Diversification of Intensively Used Grassland: Resilience and Good Fodder Quality across Different Soil Types
Abstract
1. Introduction
2. Materials and Methods
3. Results
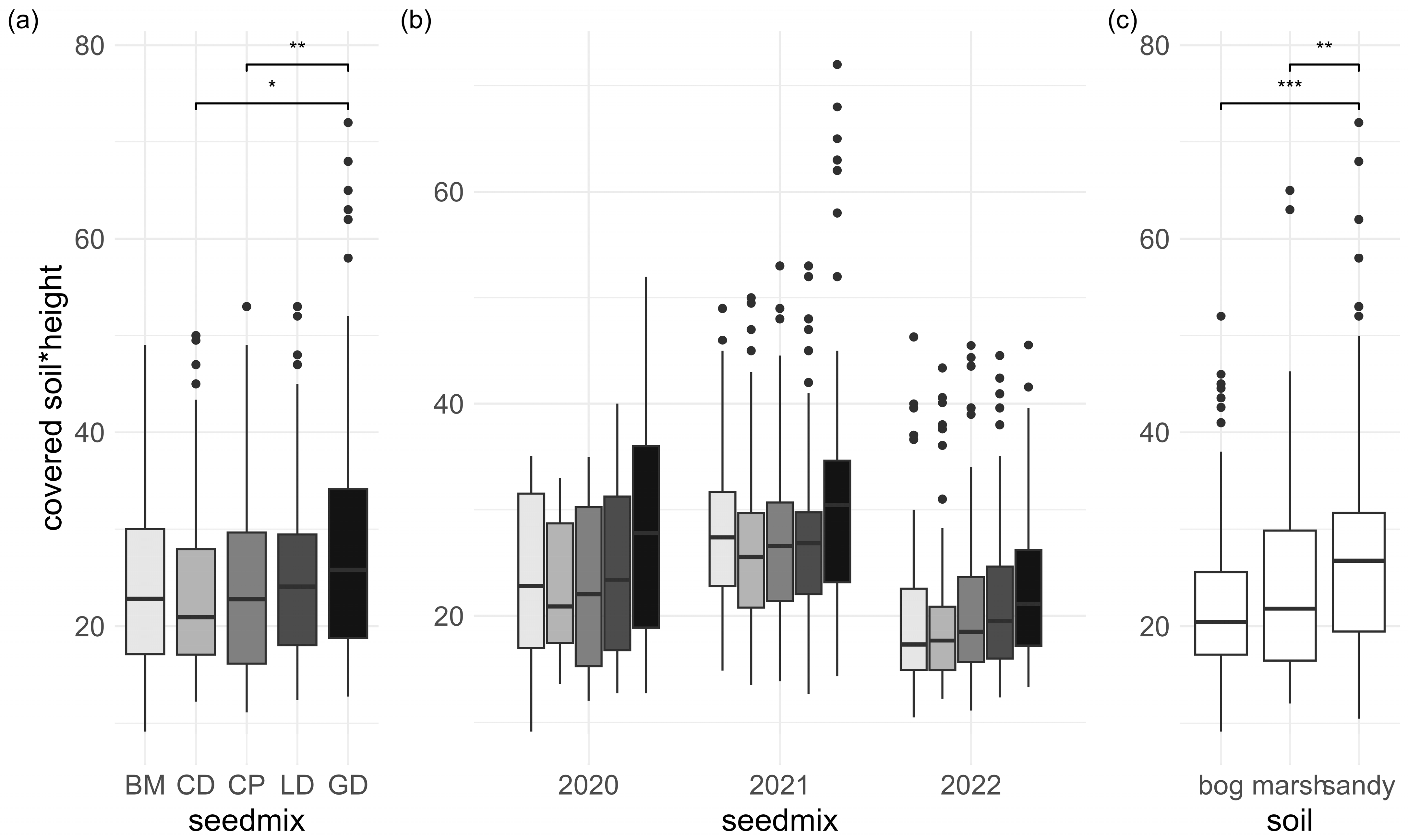
3.1. Establishment
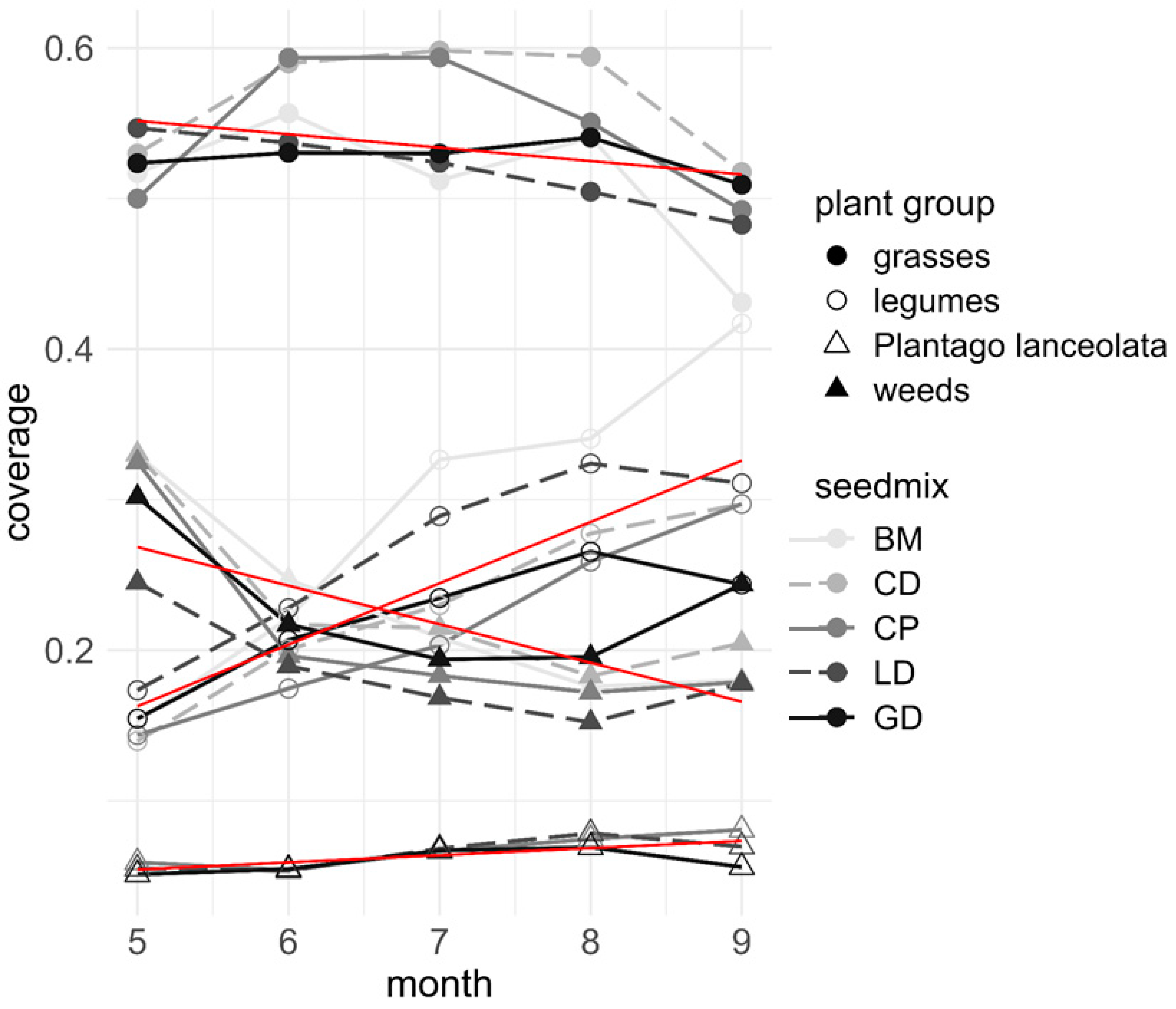
3.2. Plant Establishment
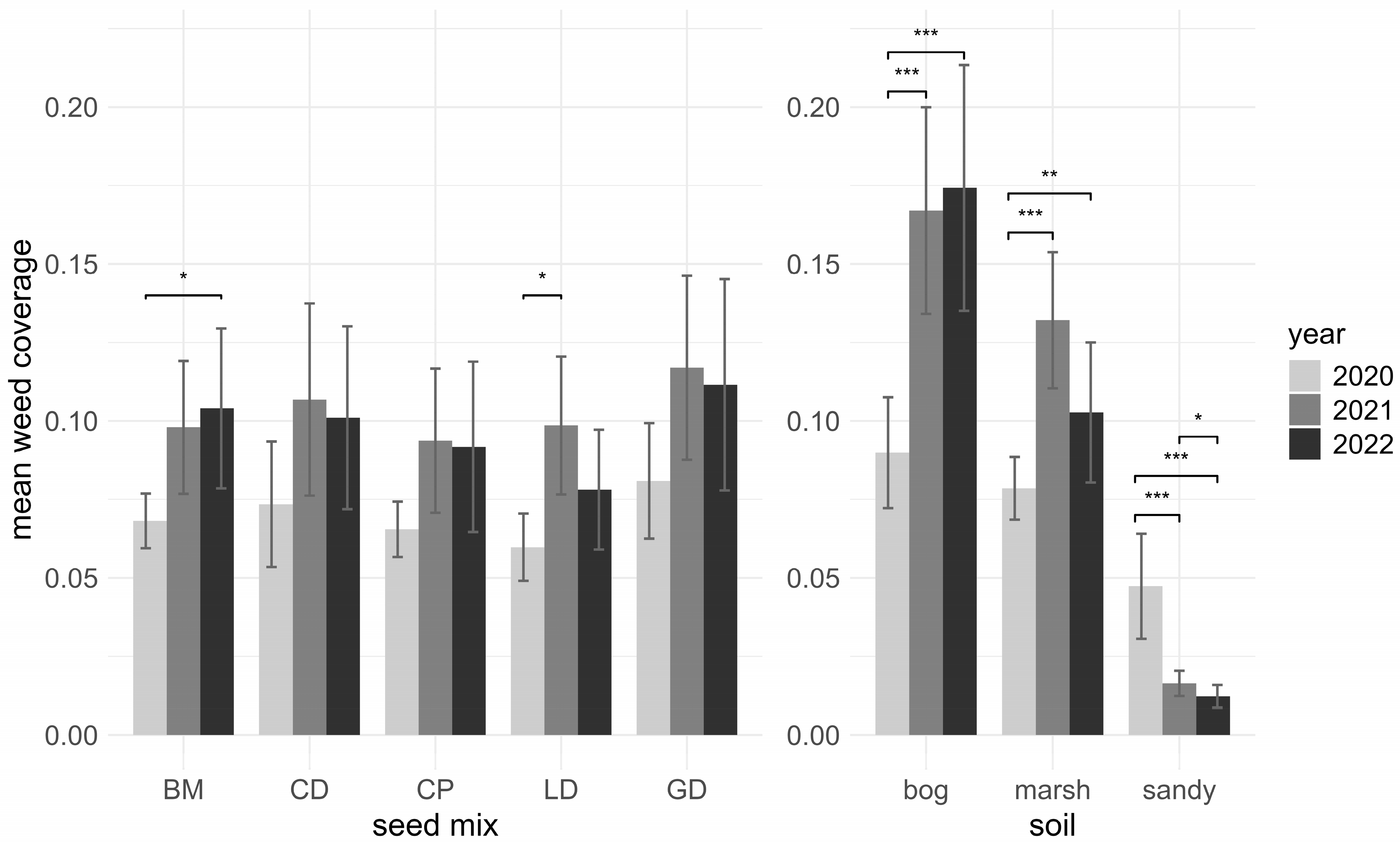
3.3. Weeds
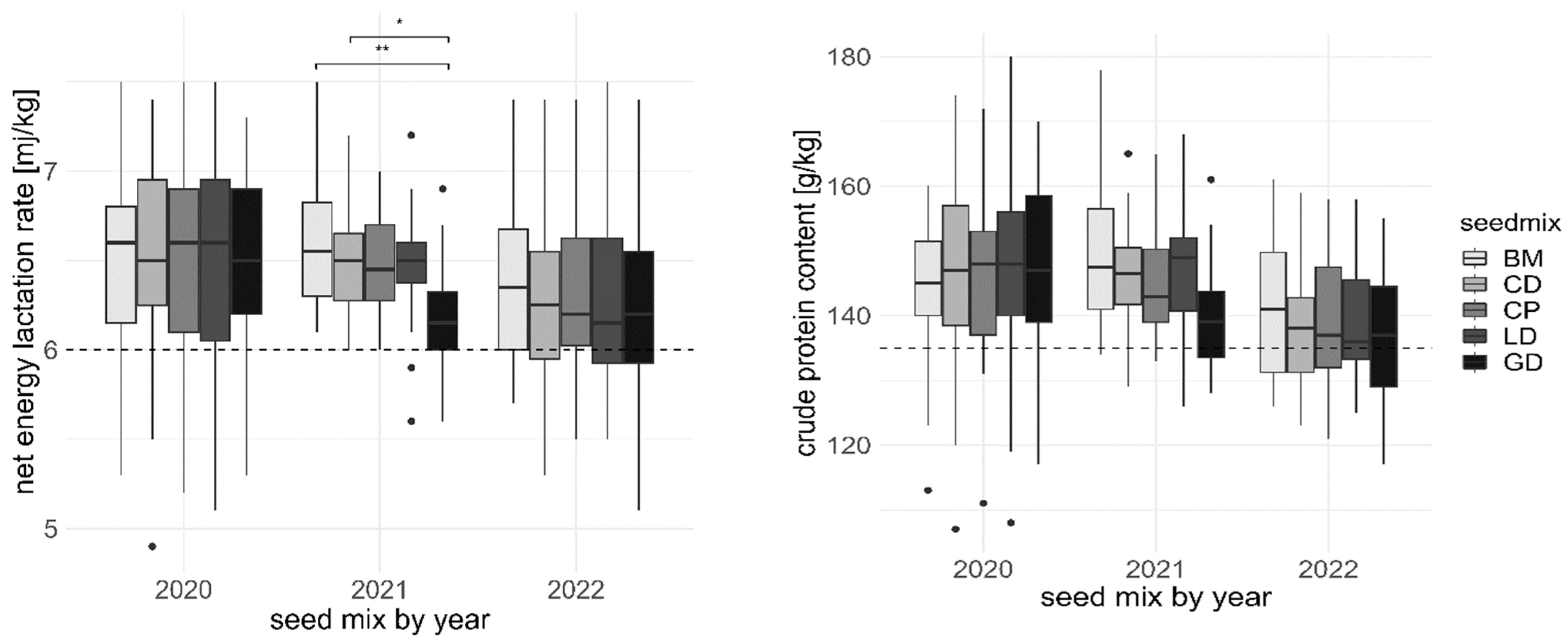
3.4. Fodder Quality
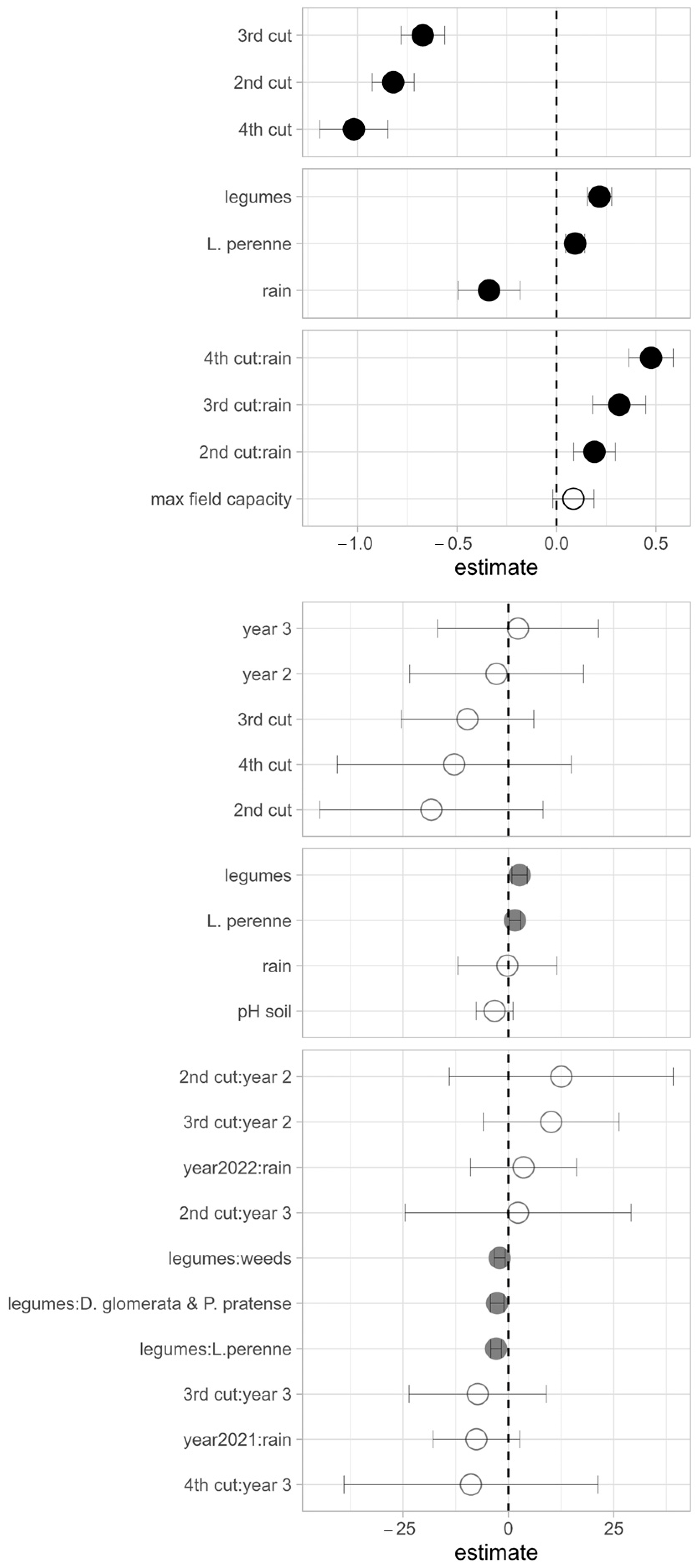
3.5. LMMs
4. Discussion
4.1. Mix and Species Performance
4.2. Weeds
4.3. Fodder Quality
4.4. Drought
4.5. Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutiérrez, J.; Ellis, W.N.; Fox, R.; Groom, Q.; Hennekens, S.; Van Landuyt, W.; Maes, D.; et al. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 2013, 16, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Virués, S.; Perović, D.J.; Gossner, M.M.; Börschig, C.; Blüthgen, N.; de Jong, H.; Simons, N.K.; Klein, A.-M.; Krauss, J.; Maier, G.; et al. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 2015, 6, 8568. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lewinsohn, T.M.; Kahl, T.; Grassein, F.; Boch, S.; Prati, D.; Birkhofer, K.; Renner, S.C.; Sikorski, J.; Wubet, T.; et al. Land-use intensification causes multitrophic homogenization of grassland communities. Nature 2016, 540, 266–269. [Google Scholar] [CrossRef]
- Grass, I.; Loos, J.; Baensch, S.; Batáry, P.; Librán-Embid, F.; Ficiciyan, A.; Klaus, F.; Riechers, M.; Rosa, J.; Tiede, J.; et al. Land-sharing/-sparing connectivity landscapes for ecosystem services and biodiversity conservation. People Nat. 2019, 1, 262–272. [Google Scholar] [CrossRef]
- Beckmann, M.; Gerstner, K.; Akin-Fajiye, M.; Ceaușu, S.; Kambach, S.; Kinlock, N.L.; Phillips, H.R.P.; Verhagen, W.; Gurevitch, J.; Klotz, S.; et al. Conventional land-use intensification reduces species richness and increases production: A global meta-analysis. Glob. Change Biol. 2019, 25, 1941–1956. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Pérez-Méndez, N.; Garratt, M.P.D.; Gemmill-Herren, B.; Miguez, F.E.; Dicks, L.V. Policies for Ecological Intensification of Crop Production. Trends Ecol. Evol. 2019, 34, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; van der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef]
