Land Degradation Affects Soil Microbial Properties, Organic Matter Composition, and Maize Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Soil Analyses
2.3.1. Soil Basic Physiochemical Properties Analysis
2.3.2. FTIR Analyses of SOM Spectroscopic Properties
2.3.3. Soil Microbial Properties Analyses
2.4. Maize Yield Measurement
2.5. Statistical Analyses
3. Results
3.1. Response of Soil Physiochemical Properties to Land Degradation
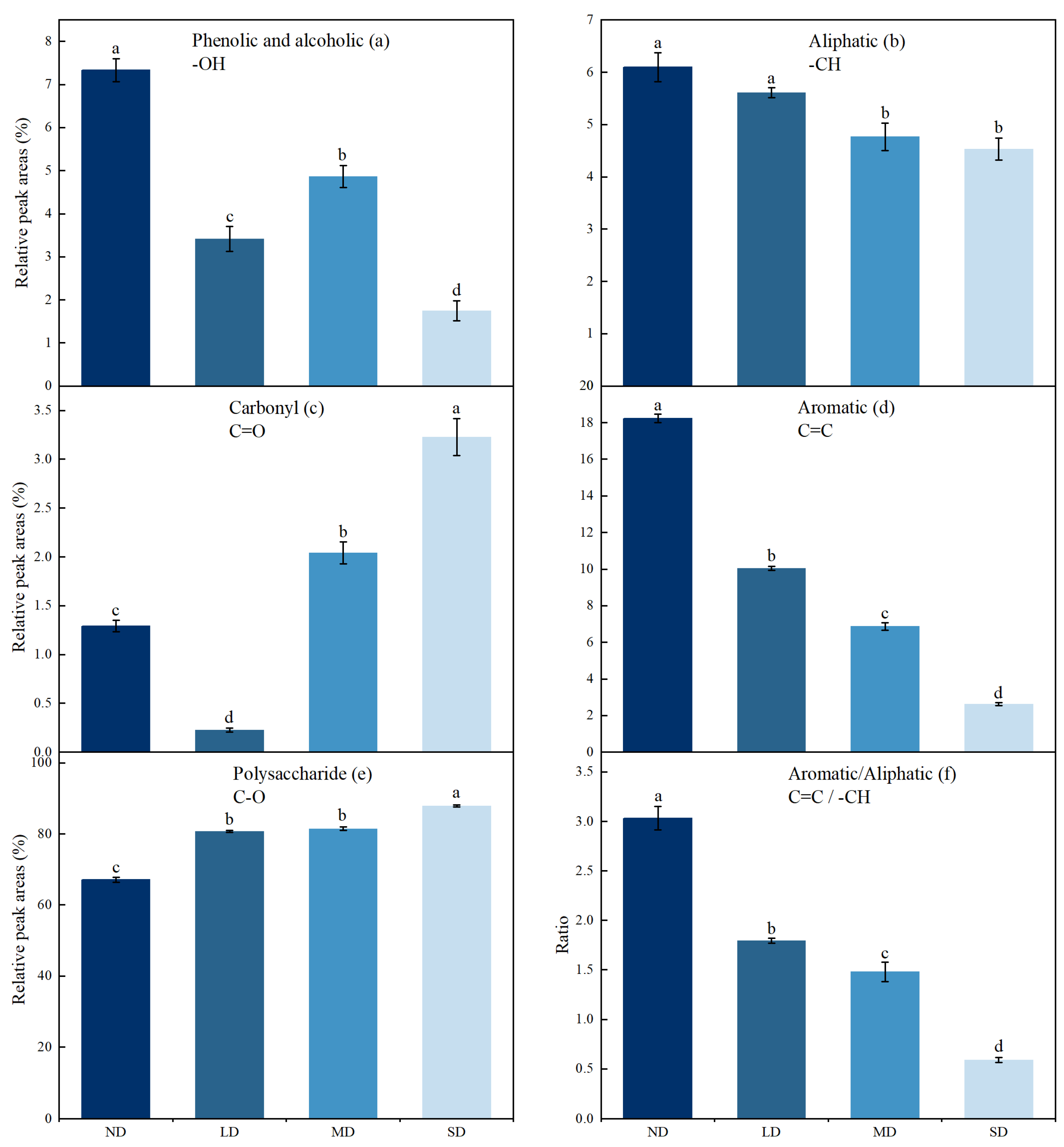
3.2. Response of SOM Functional Groups from FTIR to Land Degradation
3.3. Response of Soil Microbial Properties to Land Degradation
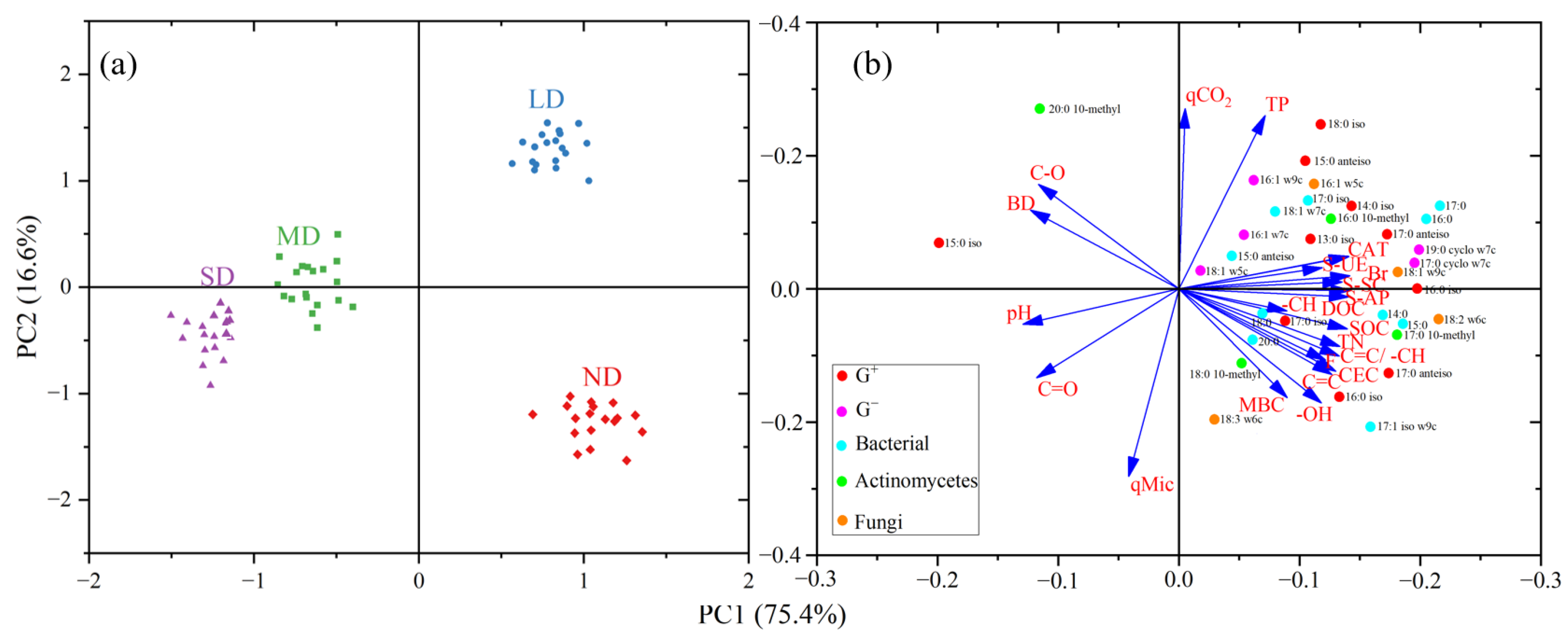
3.4. Relationship between Microbial Properties and SOM Composition
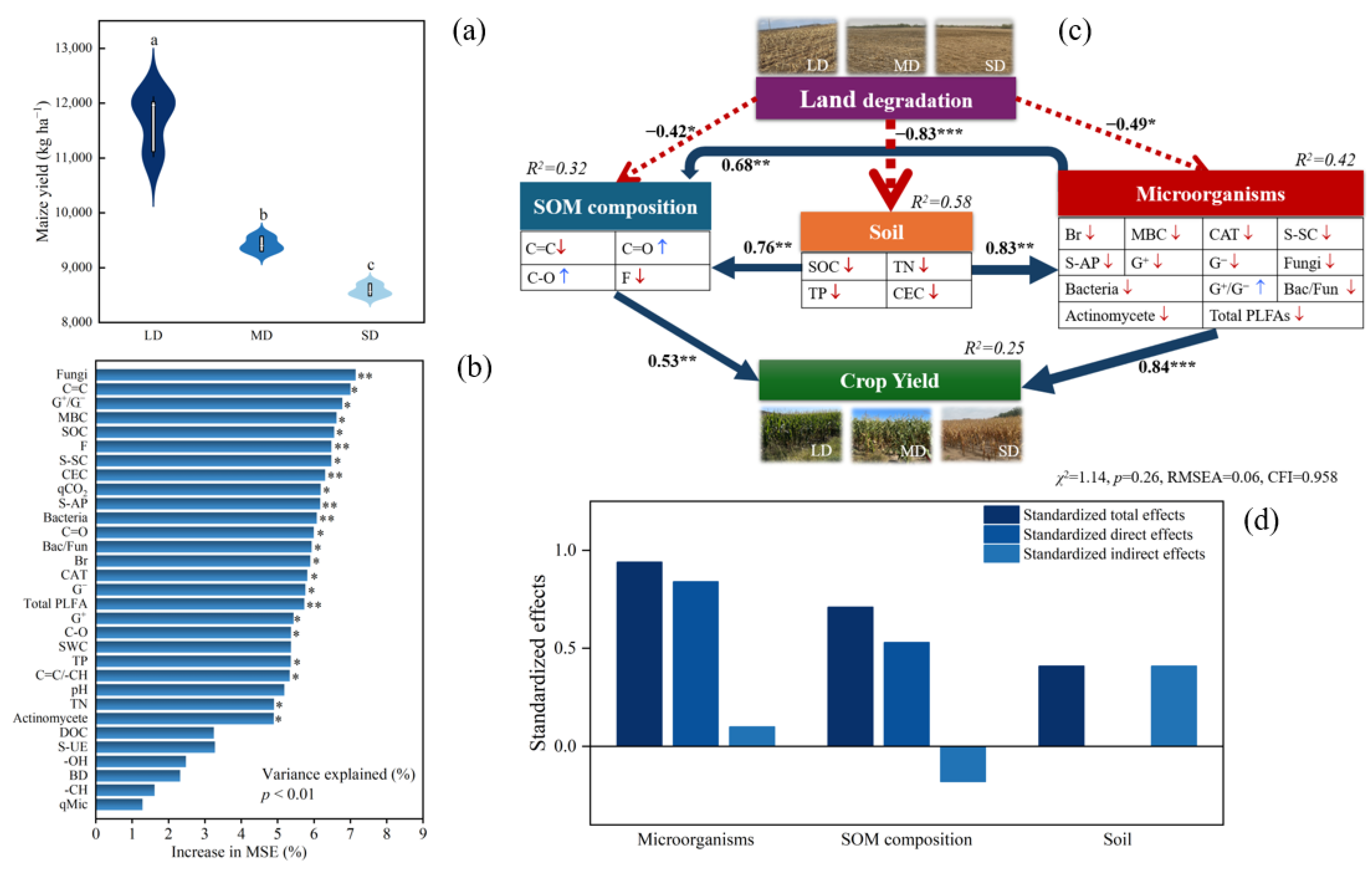
3.5. Maize Yield and Its Influencing Factors
4. Discussion
4.1. Changes in SOM Composition under Land Degradation
4.2. Microbial Property Responses to Land Degradation
4.3. Relationships among SOM Composition, Microbial Properties, and Maize Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil Microbiota as Game-Changers in Restoration of Degraded Lands. Science 2022, 375, eabe0725. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- United Nations. World Soil Day. Available online: https://www.un.org/en/observances/world-soil-day (accessed on 6 June 2024).
- Lal, R. Soil Erosion and the Global Carbon Budget. Environ. Int. 2003, 29, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Speth, J.G. Towards an Effective and Operational International Convention on Desertification; ESQUEL/PNUD/Governo do Ceará/BNB: Fortaleza, Brazil, 1994. [Google Scholar]
- Jiang, G.; Zhang, Y.; Wei, X.; Zhang, D.; Liu, S. The soil infrared spectral characteristics of soil organic matter under different Carbon saturation levels. Sci. Agric. Sin. 2018, 51, 3117–3129. [Google Scholar]
- He, Y.; Zhang, F.; Yang, M.; Li, X.; Wang, Z. Insights from Size Fractions to Interpret the Erosion-Driven Variations in Soil Organic Carbon on Black Soil Sloping Farmland, Northeast China. Agric. Ecosyst. Environ. 2023, 343, 108283. [Google Scholar] [CrossRef]
