Cereal-Legume Mixed Residue Addition Increases Yield and Reduces Soil Greenhouse Gas Emissions from Fertilized Winter Wheat in the North China Plain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Gas Flux Measurements
2.4. Soil and Plant Sampling and Analysis
2.4.1. Soil Sampling
2.4.2. Analysis of Soil Physical and Chemical Properties
2.4.3. Yield and Biomass Analysis
2.5. Statistical Analysis
3. Results
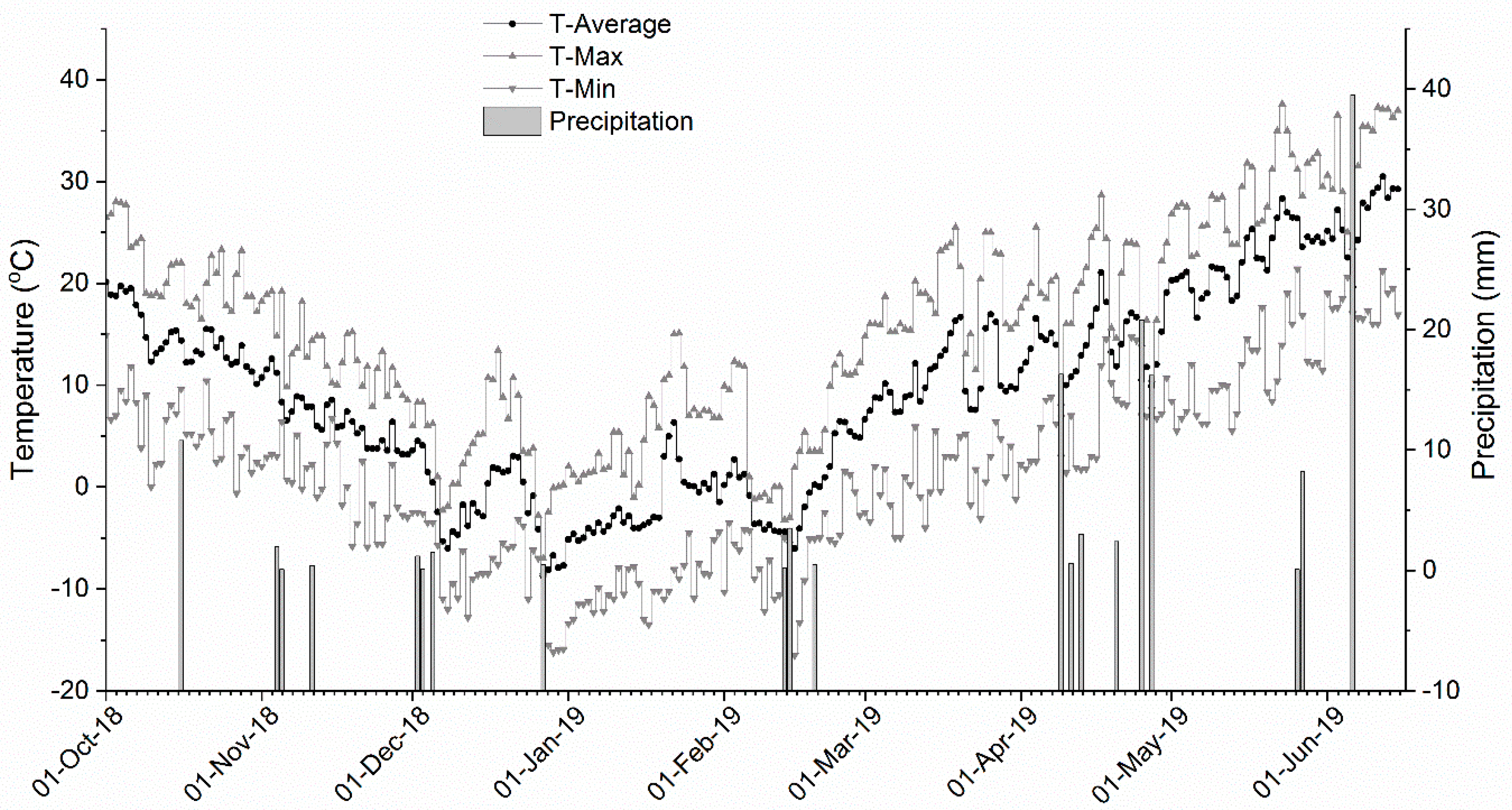
3.1. Weather Conditions
3.2. Grain Yield and Biomass Dry Matter Production
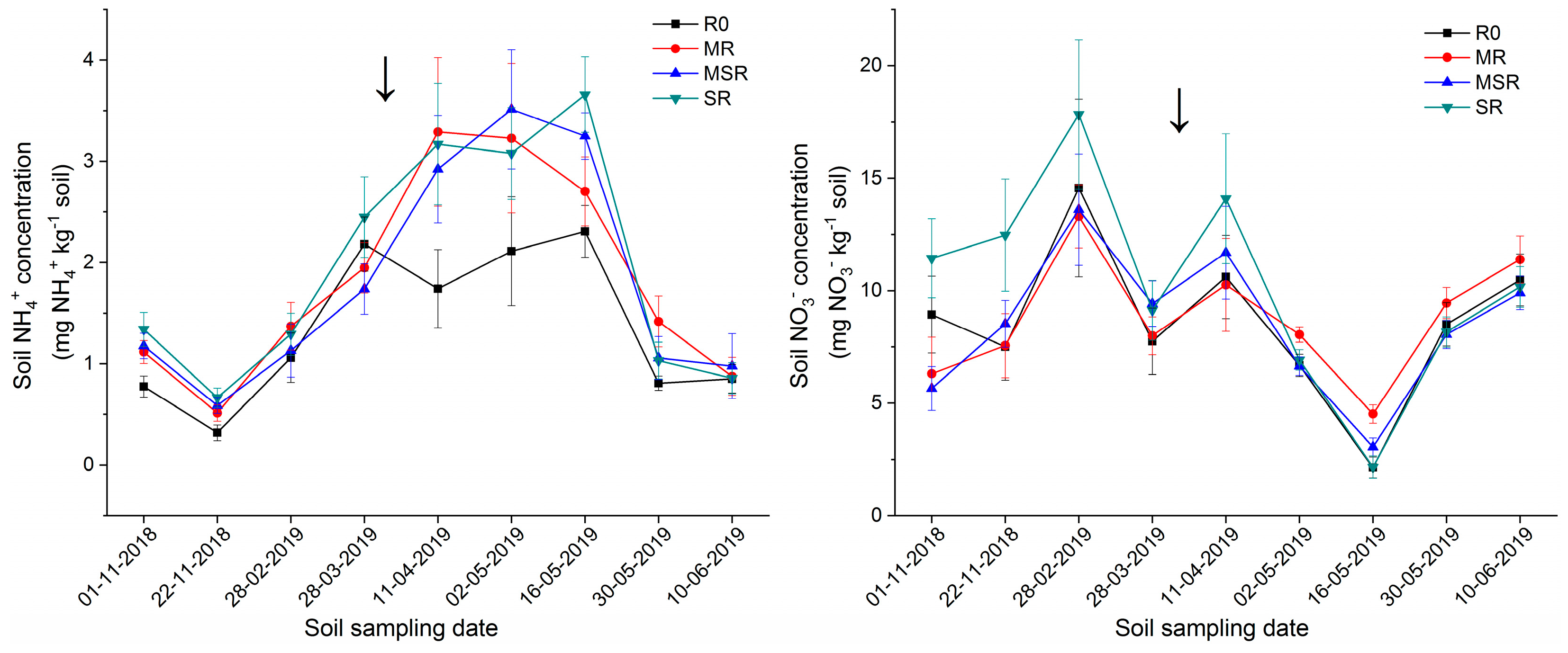
3.3. Crop Residue Effect on Soil Mineral N Concentration
3.4. Soil N2O Emissions
3.5. Soil CO2 Emissions
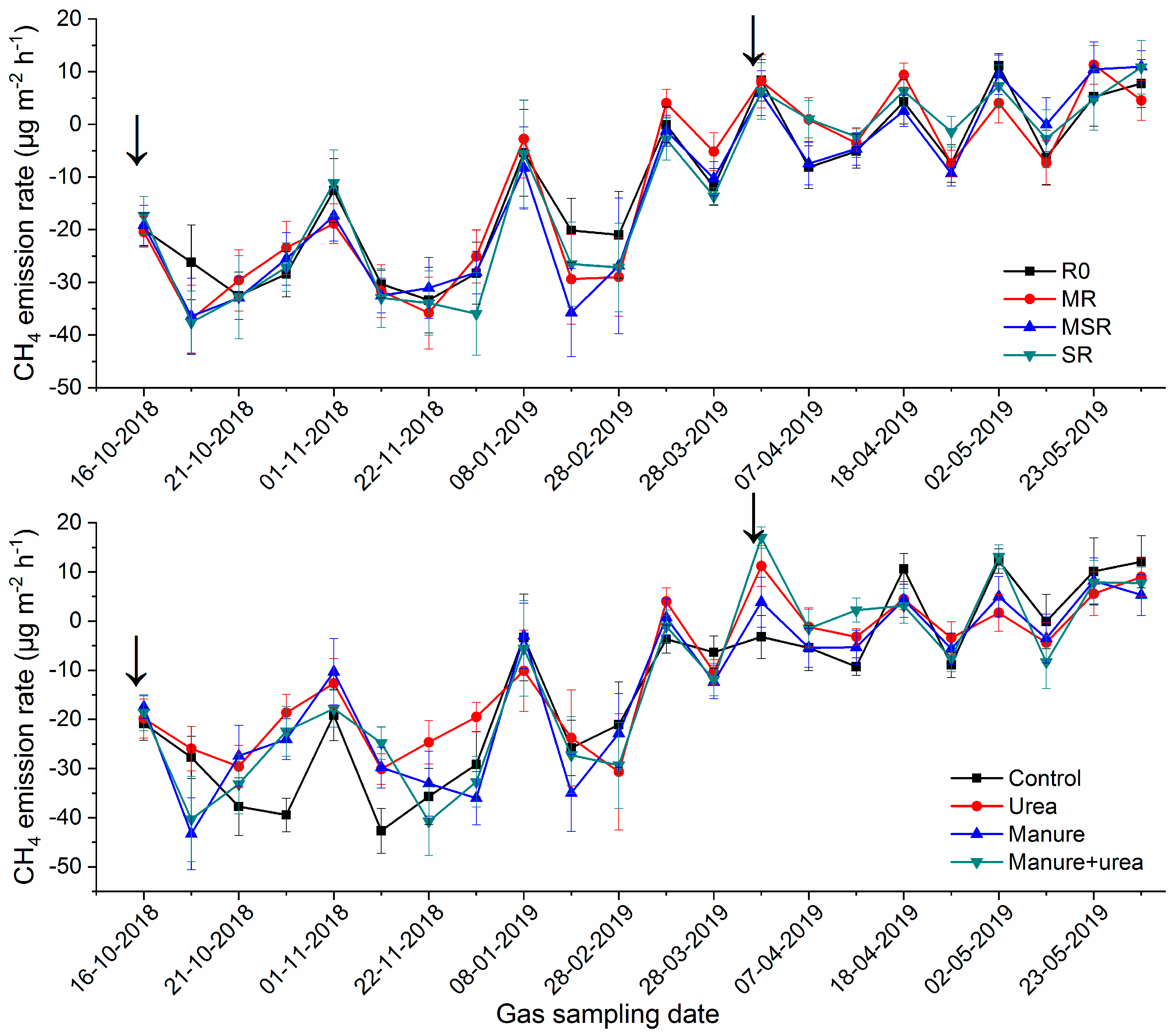
3.6. Soil CH4 Emissions
3.7. Global Warming Potential and Yield-Scaled GHG Emissions
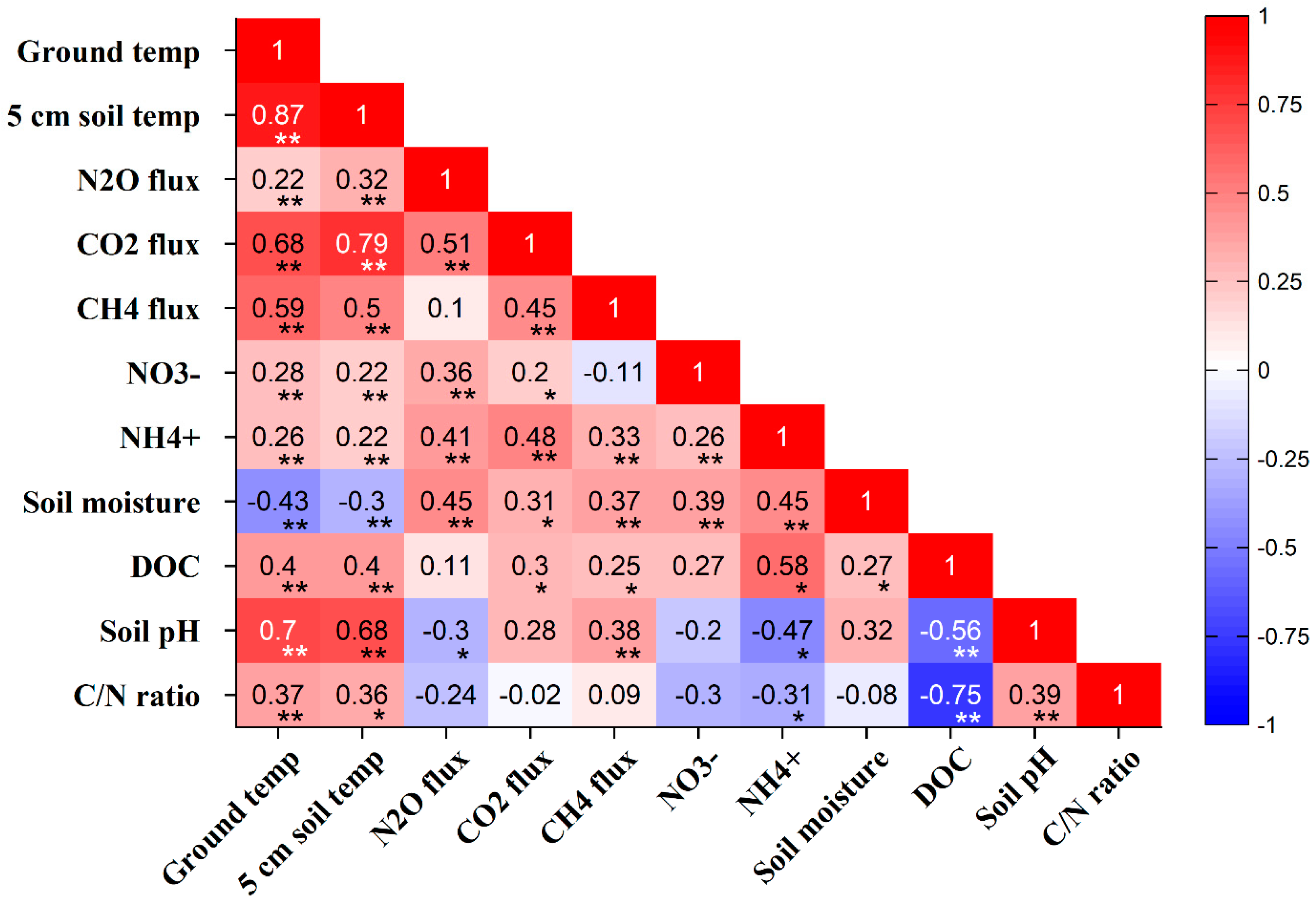
3.8. Crop Residue and N Fertilizer Effect on Soil Physicochemical Properties
4. Discussions
4.1. Crop Residue and Fertilizer Effect on Soil N Dynamics and N2O Emissions
4.2. Crop Residue and N Fertilizer Effect on Soil CO2 Emissions
4.3. Crop Residue and N Fertilizer Effect on CH4 Emissions
4.4. Yield-Scaled Emissions and Potentials of Maize-Soybean Mixed Residue
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, Z.; Li, C.; Li, J.; Ding, Z.; Xu, J.; Sun, X.; Zhou, P.; Zhao, M. Tillage and straw mulching impacts on grain yield and water use efficiency of spring maize in Northern Huang–Huai–Hai Valley. Crop J. 2015, 3, 445–450. [Google Scholar] [CrossRef]
- Lugato, E.; Bampa, F.; Panagos, P.; Montanarella, L.; Jones, A. Potential carbon sequestration of European arable soils estimated by modelling a comprehensive set of management practices. Glob. Chang. Biol. 2014, 20, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, S.; Sarkar, D.; Sinha, A.K.; Shikha; Mukhopadhyay, P.; Danish, S.; Fahad, S.; Datta, R. Carbon mineralization rates and kinetics of surface-applied and incorporated rice and maize residues in Entisol and Inceptisol soil types. Sustainability 2021, 13, 7212. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Chang. Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, R.; Liu, W.; Liu, W.; Xue, Y.; Sun, R.; Wei, Y.; Chen, Z.; Lal, R.; Dang, Y.P.; et al. Estimation of crop residue production and its contribution to carbon neutrality in China. Resour. Conserv. Recycl. 2024, 203, 107450. [Google Scholar] [CrossRef]
- Chen, J.; Gong, Y.; Wang, S.; Guan, B.; Balkovic, J.; Kraxner, F. To burn or retain crop residues on croplands? An integrated analysis of crop residue management in China. Sci. Total Environ. 2019, 662, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, Y.; Zhang, W. Projected changes in soil organic carbon stocks of China’s croplands under different agricultural managements, 2011–2050. Agric. Ecosyst. Environ. 2013, 178, 109–120. [Google Scholar] [CrossRef]
- Sun, H.; Wang, E.; Li, X.; Cui, X.; Guo, J.; Dong, R. Potential biomethane production from crop residues in China: Contributions to carbon neutrality. Renew. Sustain. Energy Rev. 2021, 148, 111360. [Google Scholar] [CrossRef]
- Shu, X.; Zou, Y.; Shaw, L.J.; Todman, L.; Tibbett, M.; Sizmur, T. Applying cover crop residues as diverse mixtures increases initial microbial assimilation of crop residue-derived carbon. Eur. J. Soil Sci. 2022, 73, e13232. [Google Scholar] [CrossRef]
