Selection of Improved Banana Diploid Resistant to Fusarium oxysporum f. sp. cubense Races 1 and Subtropical 4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fusarium Strains
2.2. Plant Materials and Growth
2.3. Plant Inoculation
2.4. Assessment of Symptoms
2.5. Histological and Histochemical Analysis
2.5.1. Whitening and Staining of Fungal Structures in Roots
2.5.2. Histochemical Analysis
2.5.3. Scanning Electron Microscopy
3. Results
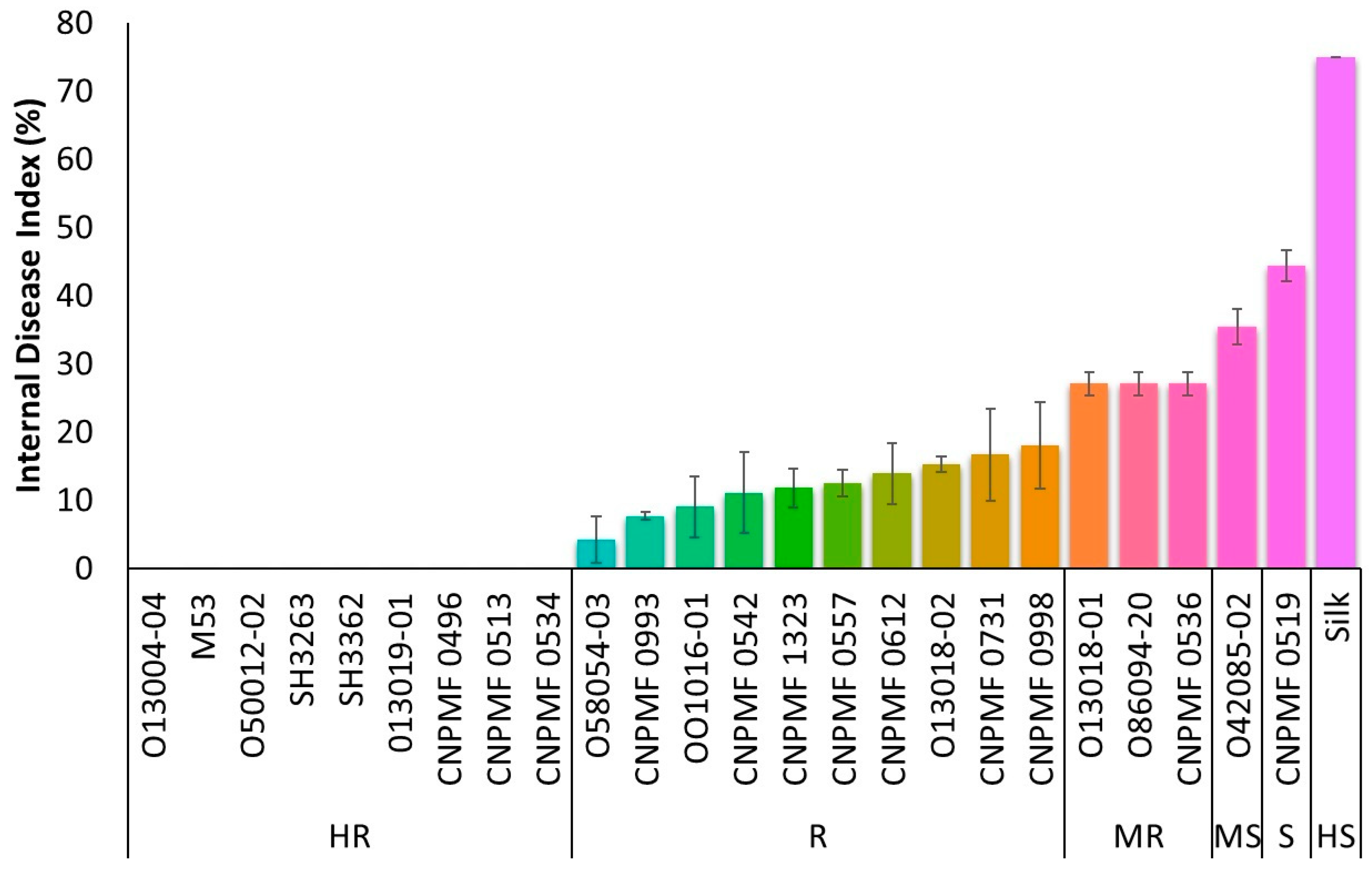
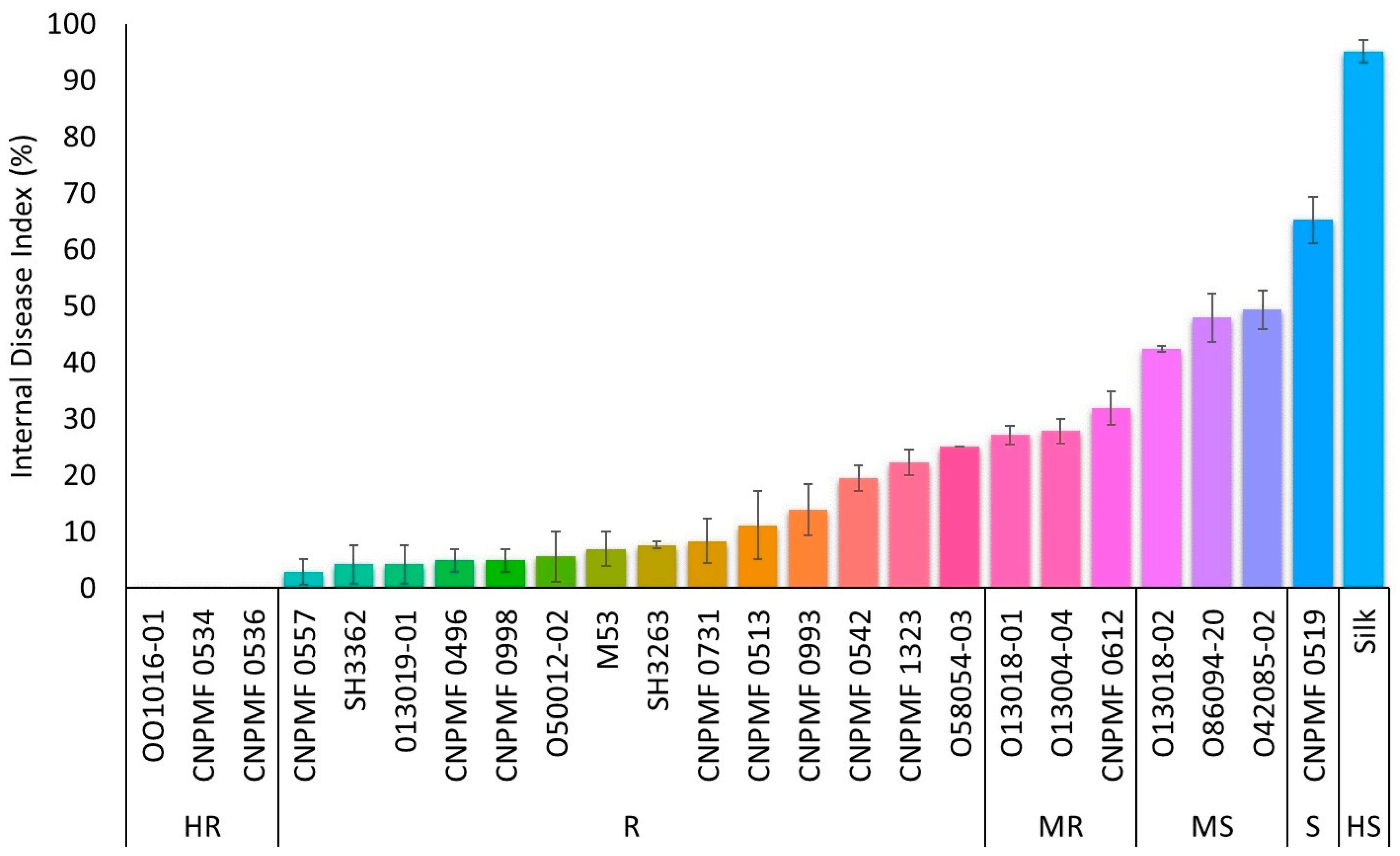
3.1. Screening for Resistance to Foc R1
3.2. Screening for Resistance to Foc ST4
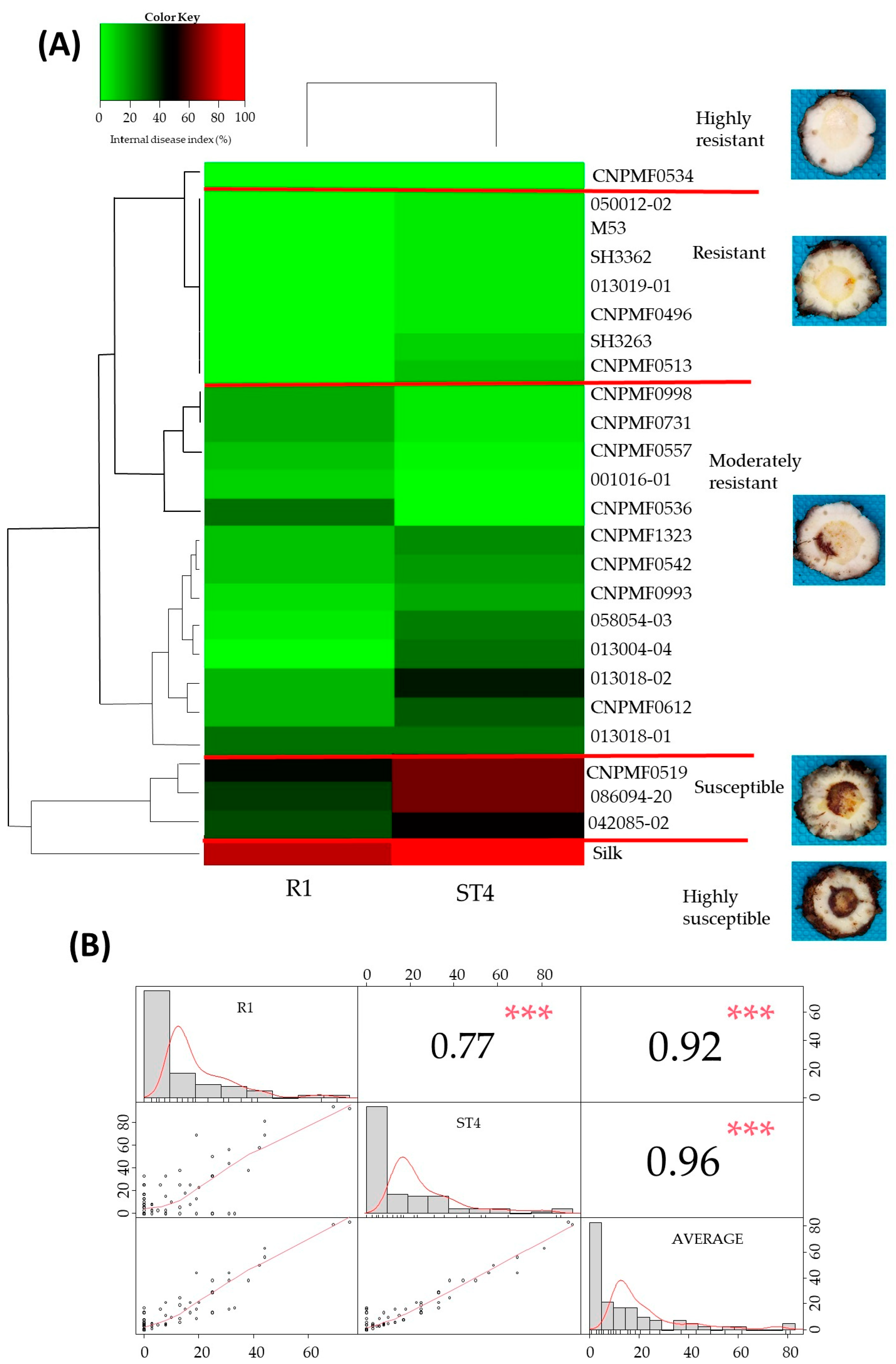
3.3. Cluster Analysis for Responses to Foc R1 and Foc ST4
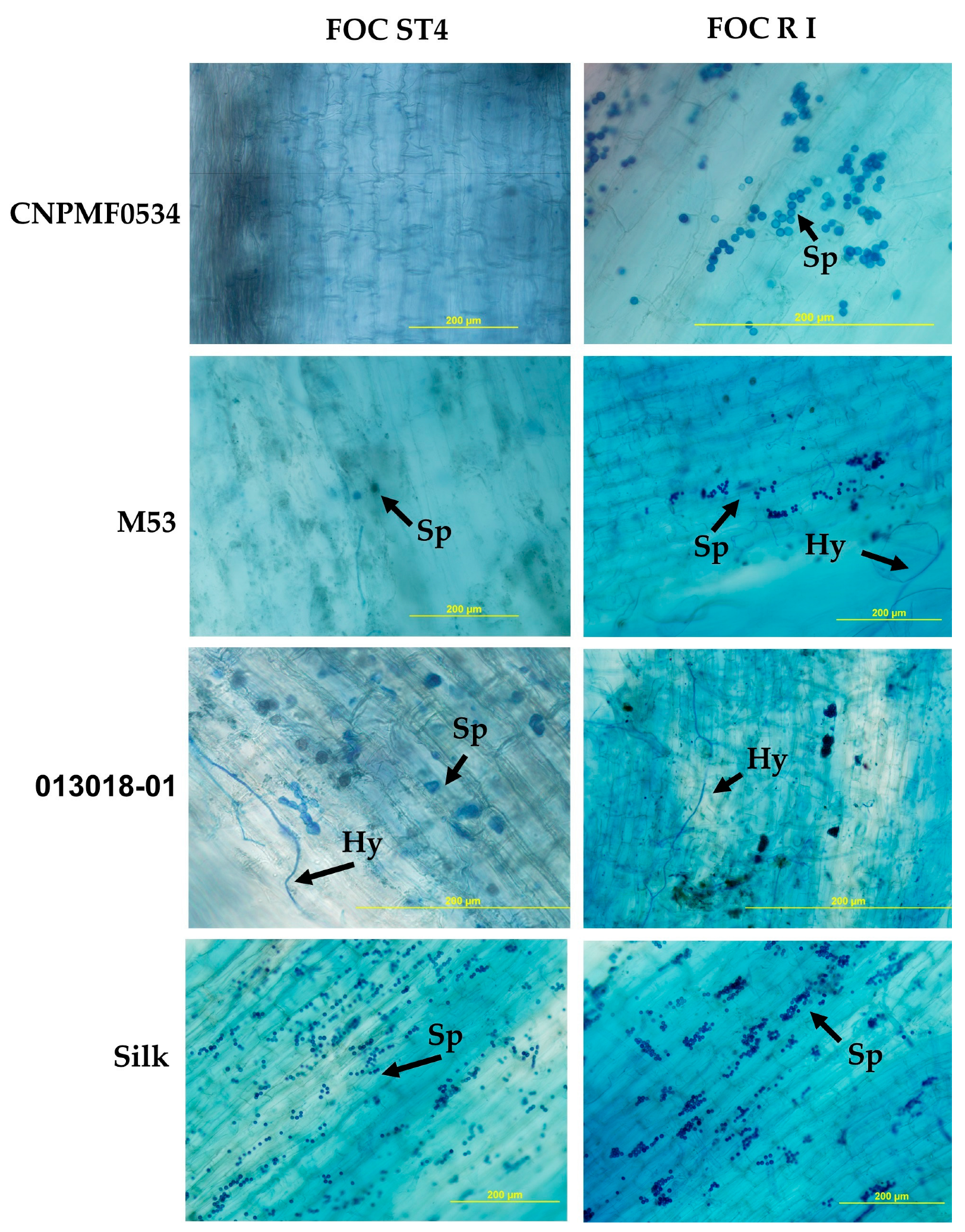
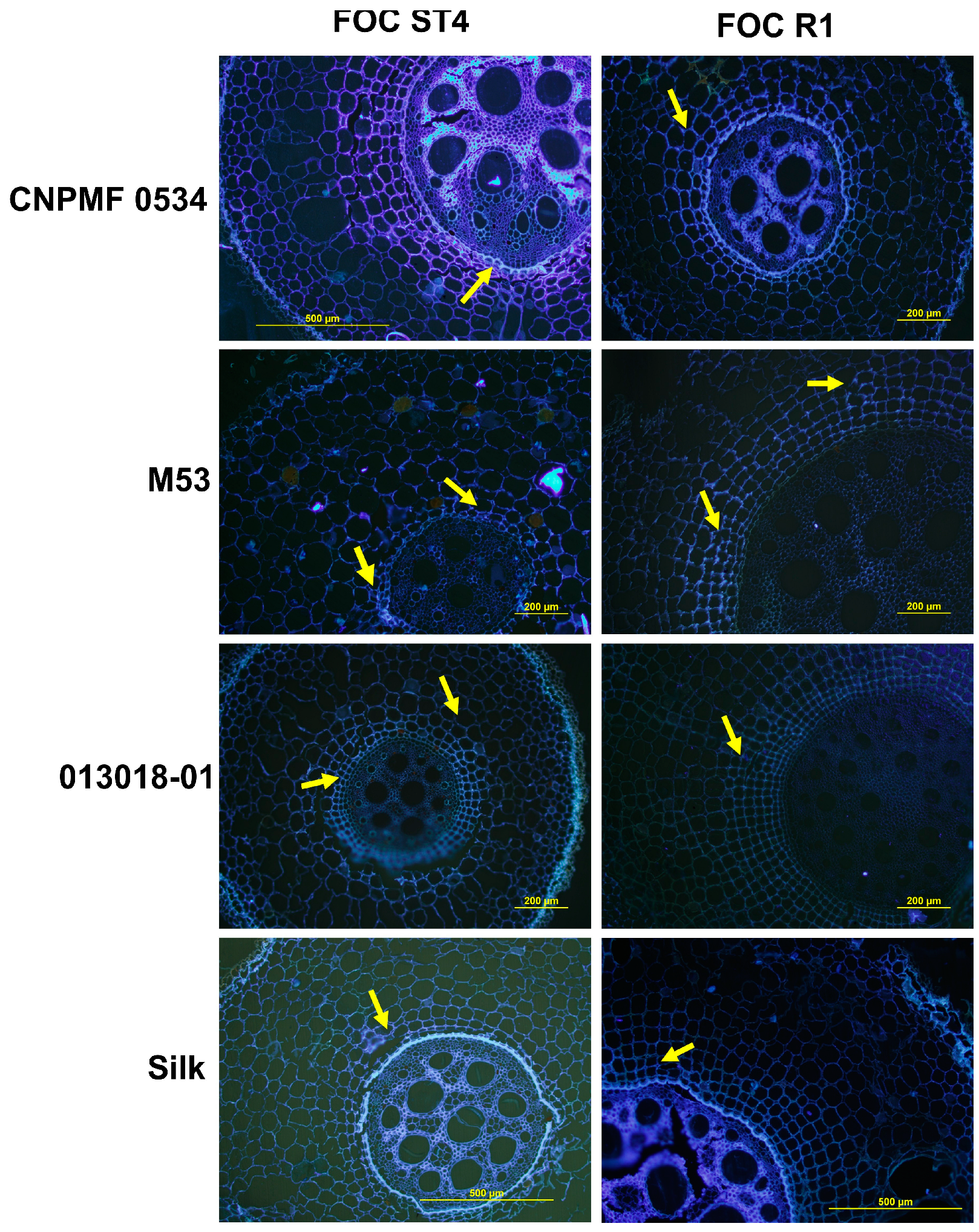
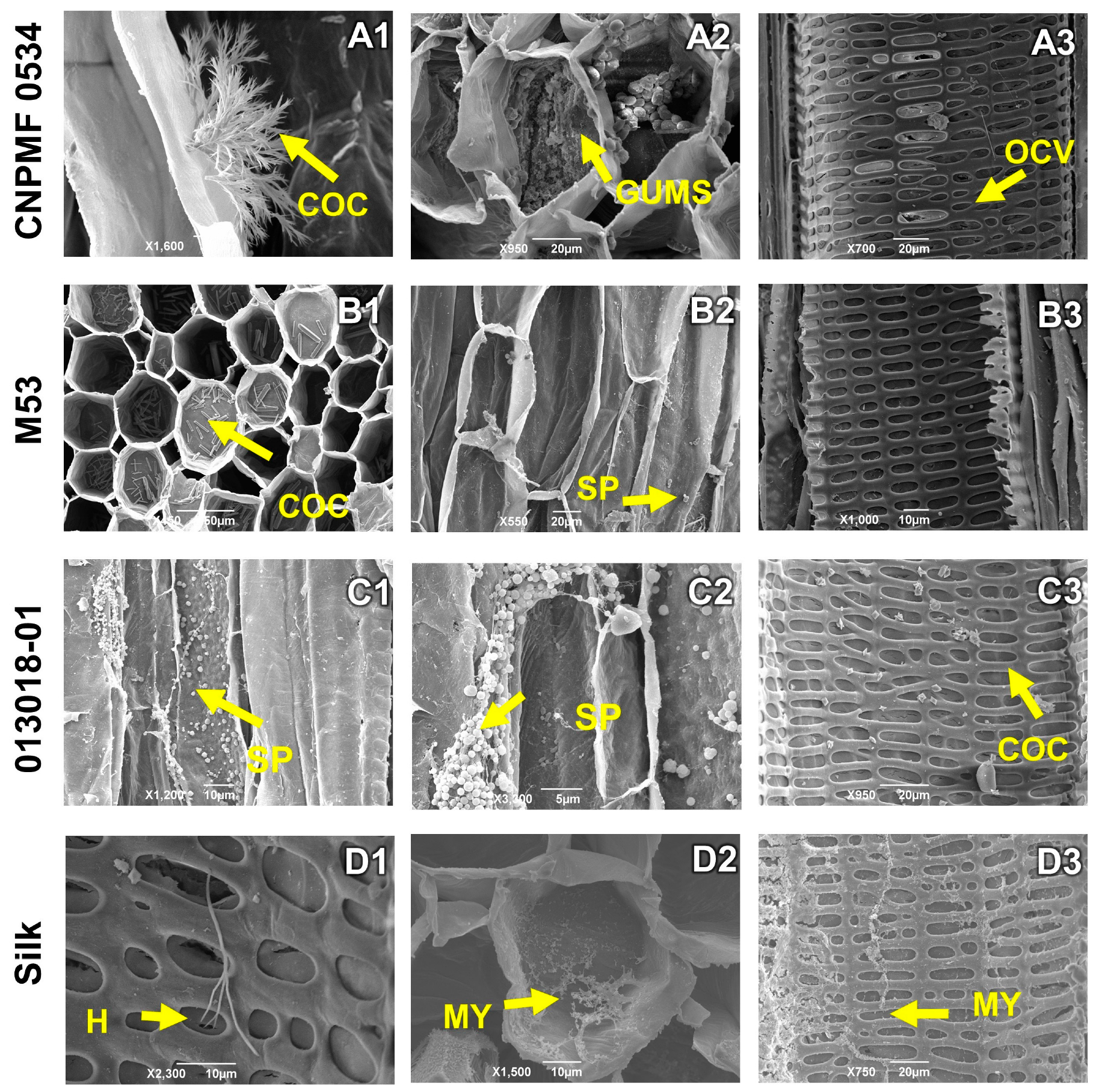
3.4. Histological and Histochemical Analyses
4. Discussion
4.1. Selection of Improved DIPLOIDS Resistant to Foc R1 and Foc ST4
4.2. Histological and Histochemical Responses of Improved Diploids Inoculated with Foc R1 and Foc ST4
4.3. Associations Based on Improved Diploid Genealogy and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2024. Available online: https://www.fao.org/faostat/en/#data (accessed on 19 March 2024).
