Revisiting the Potential of Seed Nutri-Priming to Improve Stress Resilience and Nutritive Value of Cereals in the Context of Current Global Challenges
Abstract
:1. Introduction
2. The 21st Century, an Era of Global Climate Change Threatening Plant Productivity and Consequently Food Security
3. Significance of Micronutrients in Plant Physiology and Biochemistry
3.1. What Is the Difference between Micronutrients and Heavy Metal Polluants?
3.2. Specific Roles of Micronutrients in Plant Metabolism
4. Factors Affecting Crop Nutritional Characteristics with Special Consideration on Cereals with Importance in Human Diet
5. Main Biofortification Strategies Employed to Counteract Nutrient Deficiencies in Plants Including Cereals
5.1. Seed Nutri-Priming Methodology
5.1.1. Inorganic Agents
5.1.2. Organic Micronutrient Chelators
5.1.3. Synthetic Microelements Chelators
5.1.4. Nanoparticles (NPs)
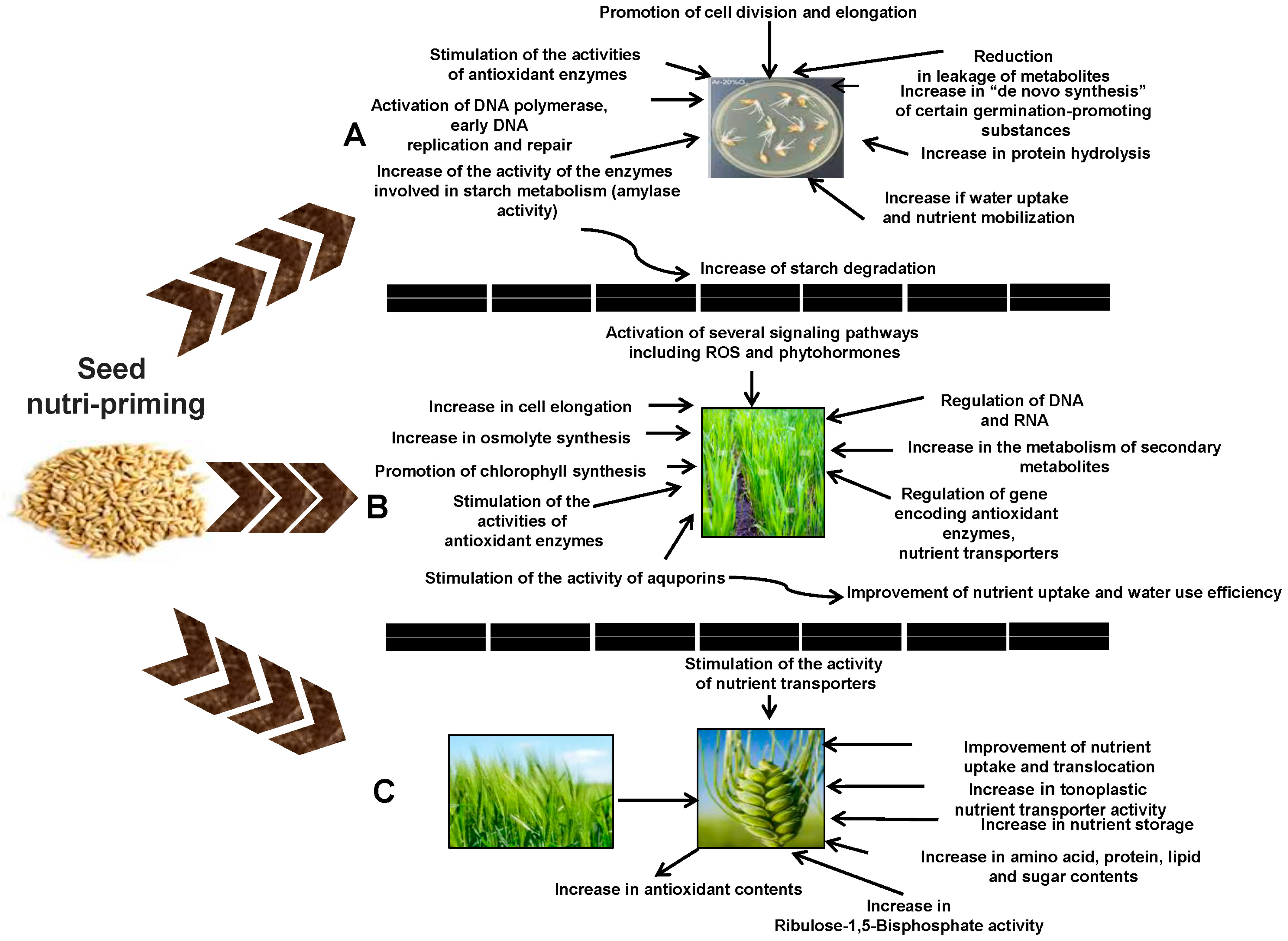
6. Key Mechanisms of Seed Nutri-Priming for Multiple Environmental Stress Mitigation
| Priming Agent | Plant Species | Stress | Reference |
|---|---|---|---|
| FeSO4 | Beta vulgaris Vigna Radiata Glycine max Stevia rebaudiana | Salinity Salinity Iron deficiency Drought | [170] [171] [172] [173,174] |
| ZnSO4 | Zea mays Triticum aestivum Glycine max Green bean Avena sativa | Salinity | [149] [98] [156] [175] [176] |
| Zea mays Hordeum vulgare Glycine max Cicer arietinum | Zn deficiency | [17] [148] [172] [177] | |
| Zea mays Spinacia oleracea | Low temperature | [150] [178] | |
| Zea mays Triticum durum Stevia rebaudiana Nigella sativa Vicia faba | Drought | [179] [180] [173,174] [181] [182] | |
| MnSO4 | Glycine max | Zn and Mn deficiency | [183] |
| Na2SeO4 | Oryza sativa Zea mays, Triticum durum Oryza sativa | Drought Drought Salinity | [184] [179,185,186] [155] |
| CuSO4 | Brassica rapa Avena sativa | Salinity | [187] [176] |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khanal, S.; Lutz, A.F.; Kraaijenbrink, P.D.A.; Van Den Hurk, B.; Yao, T.; Immerzeel, W.W. Variable 21st Century Climate Change Response for Rivers in High Mountain Asia at Seasonal to Decadal Time Scales. Water Resour. Res. 2021, 57, e2020WR029266. [Google Scholar] [CrossRef]
- Fan, Y.; Tjiputra, J.; Muri, H.; Lombardozzi, D.; Park, C.-E.; Wu, S.; Keith, D. Solar Geoengineering Can Alleviate Climate Change Pressures on Crop Yields. Nat. Food 2021, 2, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, Y.; Ibarra, D.E.; Du, X.; Dong, T.; Liu, Z.; Wang, C. Clay Mineralogical Evidence for Mid-Latitude Terrestrial Climate Change from the Latest Cretaceous through the Earliest Paleogene in the Songliao Basin, NE China. Cretac. Res. 2021, 124, 104827. [Google Scholar] [CrossRef]
- Mbah, R.E.; Wasum, D. Russian-Ukraine 2022 War: A Review of the Economic Impact of Russian-Ukraine Crisis on the USA, UK, Canada, and Europe. Adv. Soc. Sci. Res. J. 2022, 9, 144–153. [Google Scholar] [CrossRef]
- Koç, E.; Karayiğit, B. Assessment of biofortification approaches used to improve micronutrient-dense plants that are a sustainable solution to combat hidden hunger. J. Soil. Sci. Plant Nutr. 2022, 22, 475–500. [Google Scholar] [CrossRef]
- Raza, Q.; Azhar, M.T.; Rana, I.A.; Ahmad, M.Q.; Atif, R.M. Biofortification of crops to achieve food and nutritional security. In Biofortification of Grain and Vegetable Crops; Academic Press: Cambridge, MA, USA, 2024; pp. 1–17. [Google Scholar]
- Zaib, M.; Hussain, M.; Mumtaz, S.; Khalid, M.; Raza, I.; Abbas, S.; Danish, M.; Abbas, R.; Muhammad, N.; Bano, S. Micronutrients and Their significance in Agriculture: A Mini Review with Future Prospects. Int. Res. J. Edu. Technol. 2023, 5, 234–252. [Google Scholar]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 Threatens Human Nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Kihara, J.; Bolo, P.; Kinyua, M.; Rurinda, J.; Pikki, K. Micronutrient Deficiencies in African Soils and the Human Nutritional Nexus: Opportunities with Staple Crops. Environ. Geochem. Health 2020, 42, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Houmani, H.; Debez, A.; Slatni, T.; Yousfi, S.; Jellali, N.; M’sehli, W.; Abdelly, C.; Gharsalli, M. Insights into Physiological Responses of the Halophyte Suaeda fruticosa to Simultaneous Salinity and Iron Deficiency. Clean–Soil Air Water 2015, 43, 382–390. [Google Scholar] [CrossRef]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Krasilnikov, P.; Taboada, M.A. Amanullah Fertilizer Use, Soil Health and Agricultural Sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Ellouzi, H.; Zorrig, W.; Amraoui, S.; Oueslati, S.; Abdelly, C.; Rabhi, M.; Siddique, K.H.M.; Hessini, K. Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming. Antioxidants 2023, 12, 1779. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singhal, R.K.; Kumar, N.; Bose, B. Micro-Nutrient Seed Priming: A Pragmatic Approach Towards Abiotic Stress Management. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 231–255. ISBN 9789811513213. [Google Scholar]
- Liu, X.; Quan, W.; Bartels, D. Stress Memory Responses and Seed Priming Correlate with Drought Tolerance in Plants: An Overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef] [PubMed]