- Zhang, M. Physicochemical Properties and Spectral Characteristics of Soluble Organic Matter of Main Grassland Soils and Their Responses to the Utilization Patterns in Qinghai Province. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2020. [Google Scholar]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of Straw Carbon Input on Carbon Dynamics in Agricultural Soils: A Meta-Analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Ellerbrock, R.H.; Gerke, H.H. Chapter Three—Characterization of Organic Matter Composition of Soil and Flow Path Surfaces Based on Physicochemical Principles—A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 121, pp. 117–177. [Google Scholar]
- Linker, R. Application of FTIR Spectroscopy to Agricultural Soils Analysis. In Fourier Transforms—New Analytical Approaches and FTIR Strategies; IntechOpen Limited: London, UK, 2011; ISBN 978-953-307-232-6. [Google Scholar]
- Margenot, A.J.; Calderón, F.J.; Bowles, T.M.; Parikh, S.J.; Jackson, L.E. Soil Organic Matter Functional Group Composition in Relation to Organic Carbon, Nitrogen, and Phosphorus Fractions in Organically Managed Tomato Fields. Soil Sci. Soc. Am. J. 2015, 79, 772–782. [Google Scholar] [CrossRef]
- Pandey, D.; Agrawal, M.; Singh Bohra, J.; Adhya, T.K.; Bhattacharyya, P. Recalcitrant and Labile Carbon Pools in a Sub-Humid Tropical Soil under Different Tillage Combinations: A Case Study of Rice–Wheat System. Soil Tillage Res. 2014, 143, 116–122. [Google Scholar] [CrossRef]
- Sun, T.; Mao, X.; Ma, Q.; Han, K.; Wang, X.; Cheng, Q.; Sheng, X.; Mi, W.; Wu, L. Response of Rice Yield to Organic Amendments Was Regulated by Soil Chemical Properties, Microbial Functional Genes and Bacterial Community Rather than Fungal Community. Appl. Soil Ecol. 2023, 188, 104923. [Google Scholar] [CrossRef]
- Gao, T.; Tian, H.; Wang, Z.; Shi, J.; Yang, R.; Wang, F.; Xiang, L.; Dai, Y.; Megharaj, M.; He, W. Effects of Atrazine on Microbial Metabolic Limitations in Black Soils: Evidence from Enzyme Stoichiometry. Chemosphere 2023, 334, 139045. [Google Scholar] [CrossRef]
- Mandal, D.; Chandrakala, M.; Alam, N.M.; Roy, T.; Mandal, U. Assessment of Soil Quality and Productivity in Different Phases of Soil Erosion with the Focus on Land Degradation Neutrality in Tropical Humid Region of India. Catena 2021, 204, 105440. [Google Scholar] [CrossRef]