- Surey, R.; Schimpf, C.M.; Sauheitl, L.; Mueller, C.W.; Rummel, P.S.; Dittert, K.; Kaiser, K.; Böttcher, J.; Mikutta, R. Potential denitrification stimulated by water-soluble organic carbon from plant residues during initial decomposition. Soil Biol. Biochem. 2020, 147, 107841. [Google Scholar] [CrossRef]
- Basalirwa, D.; Sudo, S.; Wacal, C.; Akae, F.; Oo, A.Z.; Koyama, S.; Sasagawa, D.; Yamamoto, S.; Masunaga, T.; Nishihara, E. Assessment of crop residue and palm shell biochar incorporation on greenhouse gas emissions during the fallow and crop growing seasons of broccoli (Brassica oleracea var. Italics). Soil Tillage Res. 2020, 196, 104435. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Zhou, F.; Zhang, X.; Luo, J.; Li, Y.; Lindsey, S.; Shi, Y.; He, H.; Zhang, X. Effects of maize residue return rate on nitrogen transformations and gaseous losses in an arable soil. Agric. Water Manag. 2019, 211, 132–141. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Hu, Z.; Gao, H. CO2 emissions from a forest soil as influenced by amendments of different crop straws: Implications for priming effects. Catena 2015, 131, 56–63. [Google Scholar] [CrossRef]
- Hu, N.; Chen, Q.; Zhu, L. The Responses of Soil N2O Emissions to Residue Returning Systems: A Meta-Analysis. Sustainability 2019, 11, 748. [Google Scholar] [CrossRef]
- Akiyama, H.; Yamamoto, A.; Uchida, Y.; Hoshino, Y.T.; Tago, K.; Wang, Y.; Hayatsu, M. Effect of low C/N crop residue input on N2O, NO, and CH4 fluxes from Andosol and Fluvisol fields. Sci. Total Environ. 2020, 713, 136677. [Google Scholar] [CrossRef]
- Li, F.; Sørensen, P.; Li, X.; Olesen, J.E. Carbon and nitrogen mineralization differ between incorporated shoots and roots of legume versus non-legume based cover crops. Plant Soil 2020, 446, 243–257. [Google Scholar] [CrossRef]
- Raiesi, F. Carbon and N mineralization as affected by soil cultivation and crop residue in a calcareous wetland ecosystem in Central Iran. Agric. Ecosyst. Environ. 2006, 112, 13–20. [Google Scholar] [CrossRef]
- Köbke, S.; Senbayram, M.; Pfeiffer, B.; Nacke, H.; Dittert, K. Post-harvest N2O and CO2 emissions related to plant residue incorporation of oilseed rape and barley straw depend on soil NO3− content. Soil Tillage Res. 2018, 179, 105–113. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.; Van Beusichem, M.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- García-Marco, S.; Ravella, S.R.; Chadwick, D.; Vallejo, A.; Gregory, A.S.; Cárdenas, L.M. Ranking factors affecting emissions of GHG from incubated agricultural soils. Eur. J. Soil Sci. 2014, 65, 573–583. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Q.; George, T.S.; Yin, H.; Wang, S.; Bi, J.; Zhang, J.; Liu, X.; Song, A.; Fan, F. Investigating bacterial coupled assimilation of fertilizer-nitrogen and crop residue-carbon in upland soils by DNA-qSIP. Sci. Total Environ. 2022, 845, 157279. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Zhang, Y.; Qin, A.; Gao, Y.; Duan, A.; Zhang, J.; Liu, Z.; Zhao, B.; Liu, Z. Interactive effects of irrigation system and level on grain yield, crop water use, and greenhouse gas emissions of summer maize in North China Plain. Sci. Total Environ. 2023, 864, 161165. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, K.A.; Yawson, D.O.; Baggs, E.M.; Agyarko, K. Does incorporation of cowpea-maize residue mixes influence nitrous oxide emission and mineral nitrogen release in a tropical luvisol? Nutr. Cycl. Agroecosyst. 2011, 91, 281–292. [Google Scholar] [CrossRef]
