken, L.R.; Frostegård, Å. Competition for Electrons Favours N2O Reduction in Denitrifying Bradyrhizobium Isolates. Environ. Microbiol. 2021, 23, 2244–2259. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Han, H.; Yun, T.; Song, M.J.; Terada, A.; Laureni, M.; Yoon, S. Identification of nosZ-Expressing Microorganisms Consuming Trace N2O in Microaerobic Chemostat Consortia Dominated by an Uncultured Burkholderiales. ISME J. 2022, 16, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Watson, C.J.; Yan, M.J.; Lalor, S.; Rafique, R.; Hyde, B.; Lanigan, G.; Richards, K.G.; Holden, N.M.; Humphreys, J. A Review of Nitrous Oxide Mitigation by Farm Nitrogen Management in Temperate Grassland-Based Agriculture. J. Environ. Manag. 2013, 128, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Espinoza, N.; Franklin, D.H.; Cabrera, M.; Hinkle, N.C.; Stewart, L.; Subedi, A. Interaction of Filth Flies and Epigeal Arthropods with Soil Nitrogen and Gas Emissions in Grazing Systems under a Legacy of Low Fertilization. Sustainability 2023, 15, 12572. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wang, H.; Fan, B.; Bashir, M.A.; Jin, K.; Liu, H. Legacy Effect of Long-Term Rice–Crab Co-Culture on NO Emissions in Paddy Soils. Appl. Soil Ecol. 2024, 196, 105251. [Google Scholar] [CrossRef]
- Qin, S.; Hu, C.; Clough, T.J.; Luo, J.; Oenema, O.; Zhou, S. Irrigation of DOC-Rich Liquid Promotes Potential Denitrification Rate and Decreases N2O/(N2O + N2) Product Ratio in a 0–2 m Soil Profile. Soil Biol. Biochem. 2017, 106, 1–8. [Google Scholar] [CrossRef]
- Thuan, N.C.; Koba, K.; Yano, M.; Makabe, A.; Kinh, C.T.; Terada, A.; Toyoda, S.; Yoshida, N.; Tanaka, Y.; Katsuyama, M.; et al. N2O Production by Denitrification in an Urban River: Evidence from Isotopes, Functional Genes, and Dissolved Organic Matter. Limnology 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Lee, J.-M.; Park, D.-G.; Kang, S.-S.; Choi, E.-J.; Gwon, H.-S.; Lee, H.-S.; Lee, S.-I. Short-Term Effect of Biochar on Soil Organic Carbon Improvement and Nitrous Oxide Emission Reduction According to Different Soil Characteristics in Agricultural Land: A Laboratory Experiment. Agronomy 2022, 12, 1879. [Google Scholar] [CrossRef]
- Li, H.; Song, X.; Wu, D.; Wei, D.; Li, Y.; Ju, X. Partial Substitution of Manure Increases N2O Emissions in the Alkaline Soil but Not Acidic Soils. J. Environ. Manag. 2024, 359, 120993. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Blankinship, J.C.; Niboyet, A.; van Groenigen, K.J.; Dijkstra, P.; Le Roux, X.; Leadley, P.W.; Hungate, B.A. Effects of Multiple Global Change Treatments on Soil N2O Fluxes. Biogeochemistry 2012, 109, 85–100. [Google Scholar] [CrossRef]
- Barrat, H.A.; Evans, J.; Chadwick, D.R.; Clark, I.M.; Le Cocq, K.; Cardenas, L.M. The Impact of Drought and Rewetting on N2O Emissions from Soil in Temperate and Mediterranean Climates. Eur. J Soil Sci. 2021, 72, 2504–2516. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Y.; Qi, Y.; Peng, Q.; He, Y.; Sun, L.; Jia, J.; Guo, S.; Cao, C.; Yan, Z.; et al. Response of N2O Emission to Water and Nitrogen Addition in Temperate Typical Steppe Soil in Inner Mongolia, China. Soil Tillage Res. 2015, 151, 9–17. [Google Scholar] [CrossRef]
- Koponen, H.T.; Martikainen, P.J. Soil Water Content and Freezing Temperature Affect Freeze–Thaw Related N2O Production in Organic Soil. Nutr. Cycl. Agroecosystems 2004, 69, 213–219. [Google Scholar] [CrossRef]
