Potential of Thermal and RGB Imaging Combined with Artificial Neural Networks for Assessing Salt Tolerance of Wheat Genotypes Grown in Real-Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design, Growth Conditions, and Salinity Treatment
2.2. Measurements
2.2.1. Measurements of Relative Water Content, Chlorophyll Contents and Biomass
2.2.2. Thermal Imaging
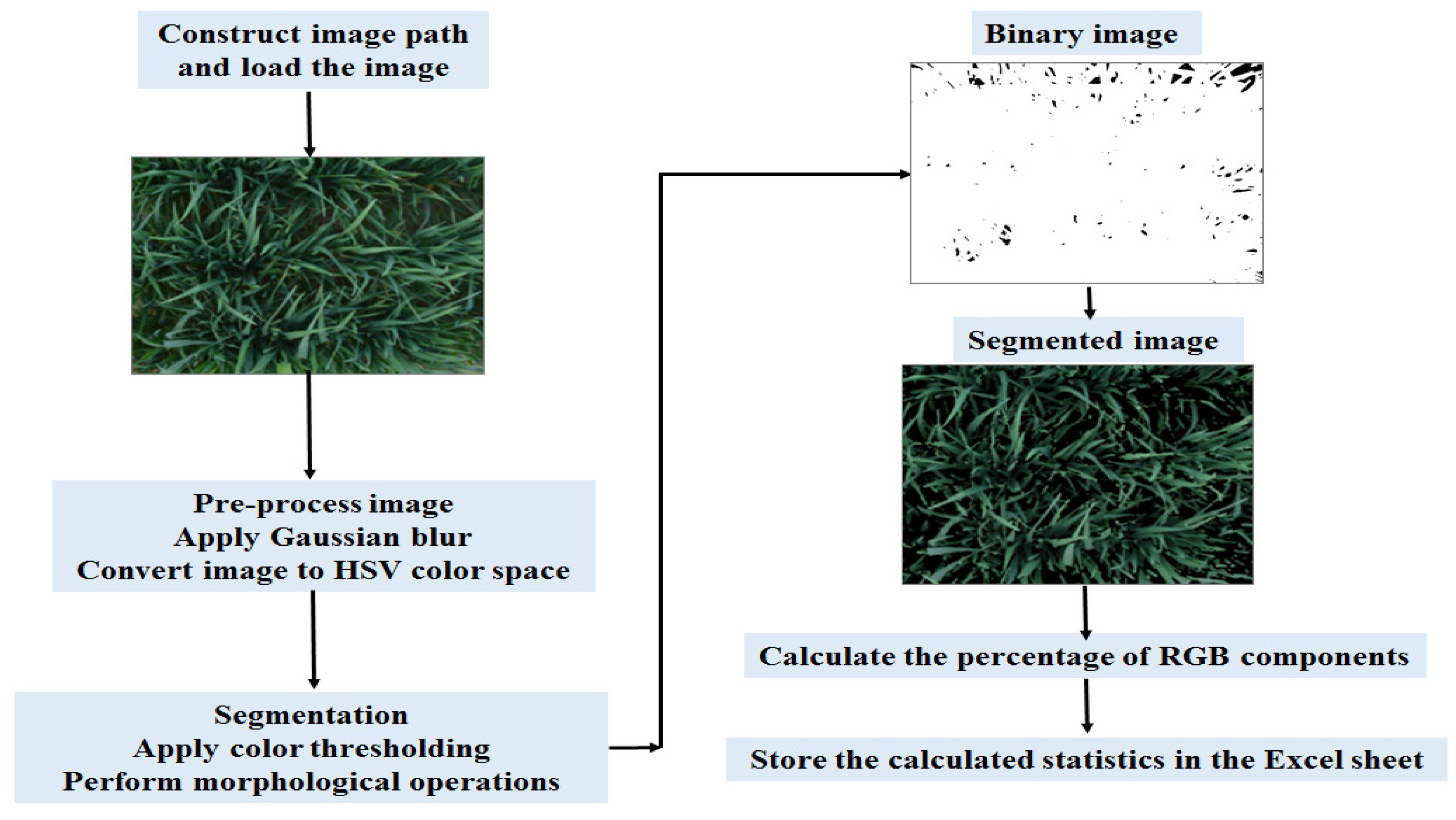
2.2.3. Digital RGB Imaging
Stages of Image Processing Pipeline for Vegetation Extraction
2.3. Back-Propagation Neural Network (BPNN)
2.4. Datasets and Software for Data Analysis
2.5. Model Evaluation
2.6. Statistical Analyses
3. Results and Discussion
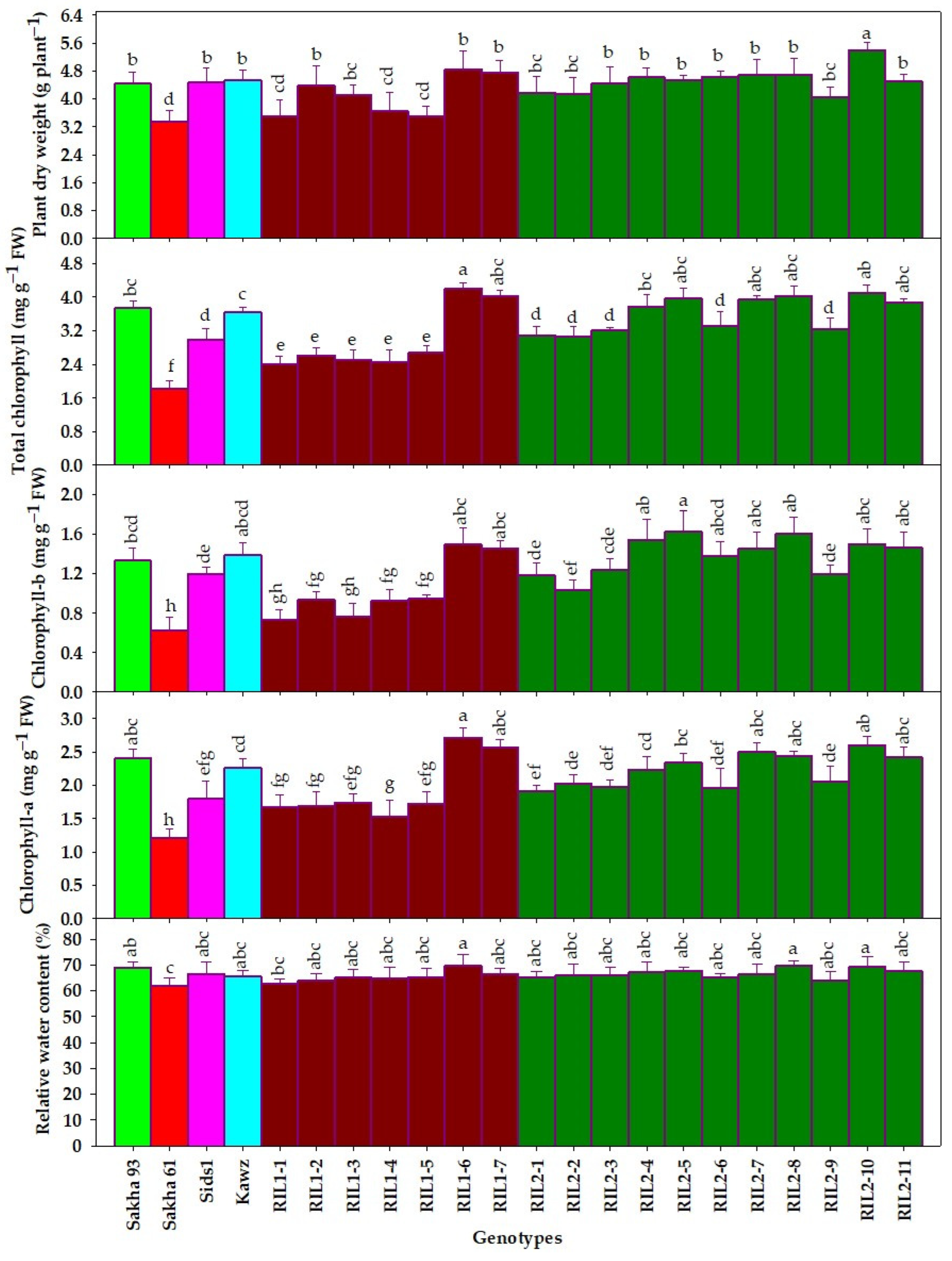
3.1. Genotypic Variations for Measured Destructive Traits
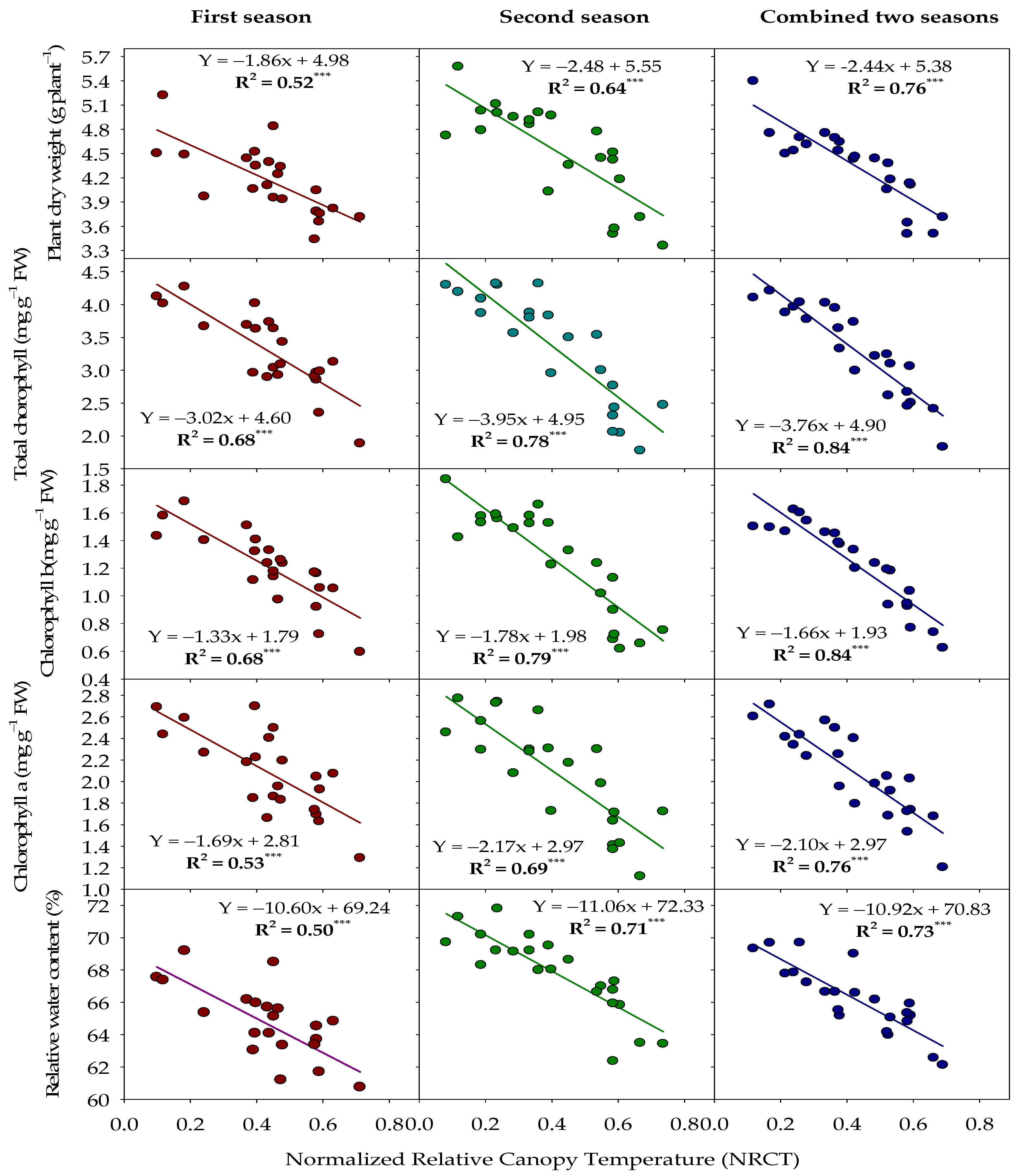
3.2. Performance of Thermal Index to Assess Measured Traits
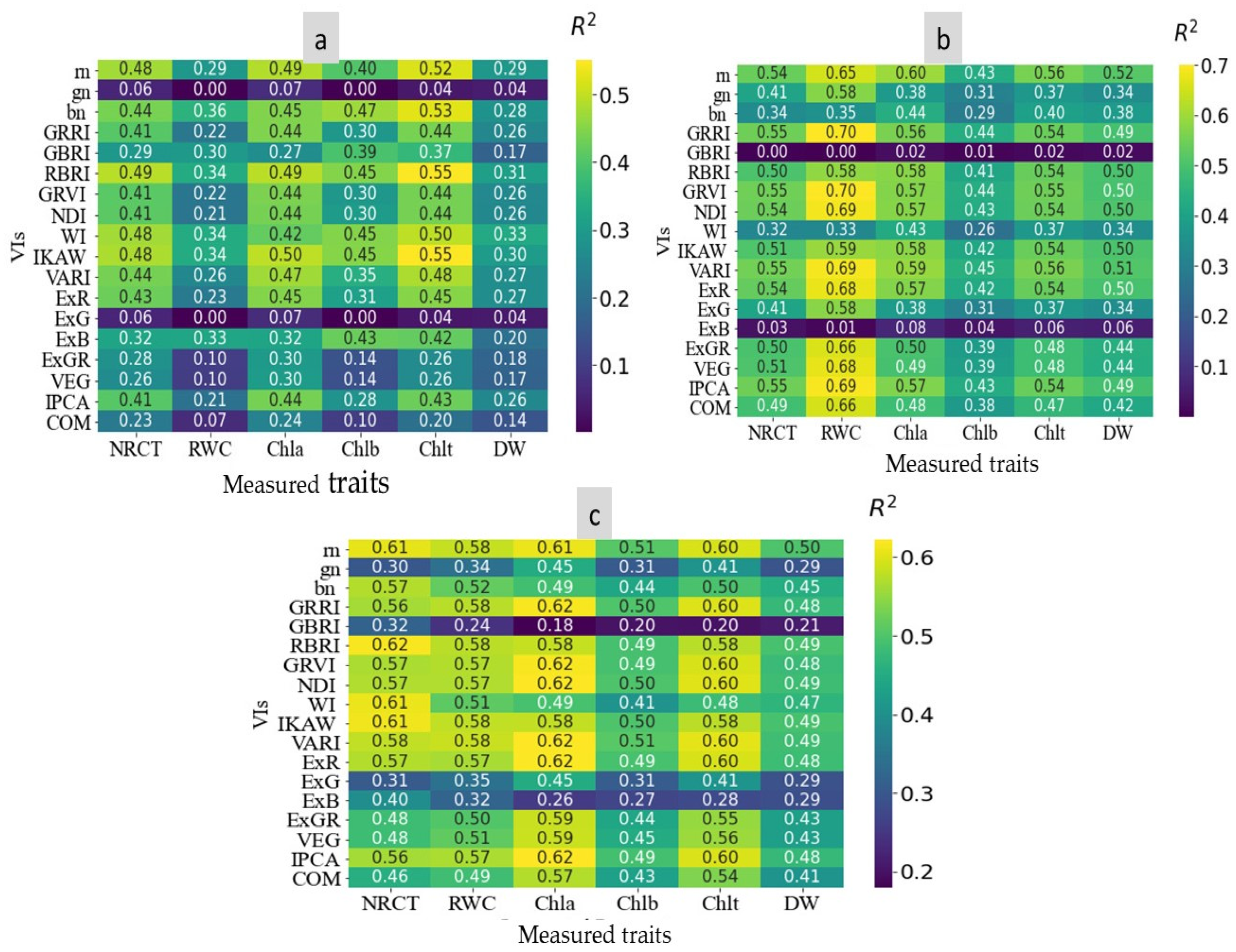
3.3. The Variation of RGB Indices and the Performance of Indices to Assess Wheat Genotypes Traits under Salinity Conditions
3.4. The Performance of ANN Model to Predict Measured Traits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaurasia, S.; Kumar, A.; Singh, A.K. Comprehensive evaluation of morpho-physiological and ionic traits in wheat (Triticum aestivum L.) genotypes under salinity stress. Agriculture 2022, 12, 1765. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hassan, W.M.; Al-Suhaibani, N.A.; Refay, Y.; Abdella, K.A. Comparative performance of multivariable agro-physiological parameters for detecting salt tolerance of wheat cultivars under simulated saline field growing conditions. Front. Plant. Sci. 2017, 8, 435. [Google Scholar] [CrossRef]
- Oyiga, B.C.; Sharma, R.; Shen, J.; Baum, M.; Ogbonnaya, F.; Léon, J.; Ballvora, A. Identification and characterization of salt tolerance of wheat germplasm using a multivariable screening approach. J. Agron. Crop Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.A.; Desoky, E.M.; Ali, M.M.A.; Yasin, M.A.T.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S.E. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. past successes and future challenges to the role played by wheat in global food security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Zada, A.; Ali, A.; Binjawhar, D.N.; Abdel-Hameed, U.K.; Shah, A.H.; Gill, S.M.; Hussain, I.; Abbas, Z.; Ullah, Z.; Sher, H.; et al. Molecular and physiological evaluation of bread wheat (Triticum aestivum L.) genotypes for stay green under drought stress. Genes 2022, 13, 2261. [Google Scholar] [CrossRef]
- Reynolds, M.; Pask, A.; Mullan, D. Physiological Breeding I: Interdisciplinary Approaches to Improve Crop Adaptation; International Maize and Wheat Improvement Center (CIMMYT): Texcoco, Mexico, 2012. [Google Scholar]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Mubushar, M.; Tahir, M.U.; Marey, S.; Refay, Y.; Tola, E. Combining hyperspectral reflectance and multivariate regression models to estimate plant biomass of advanced spring wheat lines in diverse phenological stages under salinity conditions. Appl. Sci. 2022, 12, 1983. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A Novel combined Spectral Index for Estimating the Ratio of Carotenoid to Chlorophyll Content to Monitor Crop Physiological and Phenological Status. Int. J. Appl. Earth Obs. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, H.; Chen, T.; Pen, J.; Yu, S.; Zhao, X. Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. PLoS ONE 2014, 9, e112807. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Sanjani, S.; Nikkhah-Chamanabad, H.; Mehrvar, M.R.; Asadi, A.; Amini, A. Identification of salt-tolerant barley genotypes using multiple-traits index and yield performance at the early growth and maturity stages. Bull. Natl. Res. Cent. 2021, 45, 117. [Google Scholar] [CrossRef]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and screening of agro-physiological indices for salinity stress tolerance in wheat at the seedling stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salt tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Radi, A.A.; Farghaly, F.A.; Hamada, A.M. Physiological and biochemical responses of salt-tolerant and salt-sensitive wheat and bean genotypes to salinity. J. Biol. Earth Sci. 2013, 2, 72–88. [Google Scholar]