- Uchida, Y.; Akiyama, H. Mitigation of postharvest nitrous oxide emissions from soybean ecosystems: A review. Soil Sci. Plant Nutr. 2013, 59, 477–487. [Google Scholar] [CrossRef]
- Wang, X.; He, C.; Cheng, H.; Liu, B.; Li, S.; Wang, Q.; Liu, Y.; Zhao, X.; Zhang, H. Responses of greenhouse gas emissions to residue returning in China’s croplands and influential factors: A meta-analysis. J. Environ. Manag. 2021, 289, 112486. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Wu, H.; MacDonald, G.K.; Galloway, J.N.; Zhang, L.; Gao, L.; Yang, L.; Yang, J.; Li, X.; Li, H.; Yang, T. The influence of crop and chemical fertilizer combinations on greenhouse gas emissions: A partial life-cycle assessment of fertilizer production and use in China. Resour. Conserv. Recycl. 2021, 168, 105303. [Google Scholar] [CrossRef]
- Mairura, F.S.; Musafiri, C.M.; Macharia, J.M.; Kiboi, M.N.; Ng’etich, O.K.; Shisanya, C.A.; Okeyo, J.M.; Okwuosa, E.A.; Ngetich, F.K. Yield-scaled and area-scaled greenhouse gas emissions from common soil fertility management practices under smallholder maize fields in Kenya. Sustain. Prod. Consum. 2023, 36, 292–307. [Google Scholar] [CrossRef]
- Janz, B.; Havermann, F.; Lashermes, G.; Zuazo, P.; Engelsberger, F.; Torabi, S.M.; Butterbach-Bahl, K. Effects of crop residue incorporation and properties on combined soil gaseous N2O, NO, and NH3 emissions—A laboratory-based measurement approach. Sci. Total Environ. 2022, 807, 151051. [Google Scholar] [CrossRef]
- Bichel, A.; Oelbermann, M.; Echarte, L. Impact of residue addition on soil nitrogen dynamics in intercrop and sole crop agroecosystems. Geoderma 2017, 304, 12–18. [Google Scholar] [CrossRef]
- Qin, S.; Hu, C.; Clough, T.J.; Luo, J.; Oenema, O.; Zhou, S. Irrigation of DOC-rich liquid promotes potential denitrification rate and decreases N2O/(N2O + N2) product ratio in a 0–2 m soil profile. Soil Biol. Biochem. 2017, 106, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Zhen, L.; Hu, Y. Crop Production and Security in Ningjin County of the North China Plain. Foods 2023, 12, 2196. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, N.; Rosa, V.; Fernandez, P.L.; Figueiro Aureggi, S.A.; Taboada, M.A. N2O emissions from a cultivated mollisol: Optimal time of day for sampling and the role of soil temperature. Rev. Bras. Ciênc. Solo 2012, 36, 1814–1819. [Google Scholar] [CrossRef]
- Zhai, L.M.; Liu, H.B.; Zhang, J.Z.; Huang, J.; Wang, B.R. Long-term application of organic manure and mineral fertilizer on N2O and CO2 emissions in a red soil from cultivated maize-wheat rotation in China. Agric. Sci. China 2011, 10, 1748–1757. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Ghimire, R.; Norton, U.; Bista, P.; Obour, A.K.; Norton, J.B. Soil organic matter, greenhouse gases and net global warming potential of irrigated conventional, reduced-tillage and organic cropping systems. Nutr. Cycl. Agroecosyst. 2017, 107, 49–62. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Norton, U.; Norton, J.B.; Odhiambo, J.; Del Grosso, S.J.; Hergert, G.W.; Lyon, D.J. Dryland Soil Greenhouse Gases and Yield-Scaled Emissions in No-Till and Organic Winter Wheat–Fallow Systems. Soil Sci. Soc. Am. J. 2016, 80, 178–192. [Google Scholar] [CrossRef]
