Research Progress on Diseases Caused by the Soil-Borne Fungal Pathogen Rhizoctonia solani in Alfalfa
Abstract
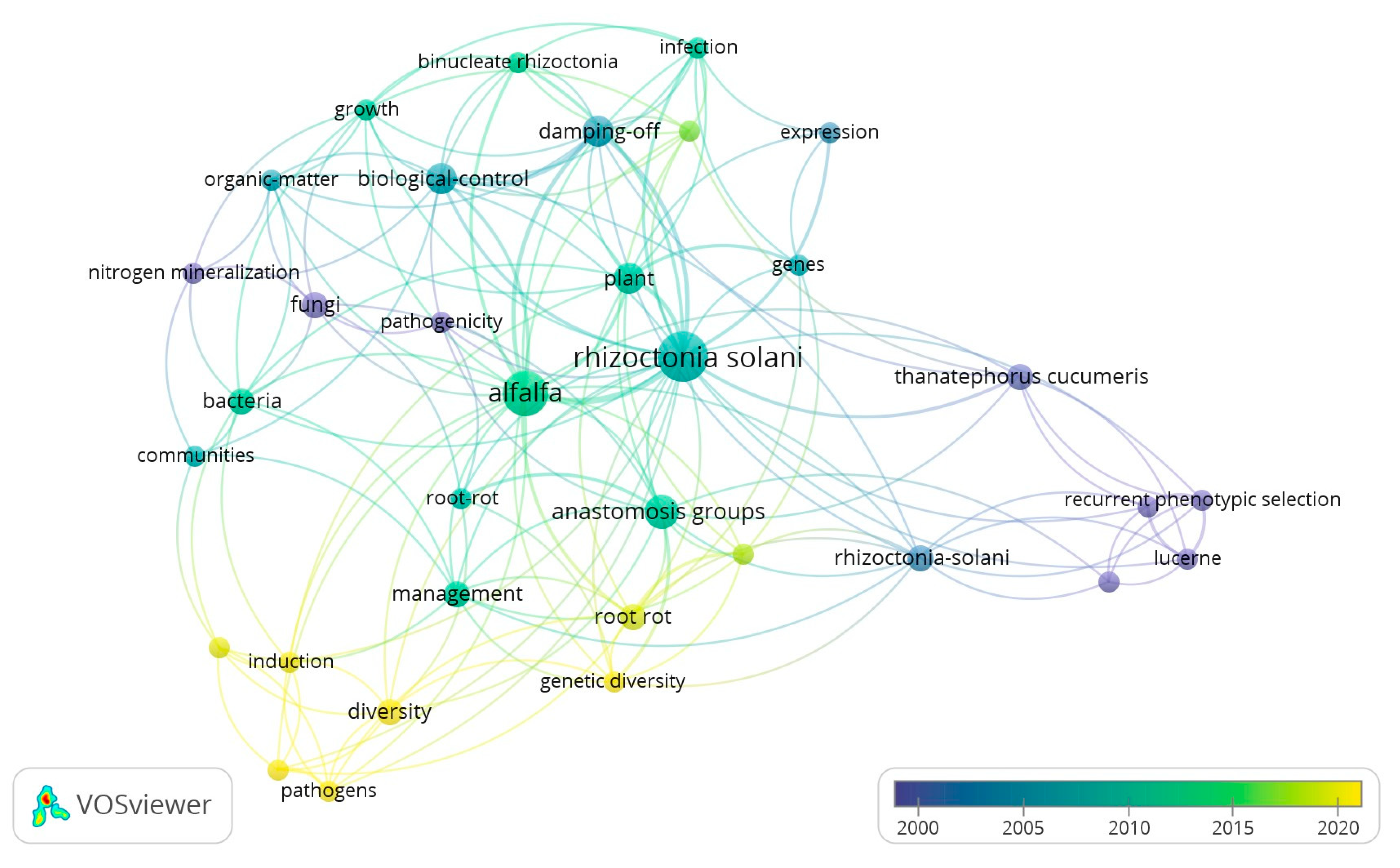
:1. Introduction
2. Diseases Caused by R. solani and Worldwide Distribution
2.1. Damping-off
2.2. Root Rot
2.3. Root Canker
2.4. Crown Rot
2.5. Crown Bud Rot
2.6. Stem Canker
2.7. Blights
2.8. Global Distribution
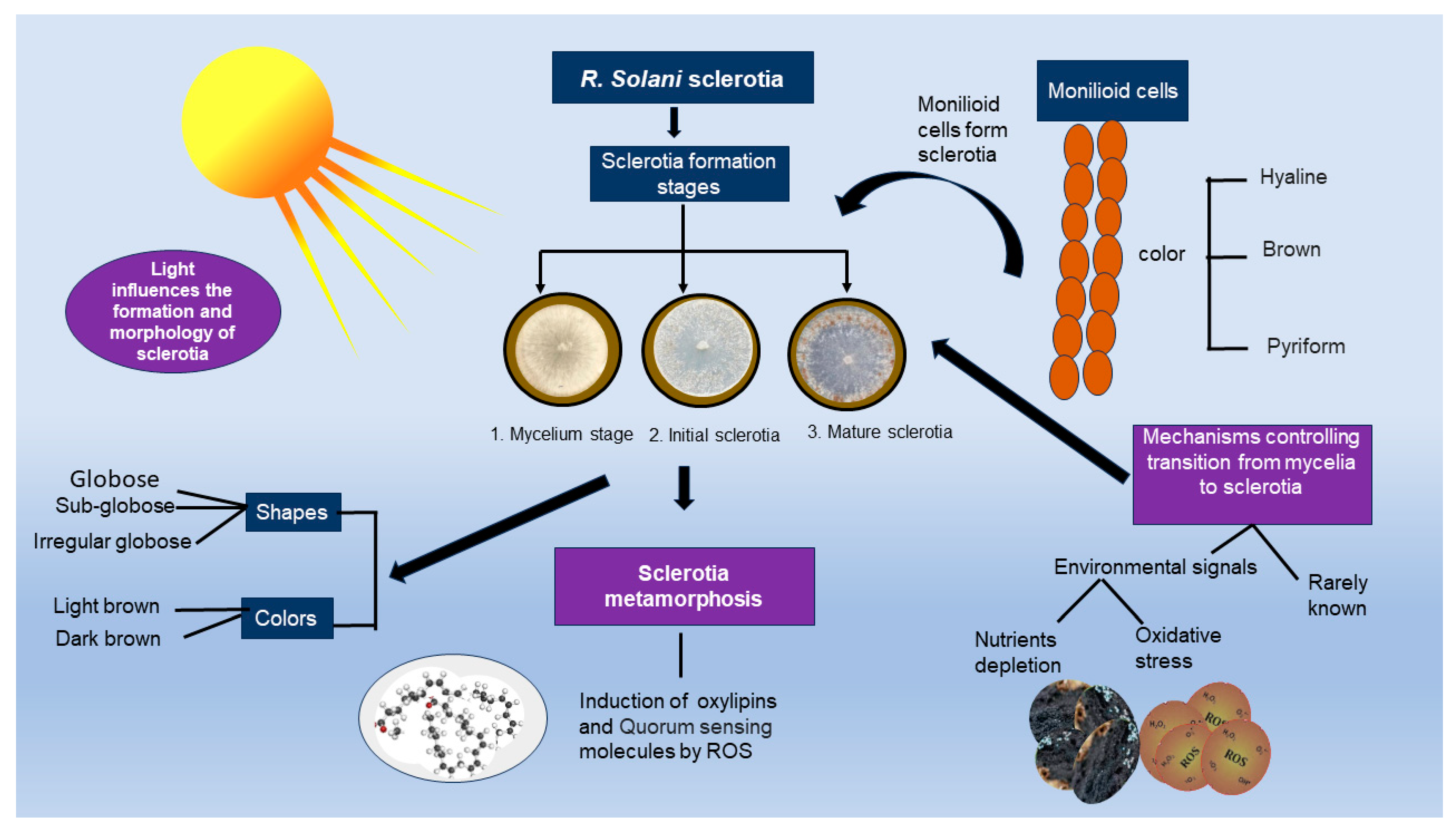
3. Survival Structure of R. solani
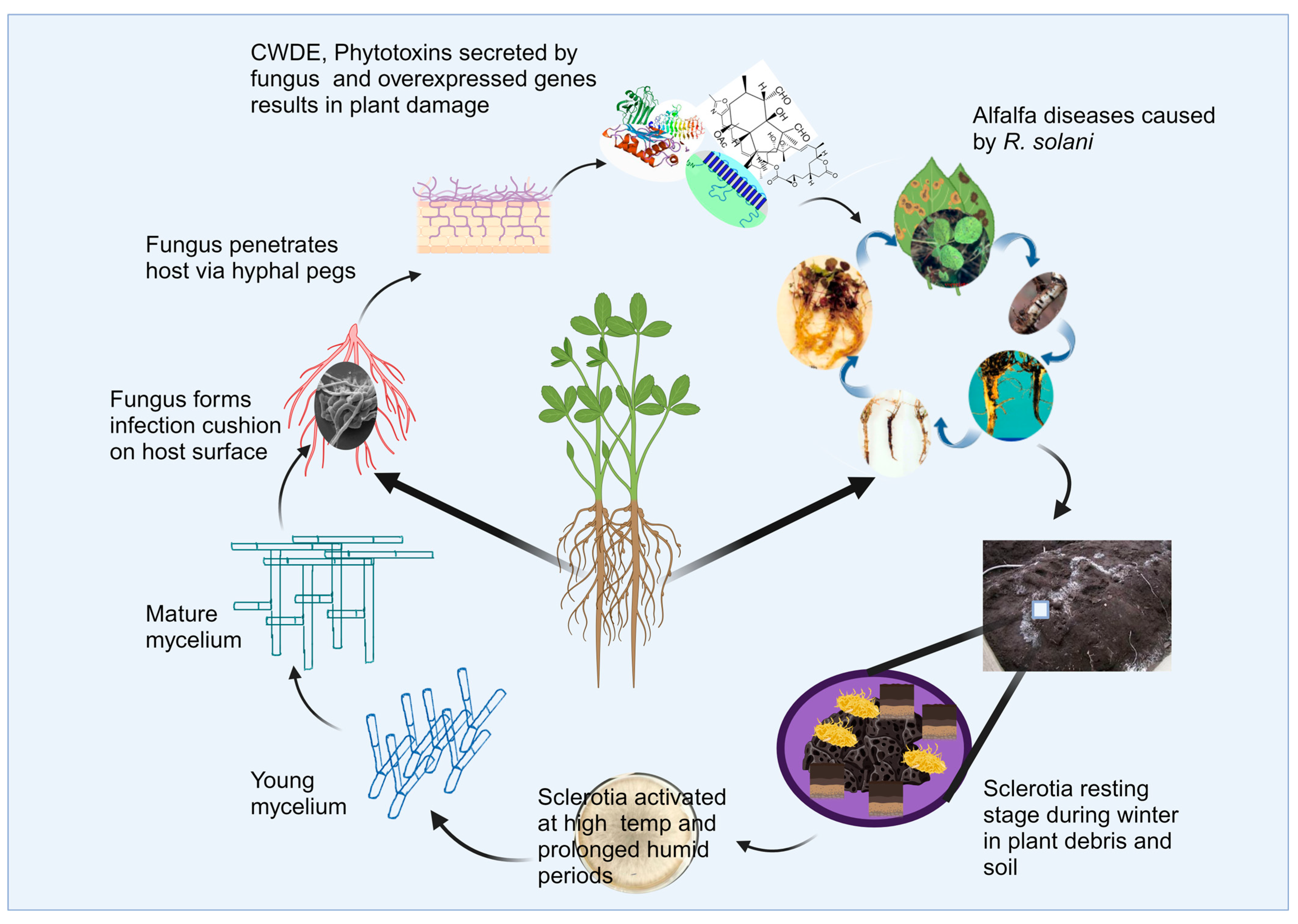
Disease Cycle of R. solani in Alfalfa
4. Genetic Diversity and Pathogenic Mechanism of R. solani
4.1. Genetic Diversity
4.2. Pathogenic Mechanism of R. solani
5. Detection and Quantification of R. solani
5.1. Selective Mediums
5.2. Conventional PCR
5.3. Real-Time Quantitative PCR (qPCR)
| Crop | AG | Primer and Sequence | Targeted Region | Annealing T (°C) | Purpose | Ref. |
|---|---|---|---|---|---|---|
| Wheat and barley | AG-2-1 and AG-8 | Rs2.1/8F(GTTGTAGCTGGCCCATTCATTTG) | ITS1 and ITS2 | 63 | Detection and quantification of Rhizoctonia species from plant and soil | [138] |
| Rs2.1/8R (AGCAGGTGTGAAGCTGCAAAAG) | ||||||
| AG-8 | Rs8F(GGGGGAATTTATTCATTTATTGGAC) | 58 | ||||
| Rs8R (GGTGTGAAGCTGCAAAAG) | ||||||
| AG-10 | Rs10F (GTAGCTGGCCTCTTAATTTG) | 60 | ||||
| Rs10R (CAAGTGTGAACCTGCAAGAC) | ||||||
| Rice | AG-1-IA | Rs1F(GCCTTTTCTACCTTAATTTGGCAG) Rs2R(GTGTGTAAATTAAGTAGACAGCAAATG) | ITS | 60 | Detection and quantification of R. solani AG-1 IA from plant | [139] |
| Mung bean Soy bean | AG-1, AG-2-2, AG-2-2LP, AG-2-3, AG-3, AG-4, AG-5 unknown | ARS (F1: GAGTTGTTGCTGGCCTTTTC) ARS (R1: TTTTTACGGGTGTCCTCAGC) ARS (F4: CAACGGATCTCTTGGCTCTC) ARS (R4: GGTGTCCTCGGCGATAGATA ARS (F5: ACTAAGTTTCAACAACGGAT) ARS (R5: TTACTTTGAAGATTTCATGA) Rso1: RsolF-(GTGAACCAAATCAGACAGA) Rso1R-(CTACTCTACTGCTTACAG) | ITS IGS | 71 67 52 45.2 | Detection and quantification of R. solani from plant Detection and quantification of R. solani from soil and plant | [131] [140] |
| Tomato | unknown | ST-RS1-F: (AGTGTTATGCTTGGTTCCACT) ST-RS1-R: (TCCTCCGCTTATTGATATGC) | ITS2 | 59.5 | Detection and quantification of R. solani from soil | [141] |
| Potato | AG-1-IA | AG-1-1A_F(TTGTTGCTGGCCTTTTCTACCT) AG-1-A_R (ATGGAATTAAATCCACCAACTATTGC) | ITS1 | 50 | Detection of pathogenic AGs and spatial distribution of R. solani in fields | [132] |
| AG-3 | AG-3_F (TCTACAGGGATTCCAGATTACGC) AG-3_R (TCACGGATCTTGGAAATCAACA) | β-tubulin | ||||
| Lettuce Sugar beet | AG-1-IB AG-2-2 IIIB | AG1-IB-F3(TGGCCTTTTAACATTGGCATGT) AG1-IB-R(CCAACCCCAAAGGACCTTGA) AG22sp2-F(TAGCTGGATCCATTAGTTTG) 5.8SKhot-R(GTTCAAAGATCGATGATTCAC) | ITS ITS | 62 55 | Detection and quantification of R. solani and R. solani AG1-IB from soil and plant Detection and quantification of R. solani AG2-2IIIB from soil and plant | [142] [143] |
| Tobacco | AG-3 | RsTqF1(AGAGTTTGGTTGTAGCTGGTCTATTT) RsTqR4(AGACAGAAGGGTTCAATGACTTATTATA) | ITS | 60 | Detection and quantification of R. solani AG-3 from plant | [144] |
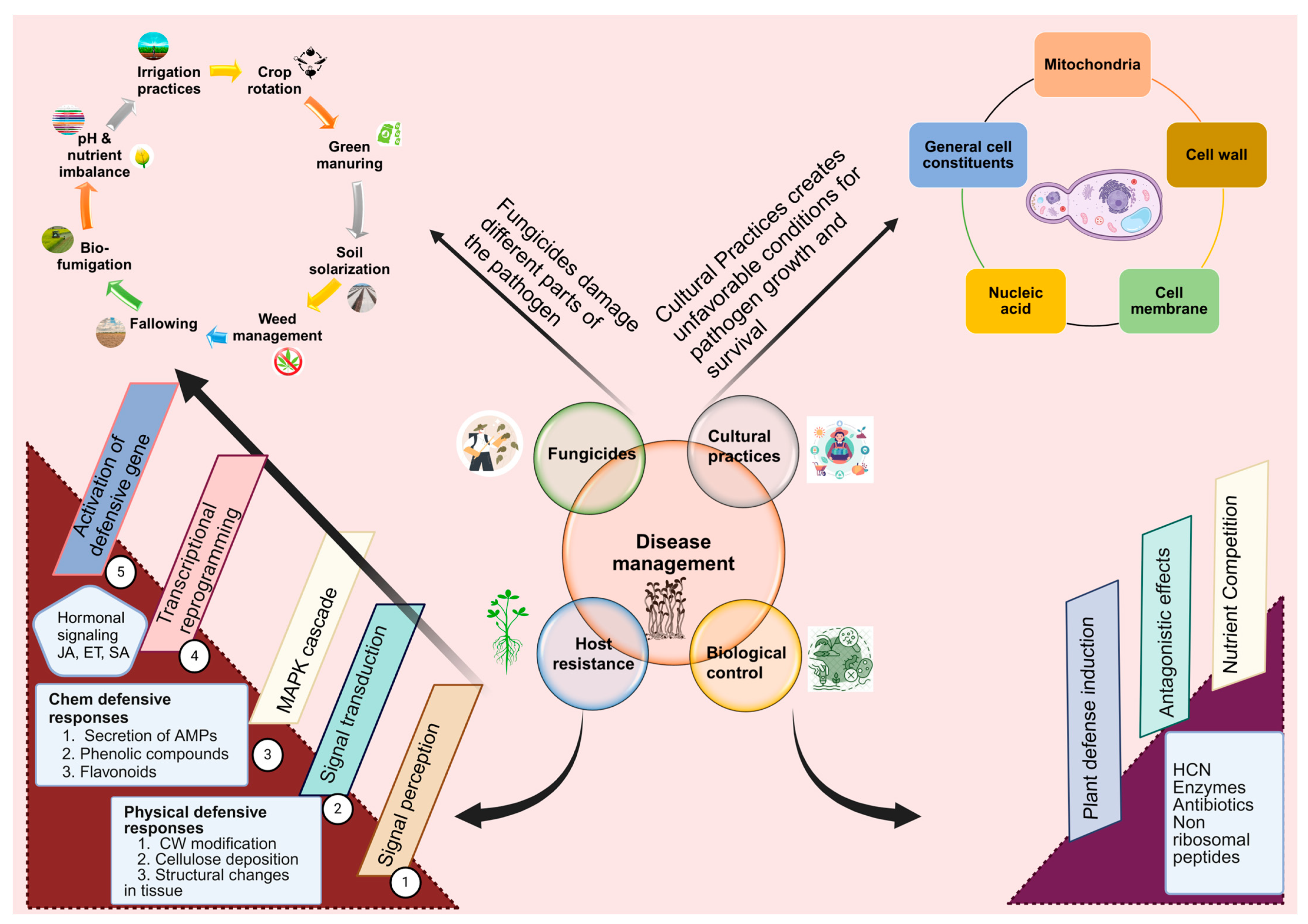
6. Management Approaches
6.1. Host Resistance
6.2. Cultural Control
6.3. Biological Control
6.4. Fungicides
7. Future Directions in Alfalfa Resistance Research
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, J.T.; Shannon, J.G.; Lee, J.-D. Harnessing the potential of forage legumes, alfalfa, soybean, and cowpea for sustainable agriculture and global food security. Front. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef]
- Ćupina, B.; Mikić, A.; Stoddard, F.L.; Krstić, Đ.; Justes, E.; Bedoussac, L.; Fustec, J.; Pejić, B. Mutual legume intercropping for forage production in temperate regions. Genet. Biofuels Local Farming Syst. 2011, 7, 347–365. [Google Scholar]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Putnam, D.; Meccage, E. Profitable alfalfa production sustains the environment. In Proceedings of the 2022 World Alfalfa Congress, San Diego, CA, USA, 14–17 November 2022; pp. 14–17. [Google Scholar]