- Genc, Y.; Taylor, J.; Lyons, G.H.; Li, Y.; Cheong, J.; Appelbee, M.; Oldach, K.; Sutton, T. Bread wheat with high salinity and sodicity tolerance. Front. Plant Sci. 2019, 10, 280. [Google Scholar] [CrossRef]
- Orzechowska, A.; Trtílek, M.; Tokarz, K.M.; Szymańska, R.; Niewiadomska, E.; Rozpądek, P.; Wątor, K. Thermal analysis of stomatal response under salinity and high light. Int. J. Mol. Sci. 2021, 22, 4663. [Google Scholar] [CrossRef]
- Sirault, X.R.R.; James, R.A.; Furbank, R.T.; Sirault, X.R.R.; James, R.A.; Furbank, R.T. A New screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Funct. Plant Biol. 2009, 36, 970–977. [Google Scholar] [CrossRef]
- Banerjee, B.P.; Joshi, S.; Thoday-Kennedy, E.; Pasam, R.K.; Tibbits, J.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping using digital and hyperspectral imaging-derived biomarkers for genotypic nitrogen response. J. Exp. Bot. 2020, 71, 4604–4615. [Google Scholar] [CrossRef]
- Kim, S.L.; Lee, N.; Lee, H.; Lee, E.; Cheon, K.-S.; Kim, M.; Baek, J.-H.; Choi, I.; Ji, H.; Yoon, I.S.; et al. High-throughput phenotyping platform for analyzing drought tolerance in rice. Planta 2020, 252, 38. [Google Scholar] [CrossRef]
- Rufo, R.; Soriano, J.M.; Villegas, D.; Royo, C.; Bellvert, J. Using unmanned aerial vehicle and ground-based RGB indices to assess agronomic performance of wheat landraces and cultivars in a Mediterranean-type environment. Remote Sens. 2021, 13, 1187. [Google Scholar] [CrossRef]
- Hasan, U.; Sawut, M.; Chen, S. Estimating the leaf area index of winter wheat based on unmanned aerial vehicle RGB-image parameters. Sustainability 2019, 11, 6829. [Google Scholar] [CrossRef]
- Thoday-Kennedy, E.; Joshi, S.; Daetwyler, H.D.; Hayden, M.; Hudson, D.; Spangenberg, G.; Kant, S. Digital phenotyping to delineate salinity response in safflower genotypes. Front. Plant Sci. 2021, 12, 662498. [Google Scholar] [CrossRef]
- Duan, L.; Han, J.; Guo, Z.; Tu, H.; Yang, P.; Zhang, D.; Fan, Y.; Chen, G.; Xiong, L.; Dai, M. Novel digital features discriminate between drought resistant and drought sensitive rice under controlled and field conditions. Front. Plant Sci. 2018, 9, 492. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, W.; Chang, Y.; Ma, X.; Tu, H.; Xiong, F.; Jiang, N.; Feng, H.; Huang, C.; Yang, P. Genome-wide association studies of image traits reveal genetic architecture of drought resistance in rice. Mol. Plant 2018, 11, 789–805. [Google Scholar] [CrossRef]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesic, A.; Peratab, P.; Cellini, F. Physiological responses to Megafol treatments in tomato plants under drought stress: A phenomic and molecular approach. Sci. Hortic. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Elsayed, S.; Barmeier, G.; Schmidhalter, U. Passive reflectance sensing and digital image analysis allows for assessing the biomass and nitrogen status of wheat in early and late tillering stages. Front. Plant Sci. 2018, 9, 1478. [Google Scholar] [CrossRef]
- Lapidot, O.; Ignat, T.; Rud, R.; Rog, I.; Alchanati, V.; Klein, T. Use of thermal imaging to detect evaporative cooling in coniferous and broadleaved tree species of the Mediterranean maquis. Agric. For. Meteorol. 2019, 271, 285–294. [Google Scholar]