- Liu, L.; Hu, C.; Yang, P.; Ju, Z.; Olesen, J.E.; Tang, J. Experimental warming-driven soil drying reduced N2O emissions from fertilized crop rotations of winter wheat–soybean/fallow, 2009–2014. Agric. Ecosyst. Environ. 2016, 219, 71–82. [Google Scholar] [CrossRef]
- Li, L.J.; Han, X.Z.; You, M.Y.; Yuan, Y.R.; Ding, X.L.; Qiao, Y.F. Carbon and nitrogen mineralization patterns of two contrasting crop residues in a Mollisol: Effects of residue type and placement in soils. Eur. J. Soil Biol. 2013, 54, 1–6. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol. Fertil. Soils 2016, 52, 165–176. [Google Scholar] [CrossRef]
- Muhammad, W.; Vaughan, S.M.; Dalal, R.C.; Menzies, N.W. Crop residues and fertilizer nitrogen influence residue decomposition and nitrous oxide emission from a Vertisol. Biol. Fertil. Soils 2011, 47, 15–23. [Google Scholar] [CrossRef]
- Schmatz, R.; Recous, S.; Aita, C.; Tahir, M.M.; Schu, A.L.; Chaves, B.; Giacomini, S.J. Crop residue quality and soil type influence the priming effect but not the fate of crop residue C. Plant Soil 2017, 414, 229–245. [Google Scholar] [CrossRef]
- Xu, C.; Han, X.; Ru, S.; Cardenas, L.; Rees, R.M.; Wu, D.; Wu, W.; Meng, F. Crop straw incorporation interacts with N fertilizer on N2O emissions in an intensively cropped farmland. Geoderma 2019, 341, 129–137. [Google Scholar] [CrossRef]
- Badagliacca, G.; Ruisi, P.; Rees, R.M.; Saia, S. An assessment of factors controlling N2O and CO2 emissions from crop residues using different measurement approaches. Biol. Fertil. Soils 2017, 53, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Abalos, D.; Rittl, T.F.; Recous, S.; Thiébeau, P.; Topp, C.F.; van Groenigen, K.J.; Butterbach-Bahl, K.; Thorman, R.E.; Smith, K.E.; Ahuja, I.; et al. Predicting field N2O emissions from crop residues based on their biochemical composition: A meta-analytical approach. Sci. Total Environ. 2022, 812, 152532. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Chang. Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, K.A.; Baggs, E.M. Do combined applications of crop residues and inorganic fertilizer lower emission of N2O from soil? Soil Use Manag. 2010, 26, 412–424. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Swank, W.T. Using soil temperature and moisture to predict forest soil nitrogen mineralization. Biol. Fertil. Soils 2002, 36, 177–182. [Google Scholar] [CrossRef]
- Thapa, R.; Tully, K.L.; Cabrera, M.L.; Dann, C.; Schomberg, H.H.; Timlin, D.; Reberg-Horton, C.; Gaskin, J.; Davis, B.W.; Mirsky, S.B. Effects of moisture and temperature on C and N mineralization from surface-applied cover crop residues. Biol. Fertil. Soils 2021, 57, 485–498. [Google Scholar] [CrossRef]
- Miller, K.S.; Geisseler, D. Temperature sensitivity of nitrogen mineralization in agricultural soils. Biol. Fertil. Soils 2018, 54, 853–860. [Google Scholar] [CrossRef]