- Fang, X.; Zhang, C.; Wang, Z.; Duan, T.; Yu, B.; Jia, X.; Pang, J.; Ma, L.; Wang, Y.; Nan, Z. Co-infection by soil-borne fungal pathogens alters disease responses among diverse alfalfa varieties. Front. Microbiol. 2021, 12, 664385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, S.; Hui, T.; Wang, Z.; Yu, B.; Ma, L.; Nan, Z.; Fang, X. Varieties with a high level of resistance provide an opportunity to manage root rot caused by Rhizoctonia solani in alfalfa. Eur. J. Plant Pathol. 2021, 160, 1–7. [Google Scholar] [CrossRef]
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Ali, G.; Wang, Z.; Li, X.; Jin, N.; Chu, H.; Jing, L. Deep soil water deficit and recovery in alfalfa fields of the Loess Plateau of China. Field Crops Res. 2021, 260, 107990. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, C.; Nan, Z. Research advances in Fusarium root rot of alfalfa (Medicago sativa). Acta Prataculturae Sin. 2019, 28, 169–183. [Google Scholar]
- Blume, L.; Hoischen-Taubner, S.; Sundrum, A. Alfalfa-a regional protein source for all farm animals. Landbauforschung 2021, 71, 1–13. [Google Scholar]
- Yang, Q.; Kang, J.; Zhang, T.; Liu, F.; Long, R.; Sun, Y. Distribution, breeding and utilization of alfalfa germplasm resources. Chin. Sci. Bull 2016, 61, 261–270. [Google Scholar]
- Wang, T.; Zhang, W.-H. Priorities for the development of alfalfa pasture in northern China. Fundam. Res. 2023, 3, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Drizou, F.; Graham, N.S.; Bruce, T.J.; Ray, R.V. Development of high-throughput methods to screen disease caused by Rhizoctonia solani AG 2-1 in oilseed rape. Plant Methods 2017, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant–microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.; Ellur, R.; Bhowmick, P.; Bollineni, H.; Kurungara, V.; Singh, A.; et al. Rhizoctonia solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef] [PubMed]
- Hane, J.K.; Anderson, J.P.; Williams, A.H.; Sperschneider, J.; Singh, K.B. Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet. 2014, 10, e1004281. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Gónzalez, D.; Rodriguez-Carres, M.; Boekhout, T.; Stalpers, J.; Kuramae, E.E.; Nakatani, A.K.; Vilgalys, R.; Cubeta, M.A. Phylogenetic relationships of Rhizoctonia fungi within the Cantharellales. Fungal Biol. 2016, 120, 603–619. [Google Scholar] [CrossRef]
- Broders, K.; Parker, M.; Melzer, M.; Boland, G. Phylogenetic diversity of Rhizoctonia solani associated with canola and wheat in Alberta, Manitoba, and Saskatchewan. Plant Dis. 2014, 98, 1695–1701. [Google Scholar] [CrossRef]
- Basbagci, G.; Dolar, F.S. First report of binucleate Rhizoctonia AG-K causing root rot on chickpea. Arch. Phytopathol. Plant Protect. 2020, 53, 640–652. [Google Scholar] [CrossRef]
- Fowler, M.C.; Miller-Garvin, J.; Regulinski, D.; Viands, D. Association of Alfalfa Radicle Lenght with Rhizoctonia Damping-Off. Crop Sci. 1999, 39, 659–661. [Google Scholar] [CrossRef]
- Gondal, A.S.; Rauf, A.; Naz, F. Anastomosis Groups of Rhizoctonia solani associated with tomato foot rot in Pothohar Region of Pakistan. Sci. Rep. 2019, 9, 3910. [Google Scholar] [CrossRef]
- Smith, O.F. Rhizoctonia root canker of Alfalfa (Medicago sativa). Phytopathology 1943, 33, 1081–1085. [Google Scholar]
- Smith, O.F. Effect of soil temperature on the development of Rhizoctonia root canker of alfalfa. Phytopathology 1946, 36, 638–642. [Google Scholar]
- Irvine, W.A. Interaction of Meloidogyne hapla and Rhizoctonia solani in Alfalfa. Ph.D. Thesis, Iowa State University of Science and Techonlogy, Ames, IA, USA, 1964. [Google Scholar]
- Samac, D.A.; Rhodes, L.H.; Lamp, W.O. Compendium of Alfalfa Diseases and Pests; The American Phytopathological Society: St. Paul, MN, USA, 2015. [Google Scholar]
- Eken, C.; Demirci, E. Identification and pathogenicity of Rhizoctonia solani and binucleate Rhizoctonia anastomosis groups isolated from forage legumes in Erzurum, Turkey. Phytoparasitica 2003, 31, 76–80. [Google Scholar] [CrossRef]
- EL-Garhy, A.M.; El-Wakil, D.A. Fungal diseases of alfalfa in Ismailia governorate. Egypt. J. Agric. Res. 2014, 92, 1219–1231. [Google Scholar] [CrossRef]