- Stutsel, B.; Johansen, K.; Malbéteau, Y.M.; McCabe, M.F. Detecting plant stress using thermal and optical imagery from an unoccupied aerial vehicle. Front. Plant Sci. 2021, 12, 734944. [Google Scholar]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crop. Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- Singh, R.N.; Krishnan, P.; Singh, V.K.; Sah, S.; Das, B. Combining biophysical parameters with thermal and RGB indices using machine learning models for predicting yield in yellow rust affected wheat crop. Sci. Rep. 2023, 13, 18814. [Google Scholar] [CrossRef]
- Ribeiro, J.E.; Coêlho, E.S.; de Oliveira, A.K.; da Silva, G.C.; Lopes, W.A.R.; Oliveira, P.H.; da Silva, E.F.; Júnior, A.P.B.; da Silveira, L.M. Artificial neural network approach for predicting the sesame (Sesamum indicum L.) leaf area: A non-destructive and accurate method. Heliyon 2023, 9, e17834. [Google Scholar]
- Kim, N.; Ha, K.-J.; Park, N.-W.; Cho, J.; Hong, S.; Lee, Y.-W. A Comparison between major artificial intelligence models for crop yield prediction: Case study of the Midwestern United States, 2006–2015. ISPRS Int. J. Geo Inf. 2019, 8, 240. [Google Scholar] [CrossRef]
- Khaki, S.; Wang, L. Crop Yield Prediction Using Deep Neural Networks. In Smart Service Systems, Operations Management, and Analytics; Yang, H., Qiu, R., Chen, W., Eds.; Springer Proceedings in Business and Economics; Springer: Berlin, Germany, 2020; pp. 139–147. [Google Scholar]
- García-Martínez, H.; Flores-Magdaleno, H.; Ascencio-Hern’andez, R.; Khalil-Gardezi, A.; Tijerina-Ch’avez, L.; Mancilla-Villa, O.R.; Vázquez-Peña, M.A. Corn grain yield estimation from vegetation indices, canopy cover, plant density, and a neural network using multispectral and RGB images acquired with unmanned aerial vehicles. Agriculture. 2020, 10, 10070277. [Google Scholar]
- Salem, S.B.H.; Gaagai, A.; Ben Slimene, I.; Moussa, A.B.; Zouari, K.; Yadav, K.K.; Eid, M.H.; Abukhadra, M.R.; El-Sherbeeny, A.M.; Gad, M.; et al. Applying Multivariate Analysis and Machine Learning Approaches to Evaluating Groundwater Quality on the Kairouan Plain, Tunisia. Water 2023, 15, 3495. [Google Scholar] [CrossRef]
- Schuize, F.H.; Wolf, H.; Jansen, H.; Vander, V.P. Applications of artificial neural networks in integrated water management: Fiction or future? Water Sci. Technol. 2005, 52, 21–31. [Google Scholar] [CrossRef]
- EIMasry, G.; Sun, D.W.; Allen, P. Near-infrared hyperspectral imaging for predicting colour, pH and tenderness of fresh beef. J. Food Eng. 2012, 110, 127–140. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional variable importance for random forests. BMC Bioinform. 2008, 9, 307. [Google Scholar] [CrossRef]
- Glorfeld, L.W. A methodology for simplification and interpretation of backpropagation-based neural network models. Expert Syst. 1996, 10, 37–54. [Google Scholar] [CrossRef]
- Melis, G.; Dyer, C.; Blunsom, P. On the state of the art of evaluation in neural language models. arXiv 2017, arXiv:1707.05589. [Google Scholar]
- Bergstra, J.; Yamins, D.; Cox, D. Making a science of model search: Hyperparameter optimization in hundreds of dimensions for vision architectures. In Proceedings of the 30th International Conference on Machine Learning (ICML 2013), Atlanta, Gerorgia, 16–21 June 2013; pp. 115–123. [Google Scholar]
- Wu, J.; Chen, X.-Y.; Zhang, H.; Xiong, L.-D.; Lei, H.; Deng, S.-H. Hyperparameter optimization for machine learning models based on Bayesian optimization. J. Electron. Sci. Technol. 2019, 17, 26–40. [Google Scholar]
- El-Hendawy, S.E.; Hu, Y.C.; Yakout, G.M.; Awad, A.M.; Hafiz, S.E.; Schmidhalter, U. Evaluating salt tolerance of wheat genotypes using multiple parameters. Euro. J. Agron. 2005, 22, 243–253. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Ruan, Y.; Hu, Y.; Schmidhalter, U. A Comparison of screening criteria for salt tolerance in wheat under field and environment controlled conditions. J. Agron. Crop Sci. 2009, 49, 1–9. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and eco-physiology. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2004; pp. 107–163. [Google Scholar]