- Lüscher, A.; Mueller-Harvey, I.; Soussana, J.F.; Rees, R.M.; Peyraud, J.L. Potential of legume-based grassland–livestock systems in Europe: A review. Grass Forage Sci. 2014, 69, 206–228. [Google Scholar] [CrossRef]
- Taube, F.; Gierus, M.; Hermann, A.; Loges, R.; Schönbach, P. Grassland and globalization—Challenges for north-west European grass and forage research. Grass Forage Sci. 2014, 69, 2–16. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Savage, J.; Bullock, J.M.; Nowakowski, M.; Orr, R.; Tallowin, J.R.B.; Pywell, R.F. Enhancing floral resources for pollinators in productive agricultural grasslands. Biol. Conserv. 2014, 171, 44–51. [Google Scholar] [CrossRef]
- Barneze, A.S.; Whitaker, J.; McNamara, N.P.; Ostle, N.J. Legumes increase grassland productivity with no effect on nitrous oxide emissions. Plant Soil 2020, 446, 163–177. [Google Scholar] [CrossRef]
- Barot, S.; Allard, V.; Cantarel, A.; Enjalbert, J.; Gauffreteau, A.; Goldringer, I.; Lata, J.-C.; Le Roux, X.; NIBOYET, A.; Porcher, E. Designing mixtures of varieties for multifunctional agriculture with the help of ecology. A review. Agron. Sustain. Dev. 2017, 37, 13. [Google Scholar] [CrossRef]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.; Allan, E.; Beßler, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic Appl. Ecol. 2017, 23, 1–73. [Google Scholar] [CrossRef]
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadottir, A.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-year continental-scale field experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef]
- Helgadóttir, Á.; Suter, M.; Gylfadóttir, T.Ó.; Kristjánsdóttir, T.A.; Lüscher, A. Grass–legume mixtures sustain strong yield advantage over monocultures under cool maritime growing conditions over a period of 5 years. Ann. Bot. 2018, 122, 337–348. [Google Scholar] [CrossRef]
- Peyraud, J.L.; Le Gall, A.; Lüscher, A. Potential food production from forage legume-based-systems in Europe: An overview. Ir. J. Agric. Food Res. 2009, 48, 115–135. [Google Scholar]