- Mintoff, S.J.L.; Nguyen, T.V.; Kelly, C.; Cullen, S.; Hearnden, M.; Williams, R.; Daniells, J.W.; Tran-Nguyen, L.T.T. Banana Cultivar Field Screening for Resistance to Fusarium oxysporum f. sp. cubense Tropical Race 4 in the Northern Territory. J. Fungi 2021, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Sun, J.; Matthews, A.; Armas-Egas, L.; Chen, N.; Hamill, S.; Mintoff, S.; Tran-Nguyen, L.T.; Batley, J.; Aitken, E.A. Assessing Variations in Host Resistance to Fusarium oxysporum f. sp. cubense Race 4 in Musa Species, with a Focus on the Subtropical Race 4. Front. Microbiol. 2019, 10, 1062. [Google Scholar]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.C.; Heck, D.W.; Santos, A.; Alves, G.; Ferro, C.G.; Dita, M.; Mizubuti, E.S. The Brazilian population of Fusarium oxysporum f. sp. cubense is not structured by VCG or by geographic origin. bioRxiv 2022, 112, 11. [Google Scholar] [CrossRef]
- Buddenhagen, I. Understanding Strain Diversity in Fusarium oxysporum f. sp. cubense and History of Introduction of ‘Tropical Race 4’ to Better Manage Banana Production. Acta Hortic. 