- Liu, K.; Li, T.; Duan, X.; Zhang, S.; Chen, M.; Hou, H.; Wang, Z.; Yu, A.; Chen, D.; Zhang, X.; et al. The Degradation of Subalpine Meadows Significantly Changed the Soil Microbiome. Agric. Ecosyst. Environ. 2023, 349, 108470. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil Moisture Mediates Microbial Carbon and Phosphorus Metabolism during Vegetation Succession in a Semiarid Region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Yao, Y. Soil Aggregates Stability and Organic Carbon Content Changes and Its Mechanism in Horqin Degraded Grassland. Master’s Thesis, Northeast Normal University, Changchun, China, 2021. (In Chinese). [Google Scholar]
- Kasel, S.; Bennett, L.T. Land-Use History, Forest Conversion, and Soil Organic Carbon in Pine Plantations and Native Forests of South Eastern Australia. Geoderma 2007, 137, 401–413. [Google Scholar] [CrossRef]
- Breidenbach, A.; Schleuss, P.-M.; Liu, S.; Schneider, D.; Dippold, M.A.; de la Haye, T.; Miehe, G.; Heitkamp, F.; Seeber, E.; Mason-Jones, K.; et al. Microbial Functional Changes Mark Irreversible Course of Tibetan Grassland Degradation. Nat. Commun. 2022, 13, 2681. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Chen, Y.; Luo, Y.; Lian, J.; Niu, Y.; Gong, X.; Yang, H.; Yu, P. Changes in Surface Soil Organic Carbon in Semiarid Degraded Horqin Grassland of Northeastern China between the 1980s and the 2010s. Catena 2019, 174, 217–226. [Google Scholar] [CrossRef]
- Li, N.; Lei, W.; Sheng, M.; Long, J.; Han, Z. Straw Amendment and Soil Tillage Alter Soil Organic Carbon Chemical Composition and Are Associated with Microbial Community Structure. Eur. J. Soil Biol. 2022, 110, 103406. [Google Scholar] [CrossRef]
- Yang, F.; Tian, J.; Fang, H.; Gao, Y.; Xu, M.; Lou, Y.; Zhou, B.; Kuzyakov, Y. Functional Soil Organic Matter Fractions, Microbial Community, and Enzyme Activities in a Mollisol Under 35 Years Manure and Mineral Fertilization. J. Soil Sci. Plant Nutr. 2019, 19, 430–439. [Google Scholar] [CrossRef]