- Cong, W.-F.; Suter, M.; Lüscher, A.; Eriksen, J. Species interactions between forbs and grass-clover contribute to yield gains and weed suppression in forage grassland mixtures. Agric. Ecosyst. Environ. 2018, 268, 154–161. [Google Scholar] [CrossRef]
- Isbell, F.; Cowles, J.; Dee, L.E.; Loreau, M.; Reich, P.B.; Gonzalez, A.; Hector, A.; Schmid, B. Quantifying effects of biodiversity on ecosystem functioning across times and places. Ecol. Lett. 2018, 21, 763–778. [Google Scholar] [CrossRef]
- Humphreys, J.; Phelan, P.; Li, D.; Burchill, W.; Eriksen, J.; Casey, I.; Enriquez-Hidalgo, D.; Søegaard, K. White clover supported pasture-based systems in North-West Europe. In Legumes in Cropping Systems; Murphy-Bokern, D., Stoddard, F., Watson, C., Eds.; CABI: Wallingford, UK, 2017. [Google Scholar]
- Schaub, S.; Finger, R.; Buchmann, N.; Steiner, V.; Klaus, V.H. The costs of diversity: Higher prices for more diverse grassland seed mixtures. Environ. Res. Lett. 2021, 16, 094011. [Google Scholar] [CrossRef]
- Allen, V.G.; Batello, C.; Berretta, E.J.; Hodgson, J.; Kothmann, M.; Li, X.; McIvor, J.; Milne, J.; Morris, C.; Peeters, A.; et al. An international terminology for grazing lands and grazing animals. Grass Forage Sci. 2011, 66, 2–28. [Google Scholar] [CrossRef]
- Reichelt, G.; Wilmanns, O. Vegetationsgeographie; Westermann: Braunschweig, Germany, 1973. [Google Scholar]
- VDLUFA. Die Chemische Untersuchung von Futtermitteln. Methodenbuch Band III; VDLUFA-Verlag: Darmstadt, Germany, 2007. [Google Scholar]
- Delta-T Devices Ltd. SunScan Anaysis System Type SS1; Delta-T Devices Ltd.: Burwell, UK, 2016. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. Package ‘Car’: Companion to Applied Regression; 2020. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 15 April 2024).
