Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Condition and Treatment of Plants
2.2. Measurement of Shoot Dry Matter Accumulation and Relative Water Content
2.3. Measurement of Light-Harvesting Pigment
2.4. Observation of Stomata Aperture and Guard Cells by Scanning Electron Microscopy
2.5. Determination of Ultrastructural Changes by Transmission Electron Microscopy
2.6. Quantification of Electrolyte Leakage, H2O2, O2•− Generation, and MDA Content
2.7. Determination of Antioxidant Enzyme Activities
2.8. Measurement of Non-Enzymatic Antioxidants
2.9. Determination of Proline and Glycine Betaine Contents
2.10. Determination of MDHAR and GST Activities
2.11. Statistical Analysis
3. Results
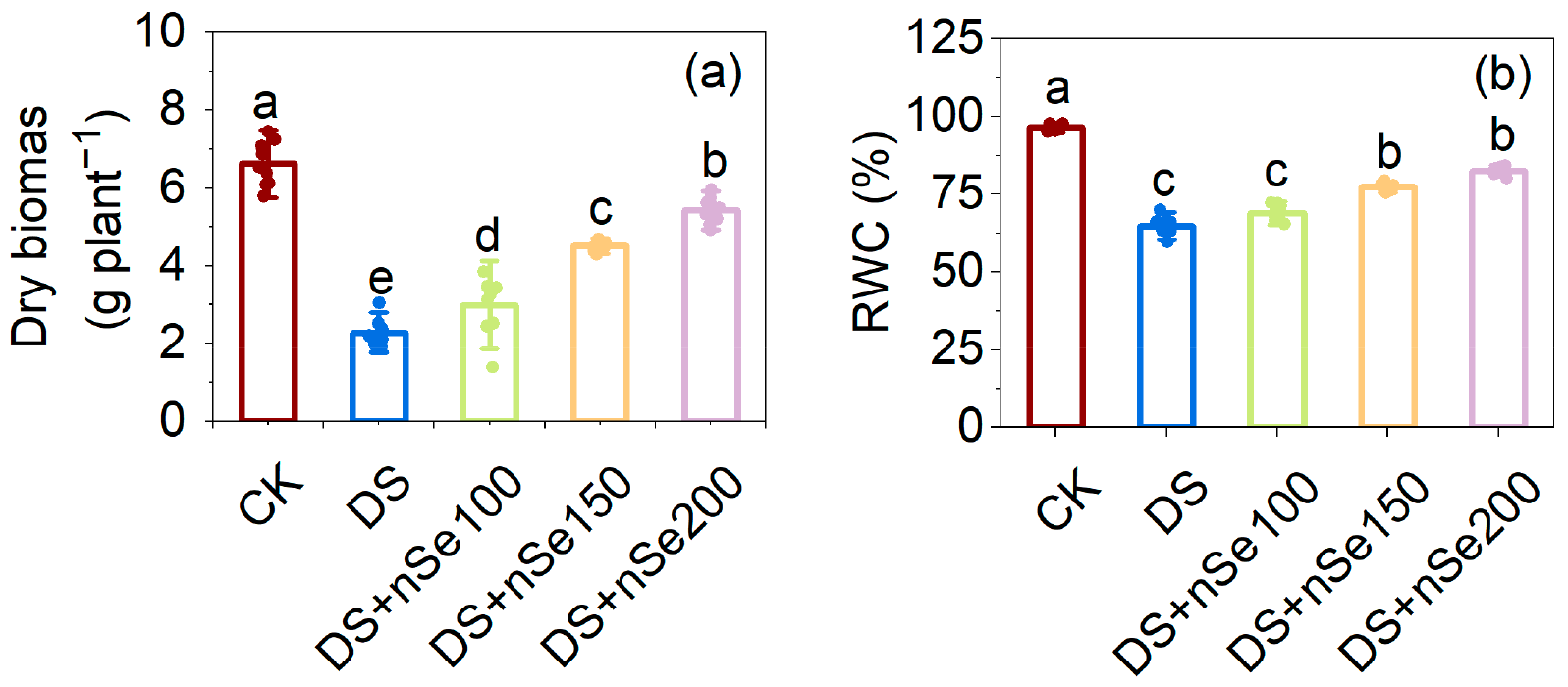
3.1. Above-Ground Dry Biomass and Relative Water Content
3.2. Effect of nSe on Light-Harvesting Pigment Contents under Drought Stress
3.3. Effect of nSe on Stomata Aperture and Morphological Traits under Drought Stress
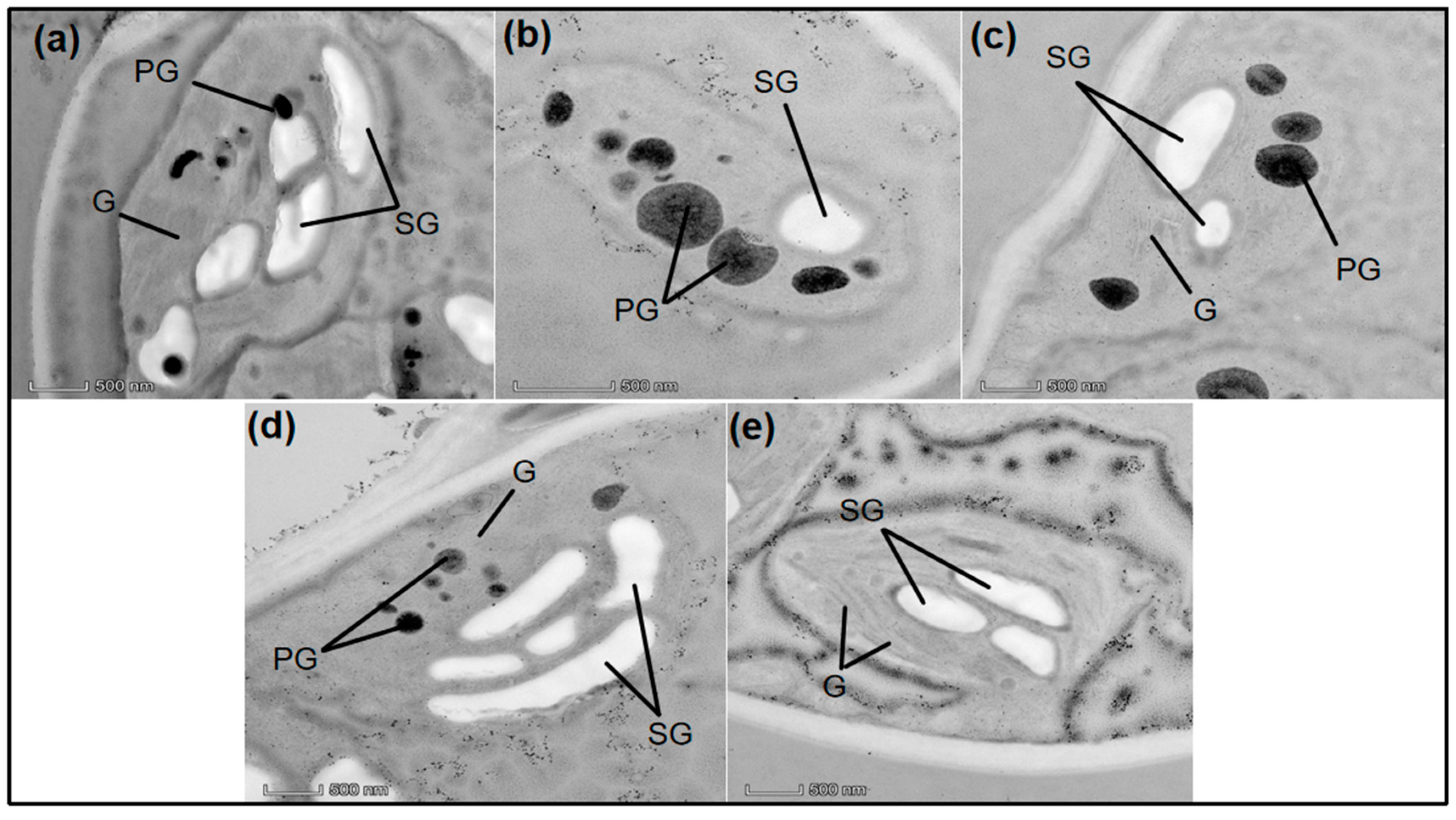
3.4. Effect of nSe on Ultrastructural Changes under Drought Stress
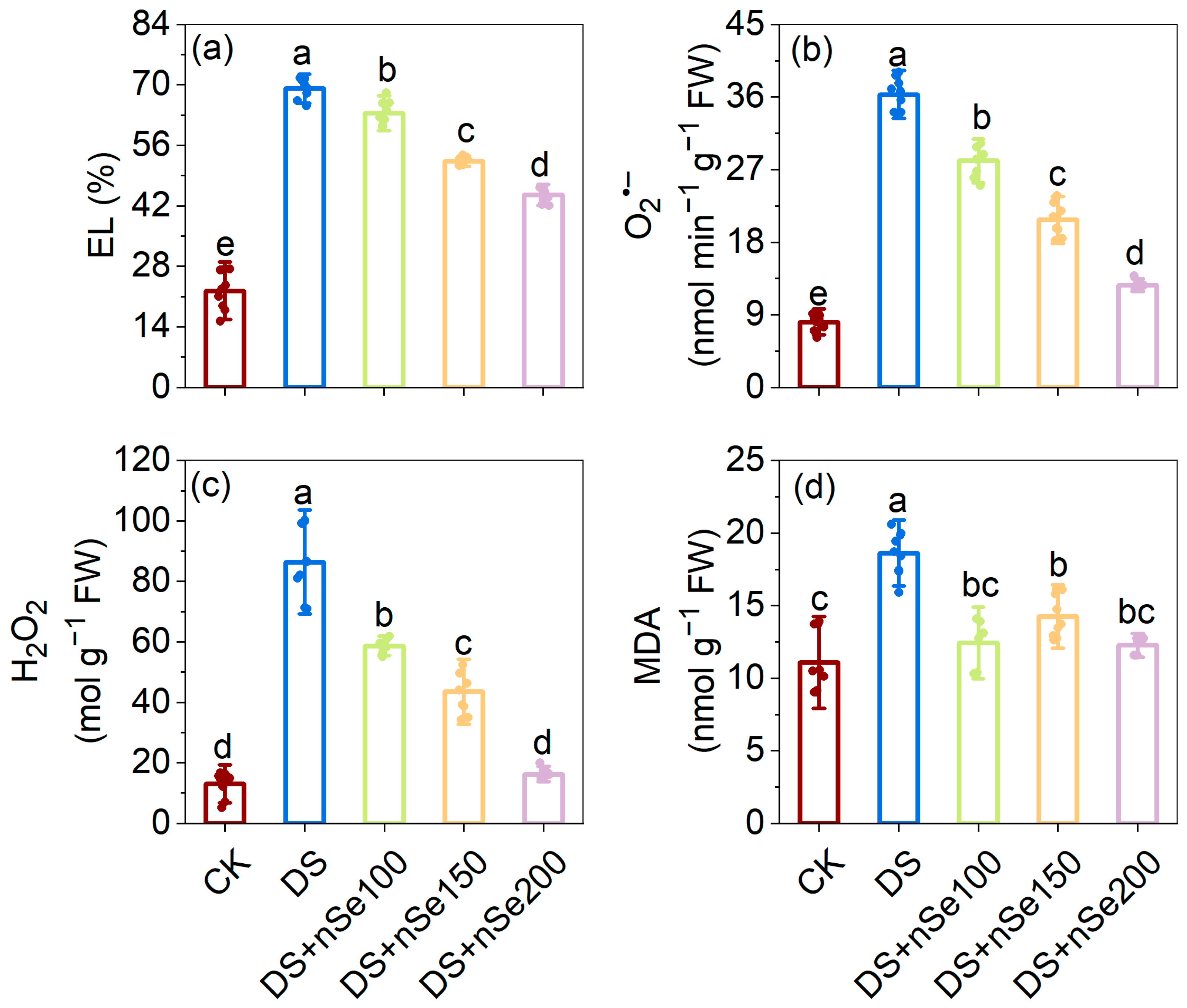
3.5. Effect of nSe Spraying on Electrolyte Leakage and ROS Accumulation under Drought Stress
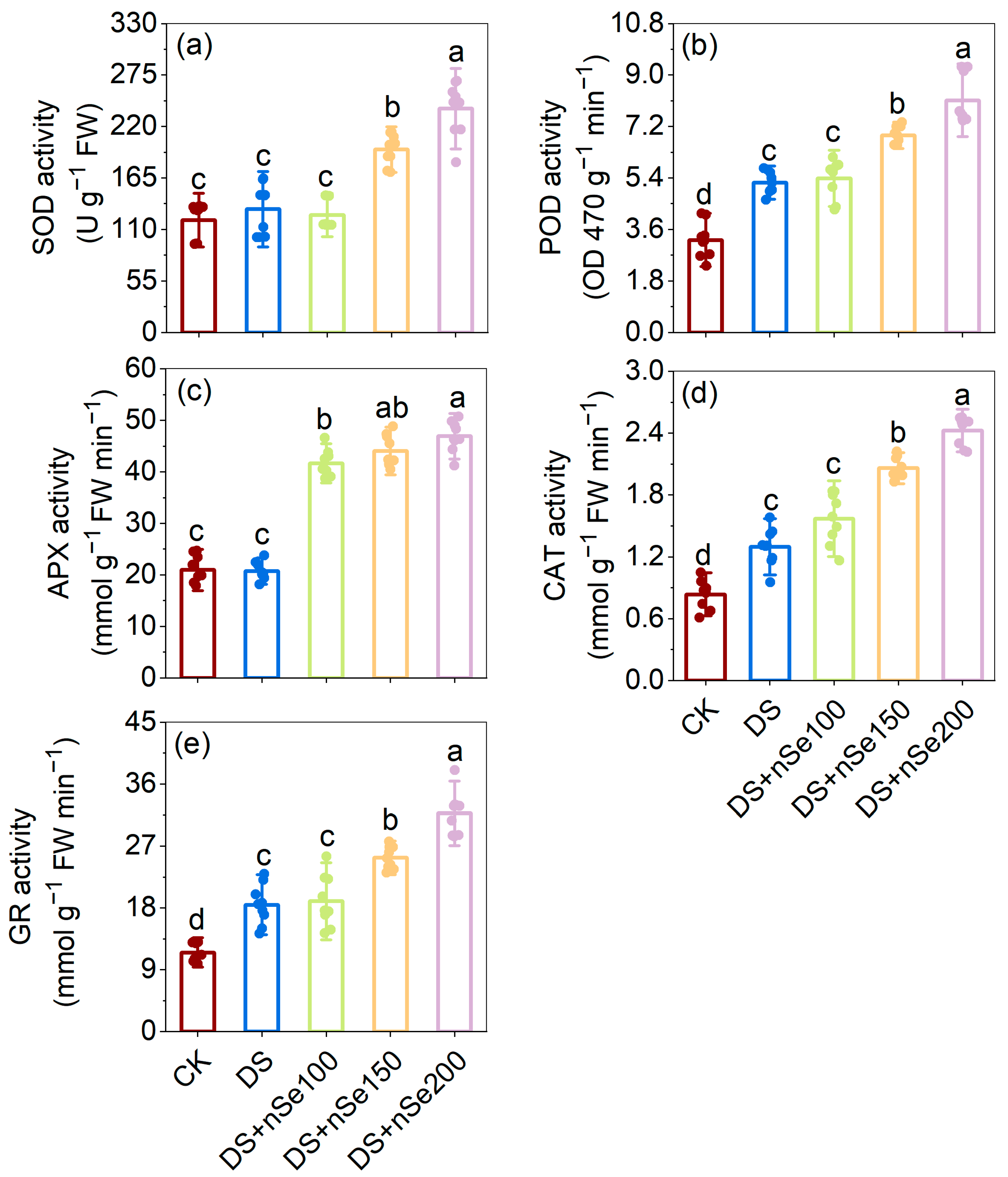
3.6. Effect of nSe Spraying on Antioxidant Enzyme Activities under Drought Stress
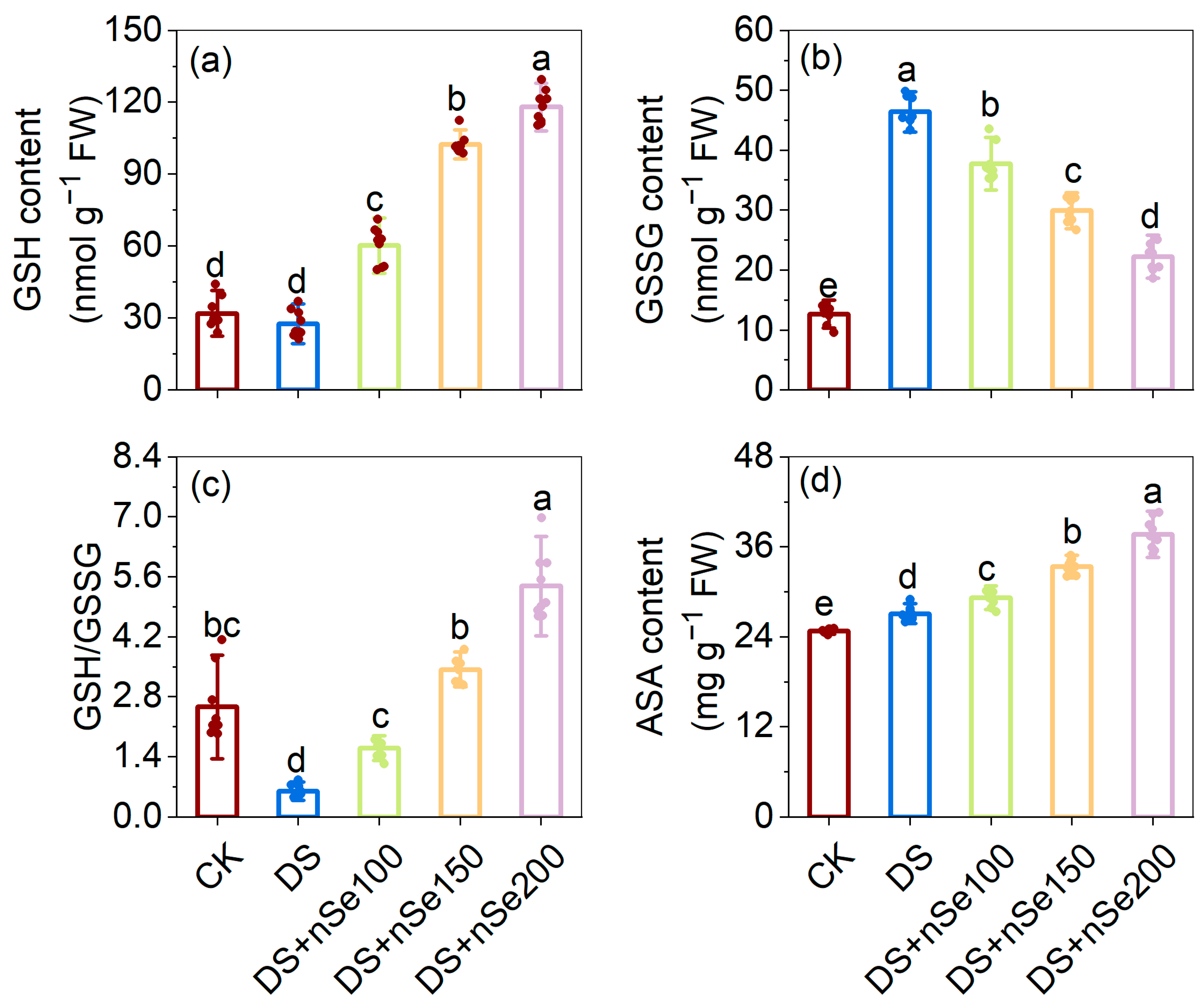
3.7. Effect of nSe on Glutathione Pool under Drought Stress
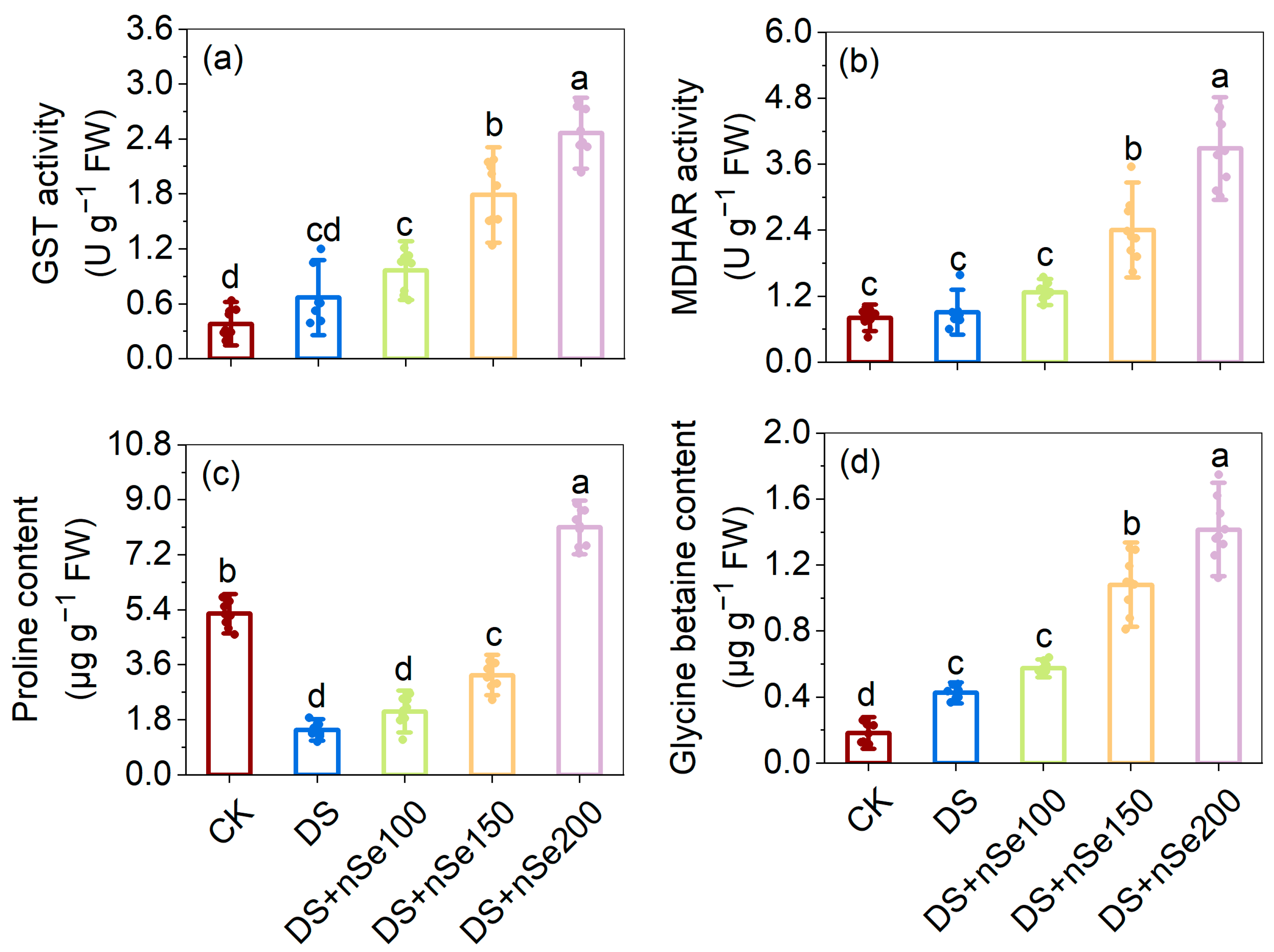
3.8. Effect of nSe on GST and MDHAR Enzyme Activities under Drought Stress
3.9. Effect of nSe on Proline and GB Contents under Drought Stress
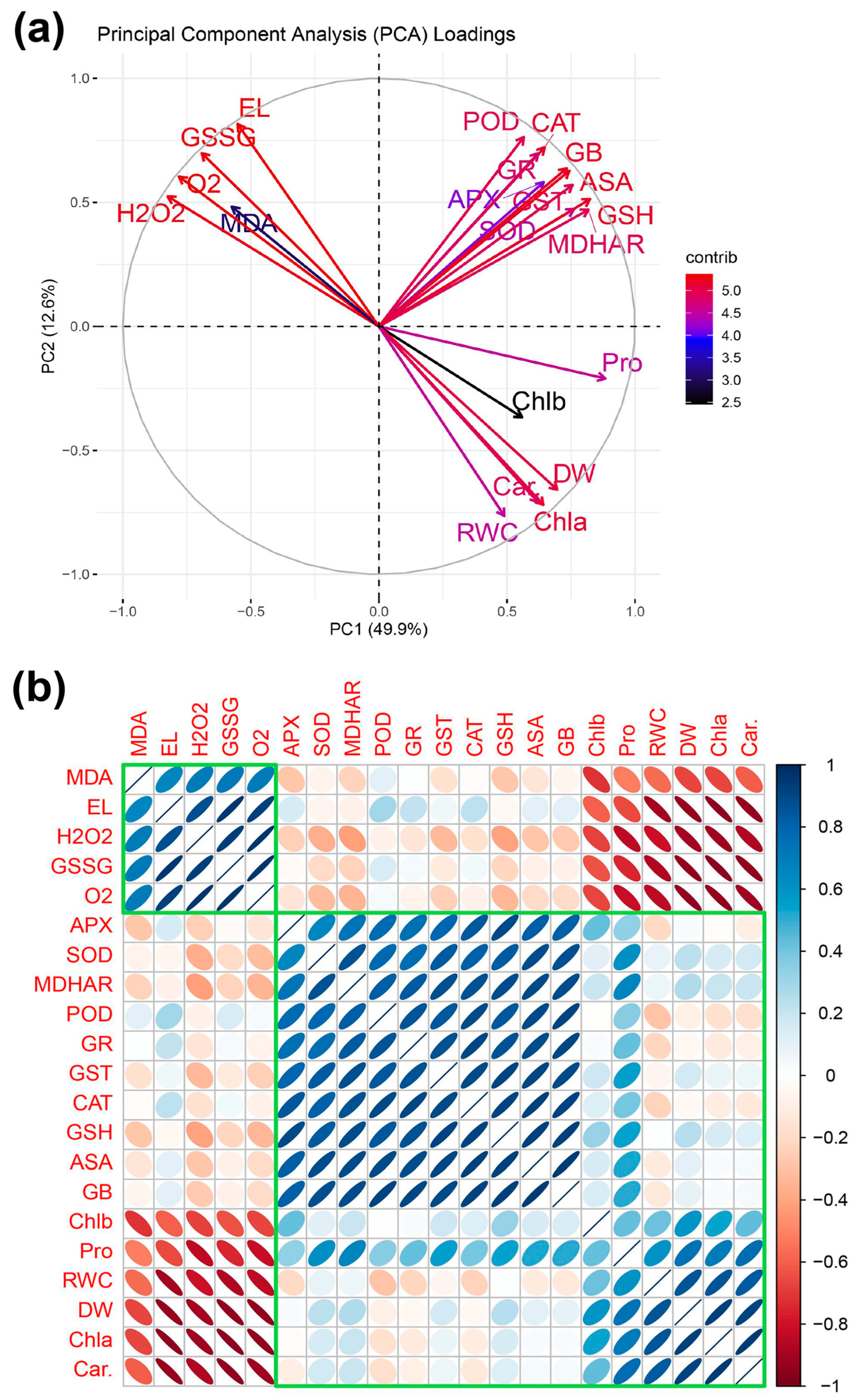
3.10. Principal Component Analysis and Pearson Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Proactive Approaches to Drought Preparedness—Where Are We Now and Where Do We Go from Here? FAO: Rome, Italy, 2019. [Google Scholar]
- Hu, Y.; Zeeshan, M.; Wang, G.; Pan, Y.; Liu, Y.; Zhou, X. Supplementary Irrigation and Varying Nitrogen Fertilizer Rate Mediate Grain Yield, Soil-Maize Nitrogen Accumulation and Metabolism. Agric. Water Manag. 2023, 276, 108066. [Google Scholar] [CrossRef]