- Kochetov, I.S.; Belolyubtsev, A.I.; Chebanenko, S.I.; Grigorov, A.A. Influence of Soil-Protecting Treatment Procedures on the Dynamics, Composition of the Organic Matter of the Soil, and the Formation of the Farm Crop Yield. Russ. Agric. Sci. 2000, 5, 33–38. [Google Scholar]
- Mahmood, S.; Nunes, M.R.; Kane, D.A.; Lin, Y. Soil Health Explains the Yield-Stabilizing Effects of Soil Organic Matter under Drought. Soil Environ. Health 2023, 1, 100048. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-Y.; Zhang, X.-Y.; Dai, X.-Q.; Fu, X.-L.; Yang, F.-T.; Liu, X.-Y.; Sun, X.-M.; Wen, X.-F.; Schaeffer, S. Changes in Soil Microbial Community Composition in Response to Fertilization of Paddy Soils in Subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Li, Y.; Han, X.; Li, B.; Zhang, X.; Li, Q.; Liang, W. Organic Substitutions Improve Soil Quality and Maize Yield through Increasing Soil Microbial Diversity. J. Clean. Prod. 2022, 347, 131323. [Google Scholar] [CrossRef]
- Li, M.; He, P.; Guo, X.-L.; Zhang, X.; Li, L.-J. Fifteen-Year No Tillage of a Mollisol with Residue Retention Indirectly Affects Topsoil Bacterial Community by Altering Soil Properties. Soil Tillage Res. 2021, 205, 104804. [Google Scholar] [CrossRef]
- Wan, W.; Liu, Z.; Li, B.; Fang, H.; Wu, H.; Yang, H. Evaluating Soil Erosion by Introducing Crop Residue Cover and Anthropogenic Disturbance Intensity into Cropland C-Factor Calculation: Novel Estimations from a Cropland-Dominant Region of Northeast China. Soil Tillage Res. 2022, 219, 105343. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, F.; Ding, J.; Bai, H.; Li, Y. Soil Respiration May Be Reduced by Wind via the Suppressing of Root Respiration: Field Observation in Maize Farmland in the Agro-Pastoral Transitional Zone, Northeastern China. Ecol. Indic. 2023, 146, 109824. [Google Scholar] [CrossRef]
- Walling, D.E.; He, Q. Mproved Models for Estimating Soil Erosion Rates from Cesium-137 Measurements. J. Environ. Qual. 1999, 28, 611–622. [Google Scholar] [CrossRef]
- Forestry Industry Standard of the People’s Republic of China Types and Degrees of Land Degradation 2022. Available online: https://www.samr.gov.cn (accessed on 18 June 2024).