- Ribeiro, P.J., Jr.; Diggle, P.J.; Christensen, O.; Schlather, M.; Bivand, R.; Ripley, B. ‘geoR’: Analysis of Geostatical Data. R Package Version 1.9-2. 2022. Available online: https://cran.r-project.org/web/packages/geoR/index.html (accessed on 15 April 2024).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics; 2021. Available online: https://cran.r-project.org/web/packages/DescTools/index.html (accessed on 15 April 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Voeten, C.C.; Voeten, M.C.C. Buildmer: Stepwise Elimination and Term Reordering for Mixed-Effects Regression; R Package Version 2.11. 2023. Available online: https://cran.r-project.org/web/packages/buildmer/buildmer.pdf (accessed on 15 April 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Lüscher, A.; Barkaoui, K.; Finn, J.A.; Suter, D.; Suter, M.; Volaire, F. Using plant diversity to reduce vulnerability and increase drought resilience of permanent and sown productive grasslands. Grass Forage Sci. 2022, 77, 235–246. [Google Scholar] [CrossRef]
- Grange, G.; Finn, J.A.; Brophy, C. Plant diversity enhanced yield and mitigated drought impacts in intensively managed grassland communities. J. Appl. Ecol. 2021, 58, 1864–1875. [Google Scholar] [CrossRef]
- Binder, S.; Isbell, F.; Polasky, S.; Catford, J.A.; Tilman, D. Grassland biodiversity can pay. Proc. Natl. Acad. Sci. USA 2018, 115, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Pol, M.; Schmidtke, K.; Lewandowska, S. Plantago lanceolata–An overview of its agronomically and healing valuable features. Open Agric. 2021, 6, 479–488. [Google Scholar] [CrossRef]
- Navarrete, S.; Kemp, P.D.; Pain, S.J.; Back, P.J. Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2016, 222, 158–167. [Google Scholar] [CrossRef]
- Cranston, L.M.; Kenyon, P.R.; Morris, S.T.; Lopez-Villalobos, N.; Kemp, P.D. Morphological and Physiological Responses of Plantain (Plantago lanceolata) and Chicory (Cichorium intybus) to Water Stress and Defoliation Frequency. J. Agron. Crop Sci. 2015, 202, 13–24. [Google Scholar] [CrossRef]
- Carlton, A.J.; Cameron, K.C.; Di, H.J.; Edwards, G.R.; Clough, T.J. Nitrate leaching losses are lower from ryegrass/white clover forages containing plantain than from ryegrass/white clover forages under different irrigation. N. Z. J. Agric. Res. 2019, 62, 150–172. [Google Scholar] [CrossRef]