- Varoquaux, N.; Cole, B.; Gao, C.; Pierroz, G.; Baker, C.R.; Patel, D.; Madera, M.; Jeffers, T.; Hollingsworth, J.; Sievert, J.; et al. Transcriptomic Analysis of Field-Droughted Sorghum from Seedling to Maturity Reveals Biotic and Metabolic Responses. Proc. Natl. Acad. Sci. USA 2019, 116, 27124–27132. [Google Scholar] [CrossRef]
- Mutava, R.N.; Prince, S.J.K.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding Abiotic Stress Tolerance Mechanisms in Soybean: A Comparative Evaluation of Soybean Response to Drought and Flooding Stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative Effect of Melatonin Improves Drought Tolerance by Regulating Growth, Photosynthetic Traits and Leaf Ultrastructure of Maize Seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef]
- Tardieu, F.; Parent, B.; Caldeira, C.F.; Welcker, C. Genetic and Physiological Controls of Growth under Water Deficit. Plant Physiol. 2014, 164, 1628–1635. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the Effect of Drought on Growth and Yield of Pomegranates by Foliar Spraying of Different Sizes of Selenium Nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef]
- Xu, Y.; Song, D.; Qi, X.; Asad, M.; Wang, S.; Tong, X.; Jiang, Y.; Wang, S. Physiological Responses and Transcriptome Analysis of Soybean under Gradual Water Deficit. Front. Plant Sci. 2023, 14, 1269884. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Farooq, S.; Kamran, M.; Ahmad, I.; Zeeshan, M.; Javed, T.; Ullah, S.; Huang, J.H.; et al. Application of Melatonin-Mediated Modulation of Drought Tolerance by Regulating Photosynthetic Efficiency, Chloroplast Ultrastructure, and Endogenous Hormones in Maize. Chem. Biol. Technol. Agric. 2022, 9, 5. [Google Scholar] [CrossRef]
- Ngara, R.; Ramulifho, E.; Movahedi, M.; Shargie, N.G.; Brown, A.P.; Chivasa, S. Identifying Differentially Expressed Proteins in Sorghum Cell Cultures Exposed to Osmotic Stress. Sci. Rep. 2018, 8, 8671. [Google Scholar] [CrossRef]