- Heumann, S.; Böttcher, J. Temperature functions of the rate coefficients of net N mineralization in sandy arable soils. Part I. Derivation from laboratory incubations. J. Plant Nutr. Soil Sci. 2004, 167, 381–389. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Lashermes, G.; Recous, S.; Alavoine, G.; Janz, B.; Butterbach-Bahl, K.; Ernfors, M.; Laville, P. N2O emissions from decomposing crop residues are strongly linked to their initial soluble fraction and early C mineralization. Sci. Total Environ. 2022, 806, 150883. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, X.; Song, C.; Li, M.; Gao, S.; Liu, Z.; Gao, J.; Wang, X. Soil CO2 and N2O emissions and microbial abundances altered by temperature rise and nitrogen addition in active-layer soils of permafrost peatland. Front. Microbiol. 2022, 13, 1093487. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.X.; Brookes, P.C.; Dahlgren, R.A.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef]
- Xue, K.; Yuan, M.M.; Shi, Z.J.; Qin, Y.; Deng, Y.; Cheng, L.; Wu, L.; He, Z.; Van Nostrand, J.D.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 2016, 6, 595–600. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Hassan, W.; Bashir, S.; Ahmed, N.; Tanveer, M.; Shah, A.N.; Bano, R.; David, J. Labile Organic Carbon Fractions, Regulator of CO2 Emission: Effect of Plant Residues and Water Regimes. Clean-Soil Air Water 2016, 44, 1358–1367. [Google Scholar] [CrossRef]
- Zou, X.; Ruan, H.; Fu, Y.; Yang, X.; Sha, L. Estimating soil labile organic carbon and potential turnover rates using a sequential fumigation–incubation procedure. Soil Biol. Biochem. 2005, 37, 1923–1928. [Google Scholar] [CrossRef]
- Ma, R.; Yu, K.; Xiao, S.; Liu, S.; Ciais, P.; Zou, J. Data-driven estimates of fertilizer-induced soil NH3, NO and N2O emissions from croplands in China and their climate change impacts. Glob. Chang. Biol. 2022, 28, 1008–1022. [Google Scholar] [CrossRef]
- Tanahashi, T.; Murase, J.; Matsuya, K.; Asakawa, S.; Kimura, M. Microbial communities responsible for the decomposition of rice straw compost in a Japanese rice paddy field determined by phospholipid fatty acid (PLFA) analysis. Soil Sci. Plant Nutr. 2004, 50, 1229–1236. [Google Scholar] [CrossRef]
- Lehrsch, G.A.; Brown, B.; Lentz, R.D.; Johnson-Maynard, J.L.; Leytem, A.B. Winter and growing season nitrogen mineralization from fall-applied composted or stockpiled solid dairy manure. Nutr. Cycl. Agroecosyst. 2016, 104, 125–142. [Google Scholar] [CrossRef]
- Gaudel, G.; Xing, L.; Raseduzzaman, M.; Poudel, M.; Dong, W.; Hu, C. Soil microbes, carbon, nitrogen, and the carbon to nitrogen ratio indicate priming effects across terrestrial ecosystems. J. Soils Sediments 2024, 24, 307–322. [Google Scholar] [CrossRef]
- Lenka, S.; Trivedi, P.; Singh, B.; Singh, B.P.; Pendall, E.; Bass, A.; Lenka, N.K. Effect of crop residue addition on soil organic carbon priming as influenced by temperature and soil properties. Geoderma 2019, 347, 70–79. [Google Scholar] [CrossRef]
- Fang, Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Chang. Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef]
- Gaudel, G.; Xing, L.; Shrestha, S.; Poudel, M.; Sherpa, P.; Raseduzzaman, M.; Zhang, X. Microbial mechanisms regulate soil organic carbon mineralization under carbon with varying levels of nitrogen addition in the above-treeline ecosystem. Sci. Total Environ. 2024, 917, 170497. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Li, Y.; Wang, G.; Liu, X.; Tang, C.; Lian, T.; Adams, J.; Liu, J.; Liu, J.; et al. Soil microbial metabolism on carbon and nitrogen transformation links the crop-residue contribution to soil organic carbon. NPJ Biofilms Microbiomes 2022, 8, 14. [Google Scholar] [CrossRef]