- Nicholls, E.; Rands, S.A.; Botías, C.; Hempel de Ibarra, N. Flower sharing and pollinator health: A behavioural perspective. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210157. [Google Scholar] [CrossRef] [PubMed]
- Federal Plant Variety Office. Beschreibende Sortenliste: Futtergräser, Esparsette, Klee, Luzerne. In Beschreibende Sortenlisten; Federal Plant Variety Office: Hannover, Germany, 2020. [Google Scholar]
- Paplauskiené, V.; Dabkeviciené, G. A study of genetic diversity in Trifolium hybridum varieties using morphological characters and ISSR markers. Žemdirbystė 2012, 99, 313–318. [Google Scholar]
- Boschma, S.; Lodge, G.; Harden, S. Seedling competition of lucerne in mixtures with temperate and tropical pasture species. Crop Pasture Sci. 2010, 61, 411–419. [Google Scholar] [CrossRef]
- Marley, C.L.; Fychan, R.; Jones, R. Yield, persistency and chemical composition of Lotus species and varieties (birdsfoot trefoil and greater birdsfoot trefoil) when harvested for silage in the UK. Grass Forage Sci. 2006, 61, 134–145. [Google Scholar] [CrossRef]
- Taylor, N.L. A Century of Clover Breeding Developments in the United States. Crop Sci. 2008, 48, 1–13. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Harris, S.; Clark, D.; Auldist, M.; Waugh, C.; Laboyrie, P. Optimum white clover content for dairy pastures. Proc. N. Z. Grassl. Assoc. 1997, 59, 29–33. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Delaby, L.; Moloney, A.; Boland, T.; Lewis, E. Nutritive value of forage legumes used for grazing and silage. Ir. J. Agric. Food Res. 2009, 48, 167–187. [Google Scholar]
- Moloney, T.; Sheridan, H.; Grant, J.; O’Riordan, E.G.; O’Kiely, P. Conservation efficiency and nutritive value of silages made from grass-red clover and multi-species swards compared with grass monocultures. Ir. J. Agric. Food Res. 2020, 59, 150–166. [Google Scholar] [CrossRef]
- Rochon, J.J.; Doyle, C.J.; Greef, J.M.; Hopkins, A.; Molle, G.; Sitzia, M.; Scholefield, D.; Smith, C.J. Grazing legumes in Europe: A review of their status, management, benefits, research needs and future prospects. Grass Forage Sci. 2004, 59, 197–214. [Google Scholar] [CrossRef]