- Lisar, S.Y.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Alghabari, F.; Ihsan, M.Z.; Khaliq, A.; Hussain, S.; Daur, I.; Fahad, S.; Nasim, W. Gibberellin-Sensitive Rht Alleles Confer Tolerance to Heat and Drought Stresses in Wheat at Booting Stage. J. Cereal Sci. 2016, 70, 72–78. [Google Scholar] [CrossRef]
- Ikan, C.; Ben-Laouane, R.; Ouhaddou, R.; Ghoulam, C.; Meddich, A. Co-Inoculation of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Can Mitigate the Effects of Drought in Wheat Plants (Triticum durum). Plant Biosyst. 2023, 157, 907–919. [Google Scholar] [CrossRef]
- Ullah, A.; Tian, Z.; Xu, L.; Abid, M.; Lei, K.; Khanzada, A.; Zeeshan, M.; Sun, C.; Yu, J.; Dai, T. Improving the Effects of Drought Priming against Post-Anthesis Drought Stress in Wheat (Triticum aestivum L.) Using Nitrogen. Front. Plant Sci. 2022, 13, 965996. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Silicon and selenium: Two vital trace elements that confer abiotic stress tolerance to plants. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 377–422. [Google Scholar]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; Reis, A.R.D. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed Priming with Zinc Oxide Nanoparticles Downplayed Ultrastructural Damage and Improved Photosynthetic Apparatus in Maize under Cobalt Stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. Nano-Pesticides: A Great Challenge for Biodiversity? The Need for a Broader Perspective. Nano Today 2020, 30, 100808. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a New Sustainable Approach for Controlling Crop Diseases and Increasing Agricultural Production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc Oxide Nanoparticles Alleviate Drought-Induced Alterations in Sorghum Performance, Nutrient Acquisition, and Grain Fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Hu, Y.X.; Guo, X.H.; Sun, C.Y.; Salam, A.; Ahmad, S.; Muhammad, I.; Nasar, J.; Jahan, M.S.; Fahad, S.; et al. Physiological and Transcriptomic Study Reveal SeNPs-Mediated AsIII Stress Detoxification Mechanisms Involved Modulation of Antioxidants, Metal Transporters, and Transcription Factors in Glycine max L. (Merr.) Roots. Environ. Pollut. 2023, 317, 120637. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Li, Y.; Li, Z.; Lu, C.; Li, P.; et al. Silica Nanoparticles Protect Rice against Biotic and Abiotic Stresses. J. Nanobiotechnol. 2022, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and Silica Nanostructure-Based Recovery of Strawberry Plants Subjected to Drought Stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.Z.; Fu, J.Y.; Du, Y.Y.; Qu, J.; Song, Y.; Wang, P.W. The GmXTH1 Gene Improves Drought Stress Resistance of Soybean Seedlings. Mol. Breed. 2022, 42, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Hu, Y.X.; Iqbal, A.; Salam, A.; Liu, Y.X.; Muhammad, I.; Ahmad, S.; Khan, A.H.; Hale, B.; Wu, H.Y.; et al. Amelioration of AsV Toxicity by Concurrent Application of ZnO-NPs and Se-NPs Is Associated with Differential Regulation of Photosynthetic Indexes, Antioxidant Pool and Osmolytes Content in Soybean Seedling. Ecotoxicol. Environ. Saf. 2021, 225, 112738. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Flexas, J.; Savé, R.; Medrano, H. Water Relations and Stomatal Characteristics of Mediterranean Plants with Different Growth Forms and Leaf Habits: Responses to Water Stress and Recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Lichtenthaler, H.A.W. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochemical Society Transactions. Water Sci. Technol. 1983, 11, 591–592. [Google Scholar]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Lü, B.; Xu, L.L. An Improved Method for the Determination of Hydrogen Peroxide in Leaves. Progr. Biochem. Biophys. 2000, 27, 548–551. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Kubiś, J. Exogenous Spermidine Differentially Alters Activities of Some Scavenging System Enzymes, H2O2 and Superoxide Radical Levels in Water-Stressed Cucumber Leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, G.; Dominy, P. Four Barley Genotypes Respond Differently to Cadmium: Lipid Peroxidation and Activities of Antioxidant Capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhang, F.; Raziuddin, R.; Gong, H.J.; Yang, Z.M.; Lu, L.; Ye, Q.F.; Zhou, W.J. Effects of 5-Aminolevulinic Acid on Oilseed Rape Seedling Growth under Herbicide Toxicity Stress. J. Plant Growth Regul. 