- Tamindžić, G.; Ignjatov, M.; Milošević, D.; Nikolić, Z.; Kostić Kravljanac, L.; Jovičić, D.; Dolijanović, Ž.; Savić, J. Seed Priming with Zinc Improves Field Performance of Maize Hybrids Grown on Calcareous Chernozem. Ital. J. Agron. 2021, 16, 1795. [Google Scholar] [CrossRef]
- Zafar, S.; Hussain, Z.; Perveen, S.; Iqbal, N.; Zafar, M.A. Deciphering Physio-Biochemical Characteristics of ZnSO4 Primed Wheat (Triticum aestivum L.) Plants Grown under Salt Stress. Pak. J. Bot. 2021, 53, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wei, J.; Han, G.; Sun, X.; Yang, X. Seed Osmopriming with Polyethylene Glycol (PEG) Enhances Seed Germination and Seedling Physiological Traits of Coronilla varia L. Under Water Stress. PLoS ONE 2024, 19, e0303145. [Google Scholar] [CrossRef] [PubMed]
- George, N.M.; Mohammed, E.A.; Muhammad, G.; Bolbol, A.A. Drought Stress Impacts and the Role of Endophytic Fungi Combating Abiotic Stress on Wheat. Bull. Fac. Sci. Zagazig Univ. 2024, 2023, 93–108. [Google Scholar] [CrossRef]
- Kidane, W.; Maetz, M.; Dardel, P. Food Security and Agricultural Development in Sub-Saharan Africa: Building a Case for More Public Support; Main Report; Policy Assistance Series; FAO [u.a.]: Rome, Italy, 2006; ISBN 978-92-5-105544-1. [Google Scholar]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The Impact of Multifactorial Stress Combination on Plant Growth and Survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef]
- Jain, S.K.; Wettberg, E.J.V.; Punia, S.S.; Parihar, A.K.; Lamichaney, A.; Kumar, J.; Gupta, D.S.; Ahmad, S.; Pant, N.C.; Dixit, G.P.; et al. Genomic-mediated breeding strategies for global warming in chickpeas (Cicer arietinum L.). Agriculture 2023, 13, 1721. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant Responses to Climate Change: Metabolic Changes under Combined Abiotic Stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the Nutritional Quality of Crop Plants under a Changing Climate: Importance and Strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Fischer, S.; Hilger, T.; Piepho, H.-P.; Jordan, I.; Cadisch, G. Do We Need More Drought for Better Nutrition? The Effect of Precipitation on Nutrient Concentration in East African Food Crops. Sci. Total Environ. 2019, 658, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Saqib, M.; Akhtar, J.; Haq, M.A.U. Interactive Effects of Salinity and Iron Deficiency on Different Rice Genotypes. J. Plant Nutr. Soil. Sci. 2015, 178, 306–311. [Google Scholar] [CrossRef]
- El-Fouly, M.M.; Salama, Z. Micronutrients (Fe, Mn, Zn) Foliar Spray for Increasing Salinity Tolerance in Wheat Triticum aestivum L. Afr. J. Plant Sci. 2011, 5, 314–322. [Google Scholar]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential Impacts of Climate Change on Vegetable Production and Product Quality—A Review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Bahuguna, R.N.; Pal, M.; Shah, D.; Maurya, S.; Jagadish, K.S.V. Elevated CO2 and Heat Stress Interactions Affect Grain Yield, Quality and Mineral Nutrient Composition in Rice under Field Conditions. Field Crops Res. 2017, 206, 149–157. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Neumann, N.; Fitzgerald, G.J.; Seneweera, S. Elevated CO2 Alters Grain Quality of Two Bread Wheat Cultivars Grown under Different Environmental Conditions. Agric. Ecosyst. Environ. 2014, 185, 24–33. [Google Scholar] [CrossRef]
- Medek, D.E.; Schwartz, J.; Myers, S.S. Estimated Effects of Future Atmospheric CO2 Concentrations on Protein Intake and the Risk of Protein Deficiency by Country and Region. Environ. Health Perspect. 2017, 125, 087002. [Google Scholar] [CrossRef]
- Leisner, C.P. Review: Climate Change Impacts on Food Security- Focus on Perennial Cropping Systems and Nutritional Value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of Elevated CO2 on Nutritional Quality of Vegetables: A Review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of Climate Changes on Crop Physiology and Food Quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate Changes and Potential Impacts on Postharvest Quality of Fruit and Vegetable Crops: A Review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Foyer, C.H. Climate Resilient Crops for Improving Global Food Security and Safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Tamindžić, G.; Azizbekian, S.; Miljaković, D.; Ignjatov, M.; Nikolić, Z.; Budakov, D.; Vasiljević, S.; Grahovac, M. Assessment of Various Nanoprimings for Boosting Pea Germination and Early Growth in Both Optimal and Drought-Stressed Environments. Plants 2024, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef]
- Appenroth, K.-J. What Are “Heavy Metals” in Plant Sciences? Acta Physiol. Plant. 2010, 32, 615–619. [Google Scholar] [CrossRef]
- Bhat, B.A.; Islam, S.T.; Ali, A.; Sheikh, B.A.; Tariq, L.; Islam, S.U.; Hassan Dar, T.U. Role of Micronutrients in Secondary Metabolism of Plants. In Plant Micronutrients; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 311–329. ISBN 978-3-030-49855-9. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The Beneficial Roles of Trace and Ultratrace Elements in Plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar] [CrossRef]