- Azzam, C.R.; Abd El-Naby, Z.M.; Abd El-Rahman, S.S.; Omar, S.A.; Ali, E.F.; Majrashi, A.; Rady, M.M. Association of saponin concentration, molecular markers, and biochemical factors with enhancing resistance to alfalfa seedling damping-off. Saudi J. Biol. Sci. 2022, 29, 2148–2162. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I. Pathological and Physiological Studies on Some Diseases of Alfalfa, Medicago sativa L. in Egypt and Their Control. Ph.D. Thesis, Azhar University, Cairo, Egypt, 1995. [Google Scholar]
- Omar, S.; Rammah, A. Evaluation of some clover and alfalfa lines to Verticillium wilt disease. Egyp. J. Agr. Res 1992, 70, 1055–1063. [Google Scholar]
- El-Morsy, G.; Belal, G. Newly recorded diseases of lucern (alfalfa) in Egypt. Foliar Egypt. J. Agric. Res 1997, 75, 543–550. [Google Scholar]
- Morsy, K.M. Biological Control of Damping-off, Root Rot and Wilt Diseases of Faba Bean by Cyanobacteria (Blue-Green Algal) Culture Filtrate. Egypt. J. Phytopathol. 2011, 39, 159–171. [Google Scholar] [CrossRef]
- Abd El-Naby Zeinab, M.; Azzam, C.R.; Abd El-Rahman, S.S. Evaluation of ten alfalfa populations for forage yield, protein content, susceptibility to seedling damping-off disease and associated biochemical markers with levels of resistance. J. Am. Sci. 2014, 10, 73–85. [Google Scholar]
- Melzer, M.S.; Yu, H.; Labun, T.; Dickson, A.; Boland, G.J. Characterization and pathogenicity of Rhizoctonia spp. from field crops in Canada. Can. J. Plant Pathol. 2016, 38, 367–374. [Google Scholar] [CrossRef]
- Addoh, P.G. Studies on Damping-Off of Alfalfa Cuttings in the Greenhouse. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 1961. [Google Scholar]
- Wang, X.; Ding, T.; Li, Y.; Guo, Y.; Li, Y.; Duan, T. Dual inoculation of alfalfa (Medicago sativa L.) with Funnelliformis mosseae and Sinorhizobium medicae can reduce Fusarium wilt. J. Appl. Microbiol. 2020, 129, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-X.; Zhang, M.; Guan, Y.-Z.; Liu, F.; Guo, Z.-P.; Zhang, H.-R.; Yang, X.-B.; Wang, C.-Z. Pathogenicity of Rhizoctonia solani to alfalfa seedlings and disease resistance of alfalfa varieties. Acta Prataculturae Sin. 2015, 24, 72. [Google Scholar]
- Luo, D.; Tian, H.; Zhang, C.; Fang, X. Advances in the research on plant root rot caused by Rhizoctonia solani. China Plant Prot. 2020, 40, 23–31. [Google Scholar]
- McKenzie, J.; Davidson, J. Prevalence of alfalfa crown and root diseases in the Peace River Region of Alberta and British Columbia. Can. Plant Dis. Surv. 1975, 55, 121–125. [Google Scholar]
- Fatemi, J. Fungi associated with alfalfa root rot in Iran. Phytopathol. Mediterr. 1972, 11, 163–165. [Google Scholar]
- Demir, D.; Eken, C.; Çelik, E.; Alkan, N. Effect of Rhizoctonia spp. on Polyphenol Oxidase Enzyme Activity in Alfalfa Seedling. Res. J. Biotechnol. 2021, 16, 47–50. [Google Scholar] [CrossRef]
- Vincelli, P.; Herr, L. 2 Diseases of Alfalfa Caused By Rhizoctonia-Solani AG-1 and AG-4. Plant Dis. 1992, 76, 1283. [Google Scholar] [CrossRef]
- Anderson, J.; Lichtenzveig, J.; Oliver, R.; Singh, K. Medicago truncatula as a model host for studying legume infecting Rhizoctonia solani and identification of a locus affecting resistance to root canker. Plant Pathol. 2013, 62, 908–921. [Google Scholar] [CrossRef]
- Naseri, B. The potential of agroecological properties in fulfilling the promise of organic farming: A case study of bean root rots and yields in Iran. Adv. Resting-State Funct. MRI, 2023; 203–236. [Google Scholar]
- Erwin, D. Important diseases of Alfalfa in southern California. Plant Dis. Rep. 1956, 40, 380–383. [Google Scholar]
- Irwin, J. Factors contributing to poor lucerne persistence in southern Queensland. Aust. J. Exp. Agric. 1977, 17, 998–1003. [Google Scholar] [CrossRef]
- Anderson, J.R.; Bentley, S.; Irwin, J.A.G.; Mackie, J.M.; Neate, S.; Pattemore, J.A. Characterisation of Rhizoctonia solani isolates causing root canker of lucerne in Australia. Australas. Plant Pathol. 2004, 33, 241–247. [Google Scholar] [CrossRef]
- Kronland, W.C.; Stanghellini, M.E. Clean slide technique for the observation of anastomosis and nuclear condition of rhizoctonia solani. Phytopathology 1988, 78, 820–822. [Google Scholar] [CrossRef]