- Mardanisamani, S.; Eramian, M. Segmentation of vegetation and microplots in aerial agriculture images. Plant Phenome J. 2022, 5, e20042. [Google Scholar] [CrossRef]
- Upendar, K.; Agrawal, K.N.; Chandel, N.S.; Singh, K. Greenness identification using visible spectral colour indices for site specific weed management. Plant Physiol. Rep. 2021, 26, 179–187. [Google Scholar] [CrossRef]
- Kumaseh, M.R.; Luther, L.; Nainggolan, N. Segmentasi citra digital ikan menggunakan metode thresholding. J. Ilm. Sains 2013, 13, 74–79. [Google Scholar] [CrossRef][Green Version]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Martin, M.L. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Woebbecke, D.; Meyer, G.; von Bargen, K.; Mortensen, D. Plant species identification, size, and enumeration using machine vision techniques on near-binary images. Int. Soc. Opt. Photonics 1993, 1836, 208–219. [Google Scholar]
- Kawashima, S.; Nakatani, M. An algorithm for estimating chlorophyll content in leaves using a video camera. Ann. Bot. 1998, 81, 49–54. [Google Scholar] [CrossRef]
- Mao, W.; Wang, Y.; Wang, Y. Real-time detection of between-row weeds using machine vision. In Proceedings of the ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; p. 1. [Google Scholar]
- Hague, T.; Tillett, N.; Wheeler, H. Automated crop and weed monitoring in widely spaced cereals. Precis. Agric. 2006, 7, 21–32. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Anuar, A.R.; Gholizadeh, A.; Wayayok, A.; Khairunniza- Bejo, S. Assessment of rice leaf chlorophyll content using visible bands at different growth stages at both the leaf and canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2014, 32, 35–45. [Google Scholar] [CrossRef]
- Guijarro, M.; Pajares, G.; Riomoros, I.; Herrera, P.J.; Burgos-Artizzu, X.P.; Ribeiro, A. Automatic segmentation of relevant textures in agricultural images. Comput. Electron. Agric. 2011, 75, 75–83. [Google Scholar] [CrossRef]
- Schalkoff, R.J. Artificial Neural Networks; McGraw-Hill Higher Education: New York, NY, USA, 1997; ISBN 0-07-057118-X. [Google Scholar]
- Haykin, S. Neural Networks: A Comprehensive Foundation, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Li, J.; Yoder, R.; Odhiambo, L.O.; Zhang, J. Simulation of nitrate distribution under drip irrigation using artificial neural networks. Irrig. Sci. 2004, 23, 29–37. [Google Scholar] [CrossRef]
- Byrd, R.H.; Lu, P.; Nocedal, J.; Zhu, C. A limited memory algorithm for bound constrained optimization. Siam J. Sci. Comput. 1995, 16, 1190–1208. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Z.H.; Sun, H.; Wang, G.X. Mapping forest ecosystem biomass density for Xiangjiang river basin by combining plot and remote sensing data and comparing spatial extrapolation methods. Remote Sens. 2017, 9, 241. [Google Scholar] [CrossRef]
- Malone, B.P.; Styc, Q.; Minasny, B.; McBratney, A.B. Digital soil mapping of soil carbon at the farm scale: A spatial downscaling approach in consideration of measured and uncertain data. Geoderma 2017, 290, 91–99. [Google Scholar] [CrossRef]
- Saggi, M.K.; Jain, S. Reference evapotranspiration estimation and modeling of the Punjab Northern India using deep learning. Comput. Electron. Agric. 2019, 156, 387–398. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, M.; McCabe, M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy. 2017, 7, 61. [Google Scholar] [CrossRef]