2009, 828, 193–204. [Google Scholar]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium wilt Tropical race 4 in Cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Martínez, G.; Olivares, B.O.; Rey, J.C.; Rojas, J.; Cardenas, J.; Muentes, C.; Dawson, C. The Advance of Fusarium Wilt Tropical Race 4 in Musaceae of Latin America and the Caribbean: Current Situation. Pathogens 2023, 12, 277. [Google Scholar] [CrossRef]
- Zheng, S.J.; García-Bastidas, F.A.; Li, X.D.; Zeng, L.; Bai, T.T.; Xu, S.T.; Yin, K.S.; Li, H.; Fu, G.; Yu, Y.C.; et al. New incursions of Fusarium oxysporum f. sp. cubense tropical Race 4 across the Greater Me kong Subregion. Front. Plant Sci. 2018, 4, 208–218. [Google Scholar]
- Rocha, A.D.J.; Soares, J.M.D.S.; Nascimento, F.D.S.; Santos, A.S.; Amorim, V.B.D.O.; Ferreira, C.F.; Haddad, F.; Santos-Serejo, J.A.D.; Amorim, E.P. Improvements in the Resistance of the Banana Species to Fusarium Wilt: A Systematic Review of Methods and Perspectives. J. Fungi 2022, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ge, X.; Wu, W.; Wang, W.; Hu, Y.; Mo, Y.; Xie, J. Identification of defense-related genes in banana roots infected by Fusarium oxysporum f. sp. cubense tropical race 4. Euphytica 2015, 205, 837–849. [Google Scholar]