- Samac, D.A.; Lamb, J.F.; Kinkel, L.L.; Hanson, L. Effect of wheel traffic and green manure treatments on forage yield and crown rot in alfalfa (Medicago sativa). Plant Soil 2013, 372, 349–359. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Velivelli, S.; Shah, D.M.; Samac, D.A. Plant Defensin Peptides have Antifungal and Antibacterial Activity Against Human and Plant Pathogens. Phytopathology 2019, 109, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Kreitlow, K.; Faulkner, L. Diseases. Alfalfa Sci. Technol. 1972, 15, 497–526. [Google Scholar]
- Velásquez-Valle, R.; Reveles-Torres, L.R.; Talavera-Correa, H. Microorganismos asociados con la pudrición de corona de alfalfa en el norte centro de México. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2018, 36, 414–422. [Google Scholar] [CrossRef]
- Lehmann, L.C. Studies of Root and Crown Rots on Alfalfa (Medicago sativa L.); The University of Arizona: Tucson, AZ, USA, 1967. [Google Scholar]
- Hawn, E.; Cormack, M. Crown bud rot of Alfalfa. Phytopathology 1952, 42, 510–515. [Google Scholar]
- Hwang, S.-F.; Howard, R.J.; Chang, K.-F. Forage and Oilseed Legume Diseases Incited by Rhizoctonia Species. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 289–301. [Google Scholar]
- Hawn, E.J. Histological Study on Crown Bud Rot of Alfalfa. Can. J. Bot. 1959, 37, 1247–1249. [Google Scholar] [CrossRef]
- McDonald, W.C. The distribution and pathogenicity of the fungi associated with crown and root rotting of alfalfa in Manitoba. Can. J. Agric. Sci. 1955, 35, 309–321. [Google Scholar]
- Houston, B.R.; Erwin, D.; Stanford, E.; Allen, M.; Hall, D.; Paulus, A. Diseases of Alfalfa in California. Gire. Calif. Agric. Ext. Serv. 1960, 485, 20. [Google Scholar]
- Eken, C.; Demirci, E. The distribution and pathogenicity of the fungal pathogens determined from alfalfa plants in Erzurum province. Ziraat Fakültesi Derg. Atatürk Üniversitesi 2001, 32, 143–150. [Google Scholar]
- Grandfield, C.; Hansing, E.; Hackerott, H. Losses Incurred in Asexual Propagation of Alfalfa Clones 1. Agron. J. 1948, 40, 804–808. [Google Scholar] [CrossRef]
- Hwang, S.-F.; Wang, H.; Gossen, B.D.; Turnbull, G.D.; Howard, R.J.; Strelkov, S.E. Effect of seed treatments and root pathogens on seedling establishment and yield of alfalfa, birdsfoot trefoil and sweetclover. Plant Pathol. J. 2006, 5, 322–328. [Google Scholar] [CrossRef]
- El-Meleigi, M.; Omar, A.; Rogaibah, A.; Ibrahim, G. Efficacy of Bacilli Strains in Growth Promotion and Biological Control of Soilborne Rhizoctonia and Fusarium on Alfalfa (Medicago sativa L.) and Potato (Solanum tuberosum L). Egypt. J. Biol. Pest Control 2017, 27, 85. [Google Scholar]
- Barnes, D.; Sarojak, D.; Frosheiser, F.; Anderson, N. Registration of alfalfa germplasm with seedling resistance to Rhizoctonia solani (Reg. Nos. GP 111 to GP 113). CABI Databases 1981, 20, 675. [Google Scholar]
- Al-Askar, A.; Ghoneem, K.; Rashad, Y. Management of some seed-borne pathogens attacking alfalfa plants in Saudi Arabia. Afr. J. Microbiol. Res. 2013, 7, 1197–1206. [Google Scholar] [CrossRef]
- ChewMadinaveitia, Y.I.; SantamariaCesar, J. Estimate of losses caused by crown rot of alfalfa (Medicago sativa L.) in the Comarca Lagunera (North of Mexico). ITEA Prod. Veg. 2000, 96, 165–172. [Google Scholar]
- Hawn, E.J. Development of Alfalfa Crown Bud Rot (Abstract); Canadian Phytopathology Society: Ottawa, ON, Canada, 1953; pp. 13–21. [Google Scholar]
- Cong, L.; Li, M.; Sun, Y.; Cong, L.; Yang, Q.; Long, R.; Kang, J.; Zhang, T. First report of root rot disease caused by Fusarium tricinctum on alfalfa in North China. Plant Dis. 2016, 100, 1503. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Rashad, Y.M. Efficacy of some plant extracts against Rhizoctonia solani on pea. J. Plant Prot. Res. 2010, 50, 239–243. [Google Scholar] [CrossRef]
- Datta, S.; Sarkar, M.; Chowdhury, A.; Rakwal, R.; Agrawal, G.K.; Sarkar, A. A comprehensive insight into the biology of Rhizoctonia solani AG1-IA Kühn, the causal organism of the sheath blight disease of rice. J. Plant Pathol. 2021, 104, 79–98. [Google Scholar] [CrossRef]
- Butler, E.; Bracker, C. Morphology and cytology of Rhizoctonia solani. Rhizoctonia Solani Biol. Pathol. 1970. [Google Scholar] [CrossRef]