- Blasco, B.; Navarro-León, E.; Ruiz, J.M. Oxidative Stress in Relation With Micronutrient Deficiency or Toxicity. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 181–194. ISBN 978-0-12-812104-7. [Google Scholar]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Ozkan, H.; Braun, H.; Sayers, Z.; Cakmak, I. Concentration and Localization of Zinc during Seed Development and Germination in Wheat. Physiol. Plant. 2006, 128, 144–152. [Google Scholar] [CrossRef]
- Cakmak, I. Zinc Deficiency in Wheat in Turkey. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 181–200. ISBN 978-1-4020-6859-1. [Google Scholar]
- Chrysargyris, A.; Höfte, M.; Tzortzakis, N.; Petropoulos, S.A.; Di Gioia, F. Editorial: Micronutrients: The Borderline Between Their Beneficial Role and Toxicity in Plants. Front. Plant Sci. 2022, 13, 840624. [Google Scholar] [CrossRef]
- Zewide, I.; Sherefu, A. Review Paper on Effect of Micronutrients for Crop Production. Nutr. Food Process 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a Nutritional Role of Iodine in Plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient Homeostasis in Plants for More Sustainable Agriculture and Healthier Human Nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Eisenhut, M.; Schneider, A. Chloroplast Transition Metal Regulation for Efficient Photosynthesis. Trends Plant Sci. 2020, 25, 817–828. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace Metal Metabolism in Plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Skrypnik, L.; Feduraev, P.; Golovin, A.; Maslennikov, P.; Styran, T.; Antipina, M.; Riabova, A.; Katserov, D. The Integral Boosting Effect of Selenium on the Secondary Metabolism of Higher Plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.U.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-Induced Anti-Oxidative Defense and Osmotic Adjustments to Enhance Drought Stress Tolerance in Sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Bandehagh, A.; Dehghanian, Z.; Gougerdchi, V.; Anwar Hossain, M. Selenium: A Game Changer in Plant Development, Growth, and Stress Tolerance, via the Modulation in Gene Expression and Secondary Metabolite Biosynthesis. Phyton 2023, 92, 2301–2324. [Google Scholar] [CrossRef]
- Biel, W.; Kazimierska, K.; Bashutska, U. Nutritional Value of Wheat, Triticale, Barley and Oat Grains. Acta Sci. Pol. Zootech. 2020, 19, 19–28. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Fulgoni, V. Certain Grain Foods Can Be Meaningful Contributors to Nutrient Density in the Diets of U.S. Children and Adolescents: Data from the National Health and Nutrition Examination Survey, 2009–2012. Nutrients 2017, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Olugbire, O.O.; Olorunfemi, S.; Oke, D.O. Global Utilisation of Cereals: Sustainability and Environmental Issues. Agro-Sci. 2021, 20, 9–14. [Google Scholar] [CrossRef]
- Uçar, Ö. Chapter 15—The Situation of Cereals Cultivation in the World and Turkey. In New Approaches and Applications in Agriculture; Baran, M.F., Ed.; Iksad Publishing House: Ankara, Turkey, 2020; pp. 328–344. [Google Scholar]
- Erenstein, O.; Poole, N.; Donovan, J. Role of Staple Cereals in Human Nutrition: Separating the Wheat from the Chaff in the Infodemics Age. Trends Food Sci. Technol. 2022, 119, 508–513. [Google Scholar] [CrossRef]
- Sarwar, H. The Importance of Cereals (Poaceae: Gramineae) Nutrition in Human Health: A Review. J. Cereals Oilseeds 2013, 4, 32–35. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Viewpoint: Agri-Nutrition Research: Revisiting the Contribution of Maize and Wheat to Human Nutrition and Health. Food Policy 2021, 100, 101976. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef]
- Apaliya, M.T.; Osae, R.; Kwaw, E.; Mahunu, G.K.; Osei-Kwarteng, M.; Ahima, J.K. Fermented African Cereal Products. In African Fermented Food Products—New Trends; Elhadi Sulieman, A.M., Adam Mariod, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 459–472. ISBN 978-3-030-82901-8. [Google Scholar]
- Brouns, F.; Van Rooy, G.; Shewry, P.; Rustgi, S.; Jonkers, D. Adverse Reactions to Wheat or Wheat Components. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1437–1452. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef]
- Janda, K.; Orłowska, A.; Watychowicz, K.; Jakubczyk, K. The Role of Oat Products in the Prevention and Therapy of Type 2 Diabetes, Hypercholesterolemia and Obesity. Pomeranian J. Life Sci. 2019, 64, 30–36. [Google Scholar] [CrossRef]
- Temple, N. Fat, Sugar, Whole Grains and Heart Disease: 50 Years of Confusion. Nutrients 2018, 10, 39. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Polonskiy, V.; Loskutov, I.; Sumina, A. Biological Role and Health Benefits of Antioxidant Compounds in Cereals. Biol. Commun. 2020, 65, 53–67. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in Cereals: Composition and Health Effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Nguyen, S.N.; Drawbridge, P.; Beta, T. Distribution of Cereal Phytochemicals and Micronutrients in Whole Grains: A Review of Nutraceutical, Industrial, and Agricultural Implications. Cereal Chem. 2024. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Khlestkina, E.K. Wheat, Barley, and Oat Breeding for Health Benefit Components in Grain. Plants 2021, 10, 86. [Google Scholar] [CrossRef]