- Rocha, A.D.J.; Soares, J.M.D.S.; Nascimento, F.D.S.; Rocha, A.d.S.; Amorim, V.B.O.D.; Ramos, E.S.T.E.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Molecular, histological and histochemical responses of banana cultivars challenged with Fusarium oxysporum f. sp. cubense with different levels of virulence. Plants 2022, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Dita, M.; Rouard, M.; Wu, W.; Roux, N.; Xie, J.H.; Ge, X.J. Deep RNA-seq analysis reveals key responding aspects of wild banana relative resistance to Fusarium oxysporum f. sp. cubense tropical race 4. Funct. Integr. Genom. 2020, 20, 551–562. [Google Scholar]
- Zuo, C.; Deng, G.; Li, B.; Huo, H.; Li, C.; Hu, C.; Kuang, R.; Yang, Q.; Dong, T.; Sheng, O.; et al. Germplasm screening of Musa spp. for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Eur. J. Plant Pathol. 2018, 151, 723–734. [Google Scholar]
- Gonçalves, Z.S.; Rocha, A.D.J.; Haddad, F.; Amorim, V.B.O.; Ferreira, C.F.; Amorim, E.P. Selection of Diploid and Tetraploid Banana Hybrids Resistant to Pseudocercospora fijiensis. Agronomy 2021, 11, 2483. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Microbiologia de Brock, 14th ed.; Artmed Editora: Porto Alegre, Brazil, 2016. [Google Scholar]