- Almagro, M.; Ruiz-Navarro, A.; Díaz-Pereira, E.; Albaladejo, J.; Martínez-Mena, M. Plant residue chemical quality modulates the soil microbial response related to decomposition and soil organic carbon and nitrogen stabilization in a rainfed Mediterranean agroecosystem. Soil Biol. Biochem. 2021, 156, 108198. [Google Scholar] [CrossRef]
- Muhammad, I.; Sainju, U.M.; Zhao, F.; Khan, A.; Ghimire, R.; Fu, X.; Wang, J. Regulation of soil CO2 and N2O emissions by cover crops: A meta-analysis. Soil Tillage Res. 2019, 192, 103–112. [Google Scholar] [CrossRef]
- Aye, N.S.; Butterly, C.R.; Sale, P.W.; Tang, C. Residue addition and liming history interactively enhance mineralization of native organic carbon in acid soils. Biol. Fertil. Soils 2017, 53, 61–75. [Google Scholar] [CrossRef]
- Brenzinger, K.; Drost, S.M.; Korthals, G.; Bodelier, P.L. Organic residue amendments to modulate greenhouse gas emissions from agricultural soils. Front. Microbiol. 2018, 9, 3035. [Google Scholar] [CrossRef]
- Liang, Z.; Rasmussen, J.; Poeplau, C.; Elsgaard, L. Priming effects decrease with the quantity of cover crop residues—Potential implications for soil carbon sequestration. Soil Biol. Biochem. 2023, 184, 109110. [Google Scholar] [CrossRef]
- Li, L.; You, M.; Shi, H.; Ding, X.; Qiao, Y.; Han, X. Soil CO2 emissions from a cultivated Mollisol: Effects of organic amendments, soil temperature, and moisture. Eur. J. Soil Biol. 2013, 55, 83–90. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kwaw-Mensah, D.; Ci, E. Effect of nitrogen fertilizer application on corn residue decomposition in Iowa. Agron. J. 2017, 109, 2415–2427. [Google Scholar] [CrossRef]
- Xu, X.; Yin, L.; Duan, C.; Jing, Y. Effect of N addition, moisture, and temperature on soil microbial respiration and microbial biomass in forest soil at different stages of litter decomposition. J. Soils Sediments 2016, 16, 1421–1439. [Google Scholar] [CrossRef]
- Irina, K.K.; Ekaterina, N.T.; Ruzalia, V.U.; Ekaterina, V.M.; Marina, V.S. Effect of temperature on litter decomposition, soil microbial community structure and biomass in a mixed-wood forest in European Russia. Curr. Sci. 2019, 116, 765–772. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, S.; Lin, J.; Zhu, B. Responses of soil microbial biomass carbon and dissolved organic carbon to drying-rewetting cycles: A meta-analysis. Catena 2021, 207, 105610. [Google Scholar] [CrossRef]
- Marzaioli, R.; Trifuoggi, M.; Rutigliano, F. Soil microbial biomass, activities and diversity in Southern Italy areas chronically exposed to trace element input from industrial and agricultural activities. Appl. Soil Ecol. 2022, 174, 104392. [Google Scholar] [CrossRef]
- Shaaban, M.; Khalid, M.S.; Hu, R.; Zhou, M. Effects of water regimes on soil N2O, CH4 and CO2 emissions following addition of dicyandiamide and N fertilizer. Environ. Res. 2022, 212, 113544. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Lu, X. A meta-analysis of the effects of crop residue return on crop yields and water use efficiency. PLoS ONE 2020, 15, e0231740. [Google Scholar] [CrossRef]