- Quan, X.; Liang, X.; Li, H.; Xie, C.; He, W.; Qin, Y. Identification and characterization of wheat germplasm for salt tolerance. Plants 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Irshad, I.; Haq, M.A.; Akhtar, J.; Maqsood, M. Effects of different organic amendments on maize (Zea mays L.) growth in salt affected soil. J. Global Innov. Agric. Sci. 2022, 10, 121–130. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Avolio, M.L.; Hoffman, A.M.; Smith, M.D. Linking gene regulation, physiology, and plant biomass allocation in Andropogon gerardii in response to drought. Plant Ecol. 2018, 219, 1–15. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, W.; Xu, Y.; Fei, H.; Zhu, Y.; Ahmad, I.; Nimir, N.E.A.; Zhou, G.; Zhu, G. Effects of Salt Stress on Physiological and Agronomic Traits of Rice Genotypes with Contrasting Salt Tolerance. Plants 2024, 13, 1157. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Rahman, M.; Hasan, M.; Amin, M.F.; Matin, M.Q.I.; Faruq, G.; Alkeridis, L.A.; Gaber, A.; Hossain, A. Assessment of the salt tolerance of diverse bread wheat (Triticum aestivum L.) genotypes during the early growth stage under hydroponic culture conditions. Heliyon 2024, 10, e29042. [Google Scholar] [CrossRef]
- Hamam, K.A.; Negim, O. Evaluation of wheat genotypes and some soil properties under saline water irrigation. Ann. Agric. Sci. 2014, 59, 165–176. [Google Scholar] [CrossRef]
- Yousfi, S.; Serret, M.D.; Araus, J.L. Shoot δ15N gives a better indication than ion concentration or D13C of genotypic differences in the response of durum wheat to salinity. Funct. Plant Biol. 2009, 36, 144–155. [Google Scholar] [CrossRef]
- Guellim, A.; Catterou, M.; Chabrerie, O.; Tetu, T.; Hirel, B.; Dubois, F.; Ahmed, H.B.; Kichey, T. Identification of Phenotypic and Physiological Markers of Salt Stress Tolerance in Durum Wheat (Triticum durum Desf.) through Integrated Analyses. Agronomy 2019, 9, 844. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mehrvar, M.R.; Sanjani, S.; Amini, A.; Nikkhah-Chamanabad, H.; Asadi, A. Effects of salinity stress on seedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiol. Plant. 2021, 43, 98. [Google Scholar] [CrossRef]
- Chen, B.; Bian, X.; Tu, M.; Yu, T.; Jiang, L.; Lu, Y.; Chen, X. moderate salinity stress increases the seedling biomass in oilseed rape (Brassica napus L.). Plants 2023, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Opportunity and challenges of phenotyping plant salt tolerance. Trends Plant Sci. 2023, 28, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Boopal, J.; Sathee, L.; Ramasamy, R.; Pandey, R.; Chinnusamy, V. Influence of incremental short term salt stress at the seedling stage on root plasticity, shoot thermal profile and ion homeostasis in contrasting wheat genotypes. Agriculture 2023, 13, 1946. [Google Scholar] [CrossRef]
- Vennam, R.R.; Bheemanahalli, R.; Reddy, K.R.; Dhillon, J. Early-season maize responses to salt stress: Morpho-physiological, leaf reflectance, and mineral composition. J. Agric. Food Res. 2024, 15, 100994. [Google Scholar] [CrossRef]
- Olsen, R.L.; Pratt, B.P.; Gump, P.; Kemper, A.; Tallman, G. Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO2 concentrations in Vicia spp. New Phytol. 2002, 153, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, E.; Talbott, L.D.; Frechilla, S.; Srivastava, A.; Zhu, J. The guard cell chloroplast: A perspective for the twenty-first century. New Phytol. 2002, 153, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.; El-Hendawy, S.; Khadr, M.; Elsherbiny, O.; Al-Suhaibani, N.; Alotaibi, M.; Tahir, M.U.; Darwish, W. Combining thermal and RGB imaging indices with multivariate and data-driven modeling to estimate the growth, water status, and yield of potato under different drip irrigation regimes. Remote Sens. 2021, 13, 1679. [Google Scholar] [CrossRef]
- Schlemmer, M.R.; Francis, D.D.; Shanahan, J.F.; Schepers, J.S. Remotely measuring chlorophyll content in corn leaves with differing nitrogen levels and relative water content. Agron. J. 2005, 97, 106–112. [Google Scholar] [CrossRef]