- Shu, C.; Chen, J.; Sun, S.; Zhang, M.; Wang, C.; Zhou, E. Two distinct classes of protein related to GTB and RRM are critical in the sclerotial metamorphosis process of Rhizoctonia solani AG-1 IA. Funct. Integr. Genom. 2015, 15, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D.R. Sclerotia formation by Rhizoctonia species and their survival. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Springer: Berlin/Heidelberg, Germany, 1996; pp. 207–215. [Google Scholar]
- Bell, D.; Sumner, D. Survival of Rhizoctonia solani and other soil-borne basidiomycetes in fallow soil. Plant Dis. 1987, 71, 911–915. [Google Scholar] [CrossRef]
- Wigg, K.S.; Brainard, S.H.; Metz, N.; Dorn, K.M.; Goldman, I.L. Novel QTL associated with Rhizoctonia solani Kühn resistance identified in two table beet× sugar beet F2: 3 populations using a new table beet reference genome. Crop Sci. 2023, 63, 535–555. [Google Scholar]
- Ritchie, F.; Bain, R.; Mcquilken, M. Survival of Sclerotia of Rhizoctonia solani AG 3 PT and Effect of Soil-Borne Inoculum Density on Disease Development on Potato. J. Phytopathol. 2013, 161, 180–189. [Google Scholar] [CrossRef]
- Pitt, D. Studies on sharp eyespot disease of cereals II. Viability of sclerotia: Persistence of the causal fungus, Rhizoctonia solani Kühn. Ann. Appl. Biol. 1964, 54, 231–240. [Google Scholar] [CrossRef]
- Coley-Smith, J.; Humphreys-Jones, D.; Gladders, P. Long-term survival of sclerotia of Rhizoctonia tuliparum. Plant Pathol. 1979, 28, 128–130. [Google Scholar] [CrossRef]
- Mustafa, H.K.; Anwer, S.S.; Zrary, T.J. Influence of pH, agitation speed, and temperature on growth of fungi isolated from Koya, Iraq. Kuwait J. Sci. 2023, 50, 657–664. [Google Scholar]
- Kwon, Y.S.; Kim, S.G.; Chung, W.S.; Bae, H.; Jeong, S.W.; Shin, S.C.; Jeong, M.-J.; Park, S.-C.; Kwak, Y.-S.; Bae, D.-W. Proteomic analysis of Rhizoctonia solani AG-1 sclerotia maturation. Fungal Biol. 2014, 118, 433–443. [Google Scholar] [CrossRef]
- Nasimi, Z.; Barriuso, J.; Keshavarz, T.; Zheng, A. Molecular, physiological, and biochemical properties of sclerotia metamorphosis in Rhizoctonia solani. Fungal Biol. Rev. 2024, 48, 100351. [Google Scholar] [CrossRef]
- Willis, W.G. Fungus Root Diseases of Alfalfa. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 1964. [Google Scholar]
- Rosewich, U.L.; Pettway, R.E.; McDonald, B.A.; Kistler, H. High levels of gene flow and heterozygote excess characterize Rhizoctonia solani AG-1 IA (Thanatephorus cucumeris) from Texas. Fungal Genet. Biol. 1999, 28, 148–159. [Google Scholar] [CrossRef]
- Singh, A.; Rohilla, R.; Singh, U.; Savary, S.; Willocquet, L.; Duveiller, E. An improved inoculation technique for sheath blight of rice caused by Rhizoctonia solani. Can. J. Plant Pathol. 2002, 24, 65–68. [Google Scholar] [CrossRef]
- Fenille, R.C.; Ciampi, M.B.; Kuramae, E.E.; Souza, N.L. Identificação de Rhizoctonia solani associada à soja no Brasil através de seqüências da região rDNA-ITS. Fitopatol. Bras. 2003, 28, 413–419. [Google Scholar] [CrossRef]
- Guleria, S.; Aggarwal, R.; Thind, T.; Sharma, T. Morphological and pathological variability in rice isolates of Rhizoctonia solani and molecular analysis of their genetic variability. J. Phytopathol. 2007, 155, 654–661. [Google Scholar] [CrossRef]
- Taheri, P.; Gnanamanickam, S.; Höfte, M. Characterization, genetic structure, and pathogenicity of Rhizoctonia spp. associated with rice sheath diseases in India. Phytopathology 2007, 97, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ceresini, P.C.; Shew, H.D.; James, T.Y.; Vilgalys, R.J.; Cubeta, M.A. Phylogeography of the Solanaceae-infecting Basidiomycota fungus Rhizoctonia solani AG-3 based on sequence analysis of two nuclear DNA loci. BMC Evol. Biol. 2007, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, M.B.; Gale, L.R.; Lemos, E.G.; Ceresini, P.C. Distinctively variable sequence-based nuclear DNA markers for multilocus phylogeography of the soybean-and rice-infecting fungal pathogen Rhizoctonia solani AG-1 IA. Genet. Mol. Biol. 2009, 32, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Padasht-Dehkaei, F.; Ceresini, P.C.; Zala, M.; Okhovvat, S.; Nikkhah, M.; McDonald, B.A. Population genetic evidence that basidiospores play an important role in the disease cycle of rice-infecting populations of Rhizoctonia solani AG-1 IA in Iran. Plant Pathol. 2013, 62, 49–58. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.M.; Wang, Z.G.; Huang, S.W. Genetic Structure and Aggressiveness of Rhizoctonia solani AG 1-IA, the Cause of Sheath Blight of Rice in Southern C hina. J. Phytopathol. 2013, 161, 753–762. [Google Scholar] [CrossRef]