- Luthria, D.L.; Lu, Y.; John, K.M.M. Bioactive Phytochemicals in Wheat: Extraction, Analysis, Processing, and Functional Properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Slama, A.; Cherif, A.; Boukhchina, S. Importance of New Edible Oil Extracted from Seeds of Seven Cereals Species. J. Food Qual. 2021, 2021, 5531414. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.K.; Pooniya, V.; Suri, V.K.; Singh, U. Soil Factors Associated with Micronutrient Acquisition in Crops- Biofortification Perspective. In Biofortification of Food Crops; Singh, U., Praharaj, C.S., Singh, S.S., Singh, N.P., Eds.; Springer: New Delhi, India, 2016; pp. 159–176. ISBN 978-81-322-2714-4. [Google Scholar]
- Hussain, S.; Maqsood, M.A.; Rengel4, Z.; Aziz, T.; Abid, M. Estimated Zinc Bioavailability in Milling Fractions of Biofortified Wheat Grains and in Flours of Different Extraction Rates. Int. J. Agric. Biol. 2013, 15, 383–388. [Google Scholar]
- Cakmak, I.; Kutman, U.B. Agronomic Biofortification of Cereals with Zinc: A Review. Eur. J. Soil. Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. REVIEW: Biofortification of Durum Wheat with Zinc and Iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Shahzad, Z.; Rouached, H.; Rakha, A. Combating Mineral Malnutrition through Iron and Zinc Biofortification of Cereals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 329–346. [Google Scholar] [CrossRef]
- Prom-u-thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous Biofortification of Rice With Zinc, Iodine, Iron and Selenium Through Foliar Treatment of a Micronutrient Cocktail in Five Countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Khalid, M.; Naveed, M.; Ahmad, R.; Shahid, M. Iron Biofortification of Wheat Grains through Integrated Use of Organic and Chemical Fertilizers in pH Affected Calcareous Soil. Plant Physiol. Biochem. 2016, 104, 284–293. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Gruissem, W.; Bhullar, N.K. Iron Biofortification in the 21st Century: Setting Realistic Targets, Overcoming Obstacles, and New Strategies for Healthy Nutrition. Curr. Opin. Biotechnol. 2017, 44, 8–15. [Google Scholar] [CrossRef]
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Metabolic Engineering of Micronutrients in Crop Plants. Ann. N. Y. Acad. Sci. 2017, 1390, 59–73. [Google Scholar] [CrossRef]
- Ngozi, U.F. The Role of Biofortification in the Reduction of Micronutrient Food Insecurity in Developing Countries. Afr. J. Biotechnol. 2013, 12, 5559–5566. [Google Scholar]
- Marques, E.; Darby, H.M.; Kraft, J. Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches. Agronomy 2021, 11, 464. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Al-Faifi, Z.; Khemira, H. Priming Improves Germination and Seed Reserve Utilization, Growth, Antioxidant Responses and Membrane Stability at Early Seedling Stage of Saudi Sorghum Varieties under Drought Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 938–953. [Google Scholar] [CrossRef]
- Hajiboland, R. Effect of Micronutrient Deficiencies on Plants Stress Responses. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 283–329. [Google Scholar] [CrossRef]
- Nakandalage, N.; Seneweera, S. Micronutrients Use Efficiency of Crop-Plants Under Changing Climate. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 209–224. ISBN 978-0-12-812104-7. [Google Scholar]
- Tiozon, R.J.N.; Fernie, A.R.; Sreenivasulu, N. Meeting Human Dietary Vitamin Requirements in the Staple Rice via Strategies of Biofortification and Post-Harvest Fortification. Trends Food Sci. Technol. 2021, 109, 65–82. [Google Scholar] [CrossRef]
- Paraman, I.; Wagner, M.E.; Rizvi, S.S.H. Micronutrient and Protein-Fortified Whole Grain Puffed Rice Made by Supercritical Fluid Extrusion. J. Agric. Food Chem. 2012, 60, 11188–11194. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern Seed Technology: Seed Coating Delivery Systems for Enhancing Seed and Crop Performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed Priming in Field Crops: Potential Benefits, Adoption and Challenges. Crop Pasture Sci. 2019, 70, 731. [Google Scholar] [CrossRef]
- Khan, R.A. Response of Wheat (Triticum aestivum L.) to Zinc Sulphate and Copper Sulphate under Salt Stress. Pure Appl. Biol. 2020, 9, 2648–2658. [Google Scholar] [CrossRef]
- Atar, B.; Uygur, V.; Sukuşu, E. Effects of Priming with Copper, Zinc and Phosphorus on Seed and Seedling Composition in Wheat and Barley. Türk Tarım Ve Doğa Bilim. Derg. 2020, 7, 104–111. [Google Scholar] [CrossRef]
- Praharaj, S.; Singh, R.; Singh, V.K.; Chandra, R.; Guru, S.K.; Chaturvedi, S. Yield and Grain Zinc Concentration of Wheat as Affected by Nutri Priming and Foliar Application of Zinc. J. Pharmacogn. Phytochem. 2019, 8, 503–505. [Google Scholar]
- Rehman, A.; Farooq, M.; Ahmad, R.; Basra, S.M.A. Seed Priming with Zinc Improves the Germination and Early Seedling Growth of Wheat. Seed Sci. Technol. 2015, 43, 262–268. [Google Scholar] [CrossRef]
- Basit, A.; Hussain, S.; Abid, M.; Zafar-ul-Hye, M.; Ahmed, N. Zinc and Potassium Priming of Maize (Zea mays L.) Seeds for Salt-Affected Soils. J. Plant Nutr. 2021, 44, 130–141. [Google Scholar] [CrossRef]