2008, 27, 159–169. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Maruta, T.; Ishikawa, T. Analysis of Ascorbate Metabolism in Arabidopsis Under High-Light Stress. In Methods in Molecular Biology; Springer: Cham, Switzerland, 2022; Volume 2526. [Google Scholar]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Raipid Determination of Free Proline for Water Studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid Assay for Determination of Water Soluble Quaternary Ammonium Compounds Programs Aimed at Plant Breeding for Salt Tolerance Are Facilitated by Rapid Screening Techniques. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Fan, S.; Wu, H.; Gong, H.; Guo, J. The Salicylic Acid Mediates Selenium-Induced Tolerance to Drought Stress in Tomato Plants. Sci. Hortic. 2022, 300, 111092. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of Zinc-Lysine on Growth, Yield and Cadmium Uptake in Wheat (Triticum aestivum L.) and Health Risk Assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Zhu, Y.M.; Chen, Y.; Qiu, C.W.; Zhu, S.; Wu, F. Genotypic Differences in Leaf Secondary Metabolism, Plant Hormones and Yield under Alone and Combined Stress of Drought and Salinity in Cotton Genotypes. Physiol. Plant 2019, 165, 343–355. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Zhang, M.; Chen, X.; Zhang, G.; Wu, F. Difference in Yield and Physiological Features in Response to Drought and Salinity Combined Stress during Anthesis in Tibetan Wild and Cultivated Barley. PLoS ONE 2013, 8, e77869. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.A.; Azooz, M.M. Insights into the Oxidative Status and Antioxidative Responses of Germinating Broccoli (Brassica oleracea Var. italica L.) Seeds in Tungstate Contaminated Water. Chemosphere 2020, 261, 127585. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Queval, G.; Foyer, C.H. The Impact of Global Change Factors on Redox Signaling Underpinning Stress Tolerance. Plant Physiol. 2013, 161, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.F.M.; Abd Elbar, O.H.