- Carter, G.A. Primary and secondary effects of the water content on the spectral reflectance of leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Taha, M.F.; Mao, H.; Wang, Y.; ElManawy, A.I.; Elmasry, G.; Wu, L.; Memon, M.S.; Niu, Z.; Huang, T.; Qiu, Z. High-Throughput analysis of leaf chlorophyll content in aquaponically grown lettuce using hyperspectral reflectance and RGB images. Plants 2024, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.; Stellacci, A.M.; Mzid, N.; Di Venosa, M.; Todorovic, M.; Cantore, V.; Albrizio, R. Comparative performance of aerial RGB vs. ground hyperspectral indices for evaluating water and nitrogen status in sweet maize. Agronomy 2024, 14, 562. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Fan, Y.; Zhou, L.; Qiu, Z. Fusion of feature selection methods and regression algorithms for predicting the canopy water content of rice based on hyperspectral data. Agriculture 2021, 11, 51. [Google Scholar] [CrossRef]
- Shen, Y.; Mercatoris, B.; Cao, Z.; Kwan, P.; Guo, L.; Yao, H.; Cheng, Q. Improving wheat yield prediction accuracy using LSTM-RF Framework based on UAV thermal infrared and multispectral imagery. Agriculture 2022, 12, 892. [Google Scholar] [CrossRef]



| RGB Indices | Formula | References |
|---|---|---|
| Red pixel percentage (rn) | R/(R + G + B) | [52] |
| Green pixel percentage (rg) | G/(R + G + B) | [52] |
| Blue pixel percentage (rb) | B/(R + G + B) | [53] |
| Green/red ratio index (GRRI) | G/R | [53] |
| Green/blue ratio index (GBRI) | G/B | [53] |
| Red/blue ratio index (RBRI) | R/B | [53] |
| Green/red vegetation index (GRVI) | (G − R)/(G + B) | [54] |
| Normalized difference index (NDI) | [55] | |
| Woebbecke index (WI) | [55] | |
| Kawashima index (IKAW) | [56] | |
| Visible atmospherically resistant index (VARI) | (G − R)/(R + G+ B) | [54] |
| Excess red vegetation index (ExR) | 1.4 × R − G | [57] |
| Excess green vegetation index (ExG) | 2 × G − R − B | [57] |
| Excess blue vegetation index (ExB) | 1.4 × B − G | [57] |
| Excess green minus excess red index (ExGR) | E × G − E × R | [57] |
| Vegetative index (VEG) | [58] | |
| Principal component analysis index (IPCA) | [59] | |
| Combination (COM) | [60] |
| Source of Variation | DF | RWC | Chla | Chlb | Chlt | PDW |
|---|---|---|---|---|---|---|
| Sum of Squares (%) | ||||||
| Blocks | 2 | 2.96 ns | 0.62 ns | 0.02 ns | 0.27 ns | 0.48 ns |
| Year (Y) | 1 | 16.32 ns | 0.00 ns | 0.53 ns | 0.12 ns | 9.03 * |
| Error-1 | 2 | 2.76 | 0.18 | 0.15 | 0.05 | 0.22 |
| Genotypes (G) | 21 | 26.85 ** | 72.23 *** | 73.33 *** | 79.89 *** | 53.78 *** |
| G × Y | 21 | 9.71 ns | 16.40 *** | 14.43 *** | 13.74 *** | 10.50 ns |
| Error-2 | 84 | 41.40 | 10.57 | 11.54 *** | 5.93 | 25.99 |
| Total | 131 | 100 | 100 | 100 | 100 | 100 |
| Genotypes | rn | gn | bn | GRRI | GBRI | RBRI | GRVI | NDI | WI |
|---|---|---|---|---|---|---|---|---|---|
| Sakha 93 | 0.291 b–e | 0.367 a–c | 0.343 a–c | 1.261 a–d | 1.071 ab | 0.850 c–f | 0.116 a–c | −0.115 c–e | −0.319 a |
| Sakha 61 | 0.286 de | 0.366 a–c | 0.349 a | 1.282 a–c | 1.049 b | 0.820 ef | 0.123 a–c | −0.122 c–e | −0.216 a |
| Sids1 | 0.299 a–d | 0.367 a–c | 0.335 a–d | 1.232 b–e | 1.099 ab | 0.893 a–e | 0.104 a–d | −0.102 a–e | −0.477 ab |
| Kawz | 0.312 a | 0.356 c | 0.332 b–d | 1.141 e | 1.072 ab | 0.942 ab | 0.066 e | −0.065 a | −0.634 ab |
| RIL1-1 | 0.280 e | 0.373 ab | 0.348 a | 1.335 a | 1.073 ab | 0.804 f | 0.143 a | −0.141 e | −0.241 a |
| RIL1-2 | 0.288 c–e | 0.375 a | 0.338 a–d | 1.304 ab | 1.111 ab | 0.853 c–f | 0.131 ab | −0.129 de | −0.427 ab |
| RIL1-3 | 0.294 b–e | 0.369 ab | 0.337 a–d | 1.257 a–d | 1.095 ab | 0.871 b–f | 0.114 a–c | −0.113 b–e | −0.425 ab |
| RIL1-4 | 0.291 b–e | 0.371 ab | 0.339 a–d | 1.27 a–c | 1.095 ab | 0.858 b–f | 0.121 a–c | −0.119 c–e | −0.396 ab |
| RIL1-5 | 0.292 b–e | 0.368 a–c | 0.340 a–c | 1.261 a–d | 1.084 ab | 0.860 b–f | 0.115 a–c | −0.113 c–e | −0.374 ab |