- Dita, M.A.; Pérez Vicente, L.; Martínez, E. Inoculation of Fusarium oxysporum f. sp. cubense causal agent of fusarium wilt in banana. In Technical Manual: Prevention and Diagnostic of Fusarium Wilt of Banana Caused by Fusarium Oxysporum f. sp. cubense Tropical Race 4 (TR4); Pérez Vicente, L., Dita, M.A., de la Parte, E.M., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 55–58. [Google Scholar]
- Mckinney, H.H. Influence of soil, temperature and on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 1923, 26, 195–217. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Karnovsk, M.J. A formaldehydeglutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137. [Google Scholar]
- Foster, A.S. Practical Plant Anatomy, 2nd ed.; Van Nostrand: Torronto, ON, Canada, 1949; p. 228. [Google Scholar]
- Ndayihanzamaso, P.; Mostert, D.; Matthews, M.C.; Mahuku, G.; Jomanga, K.; Mpanda, H.J.; Mduma, H.; Brown, A.; Uwimana, B.; Swennen, R.; et al. Evaluation of Mchare and Matooke Bananas for Resistance to Fusarium oxysporum f. sp. cubense Race 1. Plants 2020, 9, 1082. [Google Scholar] [CrossRef]
- Li, C.Q.; Yang, J.H.; Li, W.B.; Sun, J.B.; Peng, M. Direct Root Penetration and Rhizome Vascular Colonization by Fusarium oxysporum f. sp. cubense are the Key Steps in the Successful Infection of Brazil Cavendish. Plant Dis. 2017, 101, 2073–2078. [Google Scholar] [PubMed]
- Rebouças, T.A.; Haddad, F.; Ferreira, C.F.; de Oliveira, S.A.S.; Ledo, C.A.D.S.; Amorim, E.P. Identification of banana genotypes resistant to Fusarium wilt race 1 under field and greenhouse conditions. Sci. Hortic. 2018, 239, 308–313. [Google Scholar] [CrossRef]
- Nakata, P.A. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Ceita, G.D.O.; Macedo, J.N.A.; Santos, T.B.; Alemanno, L.; Gesteira, A.D.; Micheli, F.; Mariano, A.C.; Gramacho, K.P.; Silva, D.D.C.; Meinhardt, L.; et al. Involvement of calcium oxalate degradation during programmed cell death in Theobroma cacao tissues triggered by the hemibiotrophic fungus Moniliophthora pemiciosa. Plant Sci. 2007, 173, 106–117. [Google Scholar] [CrossRef]
- Zavaliev, R.; Ueki, S.; Epel, B.L.; Citovsky, V. Biology of callose (β-1, 3-glucan) turnover at plasmodesmata. Protoplasma 2011, 248, 117–130. [Google Scholar] [CrossRef]
- Dong, H.; Ye, Y.; Guo, Y.; Li, H. Comparison transcriptome analysis revealed resistance differences of Cavendish bananas to Fusarium oxysporum f. sp. cubense race1 and race 4. BMC Genom. 2020, 21, 122. [Google Scholar]
- García-Velasco, R.; Portal-González, N.; Santos-Bermúdez, R.; Rodríguez-García, A.; Companioni-González, B. Genetic improvement for resistance to Fusarium wilt in banana. Rev. Mex. Fitopatol. 2021, 39, 122–146. [Google Scholar] [CrossRef]
- Peraza-Echeverria, S.; Dale, J.L.; Harding, R.M.; Smith, M.K.; Collet, C. Characterization of disease resistance gene candidates of the nucleotide binding site (NBS) type from banana and correlation of a transcriptional polymorphism with resistance to Fusarium oxysporum f. sp. cubense race 4. Mol. Breed. 2008, 22, 565–579. [Google Scholar]
- Dale, J.; James, A.; Paul, J.Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Harding, R. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nat. Commun. 2017, 8, 1496. [Google Scholar] [CrossRef]
- D’Hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 21, 213–217. [Google Scholar] [CrossRef]
- Better Bananas. Panama TR4 Variety Screening Trial (December 2018) Sub-Trial Results (Plant and First Ratoon). Available online: https://betterbananas.com.au/2022/03/04/panama-tr4-variety-screening-trial-december-2018-sub-trial-results-plant-and-first-ratoon/ (accessed on 3 January 2023).