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; Abd El-Gawad, H.G. Melatonin Counteracts Drought Induced Oxidative Damage and Stimulates Growth, Productivity and Fruit Quality Properties of Tomato Plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical Responses and Ultrastructural Changes in Ethylene Insensitive Mutants of Arabidopsis thialiana Subjected to Bisphenol a Exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide Application Improves the Drought Tolerance in Maize Through Modulation of Enzymatic Antioxidants and Leaf Gas Exchange. J. Agron. Crop. Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Vassileva, V.; Demirevska, K.; Simova-Stoilova, L.; Petrova, T.; Tsenov, N.; Feller, U. Long-Term Field Drought Affects Leaf Protein Pattern and Chloroplast Ultrastructure of Winter Wheat in a Cultivar-Specific Manner. J. Agron. Crop. Sci. 2012, 198, 104–117. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of Drought, Heat and Their Interaction on the Growth, Yield and Photosynthetic Function of Lentil (Lens culinaris Medikus) Genotypes Varying in Heat and Drought Sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Manzoor, N.; Ali, L.; Ahmed, T.; Rizwan, M.; Ali, S.; Shahid, M.S.; Schulin, R.; Liu, Y.; Wang, G. Silicon Oxide Nanoparticles Alleviate Chromium Toxicity in Wheat (Triticum aestivum L.). Environ. Pollut. 2022, 315, 120391. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Rehman, M.; Qi, J.; Khan, A.R.; Yang, S.; Zeeshan, M.; Ulhassan, Z.; Afridi, M.S.; Yang, C.; Chen, N.; et al. Cobalt Stress Induces Photosynthetic and Ultrastructural Distortion by Disrupting Cellular Redox Homeostasis in Maize. Environ. Exp. Bot. 2024, 217, 105562. [Google Scholar] [CrossRef]
- Zhu, S.; Nong, J.; Luo, G.; Li, Q.; Wang, F.; Jiang, D.; Zhao, X. Varied Tolerance and Different Responses of Five Citrus Rootstocks to Acid Stress by Principle Component Analysis and Orthogonal Analysis. Sci. Hortic. 2021, 278, 109853. [Google Scholar] [CrossRef]