- Shen, W.; Cao, X.; Shen, F. Classification and categorization of land degradation in China. J. Ecol. Rural Environ. 2006, 22, 88–93. [Google Scholar]
- Yang, Y.; Dou, Y.; An, S. Testing Association between Soil Bacterial Diversity and Soil Carbon Storage on the Loess Plateau. Sci. Total Environ. 2018, 626, 48–58. [Google Scholar] [CrossRef]
- Sawhney, B.L. Potassium and Cesium Ion Selectivity in Relation to Clay Mineral Structure. Clays Clay Miner. 1970, 18, 47–52. [Google Scholar] [CrossRef]
- Van Oost, K.; Quine, T.A.; Govers, G.; De Gryze, S.; Six, J.; Harden, J.W.; Ritchie, J.C.; McCarty, G.W.; Heckrath, G.; Kosmas, C.; et al. The Impact of Agricultural Soil Erosion on the Global Carbon Cycle. Science 2007, 318, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, P.; Zhang, Z.; You, M.; Wu, X.; Li, L.-J. Straw Return, Rather than Warming, Alleviates Microbial Phosphorus Limitation in a Cultivated Mollisol. Appl. Soil Ecol. 2023, 186, 104821. [Google Scholar] [CrossRef]
- Pärnpuu, S.; Astover, A.; Tõnutare, T.; Penu, P.; Kauer, K. Soil Organic Matter Qualification with FTIR Spectroscopy under Different Soil Types in Estonia. Geoderma Reg. 2022, 28, e00483. [Google Scholar] [CrossRef]
- Tivet, F.; de Moraes Sá, J.C.; Lal, R.; Milori, D.M.B.P.; Briedis, C.; Letourmy, P.; Pinheiro, L.A.; Borszowskei, P.R.; da Cruz Hartman, D. Assessing Humification and Organic C Compounds by Laser-Induced Fluorescence and FTIR Spectroscopies under Conventional and No-till Management in Brazilian Oxisols. Geoderma 2013, 207–208, 71–81. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K. Carbon and Nitrogen in the Terrestrial Environment; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-8432-4. [Google Scholar]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of Organic Carbon in Deep Soil Layers Controlled by Fresh Carbon Supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Joergensen, R.G. The Fumigation-Extraction Method to Estimate Soil Microbial Biomass: Calibration of the kEC Value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The Effects of Biocidal Treatments on Metabolism in Soil—I. Fumigation with Chloroform. Soil Biol. Biochem. 1976, 8, 167–177. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Yao, Q.; Jiao, F.; Hu, X.; Liu, J.; Jin, J.; Liu, X.; Wang, G. Soil pH and Carbon Quality Index Regulate the Biogeochemical Cycle Couplings of Carbon, Nitrogen and Phosphorus in the Profiles of Isohumosols. Sci. Total Environ. 2024, 922, 171269. [Google Scholar] [CrossRef]
- Doetterl, S.; Stevens, A.; Six, J.; Merckx, R.; Van Oost, K.; Casanova Pinto, M.; Casanova-Katny, A.; Muñoz, C.; Boudin, M.; Zagal Venegas, E.; et al. Soil Carbon Storage Controlled by Interactions between Geochemistry and Climate. Nat. Geosci. 2015, 8, 780–783. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil Organic Matter as Sole Indicator of Soil Degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef] [PubMed]
- Heller, C.; Ellerbrock, R.H.; Roßkopf, N.; Klingenfuß, C.; Zeitz, J. Soil Organic Matter Characterization of Temperate Peatland Soil with FTIR-Spectroscopy: Effects of Mire Type and Drainage Intensity. Eur. J. Soil Sci. 2015, 66, 847–858. [Google Scholar] [CrossRef]