- Shu, C.-W.; Zou, C.-J.; Chen, J.-L.; Tang, F.; Yi, R.-H.; Zhou, E.-X. Genetic diversity and population structure of Rhizoctonia solani AG-1 IA, the causal agent of rice sheath blight, in South China. Can. J. Plant Pathol. 2014, 36, 179–186. [Google Scholar] [CrossRef]
- Ali, M.; Kamal, M.; Archer, S.; Buddie, A.; Rutherford, M. Anastomosis and DNA fingerprinting the rice isolates of Rhizoctonia solani Kuhn using AFLP markers. Bangladesh J. Plant Pathol. 2004, 20, 1–8. [Google Scholar]
- Moni, Z.R.; Ali, M.A.; Alam, M.S.; Rahman, M.A.; Bhuiyan, M.R.; Mian, M.S.; Iftekharuddaula, K.M.; Latif, M.A.; Khan, M.A.I. Morphological and genetical variability among Rhizoctonia solani isolates causing sheath blight disease of rice. Rice Sci. 2016, 23, 42–50. [Google Scholar] [CrossRef]
- Yi RunHua, Y.R.; Liang ChengYe, L.C.; Zhu XiRu, Z.X.; Zhou ErXun, Z.E. Genetic diversity and virulence variation of rice sheath blight strains (Rhizoctonia solani AG-1 IA) from Guangdong province. J. Trop. Subtrop. Bot. 2002, 10, 161–170. [Google Scholar]
- Wang, L.-l.; Liu, L.; Hou, Y.; Li, L.; Huang, S. Pathotypic and genetic diversity in the population of Rhizoctonia solani AG 1-IA causing rice sheath blight in China. Plant Pathol. 2015, 64, 718–728. [Google Scholar] [CrossRef]
- Keijer, J. The Initial Steps of the Infection Process in Rhizoctonia Solani. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 149–162. [Google Scholar]
- Zheng, A.; Lin, R.; Zhang, D.; Qin, P.; Xu, L.; Ai, P.; Ding, L.; Wang, Y.; Chen, Y.; Liu, Y. The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat. Commun. 2013, 4, 1424. [Google Scholar] [CrossRef]
- Nadarajah, K.; Mat Razali, N.; Cheah Boon, H.; Sahruna Nur, S.; Ismail, I.; Tathode, M.; Bankar, K. Draft Genome Sequence of Rhizoctonia solani Anastomosis Group 1 Subgroup 1A Strain 1802/KB Isolated from Rice. Genome Announc. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Das, M.M.; Haridas, M.; Sabu, A. Biological control of black pepper and ginger pathogens, Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici, using Trichoderma spp. Biocatal. Agric. Biotechnol. 2019, 17, 177–183. [Google Scholar]
- Erper, I.; Ozer, G.; Kalendar, R.; Avci, S.; Yildirim, E.; Alkan, M.; Turkkan, M. Genetic Diversity and Pathogenicity of Rhizoctonia spp. Isolates Associated with Red Cabbage in Samsun (Turkey). J. Fungi 2021, 7, 234. [Google Scholar] [CrossRef]
- Yamamoto, N.; Wang, Y.; Lin, R.; Liang, Y.; Liu, Y.; Zhu, J.; Wang, L.; Wang, S.; Liu, H.; Deng, Q. Integrative transcriptome analysis discloses the molecular basis of a heterogeneous fungal phytopathogen complex, Rhizoctonia solani AG-1 subgroups. Sci. Rep. 2019, 9, 19626. [Google Scholar] [CrossRef]
- Li, X.; An, M.; Xu, C.; Jiang, L.; Yan, F.; Yang, Y.; Zhang, C.; Wu, Y. Integrative transcriptome analysis revealed the pathogenic molecular basis of Rhizoctonia solani AG-3 TB at three progressive stages of infection. Front. Microbiol. 2022, 13, 1001327. [Google Scholar] [CrossRef]
- Kankam, F.; Long, H.-T.; He, J.; Zhang, C.-h.; Zhang, H.-X.; Pu, L.; Qiu, H. 3-Methylthiopropionic Acid of Rhizoctonia solani AG-3 and Its Role in the Pathogenicity of the Fungus. Plant Pathol. J. 2016, 32, 85. [Google Scholar] [CrossRef]
- Slot, J.C.; Rokas, A. Multiple GAL pathway gene clusters evolved independently and by different mechanisms in fungi. Proc. Natl. Acad. Sci. USA 2010, 107, 10136–10141. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Alkharouf, N.; Roberts, D.P.; Natarajan, S.S.; Mitra, A. Gene expression profiling of the plant pathogenic basidiomycetous fungus Rhizoctonia solani AG 4 reveals putative virulence factors. Mycologia 2012, 104, 1020–1035. [Google Scholar] [CrossRef]