| RIL1-6 | 0.315 a | 0.362 b–c | 0.325 d | 1.148 e | 1.115 ab | 0.971 a | 0.069 e | −0.069 a | −0.800 b |
| RIL1-7 | 0.304 a–c | 0.361 b–c | 0.335 a–d | 1.188 c–e | 1.076 ab | 0.907 a–d | 0.086 cd | −0.084 a–c | −0.458 ab |
| RIL2-1 | 0.301 a–d | 0.368 a–c | 0.331 b–d | 1.225 b–e | 1.113 ab | 0.901 a–d | 0.101 b–d | −0.099 a–d | −0.562 ab |
| RIL2-2 | 0.305 a–c | 0.362 b–c | 0.334 a–d | 1.186 c–e | 1.086 ab | 0.916 a–d | 0.085 cd | −0.084 a–c | −0.504 ab |
| RIL2-3 | 0.291 b–e | 0.371 ab | 0.338 a–d | 1.277 a–c | 1.097 ab | 0.860 b–f | 0.122 a–c | −0.119 c–e | −0.409 ab |
| RIL2-4 | 0.302 a–d | 0.367 a–c | 0.332 b–d | 1.214 b–e | 1.105 ab | 0.910 a–d | 0.097 b–d | −0.095 a–d | −0.539 ab |
| RIL2-5 | 0.305 a–c | 0.366 a–c | 0.330 b–d | 1.201 b–e | 1.111 ab | 0.926 a–c | 0.091 b–d | −0.091 a–d | −0.613 ab |
| RIL2-6 | 0.288 c–e | 0.368 a–c | 0.345 ab | 1.277 a–c | 1.069 ab | 0.837 d–f | 0.122 a–c | −0.119 c–e | −0.296 a |
| RIL2-7 | 0.305 a–c | 0.363 a–c | 0.332 b–d | 1.190 c–e | 1.093 ab | 0.919 a–d | 0.087 cd | −0.086 a–c | −0.540 ab |
| RIL2-8 | 0.301 a–d | 0.364 a–c | 0.335 a–d | 1.209 b–e | 1.086 ab | 0.899 a–e | 0.095 b–d | −0.094 a–d | −0.470 ab |
| RIL2-9 | 0.291 b–e | 0.368 a–c | 0.342 a–c | 1.264 a–c | 1.078 ab | 0.853 c–f | 0.117 a–c | −0.115 c–e | −0.346 a |
| RIL2-10 | 0.313 a | 0.363 a–c | 0.325 d | 1.159 d–e | 1.117 a | 0.965 a | 0.073 e | −0.0725 ab | −0.806 b |
| RIL2-11 | 0.306 ab | 0.367 a–c | 0.328 cd | 1.202 b–e | 1.119 a | 0.932 a–c | 0.092 b–d | −0.090 a–d | −0.637 ab |
| IKAW | VARI | ExR | ExG | ExB | ExGR | VEG | IPCA | COM | |
| Sakha 93 | −0.082 c–f | 0.241 a–c | 0.040 b–d | 0.101 a–c | 0.113 ab | 0.062 a–c | 1.194 a–c | 14.400 a–c | 6.387 ab |
| Sakha 61 | −0.100 ef | 0.266 a–c | 0.034 b–d | 0.098 a–c | 0.122 a | 0.064 a–c | 1.199 a–c | 15.23 a–c | 6.388 ab |
| Sids1 | −0.057 a–e | 0.209 b–d | 0.050 a–d | 0.102 a–c | 0.101 ab | 0.052 a–d | 1.186 a–d | 13.062 a–d | 6.383 a–c |
| Kawz | −0.032 ab | 0.133 e | 0.081 a | 0.067 c | 0.110 ab | −0.014 c | 1.117 d | 8.588 d | 6.346 d |
| Sakha 93 | −0.109 f | 0.304 a | 0.019 d | 0.118 ab | 0.115 ab | 0.099 a | 1.241 a | 17.691 a | 6.408 a |
| Sakha 61 | −0.080 c–f | 0.268 ab | 0.029 cd | 0.124 a | 0.098 ab | 0.095 a | 1.236 a | 16.43 ab | 6.408 a |
| Sids1 | −0.069 b–f | 0.233 a–c | 0.042 b–d | 0.108 ab | 0.103 ab | 0.066 a–c | 1.201 a–c | 14.303 a–c | 6.391 ab |
| Kawz | −0.077 b–f | 0.248 a–c | 0.037 b–d | 0.112 ab | 0.104 ab | 0.076 ab | 1.212 a–c | 15.15 a–c | 6.396 ab |
| RIL1-1 | −0.076 b–f | 0.238 a–c | 0.041 b–d | 0.104 a–c | 0.108 ab | 0.063 a–c | 1.199 a–c | 14.42 a–c | 6.389 ab |
| RIL1-2 | −0.015 a | 0.133 e | 0.079 a | 0.084 bc | 0.093 b | 0.005 cd | 1.137 c–d | 8.764 d | 6.359 bd |
| RIL1-3 | −0.050 a–d | 0.174 de | 0.065 ab | 0.083 bc | 0.108 ab | 0.018 b–d | 1.150 b–d | 10.804 cd | 6.364 bd |
| RIL1-4 | −0.048 a–d | 0.200 b–d | 0.054 a–c | 0.105 a–c | 0.095 ab | 0.051 a–d | 1.186 a–d | 12.715 b–d | 6.384 a–c |
| RIL1-5 | −0.044 a–d | 0.170 de | 0.066 ab | 0.085 a–c | 0.106 ab | 0.019 b–d | 1.152 b–d | 10.716 cd | 6.365 bd |
| RIL1-6 | −0.076 b–f | 0.250 a–c | 0.037 b–d | 0.113 ab | 0.103 ab | 0.077 ab | 1.214 ab | 15.238 a–c | 6.396 ab |
| RIL1-7 | −0.047 a–d | 0.193 b–e | 0.057 a–c | 0.100 a–c | 0.098 ab | 0.043 a–d | 1.177 a–d | 12.218 b–d | 6.379 a–c |
| RIL2-1 | −0.0385 a–c | 0.180 c–e | 0.060 a–c | 0.098 a–c | 0.095 ab | 0.038 a–d | 1.170 a–d | 11.529 b–d | 6.376 a–c |
| RIL2-2 | −0.089 d–f | 0.257 a–c | 0.036 b–d | 0.103 a–c | 0.115 ab | 0.067 a–c | 1.204 a–c | 15.14 a–c | 6.390 ab |
| RIL2-3 | −0.042 a–d | 0.172 de | 0.064 ab | 0.089 a–c | 0.102 ab | 0.025 b–d | 1.156 b–d | 10.901 cd | 6.369 a–c |
| RIL2-4 | −0.054 a–e | 0.191 b–e | 0.058 a–c | 0.092 a–c | 0.105 ab | 0.035 a–d | 1.167 a–d | 11.890 b–d | 6.373 a–c |
| RIL2-5 | −0.080 c–f | 0.242 a–d | 0.040 b–d | 0.104 a–c | 0.110 ab | 0.064 a–c | 1.199 a–c | 14.53 a–c | 6.389 ab |