- Hancock, K.; Collette, V.; Chapman, E.; Hanson, K.; Temple, S.; Moraga, R.; Caradus, J. Progress towards developing bloat-safe legumes for the farming industry. Crop Pasture Sci. 2014, 65, 1107–1113. [Google Scholar] [CrossRef]
- Suter, M.; Connolly, J.; Finn, J.A.; Loges, R.; Kirwan, L.; Sebastià, M.-T.; Lüscher, A. Nitrogen yield advantage from grass–legume mixtures is robust over a wide range of legume proportions and environmental conditions. Glob. Change Biol. 2015, 21, 2424–2438. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Casler, M.D. Forage quality of five cool-season grasses. I. Cultivar effects. Anim. Feed Sci. Technol. 1990, 27, 197–207. [Google Scholar] [CrossRef]
- Marcuvitz, S.; Turkington, R. Differential effects of light quality, provided by different grass neighbours, on the growth and morphology of Trifolium repens L. (white clover). Oecologia 2000, 125, 293–300. [Google Scholar] [CrossRef]
- Maldonado Peralta, M.D.L.Á.; Rojas García, A.R.; Torres Salado, N.; Herrera Pérez, J.; Joaquín Cancino, S.; Ventura Ríos, J.; Hernández Garay, A.; Hernández Guzmán, F.J. Productivity of orchard grass (Dactylis glomerata L.) alone and associated with perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.). Rev. Bras. Zootec. 2017, 46, 890–895. [Google Scholar] [CrossRef][Green Version]
- Annicchiarico, P.; Proietti, S. White clover selected for enhanced competitive ability widens the compatibility with grasses and favours the optimization of legume content and forage yield in mown clover-grass mixtures. Grass Forage Sci. 2010, 65, 318–324. [Google Scholar] [CrossRef]
- Becker, T.; Isselstein, J.; Jürschik, R.; Benke, M.; Kayser, M. Performance of Modern Varieties of Festuca arundinacea and Phleum pratense as an Alternative to Lolium perenne in Intensively Managed Sown Grasslands. Agronomy 2020, 10, 540. [Google Scholar] [CrossRef]
- Zuppinger-Dingley, D.; Schmid, B.; Petermann, J.S.; Yadav, V.; De Deyn, G.B.; Flynn, D.F.B. Selection for niche differentiation in plant communities increases biodiversity effects. Nature 2014, 515, 108–111. [Google Scholar] [CrossRef]