- Lapaz, A.D.M.; de Camargos, L.S.; Yoshida, C.H.P.; Firmino, A.C.; de Figueiredo, P.A.M.; Aguilar, J.V.; Nicolai, A.B.; Silva de Paiva, W.d.; Cruz, V.H.; Tomaz, R.S. Response of Soybean to Soil Waterlogging Associated with Iron Excess in the Reproductive Stage. Physiol. Mol. Biol. Plants 2020, 26, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide Nanoparticles Application Alleviates Arsenic (As) Toxicity in Soybean Plants by Restricting the Uptake of as and Modulating Key Biochemical Attributes, Antioxidant Enzymes, Ascorbate-Glutathione Cycle and Glyoxalase System. Plants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium Modulates Dynamics of Antioxidative Defence Expression, Photosynthetic Attributes and Secondary Metabolites to Mitigate Chromium Toxicity in Brassica juncea L. Plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.S.P. Alleviation of the Effect of Salinity on Growth and Yield of Strawberry by Foliar Spray of Selenium-Nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mondal, S.; Islam, J.; Sarkar, R.; Saha, B.; Sen, A. Rhizospheric Nano-Remediation Salvages Arsenic Genotoxicity: Zinc-Oxide Nanoparticles Articulate Better Oxidative Stress Management, Reduce Arsenic Uptake, and Increase Yield in Pisum sativum (L.). Sci. Total Environ. 2024, 913, 169493. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Dai, H.; Zheng, W.; Cao, F.; Zhang, G.; Sun, D.; Wu, F. Genotypic Differences in Physiological Characteristics in the Tolerance to Drought and Salinity Combined Stress between Tibetan Wild and Cultivated Barley. Plant Physiol. Biochem. 2013, 63, 49–60. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is Proline Accumulation per Se Correlated with Stress Tolerance or Is Proline Homeostasis a More Critical Issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative Stress and Antioxidative System in Plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Differential Biochemical Responses of Wheat Shoots and Roots to Nickel Stress: Antioxidative Reactions and Proline Accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.; Noor, Y.; Hu, W.; Hannan, F.; Zhou, W. Mitigation Effects of Exogenous Melatonin-Selenium Nanoparticles on Arsenic-Induced Stress in Brassica napus. Environ. Pollut. 