- Plante, A.F.; Sanderman, J.; Asanopoulos, C.H.; Bell, S.; Baldock, J. Interpreting Ramped Combustion Thermograms Using 13C NMR Spectroscopy to Characterize Soil Organic Matter Composition. Geoderma 2023, 432, 116415. [Google Scholar] [CrossRef]
- Guo, L.-J.; Zhang, Z.-S.; Wang, D.-D.; Li, C.-F.; Cao, C.-G. Effects of Short-Term Conservation Management Practices on Soil Organic Carbon Fractions and Microbial Community Composition under a Rice-Wheat Rotation System. Biol. Fertil. Soils 2015, 51, 65–75. [Google Scholar] [CrossRef]
- Ndzelu, B.S.; Dou, S.; Zhang, X. Corn Straw Return Can Increase Labile Soil Organic Carbon Fractions and Improve Water-Stable Aggregates in Haplic Cambisol. J. Arid Land 2020, 12, 1018–1030. [Google Scholar] [CrossRef]
- Fang, K.; Chen, L.; Qin, S.; Zhang, Q.; Liu, X.; Chen, P.; Yang, Y. Mineral and Climatic Controls Over Soil Organic Matter Stability Across the Tibetan Alpine Permafrost Region. Glob. Biogeochem. Cycles 2021, 35, e2021GB007118. [Google Scholar] [CrossRef]
- Baldock, J.A.; Preston, C.M. Chemistry of Carbon Decomposition Processes in Forests as Revealed by Solid-State Carbon-13 Nuclear Magnetic Resonance. In Carbon Forms and Functions in Forest Soils; McFee, W.W., Kelly, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 2006; pp. 89–117. ISBN 978-0-89118-869-8. [Google Scholar]
- Liu, Y.; Miao, H.; Chang, X.; Wu, G. Higher Species Diversity Improves Soil Water Infiltration Capacity by Increasing Soil Organic Matter Content in Semiarid Grasslands. Land Degrad. Dev. 2019, 30, 1599–1606. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, L.-F.; Zhang, J.-C.; Yu, Y.-C.; Deangelis, D.L. Relationship Between Vegetation Restoration and Soil Microbial Characteristics in Degraded Karst Regions: A Case Study. Pedosphere 2011, 21, 132–138. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Li, F.; Sun, M.; Yang, X.; Wang, G.; Ma, J.; Sun, W. Exchangeable Ca2+ Content and Soil Aggregate Stability Control the Soil Organic Carbon Content in Degraded Horqin Grassland. Ecol. Indic. 2022, 134, 108507. [Google Scholar] [CrossRef]
- McCulley, R.L.; Archer, S.R.; Boutton, T.W.; Hons, F.M.; Zuberer, D.A. Soil Respiration and Nutrient Cycling in Wooded Communities Developing in Grassland. Ecology 2004, 85, 2804–2817. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Hobbie, S.E.; MacDougall, A.S. Soil Nutrients Increase Long-term Soil Carbon Gains Threefold on Retired Farmland. Glob. Change Biol. 2021, 27, 4909–4920. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Zhou, R.-L.; Zhang, T.-H.; Zhao, X.-Y. Effects of Desertification on Soil and Crop Growth Properties in Horqin Sandy Cropland of Inner Mongolia, North China. Soil Tillage Res. 2006, 87, 175–185. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Franco, N.; Walter, R.; Wiesmeier, M.; Hurtarte, L.C.C.; Berauer, B.J.; Buness, V.; Zistl-Schlingmann, M.; Kiese, R.; Dannenmann, M.; Kögel-Knabner, I. Biotic and Abiotic Controls on Carbon Storage in Aggregates in Calcareous Alpine and Prealpine Grassland Soils. Biol. Fertil. Soils 2021, 57, 203–218. [Google Scholar] [CrossRef]