- Dutheil, J.Y.; Mannhaupt, G.; Schweizer, G.; MK Sieber, C.; Münsterkötter, M.; Güldener, U.; Schirawski, J.; Kahmann, R. A tale of genome compartmentalization: The evolution of virulence clusters in smut fungi. Genome Biol. Evol. 2016, 8, 681–704. [Google Scholar] [CrossRef]
- Dickman, M.B.; de Figueiredo, P. Death be not proud—Cell death control in plant fungal interactions. PLoS Path. 2013, 9, e1003542. [Google Scholar] [CrossRef]
- Li, S.; Peng, X.; Wang, Y.; Hua, K.; Xing, F.; Zheng, Y.; Liu, W.; Sun, W.; Wei, S. The effector AGLIP1 in Rhizoctonia solani AG1 IA triggers cell death in plants and promotes disease development through inhibiting PAMP-triggered immunity in Arab. Thaliana. Front. Microbiol. 2019, 10, 2228. [Google Scholar] [CrossRef]
- Harris, K.; Young, I.M.; Gilligan, C.A.; Otten, W.; Ritz, K. Effect of bulk density on the spatial organisation of the fungus Rhizoctonia solani in soil. FEMS Microbiol. Ecol. 2003, 44, 45–56. [Google Scholar] [CrossRef]
- Spurlock, T.; Rothrock, C.; Monfort, W. Evaluation of methods to quantify populations of Rhizoctonia in Soil. Plant Dis. 2015, 99, 836–841. [Google Scholar] [CrossRef]
- Paulitz, T.; Schroeder, K. A new method for the quantification of Rhizoctonia solani and R. oryzae from soil. Plant Dis. 2005, 89, 767–772. [Google Scholar] [CrossRef]
- Salamone, A.L.; Okubara, P.A. Real-time PCR quantification of Rhizoctonia solani AG-3 from soil samples. J. Microbiol. Methods 2020, 172, 105914. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Grover, R. A selective medium for isolating Rhizoctonia solani from soil. Ann. Appl. Biol. 1985, 106, 405–412. [Google Scholar] [CrossRef]
- Ko, W.-H.; Hora, F.K. A selective medium for the quantitative determination of Rhizoctonia solani in soil. Phytopathology 1971, 61, 707–710. [Google Scholar] [CrossRef]
- Haque, M.E.; Parvin, M.S. Evaluation of three forms of Rhizoctonia solani mediated pathogenicity to sugar beet cultivars in greenhouse studies. bioRxiv 2022. [Google Scholar]
- Jambhulkar, P.; Lakshman, D.; Roberts, D.; Sharma, P. The biology, identification and management of Rhizoctonia pathogens. Indian Phytopathol. 2016, 69, 93–106. [Google Scholar]
- Harries, E.; Berruezo, L.A.; Galván, M.Z.; Rajal, V.B.; Mercado Cárdenas, G.E. Soil properties related to suppression of Rhizoctonia solani on tobacco fields from northwest Argentina. Plant Pathol. 2020, 69, 77–86. [Google Scholar] [CrossRef]
- Vojvodic, M.; Lazic, D.; Mitrović, P.; Pešić, B.; Vico, I.; Bulajić, A. Conventional and real-time PCR assays for detection and identification of Rhizoctonia solani AG-2-2, the causal agent of root rot of sugar beet. Pestic. I Fitomedicina 2019, 34, 19–29. [Google Scholar] [CrossRef]
- Salazar, O.; Julian, M.; Rubio, V. Primers based on specific rDNA-ITS sequences for PCR detection of Rhizoctonia solani, R. solani AG 2 subgroups and ecological types, and binucleate Rhizoctonia. Mycol. Res. 2000, 104, 281–285. [Google Scholar] [CrossRef]
- Choudhary, P.; Rai, P.; Yadav, J.; Verma, S.; Chakdar, H.; Goswami, S.K.; Srivastava, A.K.; Kashyap, P.L.; Saxena, A.K. A rapid colorimetric LAMP assay for detection of Rhizoctonia solani AG-1 IA causing sheath blight of rice. Sci. Rep. 2020, 10, 22022. [Google Scholar] [CrossRef]
- Lees, A.; Cullen, D.; Sullivan, L.; Nicolson, M. Development of conventional and quantitative real-time PCR assays for the detection and identification of Rhizoctonia solani AG-3 in potato and soil. Plant Pathol. 2002, 51, 293–302. [Google Scholar] [CrossRef]
- Tripathi, A.; Dubey, S.C.; Akhtar, J.; Kumar, P. Development of PCR-based assays to diagnose the major fungal pathogens infecting pulse crops, potential for germplasm health certification and quarantine processing. World J. Microbiol. Biotechnol. 2023, 39, 74. [Google Scholar] [CrossRef]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. 2021, 49, 800–812. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, J.; Huff Hartz, K.E.; Lydy, M.J.; Moon, T.S.; Sander, M.; Parker, K.M. Analysis of RNA interference (RNAi) biopesticides: Double-stranded RNA (dsRNA) extraction from agricultural soils and quantification by RT-qPCR. Environ. Sci. Technol. 2020, 54, 4893–4902. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Du, X.; He, Q.; Liu, Y.; Wang, Z.; Feng, K.; Li, Y.; Deng, Y. ddPCR surpasses classical qPCR technology in quantitating bacteria and fungi in the environment. Mol. Ecol. Resour. 2022, 22, 2587–2598. [Google Scholar] [CrossRef]