- Seddigh, M.; Khoshgoftarmanesh, A.H.; Ghasemi, S. The Effectiveness of Seed Priming with Synthetic Zinc-Amino Acid Chelates in Comparison with Soil-Applied ZnSO4 in Improving Yield and Zinc Availability of Wheat Grain. J. Plant Nutr. 2016, 39, 417–427. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia Ur Rehman, M.; Waris, A.A. Zinc and Iron Oxide Nanoparticles Improved the Plant Growth and Reduced the Oxidative Stress and Cadmium Concentration in Wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Feng, X.; Ma, Q. Transcriptome and Proteome Profiling Revealed Molecular Mechanism of Selenium Responses in Bread Wheat (Triticum aestivum L.). BMC Plant Biol. 2021, 21, 584. [Google Scholar] [CrossRef]
- Karimi, N.; Goltapeh, E.M.; Amini, J.; Mehnaz, S.; Zarea, M.J. Effect of Azospirillum zeae and Seed Priming with Zinc, Manganese and Auxin on Growth and Yield Parameters of Wheat, under Dryland Farming. Agric. Res. 2021, 10, 44–55. [Google Scholar] [CrossRef]
- Rasool, T.; Ahmad, R.; Farooq, M. Seed Priming with Micronutrients for Improving the Quality and Yield of Hybrid Maize. Gesunde Pflanz. 2019, 71, 37–44. [Google Scholar] [CrossRef]
- Hadia, E.; Slama, A.; Romdhane, L.; Cheikh M’Hamed, H.; Fahej, M.A.S.; Radhouane, L. Seed Priming of Bread Wheat Varieties with Growth Regulators and Nutrients Improves Salt Stress Tolerance Particularly for the Local Genotype. J. Plant Growth Regul. 2023, 42, 304–318. [Google Scholar] [CrossRef]
- Poudel, P.; Di Gioia, F.; Lambert, J.D.; Connolly, E.L. Zinc Biofortification through Seed Nutri-priming using Alternative Zinc Sources and Concentration Levels in Pea and Sunflower Microgreens. Front. Plant Sci. 2023, 14, 1177844. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biol. Technol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Carmona, V.M.V.; Filho, A.B.C.; de Almeida, H.J.; Silva, G.C.; dos Reis, A.R. Agronomic biofortification of beet plants with zinc via seed priming. Revista Caatinga. 2020, 33, 116–123. [Google Scholar] [CrossRef]
- Sharma, V.; Kharb, V.; Verma, V.; Dhaliwal, S.S.; Kalia, A.; Behera, S.K.; Singh, P. Comparative Potential of Different Fe Sources for Seed Priming to Enhance Yield and Iron Content in Direct Seeded Aerobic Rice. Cereal Res. Commun. 2024, 1–10. [Google Scholar] [CrossRef]
- Majda, C.; Khalid, D.; Aziz, A.; Rachid, B.; Badr, A.-S.; Lotfi, A.; Mohamed, B. Nutri-Priming as an Efficient Means to Improve the Agronomic Performance of Molybdenum in Common Bean (Phaseolus vulgaris L.). Sci. Total Environ. 2019, 661, 654–663. [Google Scholar] [CrossRef]
- Mirbolook, A.; Lakzian, A.; Sadaghiani, M.R. Fortification of Bread Wheat Using Synthesized Zn- Glycine and Zn-Alanine Chelates in Comparison with ZnSO4 in a Calcareous Soil. Commun. Soil Sci. Plant Anal. 2020, 51, 1048–1064. [Google Scholar] [CrossRef]
- Mirbolook, A.; Rasouli-Sadaghiani, M.; Sepehr, E.; Lakzian, A.; Hakimi, M. Synthesized Zn(II)-Amino Acid and -Chitosan Chelates to Increase Zn Uptake by Bean (Phaseolus vulgaris) Plants. J. Plant Growth Regul. 2021, 40, 831–847. [Google Scholar] [CrossRef]
- Hussaan, M.; Abbas, S.; Ali, Q.; Akram, M.S.; Tanwir, K.; Raza, A.; Hashmat, S.; Aqeel, M.; Chaudhary, H.J.; Javed, M.T. Elucidating the mechanistic role of Zinc-Lysine to enhance cd tolerance in diverse wheat (Triticum aestivum L.) cultivars through distinct morpho-physio-biochemical improvements under cd stress. J. Soil. Sci. Plant Nutr. 2023, 23, 5419–5437. [Google Scholar] [CrossRef]
- Babadi, M.A.; Masir, M.N.; Moezzi, A.A.; Taghavi, M.; Rahnama, A.; Razavi, B.S. Evaluation the efficiency of different application method of Fe aminochelates compared to FeSO4 on yield and quality traits of oleic Sunflower (Helianthus annuus L.) in a calcareous soil. In Proceedings of the EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024. [Google Scholar] [CrossRef]
- Veena, M.; Puthur, J.T. Seed Nutripriming with Zinc Is an Apt Tool to Alleviate Malnutrition. Environ. Geochem. Health 2022, 44, 2355–2373. [Google Scholar] [CrossRef]
- Zhao, A.; Mei, S.Y.; Wang, B.; Tian, X. Effects of ZnSO4 and Zn-EDTA applied by broadcasting or by banding on soil zn fractions and zn uptake by wheat (Triticum aestivum L.) under greenhouse conditions. J. Plant Nutr. Soil. Sci. 2019, 182, 307–317. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Shivay, Y.S.; Nisar, S.; Gaber, A.; Brestic, M.; Barek, V. Biofortification—A frontier novel approach to enrich micronutrients in field crops to encounter the nutritional security. Molecules 2022, 27, 1340. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Cardarelli, M.; Rouphael, Y.; Cesco, S.; Pii, Y.; Colla, G. Iron Nutrition in Agriculture: From Synthetic Chelates to Biochelates. Sci. Hortic. 2023, 312, 111833. [Google Scholar] [CrossRef]
- Raza, A.; Tahir, M.A.; Sabah, N.-U.; Shah, S.H.; Sarwar, G.; Manzoor, M.Z. Seed Priming with Zinc Ion on Growth Performance and Nutrient Acquisition of Maize in Aridisols. Pak. J. Bot. 2023, 55, 1365–1374. [Google Scholar] [CrossRef]
- Singh, N.K.; Rai, A.K. Enhancing Crop Productivity through Nanotechnology: A Comprehensive Review of Strategies and Results. J. Exp. Agric. Int. 2024, 46, 435–458. [Google Scholar] [CrossRef]