- Wu, X.; Li, T.; Wang, D.; Wang, F.; Fu, B.; Liu, G.; Lv, Y. Soil Properties Mediate the Freeze-Thaw-Related Soil N2O and CO2 Emissions from Temperate Grasslands. Catena 2020, 195, 104797. [Google Scholar] [CrossRef]
- Holst, J.; Liu, C.; Yao, Z.; Brueggemann, N.; Zheng, X.; Giese, M.; Butterbach-Bahl, K. Fluxes of Nitrous Oxide, Methane and Carbon Dioxide during Freezing-Thawing Cycles in an Inner Mongolian Steppe. Plant Soil 2008, 308, 105–117. [Google Scholar] [CrossRef]
- Jia, Z.; Li, P.; Wu, Y.; Yang, S.; Wang, C.; Wang, B.; Yang, L.; Wang, X.; Li, J.; Peng, Z.; et al. Deepened Snow Cover Alters Biotic and Abiotic Controls on Nitrogen Loss during Non-Growing Season in Temperate Grasslands. Biol. Fertil. Soils 2021, 57, 165–177. [Google Scholar] [CrossRef]


| Growing Season | Non-Growing Season | |||||||
|---|---|---|---|---|---|---|---|---|
| Content | Water | N | Water:N | Content | Water | N | Water:N | |
| WFPS (%) | 26.53 ± 1.40 | 0.687 | 0.526 | 0.706 | 22.90 ± 1.51 | <0.001 ** | 0.652 | 0.879 |
| pH | 7.68 ± 0.02 | <0.001 ** | <0.001 ** | <0.001 ** | 7.95 ± 0.01 | 0.009 ** | <0.001 ** | <0.001 ** |
| DOC (mg C g−1 Soil) | 28.36 ± 2.73 | 0.005 ** | 0.163 | 0.211 | 22.93 ± 1.54 | 0.976 | 0.141 | 0.278 |
| MBC (mg C g−1 Soil) | 374.51 ± 24.32 | 0.459 | 0.781 | 0.009 ** | 288.48 ± 23.46 | 0.076 | 0.381 | 0.392 |
| NH4+ (mg N g−1 Soil) | 5.02 ± 0.41 | 0.131 | <0.001 ** | 0.543 | 3.58 ± 0.29 | 0.760 | 0.463 | 0.784 |
| NO3− (mg N g−1 Soil) | 2.39 ± 0.30 | 0.130 | <0.001 ** | 0.781 | 5.02 ± 0.37 | 0.578 | 0.043 * | 0.050 * |
| TN (mg N g−1 Soil) | 1.95 ± 0.02 | 0.360 | <0.001 ** | 0.113 | 2.00 ± 0.02 | 0.012 * | 0.385 | 0.388 |
| TC (mg C g−1 Soil) | 17.57 ± 0.34 | 0.978 | <0.001 ** | <0.001 ** | 18.30 ± 0.25 | 0.723 | 0.146 | <0.001 ** |
| Growing Season | Non-Growing Season | |||||
|---|---|---|---|---|---|---|
| df | P-Flux | P-Emission | df | P-Flux | P-Emission | |
| Water | 1 | 0.038 * | 0.008 ** | 1 | 0.780 | 0.003 ** |
| Nitrogen | 3 | <0.001 ** | <0.001 ** | 3 | 0.083 | 0.010 * |
| Time | 1 | <0.001 ** | 1 | <0.001 ** | ||
| Water × Nitrogen | 3 | 0.400 | 0.415 | 3 | 0.117 | 0.079 |
| Water × Time | 1 | 0.635 | 1 | <0.001 ** | ||
| Nitrogen × Time | 3 | 0.133 | 3 | 0.120 | ||
| Water × Nitrogen ×Time | 3 | 0.353 | 3 | 0.044 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Qi, Y.; Yin, F.; Guo, Y.; Dong, Y.; Liu, X.; Yuan, X.; Lv, N. The Seasonal Response of N2O Emissions to Increasing Precipitation and Nitrogen Deposition and Its Driving Factors in Temperate Semi-Arid Grassland. Agronomy 2024, 14, 1153. https://doi.org/10.3390/agronomy14061153
Peng Q, Qi Y, Yin F, Guo Y, Dong Y, Liu X, Yuan X, Lv N. The Seasonal Response of N2O Emissions to Increasing Precipitation and Nitrogen Deposition and Its Driving Factors in Temperate Semi-Arid Grassland. Agronomy. 2024; 14(6):1153. https://doi.org/10.3390/agronomy14061153
Chicago/Turabian StylePeng, Qin, Yuchun Qi, Feihu Yin, Yu Guo, Yunshe Dong, Xingren Liu, Xiujin Yuan, and Ning Lv. 2024. "The Seasonal Response of N2O Emissions to Increasing Precipitation and Nitrogen Deposition and Its Driving Factors in Temperate Semi-Arid Grassland" Agronomy 14, no. 6: 1153. https://doi.org/10.3390/agronomy14061153
APA StylePeng, Q., Qi, Y., Yin, F., Guo, Y., Dong, Y., Liu, X., Yuan, X., & Lv, N. (2024). The Seasonal Response of N2O Emissions to Increasing Precipitation and Nitrogen Deposition and Its Driving Factors in Temperate Semi-Arid Grassland. Agronomy, 14(6), 1153. https://doi.org/10.3390/agronomy14061153