- MacNaeidhe, F.S.; Curran, P.L. Weed Colonisation of Bog Taken into Cultivation and Seed Dormancy of Polygonum Invaders. Ir. J. Agric. Res. 1982, 21, 199–209. [Google Scholar]
- Hartmann, S.; Hochberg, H.; Riehl, G.; Wurth, W. Measuring the loss of dry matter yield effected by rough-stalked meadow-grass (Poa trivialis). In Proceedings of the Grassland Farming and Land Management Systems in Mountainous Regions: The 16th Symposium of the European Grassland Federation, Gumpenstein, Austria, 29–31 August 2011; Agricultural Research and Education Center (AREC) Raumberg-Gumpenstein: Raumberg-Gumpenstein, Austria, 2011; pp. 241–243. [Google Scholar]
- Connolly, J.; Sebastià, M.-T.; Kirwan, L.; Finn, J.A.; Llurba, R.; Suter, M.; Collins, R.P.; Porqueddu, C.; Helgadóttir, Á.; Baadshaug, O.H.; et al. Weed suppression greatly increased by plant diversity in intensively managed grasslands: A continental-scale experiment. J. Appl. Ecol. 2018, 55, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Robins, J.G.; Rigby, C.; Waldron, B.L. Comparative trends in forage nutritional quality across the growing season in 13 grasses. Can. J. Plant Sci. 2017, 97, 72–82. [Google Scholar] [CrossRef][Green Version]
- Komainda, M.; Muto, P.; Isselstein, J. Trade-off between forage quality and yield by adapting the harvesting regime to promote flowering in ley grassland. In Meeting the Future Demands for Grassland Production; European Grassland Federation, Natural Resources Institute Finland (Luke): Helsinki, Finnland, 2020; pp. 511–513. [Google Scholar]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Scherer-Lorenzen, M.; Weigelt, A. Grassland Resistance and Resilience after Drought Depends on Management Intensity and Species Richness. PLoS ONE 2012, 7, e36992. [Google Scholar] [CrossRef] [PubMed]
- Haughey, E.; Suter, M.; Hofer, D.; Hoekstra, N.J.; McElwain, J.C.; Lüscher, A.; Finn, J.A. Higher species richness enhances yield stability in intensively managed grasslands with experimental disturbance. Sci. Rep. 2018, 8, 15047. [Google Scholar] [CrossRef]