- Lee, J.H.; Kasote, D.M. Nano-Priming for Inducing Salinity Tolerance, Disease Resistance, Yield Attributes, and Alleviating Heavy Metal Toxicity in Plants. Plants 2024, 13, 446. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, S.K.; Kumar, R.; Singh, P.M.; Chaubey, T.; Singh, V.; Behera, T.K. Seed priming with engineered nanomaterials for mitigating abiotic stress in plants. In Nanotechnology for Abiotic Stress Tolerance and Management in Crop Plants; Academic Press: Cambridge, MA, USA, 2024; pp. 229–247. [Google Scholar]
- Gomes, D.G.; Pelegrino, M.T.; Ferreira, A.S.; Bazzo, J.H.; Zucareli, C.; Seabra, A.B.; Oliveira, H.C. Seed Priming with Copper-loaded Chitosan Nanoparticles Promotes Early Growth and Enzymatic Antioxidant Defense of Maize (Zea mays L.) Seedlings. J. Chem. Technol. Biotechnol. 2021, 96, 2176–2184. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Das, H.; Mukherjee, A.; Kundu, R. Nanopriming with Zero-valent Iron Synthesized using Pomegranate peel Waste: A “green” Approach for Yield Enhancement in Oryza sativa L. cv. Gonindobhog. Plant Physiol. Biochem. 2021, 163, 261–275. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Seed Priming with Iron Oxide Nanoparticles Modulate Antioxidant Potential and Defense-Linked Hormones in Watermelon Seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Shahverdi, M.A.; Omidi, H.; Tabatabaei, S.J. Effect of Nutri-priming on Germination Indices and Physiological Characteristics of Stevia Seedling under Salinity Stress. J. Seed Sci. 2017, 39, 353–362. [Google Scholar] [CrossRef]
- Pawar, V.A.; Laware, S.L. Seed Priming a Critical Review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.W.; DeRose, E.F.; Cavanaugh, N.A.; Beard, W.A.; Shock, D.D.; Mueller, G.A.; Wilson, S.H.; London, R.E. Metal-Induced DNA Translocation Leads to DNA Polymerase Conformational Activation. Nucleic Acids Res. 2012, 40, 2974–2983. [Google Scholar] [CrossRef]
- Bose, B.; Kumar, M.; Singhal, R.K.; Mondal, S. Impact of Seed Priming on the Modulation of Physico-Chemical and Molecular Processes During Germination, Growth, and Development of Crops. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 23–40. ISBN 9789811300318. [Google Scholar]
- Johnson, R.; Puthur, J.T. Seed Priming as a Cost Effective Technique for Developing Plants with Cross Tolerance to Salinity Stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed Priming with BABA (β-Amino Butyric Acid): A Cost-Effective Method of Abiotic Stress Tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; Lima-Brito, J. Seed Priming with Iron and Zinc in Bread Wheat: Effects in Germination, Mitosis and Grain Yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef]
- Carvalho, A.; Lino, A.; Alves, C.; Lino, C.; Vareiro, D.; Lucas, D.; Alfonso, G.; Costa, G.; Esteves, M.; Bezerra, M.; et al. Combination of Iron and Zinc Enhanced the Root Cell Division, Mitotic Regularity and Nucleolar Activity of Hexaploid Triticale. Plants 2023, 12, 2517. [Google Scholar] [CrossRef]
- Hera, M.H.R.; Hossain, M.; Paul, A.K. Effect of Foliar Zinc Spray on Growth and Yield of Heat Tolerant Wheat under Water Stress. Int. J. Biol. Environ. Eng. 2018, 1, 10–16. [Google Scholar]
- Hilo, A.; Shahinnia, F.; Druege, U.; Franken, P.; Melzer, M.; Rutten, T.; von Wirén, N.; Hajirezaei, M.R. A Specific Role of Iron in Promoting Meristematic Cell Division during Adventitious Root Formation. J. Exp. Bot. 2017, 68, 4233–4247. [Google Scholar] [CrossRef]
- Mottonen, M.; Aphalo, P.J.; Lehto, T. Role of Boron in Drought Resistance in Norway Spruce (Picea abies) Seedlings. Tree Physiol. 2001, 21, 673–681. [Google Scholar] [CrossRef]
- El-Shintinawy, F. Structural and Functional Damage Caused by Boron Deficiency in Sunflower Leaves. Photosynthetica 1999, 36, 565–573. [Google Scholar] [CrossRef]
- Salehi, H.; Cheheregani Rad, A.; Raza, A.; Djalovic, I.; Prasad, P.V.V. The Comparative Effects of Manganese Nanoparticles and Their Counterparts (Bulk and Ionic) in Artemisia annua Plants via Seed Priming and Foliar Application. Front. Plant Sci. 2023, 13, 1098772. [Google Scholar] [CrossRef]
- Mondal, S.; Bose, B. Impact of Micronutrient Seed Priming on Germination, Growth, Development, Nutritional Status and Yield Aspects of Plants. J. Plant Nutr. 2019, 42, 2577–2599. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Choukri, M.; Abouabdillah, A.; Bouabid, R.; Abd-Elkader, O.H.; Pacioglu, O.; Boufahja, F.; Bourioug, M. Zn Application through Seed Priming Improves Productivity and Grain Nutritional Quality of Silage Corn. Saudi J. Biol. Sci. 2022, 29, 103456. [Google Scholar] [CrossRef]
- Rahman, M.T.; Idid, S.Z. Can Zn Be a Critical Element in COVID-19 Treatment? Biol. Trace Elem. Res. 2021, 199, 550–558. [Google Scholar] [CrossRef]
- Ajouri, A.; Asgedom, H.; Becker, M. Seed Priming Enhances Germination and Seedling Growth of Barley under Conditions of P and Zn Deficiency. J. Plant Nutr. Soil. Sci. 2004, 167, 630–636. [Google Scholar] [CrossRef]
- Imran, M.; Boelt, B.; Mühling, K.-H. Zinc Seed Priming Improves Salt Resistance in Maize. J. Agron. Crop Sci. 2018, 204, 390–399. [Google Scholar] [CrossRef]