- Meng, H.N.; Song, C.C.; Miao, Y.Q.; Mao, R.; Wang, X.W. Response of CH4 emissions to moss removal and N addition in boreal peatland of northeast China. Biogeosciences 2014, 11, 4809–4816. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, X.; Liu, J.; Sun, N.; Wu, L.; Li, Z.; Xu, M. A synthetic analysis of greenhouse gas emissions from manure amended agricultural soils in China. Sci. Rep. 2017, 7, 8123. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zheng, C.; Guan, D.; Li, S.; Xie, F.; Chen, J.; Hang, X.; Jiang, Y.; Deng, A.; et al. Significant residual effects of wheat fertilization on greenhouse gas emissions in succeeding soybean growing season. Soil Tillage Res. 2017, 169, 7–15. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Selmants, P.C.; Hart, S.C. Does dissolved organic carbon regulate biological methane oxidation in semiarid soils? Glob. Chang. Biol. 2013, 19, 2149–2157. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-Term Effect of Manure and Fertilizer on Soil Organic Carbon Pools in Dryland Farming in Northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Gao, J.; Ouyang, H.; Lei, G.; Xu, X.; Zhang, M. Effects of temperature, soil moisture, soil type and their interactions on soil carbon mineralization in Zoigê alpine wetland, Qinghai-Tibet Plateau. Chin. Geogr. Sci. 2011, 21, 27–35. [Google Scholar] [CrossRef]
- Van Hulzen, J.; Segers, R.; Van Bodegom, P.; Leffelaar, P. Temperature effects on soil methane production: An explanation for observed variability. Soil Biol. Biochem. 1999, 31, 1919–1929. [Google Scholar] [CrossRef]
- Fan, L.; Dippold, M.A.; Thiel, V.; Ge, T.; Wu, J.; Kuzyakov, Y.; Dorodnikov, M. Temperature sensitivity of anaerobic methane oxidation versus methanogenesis in paddy soil: Implications for the CH4 balance under global warming. Glob. Chang. Biol. 2022, 28, 654–664. [Google Scholar] [CrossRef]
- Te, X.; Din, A.M.U.; Cui, K.; Raza, M.A.; Ali, M.F.; Xiao, J.; Yang, W. Inter-specific root interactions and water use efficiency of maize/soybean relay strip intercropping. Field Crops Res. 2023, 291, 108793. [Google Scholar] [CrossRef]
- Raza, M.A.; Zhiqi, W.; Yasin, H.S.; Gul, H.; Qin, R.; Rehman, S.U.; Mahmood, A.; Iqbal, Z.; Ahmed, Z.; Luo, S.; et al. Effect of crop combination on yield performance, nutrient uptake, and land use advantage of cereal/legume intercropping systems. Field Crops Res. 2023, 304, 109144. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, H.; Yang, Z.; Zhu, Q.; Yan, B.; Fei, J.; Rong, X.; Peng, J.; Luo, G. Intercropping-driven nitrogen trade-off enhances maize productivity in a long-term experiment. Field Crops Res. 2022, 287, 108671. [Google Scholar] [CrossRef]
- Gui, D.; Zhang, Y.; Lv, J.; Guo, J.; Sha, Z. Effects of intercropping on soil greenhouse gas emissions—A global meta-analysis. Sci. Total Environ. 2024, 918, 170632. [Google Scholar] [CrossRef]
- Han, F.; Javed, T.; Hussain, S.; Guo, S.; Guo, R.; Yang, L.; Liu, X.; Cai, T.; Zhang, P.; Jia, Z.; et al. Maize/peanut rotation intercropping improves ecosystem carbon budget and economic benefits in the dry farming regions of China. J. Environ. Manag. 2024, 353, 120090. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, X.; Chen, G.; Afzal, M.R.; Wei, T.; Zeng, H.; Subbarao, G.V.; Wei, Z.; Zhu, Y. Intercropping with BNI-sorghum benefits neighbouring maize productivity and mitigates soil nitrification and N2O emission. Agric. Ecosyst. Environ. 2023, 352, 108510. [Google Scholar] [CrossRef]