- Qin, C.; Zhu, K.; Chiariello, N.R.; Field, C.B.; Peay, K.G. Fire History and Plant Community Composition Outweigh Decadal Multi-factor Global Change as Drivers of Microbial Composition in an Annual Grassland. J. Ecol. 2020, 108, 611–625. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting Global Grassland Degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Z.; He, C.; Zhang, Y.; Dong, F.; Huang, W. Effect of Soil Microbial Community Structure on the Chemical Compositions of Different Soil Organic Matter Fractions in Land Uses of the Pearl River Estuary. Appl. Soil Ecol. 2024, 193, 105126. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of Microorganisms to Geotechnical Engineering for Bioclogging and Biocementation of Soil in Situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Helliwell, J.R.; Miller, A.J.; Whalley, W.R.; Mooney, S.J.; Sturrock, C.J. Quantifying the Impact of Microbes on Soil Structural Development and Behaviour in Wet Soils. Soil Biol. Biochem. 2014, 74, 138–147. [Google Scholar] [CrossRef]
- Wu, Q.-S.; He, X.-H.; Zou, Y.-N.; He, K.-P.; Sun, Y.-H.; Cao, M.-Q. Spatial Distribution of Glomalin-Related Soil Protein and Its Relationships with Root Mycorrhization, Soil Aggregates, Carbohydrates, Activity of Protease and β-Glucosidase in the Rhizosphere of Citrus unshiu. Soil Biol. Biochem. 2012, 45, 181–183. [Google Scholar] [CrossRef]
- Ma, C.; Chen, X.; Zhang, J.; Zhu, Y.; Kalkhajeh, Y.K.; Chai, R.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; et al. Linking Chemical Structure of Dissolved Organic Carbon and Microbial Community Composition with Submergence-Induced Soil Organic Carbon Mineralization. Sci. Total Environ. 2019, 692, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-G.; Chu, H. Microbial Resistance Promotes Plant Production in a Four-Decade Nutrient Fertilization Experiment. Soil Biol. Biochem. 2020, 141, 107679. [Google Scholar] [CrossRef]
- Li, M.; Wang, G.; Kang, X.; Hu, H.; Wang, Y.; Zhang, X.; Sun, X.; Zhang, H.; Hu, Z.; Xi, B. Long-term Fertilization Alters Microbial Community but Fails to Reclaim Soil Organic Carbon Stocks in a Land-use Changed Soil of the Tibetan Plateau. Land Degrad. Dev. 2020, 31, 531–542. [Google Scholar] [CrossRef]
- Zhang, Z.; He, P.; Hao, X.; Li, L.-J. Long-Term Mineral Combined with Organic Fertilizer Supports Crop Production by Increasing Microbial Community Complexity. Appl. Soil Ecol. 2023, 188, 104930. [Google Scholar] [CrossRef]
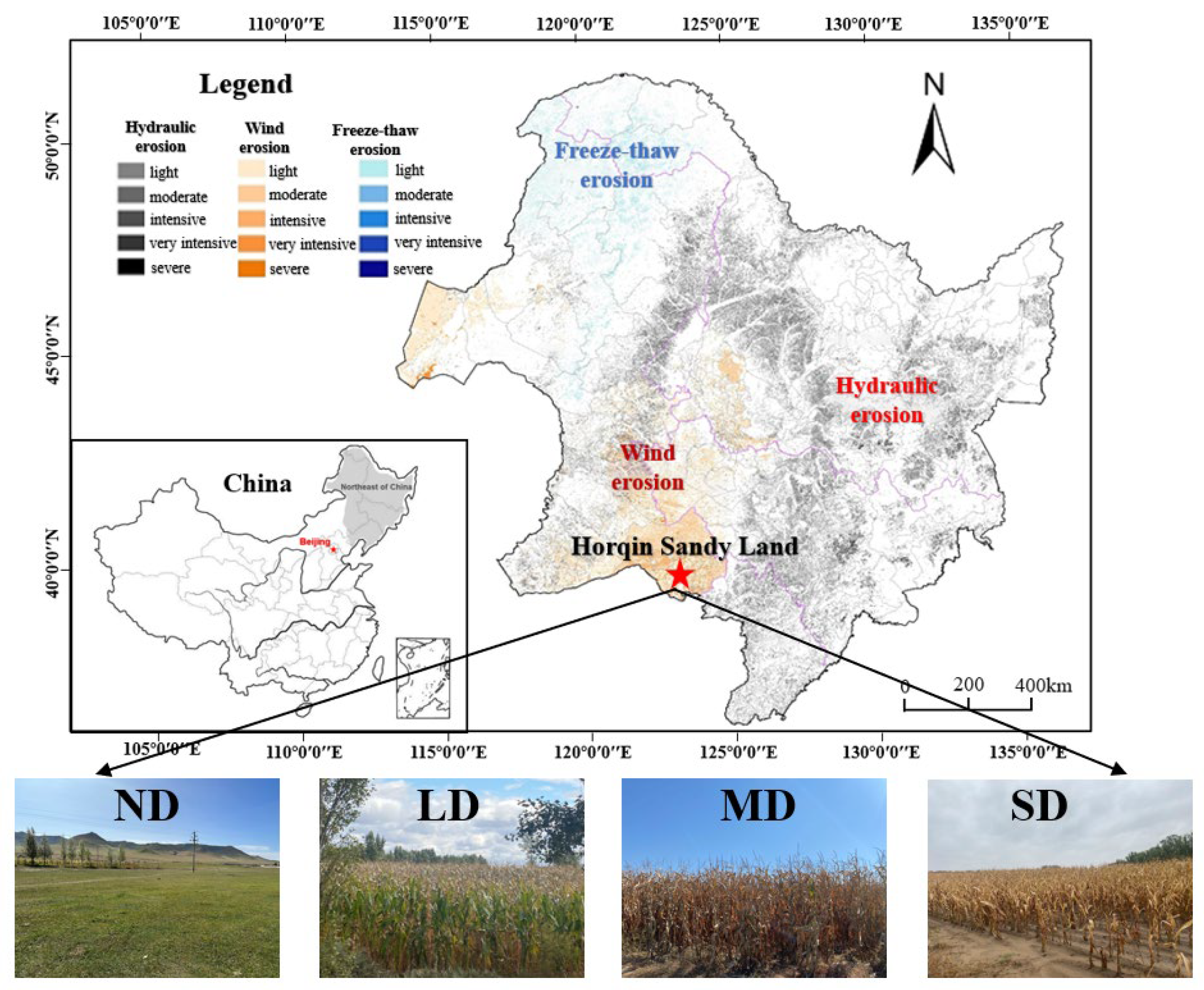

| Evaluation Factors | Degree of Land Degradation | |||
|---|---|---|---|---|
| Light Degradation | Moderate Degradation | Severe Degradation | Extremely Severe Degradation | |
| Annual rate of expansion of wind-accumulated (wind-eroded) surfaces (%) | <1 | 1~2 | 2~5 | >5 |
| Vegetation cover (%) | >50 | 30~50 | 10~30 | <10 |
| Soil loss by wind erosion (t hm−2 a−1) | <10 | 10~50 | 50~200 | >200 |
| Annual wind erosion depth (mm) | <0.5 | 0.5~3.0 | 3.0~10.0 | >10.0 |
| Reduction in biological production (%) | <15 | 15~35 | 35~75 | >75 |