- Imran, M.; Mahmood, A.; Römheld, V.; Neumann, G. Nutrient Seed Priming Improves Seedling Development of Maize Exposed to Low Root Zone Temperatures during Early Growth. Eur. J. Agron. 2013, 49, 141–148. [Google Scholar] [CrossRef]
- Rehman, A.U.; Masood, S.; Khan, N.U.; Abbasi, M.E.; Hussain, Z.; Ali, I. Molecular Basis of Iron Biofortification in Crop Plants; A Step Towards Sustainability. Plant Breed. 2021, 140, 12–22. [Google Scholar] [CrossRef]
- Iqbal, S.; Farooq, M.; Cheema, S.A.; Afzal, I. Boron Seed Priming Improves the Seedling Emergence, Growth, Grain Yield and Grain Biofortification of Bread Wheat. Int. J. Agric. Biol. 2017, 19, 177–182. [Google Scholar] [CrossRef]
- Hafeez, K.; Atif, M.; Perveen, S.; Parveen, A.; Akhtar, F.; Yasmeen, N. Unraveling the Contribution of Copper Seed Priming in Enhancing Chromium Tolerance in Wheat by Improving Germination, Growth, and Grain Yield. Environ. Sci. Pollut. Res. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Gupta, M. Micronutrient Seed Priming: New Insights in Ameliorating Heavy Metal Stress. Environ. Sci. Pollut. Res. 2022, 29, 58590–58606. [Google Scholar] [CrossRef]
- Subramanyam, K.; Du Laing, G.; Van Damme, E.J.M. Sodium Selenate Treatment Using a Combination of Seed Priming and Foliar Spray Alleviates Salinity Stress in Rice. Front. Plant Sci. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-Y.; Zhu, H.-D.; Yin, K.-D.; Du, J.-D.; Zhang, Y.-X. Seed Priming Mitigates the Effects of Saline-Alkali Stress in Soybean Seedlings. Chil. J. Agric. Res. 2017, 77, 118–125. [Google Scholar] [CrossRef]
- Padhan, B.K.; Sathee, L.; Jain, V. Nitrogen Remobilization and Its Importance in Nitrogen Use Efficiency (NUE) of Crops. Indian. J. Agric. Sci. 2021, 90, 2251–2261. [Google Scholar] [CrossRef]
- Tuiwong, P.; Lordkaew, S.; Veeradittakit, J.; Jamjod, S.; Prom-u-thai, C. Seed Priming and Foliar Application with Nitrogen and Zinc Improve Seedling Growth, Yield, and Zinc Accumulation in Rice. Agriculture 2022, 12, 144. [Google Scholar] [CrossRef]
- Ji, C.; Li, J.; Jiang, C.; Zhang, L.; Shi, L.; Xu, F.; Cai, H. Zinc and Nitrogen Synergistic Act on Root-to-Shoot Translocation and Preferential Distribution in Rice. J. Adv. Res. 2022, 35, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hussain, S.; Khan, S.; Geng, M. Seed Priming Improved Antioxidant Defense System and Alleviated Ni-Induced Adversities in Rice Seedlings Under N, P, or K Deprivation. Front. Plant Sci. 2020, 11, 565647. [Google Scholar] [CrossRef]
- Khan, E.; Gupta, M. Arsenic–Silicon Priming of Rice (Oryza sativa L.) Seeds Influence Mineral Nutrient Uptake and Biochemical Responses through Modulation of Lsi-1, Lsi-2, Lsi-6 and Nutrient Transporter Genes. Sci. Rep. 2018, 8, 10301. [Google Scholar] [CrossRef]
- Khan, F.; Hussain, S.; Tanveer, M.; Khan, S.; Hussain, H.A.; Iqbal, B.; Geng, M. Coordinated Effects of Lead Toxicity and Nutrient Deprivation on Growth, Oxidative Status, and Elemental Composition of Primed and Non-Primed Rice Seedlings. Environ. Sci. Pollut. Res. 2018, 25, 21185–21194. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.G.; Ribeiro De Lima, B.; Alves Medeiros, L.R.; Ribeiro, S.A.; Bucher, C.A.; Santos, L.A.; Fernandes, M.S.; Vieira Rossetto, C.A. Nutripriming with Ammonium Nitrate Improves Emergence and Root Architecture and Promotes an Increase in Nitrogen Content in Upland Rice Seedlings. Biocatal. Agric. Biotechnol. 2022, 42, 102331. [Google Scholar] [CrossRef]
- Singhal, R.K.; Pandey, S.; Bose, B. Seed Priming with Mg(NO3)2 and ZnSO4 Salts Triggers Physio-Biochemical and Antioxidant Defense to Induce Water Stress Adaptation in Wheat (Triticum aestivum L.). Plant Stress 2021, 2, 100037. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Afshari, F.; Nakhaei, F.; Mosavi, S.; Seghatoleslami, M. Physiological and Biochemical Responses of Stevia Rebaudiana Bertoni to Nutri-Priming and Foliar Nutrition under Water Supply Restrictions. Ind. Crops Prod. 2022, 176, 114399. [Google Scholar] [CrossRef]
- Thomas, D.T.; Puthur, J.T. Amplification of Abiotic Stress Tolerance Potential in Rice Seedlings with a Low Dose of UV-B Seed Priming. Funct. Plant Biol. 2019, 46, 455. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.; Yasin, N.A. Selenium Seed Priming Enhanced the Growth of Salt-Stressed Brassica rapa L. through Improving Plant Nutrition and the Antioxidant System. Front. Plant Sci. 2023, 13, 1050359. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Moatter, K. Effects of Seed Priming with PbSO4 and FeSO4 on Germination and Growth of Seedlings of Beta vulgaris L. under NaCl Stress. Pure Appl. Biol. 2020, 9, 1405–1423. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, A.; Qadri, T.A. Influence of Seed Priming with FeSO4 on Germination, Growth and Biochemical Aspects of Mung bean (Vigna radiata L.) Grown under NaCl Stress. J. Biosci. Appl. Res. 2019, 5, 519–532. [Google Scholar] [CrossRef]
- Goiba, P.K.; Durgude, A.; Pharande, A.; Kadlag, A.; Chauhan, M.; Nimbalkar, C. Effect of Seed Priming with Iron and Zinc on Yield Contributing Parameters as Well as the Nutrient Uptake of the Soybean (Glycine max) in Calcareous Soil. Int. J. Chem. Stud. 2018, 6, 758–760. [Google Scholar]