- Sant’Anna, S.; Martins, M.; Goulart, J.; Araújo, S.; Araújo, E.; Zaman, M.; Jantalia, C.; Alves, B.; Boddey, R.M.; Urquiaga, S. Biological nitrogen fixation and soil N2O emissions from legume residues in an Acrisol in SE Brazil. Geoderma Reg. 2018, 15, e00196. [Google Scholar] [CrossRef]
- Pimentel, L.G.; Weiler, D.A.; Pedroso, G.M.; Bayer, C. Soil N2O emissions following cover-crop residues application under two soil moisture conditions. J. Plant Nutr. Soil Sci. 2015, 178, 631–640. [Google Scholar] [CrossRef]




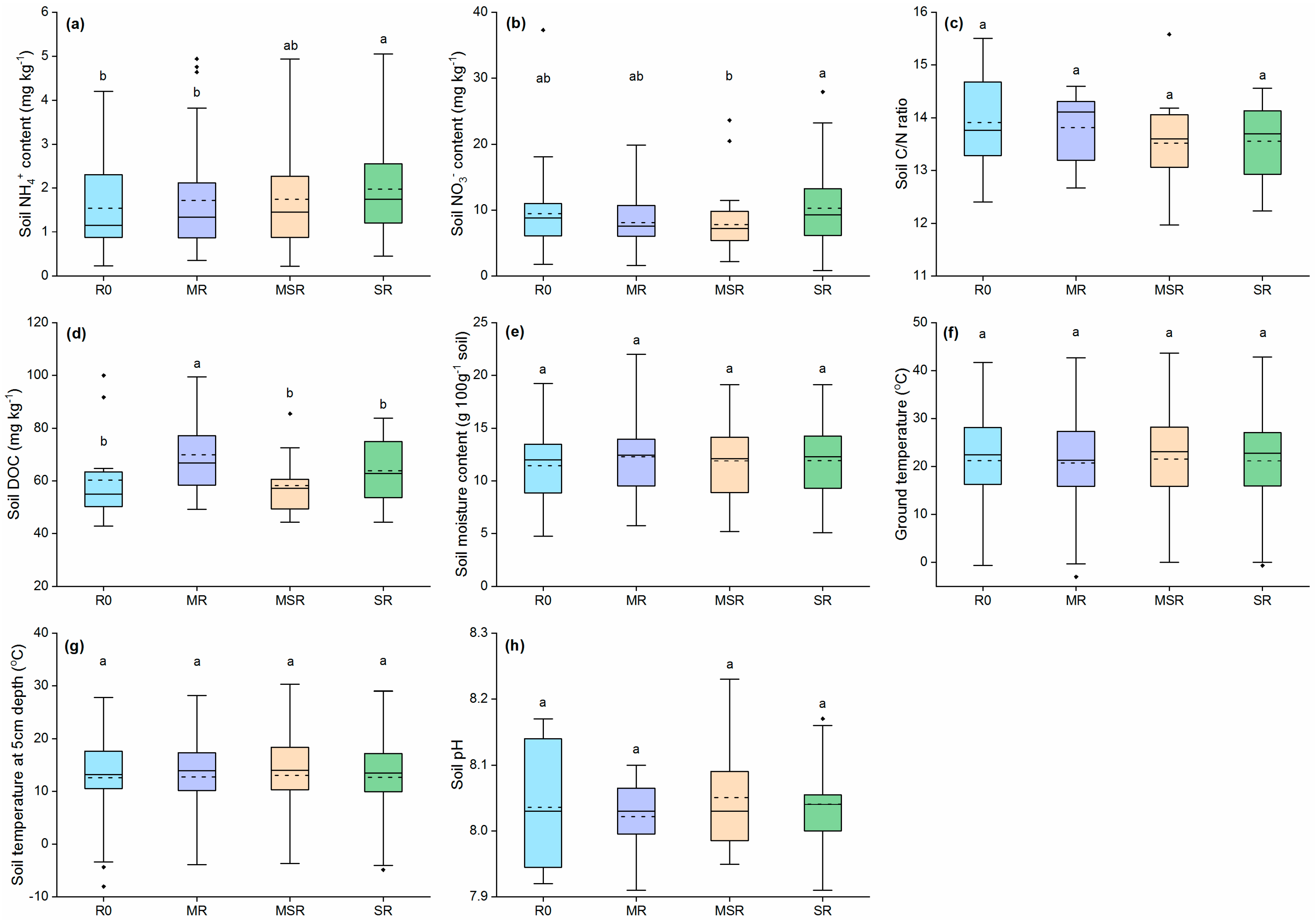

| Variable Source | d.f. | N2O Emissions | CO2 Emissions | CH4 Emissions | Global Warming Potential | Grain Yield | GHGI |
|---|---|---|---|---|---|---|---|
| Crop residue | 3 | 0.031 | 0.001 | 0.891 | 0.002 | 0.038 | 0.031 |
| N treatment | 3 | 0.000 | 0.636 | 0.796 | 0.368 | 0.000 | 0.000 |
| Crop residue × N treatment | 9 | 0.046 | 0.006 | 0.924 | 0.030 | 0.008 | 0.048 |
| Treatment | N2O Emissions | CO2 Emissions | CH4 Emissions | GWP (kg CO2-eq ha−1 season−1) | Grain Yield (kg ha−1) | GHGI (kg CO2-eq kg−1 grain) |
|---|---|---|---|---|---|---|
| kg ha−1 season−1 | ||||||
| R0 | 1.55 ± 0.26 b | 6032 ± 214 b | −0.76 ± 0.08 a | 6468 ± 264 b | 4003 ± 470 b | 1.81 ± 0.16 b |
| MR | 1.69 ± 0.30 b | 7640 ± 335 a | −0.80 ± 0.10 a | 8116 ± 336 a | 4231 ± 504 ab | 2.29 ± 0.30 a |
| MSR | 1.63 ± 0.25 b | 6969 ± 249 a | −0.88 ± 0.12 a | 7424 ± 211 a | 4843 ± 449 a | 1.71 ± 0.19 b |
| SR | 2.11 ± 0.28 a | 6884 ± 263 a | −0.87 ± 0.15 a | 7484 ± 278 a | 4946 ± 425 a | 1.70 ± 0.23 b |
| Control | 0.75 ± 0.10 c | 6751 ± 322 a | −0.83 ± 0.13 a | 6947 ± 337 a | 2818 ± 306 c | 2.71 ± 0.27 a |
| Urea | 2.45 ± 0.22 a | 6905 ± 252 a | −0.73 ± 0.11 a | 7612 ± 281 a | 5565 ± 352 a | 1.43 ± 0.10 c |
| Manure | 1.43 ± 0.11 b | 7152 ± 313 a | −0.89 ± 0.12 a | 7547 ± 312 a | 3942 ± 249 b | 1.96 ± 0.12 b |
| Manure + urea | 2.35 ± 0.26 a | 6717 ± 332 a | −0.88 ± 0.09 a | 7387 ± 315 a | 5699 ± 340 a | 1.34 ± 0.12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raseduzzaman, M.; Gaudel, G.; Ali, M.R.; Timilsina, A.; Bizimana, F.; Aluoch, S.O.; Li, X.; Zhang, Y.; Hu, C. Cereal-Legume Mixed Residue Addition Increases Yield and Reduces Soil Greenhouse Gas Emissions from Fertilized Winter Wheat in the North China Plain. Agronomy 2024, 14, 1167. https://doi.org/10.3390/agronomy14061167
Raseduzzaman M, Gaudel G, Ali MR, Timilsina A, Bizimana F, Aluoch SO, Li X, Zhang Y, Hu C. Cereal-Legume Mixed Residue Addition Increases Yield and Reduces Soil Greenhouse Gas Emissions from Fertilized Winter Wheat in the North China Plain. Agronomy. 2024; 14(6):1167. https://doi.org/10.3390/agronomy14061167
Chicago/Turabian StyleRaseduzzaman, Md, Gokul Gaudel, Md Razzab Ali, Arbindra Timilsina, Fiston Bizimana, Stephen Okoth Aluoch, Xiaoxin Li, Yuming Zhang, and Chunsheng Hu. 2024. "Cereal-Legume Mixed Residue Addition Increases Yield and Reduces Soil Greenhouse Gas Emissions from Fertilized Winter Wheat in the North China Plain" Agronomy 14, no. 6: 1167. https://doi.org/10.3390/agronomy14061167
APA StyleRaseduzzaman, M., Gaudel, G., Ali, M. R., Timilsina, A., Bizimana, F., Aluoch, S. O., Li, X., Zhang, Y., & Hu, C. (2024). Cereal-Legume Mixed Residue Addition Increases Yield and Reduces Soil Greenhouse Gas Emissions from Fertilized Winter Wheat in the North China Plain. Agronomy, 14(6), 1167. https://doi.org/10.3390/agronomy14061167