| RIL2-6 | −0.018 a | 0.142 e | 0.076 a | 0.088 a–c | 0.092 b | 0.012 b–d | 1.144 b–d | 9.348 d | 6.363 bd |
| RIL2-7 | −0.036 a–c | 0.179 de | 0.061 a–c | 0.101 a–c | 0.093 b | 0.040 a–d | 1.174 a–d | 11.609 b–d | 6.378 a–c |
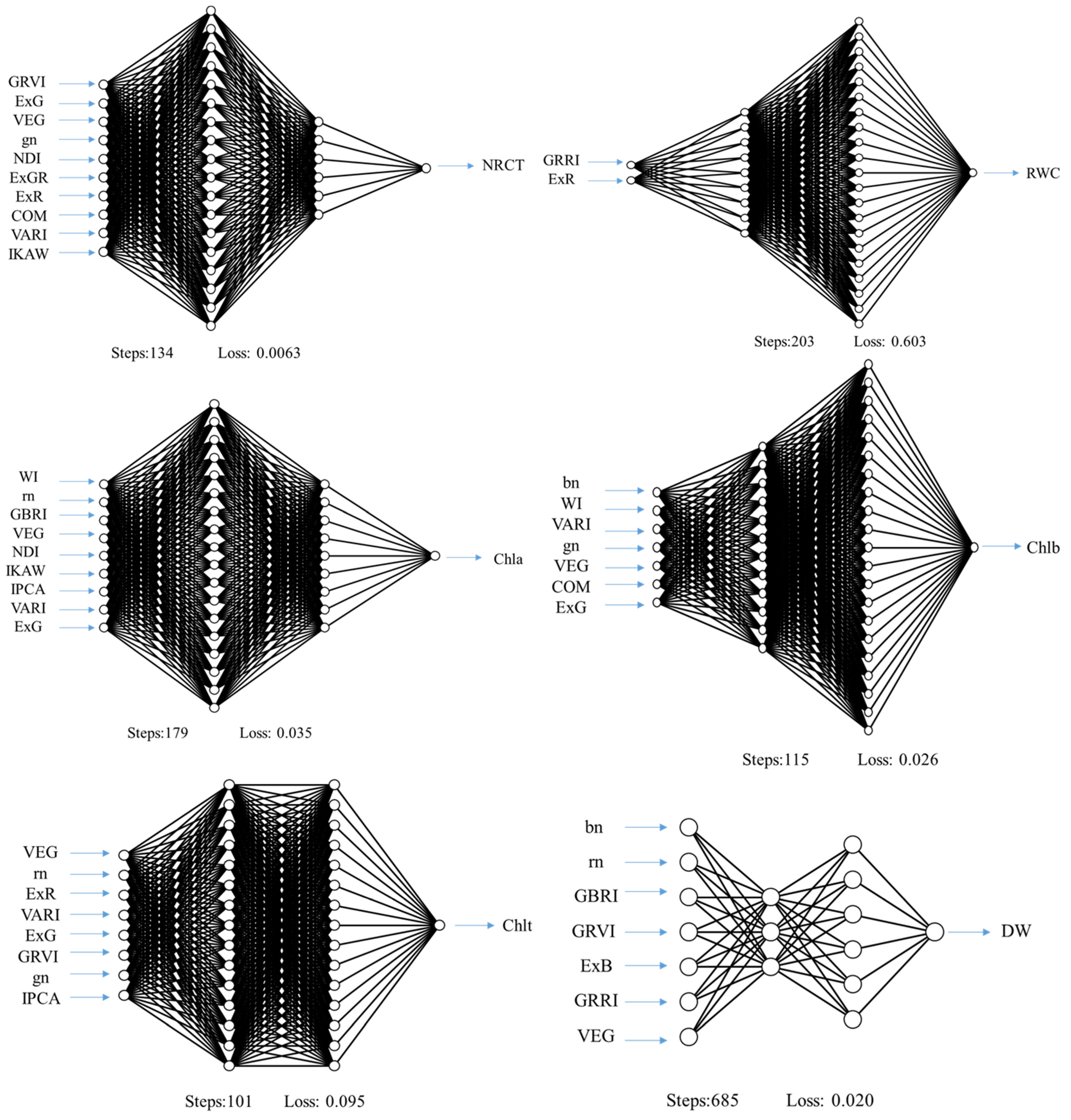
| Variable | Season | Suggested Features | Parameters | Training | Cross-Validation | ||||
|---|---|---|---|---|---|---|---|---|---|
| (h1, h2, f) | R2 | MSE | RMSE | R2 | MSE | RMSE | |||
| NRCT | S1 | GRRI, ExB, IKAW, NDI | (3, 18, relu) | 0.618 | 0.009 | 0.098 | 0.460 | 0.009 | 0.095 |
| S2 | WI, GBRI, ExB, IPCA, ExG, IKAW | (12, 12, logistic) | 0.999 | 1 × 10−6 | 0.001 | 0.581 | 0.009 | 0.093 | |
| CTS | GRVI, ExG, VEG, gn, NDI, ExGR, ExR COM, VARI, IKAW | (18, 6, identity) | 0.895 | 0.003 | 0.055 | 0.682 | 0.005 | 0.072 | |
| RWC | S1 | ExR, NDI, IKAW, RBRI, VEG, ExGR | (15, 3, relu) | 0.570 | 2.462 | 1.569 | 0.263 | 2.022 | 1.422 |
| S2 | GRRI, ExR | (9, 21, relu) | 0.806 | 1.208 | 1.099 | 0.681 | 1.171 | 1.082 | |
| CTS | RBRI, GRVI, GBRI, ExGR, VEG, VARI, NDI | (15, 3, identity) | 0.810 | 1.077 | 1.038 | 0.561 | 1.855 | 1.362 | |
| Chla | S1 | VARI | (18, 9, logistic) | 0.909 | 0.012 | 0.111 | 0.571 | 0.036 | 0.190 |
| S2 | ExB, bn, GRRI, GBRI, ExGR, NDI, VARI, ExR | (3, 3, relu) | 0.715 | 0.066 | 0.256 | 0.557 | 0.072 | 0.268 | |
| CTS | WI, rn, GBRI, VEG, NDI, IKAW, IPCA, VARI, ExG | (18, 9, identity) | 0.866 | 0.018 | 0.136 | 0.706 | 0.027 | 0.165 | |
| Chlb | S1 | NDI, gn | (18, 15, relu) | 0.534 | 0.030 | 0.174 | 0.342 | 0.024 | 0.155 |
| S2 | IKAW, RBRI, NDI, ExR, GRVI, VARI, VEG, COM, gn, ExB | (3, 3, logistic) | 0.981 | 0.003 | 0.051 | 0.284 | 0.049 | 0.221 | |
| CTS | bn, WI, VARI, gn, VEG, COM, ExG | (12, 21, identity) | 0.727 | 0.020 | 0.142 | 0.461 | 0.023 | 0.150 | |
| Chlt | S1 | COM, GRRI, IKAW, RBRI, IPCA, gn, ExG, rn, GRVI, VEG | (6, 18, relu) | 0.841 | 0.053 | 0.231 | 0.629 | 0.061 | 0.246 |
| S2 | GRVI | (3, 9, relu) | 0.692 | 0.207 | 0.455 | 0.472 | 0.190 | 0.436 | |
| CTS | VEG, rn, ExR, VARI, ExG, GRVI, gn, IPCA | (15, 15, identity) | 0.899 | 0.036 | 0.190 | 0.737 | 0.058 | 0.240 | |
| DW | S1 | bn, rn, GBRI, GRVI, ExB, GRRI, VEG | (3, 6, logistic) | 0.947 | 0.009 | 0.094 | 0.645 | 0.039 | 0.198 |
| S2 | bn, ExGR, IKAW, WI | (21, 21, relu) | 0.977 | 0.008 | 0.089 | 0.515 | 0.082 | 0.286 | |
| CTS | RBRI, gn, COM, VARI, rn, GBRI, bn, GRRI, WI | (9, 12, identity) | 0.827 | 0.034 | 0.184 | 0.338 | 0.073 | 0.270 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hendawy, S.; Tahir, M.U.; Al-Suhaibani, N.; Elsayed, S.; Elsherbiny, O.; Elsharawy, H. Potential of Thermal and RGB Imaging Combined with Artificial Neural Networks for Assessing Salt Tolerance of Wheat Genotypes Grown in Real-Field Conditions. Agronomy 2024, 14, 1390. https://doi.org/10.3390/agronomy14071390
El-Hendawy S, Tahir MU, Al-Suhaibani N, Elsayed S, Elsherbiny O, Elsharawy H. Potential of Thermal and RGB Imaging Combined with Artificial Neural Networks for Assessing Salt Tolerance of Wheat Genotypes Grown in Real-Field Conditions. Agronomy. 2024; 14(7):1390. https://doi.org/10.3390/agronomy14071390
Chicago/Turabian StyleEl-Hendawy, Salah, Muhammad Usman Tahir, Nasser Al-Suhaibani, Salah Elsayed, Osama Elsherbiny, and Hany Elsharawy. 2024. "Potential of Thermal and RGB Imaging Combined with Artificial Neural Networks for Assessing Salt Tolerance of Wheat Genotypes Grown in Real-Field Conditions" Agronomy 14, no. 7: 1390. https://doi.org/10.3390/agronomy14071390
APA StyleEl-Hendawy, S., Tahir, M. U., Al-Suhaibani, N., Elsayed, S., Elsherbiny, O., & Elsharawy, H. (2024). Potential of Thermal and RGB Imaging Combined with Artificial Neural Networks for Assessing Salt Tolerance of Wheat Genotypes Grown in Real-Field Conditions. Agronomy, 14(7), 1390. https://doi.org/10.3390/agronomy14071390