- Gorzi, A.; Omidi, H.; Bostani, A.B. Morpho-Physiological Responses of Stevia (Stevia rebaudiana Bertoni) to Various Priming Treatments Under Drought Stress. Appl. Ecol. Environ. Res. 2018, 16, 4753–4771. [Google Scholar] [CrossRef]
- Gorzi, A.; Omidi, H.; Bostani, A. Effect of Stevia (Stevia rebaudiana) Seed Priming Treatments with Salicylic Acid, Iron, and Zinc on Some Germination Traits and Photosynthetic Pigments under Drought Stress. Iran. J. Seed Res. 2020, 6, 125–135. [Google Scholar] [CrossRef]
- Gulmezoglu, N.; Aydogan, C.; Turhan, E. Physiological, Biochemical and Mineral Dimensions of Green Bean genotypes Depending on Zn Priming and Salinity. Legume Res. 2016, 39, 713–721. [Google Scholar] [CrossRef]
- Iqbal, S. Influence of Seed Priming with CuSO4 and ZnSO4 on Germination and Seedling Growth of Oat under NaCl Stress. Pure Appl. Biol. 2020, 9, 897–912. [Google Scholar] [CrossRef]
- Nautiyal, N.; Shukla, K. Evaluation of Seed Priming Zinc Treatments in Chickpeas for Seedling Establishment Under Zinc Deficient Conditions. J. Plant Nutr. 2013, 36, 251–258. [Google Scholar] [CrossRef]
- Imran, M.; Mahmood, A.; Neumann, G.; Boelt, B. Zinc Seed Priming Improves Spinach Germination at Low Temperature. Agriculture 2021, 11, 271. [Google Scholar] [CrossRef]
- Nawaz, F.; Zulfiqar, B.; Ahmad, K.S.; Majeed, S.; Shehzad, M.A.; Javeed, H.M.R.; Tahir, M.N.; Ahsan, M. Pretreatment with Selenium and Zinc Modulates Physiological Indices and Antioxidant Machinery to Improve Drought Tolerance in Maize (Zea mays L.). S. Afr. J. Bot. 2021, 138, 209–216. [Google Scholar] [CrossRef]
- Candan, N.; Cakmak, I.; Ozturk, L. Zinc-biofortified Seeds Improved Seedling Growth under Zinc Deficiency and Drought Stress in Durum Wheat. J. Plant Nutr. Soil. Sci. 2018, 181, 388–395. [Google Scholar] [CrossRef]
- Fallah, S.; Malekzadeh, S.; Pessarakli, M. Seed Priming Improves Seedling Emergence and Reduces Oxidative Stress in Nigella sativa under Soil Moisture Stress. J. Plant Nutr. 2018, 41, 29–40. [Google Scholar] [CrossRef]
- Farooq, M.; Almamari, S.A.D.; Rehman, A.; Al-Busaidi, W.M.; Wahid, A.; Al-Ghamdi, S.S. Morphological, Physiological and Biochemical Aspects of Zinc Seed Priming-Induced Drought Tolerance in Faba Bean. Sci. Hortic. 2021, 281, 109894. [Google Scholar] [CrossRef]
- Muhammad, I.; Volker, R.; Günter, N. Accumulation and Distribution of Zn and Mn in Soybean Seeds after Nutrient Seed Priming and Its Contribution to Plant Growth under Zn- and Mn-Deficient Conditions. J. Plant Nutr. 2017, 40, 695–708. [Google Scholar] [CrossRef]
- Emam, M.M.; Khattab, H.E.; Helal, N.M. Effect of Selenium and Silicon on Yield Quality of Rice Plant Grown under Drought Stress. Aust. J. Crop Sci. 2014, 8, 596–605. [Google Scholar]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Bukhari, M.A. Supplemental Selenium Improves Wheat Grain Yield and Quality through Alterations in Biochemical Processes under Normal and Water Deficit Conditions. Food Chem. 2015, 175, 350–357. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) Seed Priming Induced Growth and Biochemical Changes in Wheat Under Water Deficit Conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef]
- Begum, N.; Hamayun, M.; Rahman, I.U.; Ijaz, F.; Sohail, Z.I.; Afzal, M.; Ullah, A.; Karim, S. Influence of Seed Priming with ZnSO4 and CuSO4 on Germination and Seedling Growth of Brassica rapa under Nacl Stress. Middle East. J. Sci. Res. 2014, 22, 879–885. [Google Scholar]


| Priming Agent Form | Concentration | Duration | Reference |
|---|---|---|---|
| ZnSO4 | 100–200 ppm 0.3 and 0.5% 0.1, 0.3, 0.5, 0.7% 0.01, 0.05, 0.1, 0.5, 1.0 M 4 mM | 1 h 10 h 6 h 12 h 24 h | [98] [99] [100] [101] [102] |
| ZnCl2 | 0.01, 0.05, 0.1, 0.5, 1.0 M | 12 h | [101] |
| Zn(Gln), Zn(Arg), Zn(His) | 40 mg Zn kg−1 soil | 12 h | [103] |
| ZnO NPs | 0, 25, 50, 75, 100 mg/L | 24 h | [104] |
| CuSO4 | 100–200 ppm 0.03 and 0.06% | 1 h 10 h | [98] [99] |
| CUO-NPs | 3.33, 44.4, 5.55 mg/L | 8 h | [105] |
| Na2SeO4 | 10 μM | 30 mn, 24 h | [106] |
| MnSO4 | 0.1, 0.2, 0.3, 0.4% | 6 h | [107] |
| H3BO3 | 0.01% | 8 h | [108] |
| FeEDTA | 50 µmol/L | 12 h | [109] |
| Fe NPs | 0, 5, 10, 15, 20 mg/L | 24 h | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houmani, H.; Ben Slimene Debez, I.; Turkan, I.; Mahmoudi, H.; Abdelly, C.; Koyro, H.-W.; Debez, A. Revisiting the Potential of Seed Nutri-Priming to Improve Stress Resilience and Nutritive Value of Cereals in the Context of Current Global Challenges. Agronomy 2024, 14, 1415. https://doi.org/10.3390/agronomy14071415
Houmani H, Ben Slimene Debez I, Turkan I, Mahmoudi H, Abdelly C, Koyro H-W, Debez A. Revisiting the Potential of Seed Nutri-Priming to Improve Stress Resilience and Nutritive Value of Cereals in the Context of Current Global Challenges. Agronomy. 2024; 14(7):1415. https://doi.org/10.3390/agronomy14071415
Chicago/Turabian StyleHoumani, Hayet, Imen Ben Slimene Debez, Ismail Turkan, Henda Mahmoudi, Chedly Abdelly, Hans-Werner Koyro, and Ahmed Debez. 2024. "Revisiting the Potential of Seed Nutri-Priming to Improve Stress Resilience and Nutritive Value of Cereals in the Context of Current Global Challenges" Agronomy 14, no. 7: 1415. https://doi.org/10.3390/agronomy14071415