| Comprehensive characterization of the surface landscape | The natural landscape has not yet been destroyed, with localised areas of patchy wind erosion and quicksand. | Patchy distribution of quicksand or windswept ground with obvious signs of wind erosion and surface coarsening. | Wind-eroded depressions and remnant mounds are evident, and wind erosion marks and rough sandy surfaces are widely distributed. | Presence of dense mobile dunes or windswept areas. |
| Treatment | BD | SWC | pH | SOC | TN | TP | TK | CEC | DOC |
|---|---|---|---|---|---|---|---|---|---|
| (g cm−3) | % | (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | (cmol kg−1) | (mg kg −1) | ||
| ND | 1.21 ± 0.04 d | 26.33 ± 0.42 a | 7.83 ± 0.18 b | 13.84 ± 0.23 a | 1.92 ± 0.09 a | 0.28 ± 0.01 c | 22.50 ± 1.87 a | 23.73 ± 1.09 a | 260.26 ± 6.43 a |
| LD | 1.48 ± 0.03 c | 21.55 ± 1.86 b | 7.76 ± 0.03 b | 9.07 ± 0.29 b | 1.12 ± 0.02 b | 0.72 ± 0.03 a | 21.83 ± 0.69 a | 20.25 ± 0.19 b | 224.54 ± 4.53 b |
| MD | 1.56 ± 0.03 b | 8.01 ± 2.30 c | 8.50 ± 0.29 a | 3.72 ± 0.24 c | 0.47 ± 0.04 c | 0.34 ± 0.03 b | 22.02 ± 0.78 a | 13.56 ± 0.56 c | 134.94 ± 3.72 c |
| SD | 1.70 ± 0.02 a | 3.99 ± 0.93 d | 8.54 ± 0.07 a | 2.41 ± 0.22 d | 0.29 ± 0.02 d | 0.20 ± 0.01 d | 22.43 ± 0.43 a | 7.27 ± 0.29 d | 123.66 ± 1.21 c |
| Soil Properties | ND | LD | MD | SD |
|---|---|---|---|---|
| MBC (mg kg−1) | 518.89 ± 30.49 a | 115.31 ± 3.56 b | 103.93 ± 3.04 bc | 88.85 ± 3.81 c |
| Br (mgCO2-C kg−1 h−1) | 4.38 ± 0.42 a | 3.68 ± 0.23 b | 2.78 ± 0.16 c | 1.00 ± 0.09 d |
| qCO2 | 0.85 ± 0.08 d | 3.21 ± 0.29 a | 2.67 ± 0.10 b | 1.13 ± 0.11 c |
| qMic | 0.04 ± 0.00 a | 0.01 ± 0.00 c | 0.03 ± 0.00 b | 0.04 ± 0.00 a |
| CAT (mmol d−1 g−1) | 72.67 ± 4.24 a | 65.96 ± 4.31 a | 39.34 ± 1.39 b | 19.67 ± 2.28 c |
| S-SC (U g−1) | 14.17 ± 1.13 a | 12.32 ± 0.98 b | 4.18 ± 0.36 c | 2.38 ± 0.29 d |
| S-UE (U g−1) | 1714.99 ± 181.68 a | 1653.14 ± 61.10 a | 1333.92 ± 60.26 b | 533.62 ± 85.94 c |
| S-AP (U g−1) | 7.47 ± 0.24 a | 6.351 ± 0.22 b | 2.68 ± 0.48 c | 1.68 ± 0.13 d |
| G+ (nmol g−1) | 4.53 ± 0.10 b | 5.83 ± 0.11 a | 2.50 ± 0.09 c | 1.86 ± 0.03 d |
| G− (nmol g−1) | 5.62 ± 0.08 b | 6.77 ± 0.10 a | 2.82 ± 0.10 c | 2.01 ± 0.02 d |
| Fungi (nmol g−1) | 5.67 ± 0.09 a | 5.56 ± 0.09 a | 2.87 ± 0.05 b | 2.48 ± 0.04 c |
| Actinomycetes (nmol g−1) | 4.51 ± 0.07 a | 4.84 ± 0.04 a | 1.89 ± 0.26 b | 1.23 ± 0.14 c |
| Bacterial (nmol g−1) | 27.19 ± 0.37 a | 23.98 ± 0.37 b | 11.34 ± 0.33 c | 8.95 ± 0.09 d |
| Total PLFAs (nmol g−1) | 47.53 ± 0.61 a | 46.98 ± 0.70 a | 21.43 ± 0.66 b | 16.54 ± 0.29 c |
| G+/G− | 0.81 ± 0.01 c | 0.86 ± 0.01 b | 0.89 ± 0.02 b | 0.92 ± 0.01 a |
| Bac/Fun | 4.80 ± 0.09 a | 4.31 ± 0.02 b | 3.96 ± 0.06 c | 3.61 ± 0.06 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Li, M.; Wang, S.; Lu, X. Land Degradation Affects Soil Microbial Properties, Organic Matter Composition, and Maize Yield. Agronomy 2024, 14, 1348. https://doi.org/10.3390/agronomy14071348
Gao M, Li M, Wang S, Lu X. Land Degradation Affects Soil Microbial Properties, Organic Matter Composition, and Maize Yield. Agronomy. 2024; 14(7):1348. https://doi.org/10.3390/agronomy14071348
Chicago/Turabian StyleGao, Ming, Meng Li, Shuli Wang, and Xinchun Lu. 2024. "Land Degradation Affects Soil Microbial Properties, Organic Matter Composition, and Maize Yield" Agronomy 14, no. 7: 1348. https://doi.org/10.3390/agronomy14071348