- Schärer, M.-L.; Lüscher, A.; Kahmen, A. Post-drought compensatory growth in perennial grasslands is determined by legacy effects of the soil and not by plants. New Phytol. 2023, 240, 2265–2275. [Google Scholar] [CrossRef]
| Species | Mix 1: BM | Mix 2: CD | Mix 3: CP | Mix 4: LD | Mix 5: GD |
|---|---|---|---|---|---|
| Lolium perenne ‘Indicus’ | 25% | 25% | 25% | 18% | 5% |
| Lolium perenne ‘Soraya’ | 20% | 20% | 20% | 17% | 10% |
| Lolium perenne ‘Melpaula’ | 25% | 25% | 22% | 18% | 5% |
| Lolium perenne ‘Melfrost’ | 21% | 21% | 21% | 17% | 10% |
| Trifolium repens ‘Bombus | 9% | 2% | 2% | 2% | 2% |
| Trifolium repens ‘Liflex’ | 2% | 2% | 2% | 2% | |
| Trifolium repens ‘Silvester’ | 2% | 2% | 2% | 2% | |
| Trifolium repens ‘Jura’ | 2% | 2% | 2% | 2% | |
| Trifolium repens ‘Pipolina’ | 1% | 1% | 1% | 1% | |
| Plantago lanceolata | 3% | 4% | 4% | ||
| Lotus corniculatus | 1% | 1% | |||
| Medicago sativa | 5% | 5% | |||
| Trifolium resupinatum | 4% | 4% | |||
| Trifolium hybridum | 1% | 1% | |||
| Medicago lupulina | 1% | 1% | |||
| Trifolium pratense | 5% | 5% | |||
| Dactylis glomerata | 10% | ||||
| Festuca arundinacea | 20% | ||||
| Phleum pratense | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albers, R.; Albach, D.C. Diversification of Intensively Used Grassland: Resilience and Good Fodder Quality across Different Soil Types. Agronomy 2024, 14, 1152. https://doi.org/10.3390/agronomy14061152
Albers R, Albach DC. Diversification of Intensively Used Grassland: Resilience and Good Fodder Quality across Different Soil Types. Agronomy. 2024; 14(6):1152. https://doi.org/10.3390/agronomy14061152
Chicago/Turabian StyleAlbers, Regine, and Dirk Carl Albach. 2024. "Diversification of Intensively Used Grassland: Resilience and Good Fodder Quality across Different Soil Types" Agronomy 14, no. 6: 1152. https://doi.org/10.3390/agronomy14061152
APA StyleAlbers, R., & Albach, D. C. (2024). Diversification of Intensively Used Grassland: Resilience and Good Fodder Quality across Different Soil Types. Agronomy, 14(6), 1152. https://doi.org/10.3390/agronomy14061152