| Genotype | Genealogy |
|---|---|
| M53 | [(Malaccensis − Kedah × Banksii − Samoa)] × [(Paka × Banksii − Samoa)] |
| 001016-01 | Borneo × Guyod |
| 013004-04 | Malaccensis × Madang |
| 013018-01 | Malaccensis × Sinwobogi |
| 013018-02 | Malaccensis × Sinwobogi |
| 042085-02 | M53 × [(Madu × Calcutta 4)] |
| 050012-02 | M61 × Lidi |
| 058054-03 | [(Calcutta 4 × Pahang)] × [(Borneo × Madang)] |
| 086094-20 | [(Calcutta 4 × Galeo)] × SH3263 |
| SH3263 | - |
| SH3362 | - |
| 013019-01 | Malaccensis × Tjau Lagada |
| CNPMF 0557 | [(M61 × Pisang Lilin)] × [(Malaccensis × Tjau Lagada)] |
| CNPMF 0496 | [(M61 × Pisang Lilin)] × [(Terrinha × Calcutta 4)] |
| CNPMF 0536 | [(Calcutta 4 × Madang)] × [(Borneo × Guyod)] |
| CNPMF 0542 | [(SH3263)] × [(Malaccensis × Sinwobogi)] |
| CNPMF 0612 | [(M53 × Madu) × Madu)] × SH3263 |
| CNPMF 0731 | [(Malaccensis × Madang)] × [(Tuu Gia × Calcutta 4)] |
| CNPMF 0998 | [(Borneo × Guyod)] × [(Borneo × Guyod) × SH3263] |
| CNPMF 1323 | [(Malaccensis × Sinwobogi)] × [(Calcutta 4 × Heva)] |
| CNPMF 0513 | [(M61 × Pisang Lilin)] × [(M53 × Kumburgh) |
| CNPMF 0519 | Self-fertilization (wild diploid Tambi) |
| CNPMF 0534 | [(Calcutta 4 × Madang)] × [(Borneo × Guyod)] |
| CNPMF 0993 | [(Borneo × Guyod) × (Tuu Gia × Calcutta 4)] × [(Khai × (Calcutta 4 × Madang)] |
| Silk | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, W.S.; Rocha, A.d.J.; da Silva, W.B.; Amorim, V.B.O.d.; Ramos, A.P.d.S.; Haddad, F.; Amorim, E.P. Selection of Improved Banana Diploid Resistant to Fusarium oxysporum f. sp. cubense Races 1 and Subtropical 4. Agronomy 2024, 14, 1277. https://doi.org/10.3390/agronomy14061277
Santana WS, Rocha AdJ, da Silva WB, Amorim VBOd, Ramos APdS, Haddad F, Amorim EP. Selection of Improved Banana Diploid Resistant to Fusarium oxysporum f. sp. cubense Races 1 and Subtropical 4. Agronomy. 2024; 14(6):1277. https://doi.org/10.3390/agronomy14061277
Chicago/Turabian StyleSantana, Welly Sacramento, Anelita de Jesus Rocha, Wesley Barreto da Silva, Vanusia Batista Oliveira de Amorim, Andresa Priscila de Souza Ramos, Fernando Haddad, and Edson Perito Amorim. 2024. "Selection of Improved Banana Diploid Resistant to Fusarium oxysporum f. sp. cubense Races 1 and Subtropical 4" Agronomy 14, no. 6: 1277. https://doi.org/10.3390/agronomy14061277
APA StyleSantana, W. S., Rocha, A. d. J., da Silva, W. B., Amorim, V. B. O. d., Ramos, A. P. d. S., Haddad, F., & Amorim, E. P. (2024). Selection of Improved Banana Diploid Resistant to Fusarium oxysporum f. sp. cubense Races 1 and Subtropical 4. Agronomy, 14(6), 1277. https://doi.org/10.3390/agronomy14061277