2022, 292, 118473. [Google Scholar] [CrossRef] [PubMed]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-Selenium Nanoparticles Enhance Disease Suppressive Ability of Trichoderma against Downy Mildew Disease Caused by Sclerospora graminicola in Pearl Millet. Sci. Rep. 2017, 7, 2612. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V.V. High-Temperature Stress Alleviation by Selenium Nanoparticle Treatment in Grain Sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Fathi, E.; Allah, A.; Alqarawi, A.A.; Ahmad, P. Combined Use of Biochar and Zinc Oxide Nanoparticle Foliar Spray Improved the Plant Growth and Decreased the Cadmium Accumulation in Rice (Oryza sativa L.) Plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef]

| Treatments | SPAD | chl a mg g−1 FW | chl b mg g−1 FW | Car mg g−1 FW | a + b | a/b |
|---|---|---|---|---|---|---|
| CK | 26.11 ± 2.18 a | 12.5 ± 1.16 a | 3.38 ± 0.60 a | 8.75 ± 0.58 a | 15.88 ± 0.95 a | 3.85 ± 0.02 a |
| DS | 10.72 ± 3.71 c | 6.69 ± 0.36 e | 2.44 ± 0.34 b | 5.01 ± 0.59 d | 8.130 ± 0.43 e | 2.72 ± 1.41 c |
| DS + nSe100 | 13.62 ± 3.52 c | 7.84 ± 0.18 d | 3.00 ± 0.08 a | 5.29 ± 0.24 d | 11.50 ± 0.14 d | 2.41 ± 0.11 c |
| DS + nSe150 | 19.02 ± 2.77 b | 9.29 ± 0.53 c | 3.11 ± 0.25 a | 6.14 ± 0.33 c | 12.40 ± 0.33 c | 3.02 ± 0.40 b |
| DS + nSe200 | 22.23 ± 2.18 ab | 10.5 ± 0.27 b | 3.26 ± 0.17 a | 7.64 ± 0.44 b | 13.54 ± 0.16 b | 3.53 ± 0.28 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeeshan, M.; Wang, X.; Salam, A.; Wu, H.; Li, S.; Zhu, S.; Chang, J.; Chen, X.; Zhang, Z.; Zhang, P. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy 2024, 14, 1372. https://doi.org/10.3390/agronomy14071372
Zeeshan M, Wang X, Salam A, Wu H, Li S, Zhu S, Chang J, Chen X, Zhang Z, Zhang P. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy. 2024; 14(7):1372. https://doi.org/10.3390/agronomy14071372
Chicago/Turabian StyleZeeshan, Muhammad, Xin Wang, Abdul Salam, Hao Wu, Shengnan Li, Shiqi Zhu, Jinzhe Chang, Xiaoyuan Chen, Zhixiang Zhang, and Peiwen Zhang. 2024. "Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity" Agronomy 14, no. 7: 1372. https://doi.org/10.3390/agronomy14071372