- Bhat, A.I.; Rao, G.P.; Bhat, A.I.; Rao, G.P. Real-Time Polymerase Chain Reaction. Protocol 2020, 347–356. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Córdoba, J.J.; Andrade, M.J. Design of primers and probes for quantitative real-time PCR methods. PCR Primer Des. 2015, 1275, 31–56. [Google Scholar]
- Soheili-Moghaddam, B.; Nasr-Esfahani, M.; Mousanejad, S.; Hassanzadeh-Khankahdani, H.; Karbalaie-Khiyavie, H. Biochemical defense mechanism associated with host-specific disease resistance pathways against Rhizoctonia solani AG3-PT potatoes canker disease. Planta 2023, 257, 13. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, K.; Dubey, S.; Singh, A. Intergenic spacer region based marker for identification and quantification of Fusarium oxysporum f. sp. ciceris in chickpea plant using real time PCR assay. Res. J. Biotechnol. 2014, 9, 36–40. [Google Scholar]
- Dubey, S.C.; Tripathi, A.; Upadhyay, B.K.; Kumar, A. Development of conventional and real time PCR assay for detection and quantification of Rhizoctonia solani infecting pulse crops. Biologia 2016, 71, 133–138. [Google Scholar] [CrossRef]
- Budge, G.; Shaw, M.; Colyer, A.; Pietravalle, S.; Boonham, N. Molecular tools to investigate Rhizoctonia solani distribution in soil. Plant Pathol. 2009, 58, 1071–1080. [Google Scholar] [CrossRef]
- Agarwal, M.; Tomar, R.S.; Jyoti, A. Detection of water-borne pathogenic bacteria: Where molecular methods rule. Int. J. Multidiscip. Curr. Res. 2014, 2, 351–358. [Google Scholar]
- Sanzani, S.M.; Li Destri Nicosia, M.G.; Faedda, R.; Cacciola, S.O.; Schena, L. Use of quantitative PCR detection methods to study biocontrol agents and phytopathogenic fungi and oomycetes in environmental samples. J. Phytopathol. 2014, 162, 1–13. [Google Scholar] [CrossRef]
- Elizaquível, P.; Aznar, R.; Sánchez, G. Recent developments in the use of viability dyes and quantitative PCR in the food microbiology field. J. Appl. Microbiol. 2014, 116, 1–13. [Google Scholar] [CrossRef]
- Laidlaw, A.M.; Gänzle, M.G.; Yang, X. Comparative assessment of qPCR enumeration methods that discriminate between live and dead Escherichia coli O157: H7 on beef. Food Microbiol. 2019, 79, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.R.; Groenhof, A.C.; Forrest, R.; Lamotte, R. A One-Step, Immunochromatographic Lateral Flow Device Specific to Rhizoctonia solani and Certain Related Species, and Its Use to Detect and Quantify R. solani in Soil. Phytopathology 2004, 94, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Okubara, P.; Schroeder, K.; Paulitz, T. Identification and quantification of Rhizoctonia solani and R. oryzae using real-time polymerase chain reaction. Phytopathology 2008, 98, 837–847. [Google Scholar] [CrossRef]
- Sayler, R.J.; Yang, Y. Detection and quantification of Rhizoctonia solani AG-1 IA, the rice sheath blight pathogen, in rice using real-time PCR. Plant Dis. 2007, 91, 1663–1668. [Google Scholar] [CrossRef]
- Rocha, L.F.; Srour, A.Y.; Pimentel, M.; Subedi, A.; Bond, J.P.; Fakhoury, A.; Ammar, H.A. A panel of qPCR assays to detect and quantify soybean soil-borne pathogens. Lett. Appl. Microbiol. 2023, 76, ovac023. [Google Scholar] [CrossRef] [PubMed]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.; Lévesque, C.A.; Cammue, B.P.; Thomma, B.P. Quantitative assessment of phytopathogenic fungi in various substrates using a DNA macroarray. Environ. Microbiol. 2005, 7, 1698–1710. [Google Scholar] [CrossRef]
- Wallon, T.; Sauvageau, A.; Van der Heyden, H. Detection and quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB causing the bottom rot of lettuce in tissues and soils by multiplex qPCR. Plants 2020, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Bashir, A.; Karlovsky, P. Real-time PCR (qPCR) assay for Rhizoctonia solani anastomoses group AG2-2IIIb. Pak. J. Bot. 2014, 46, 353–356. [Google Scholar]
- Zhao, Y.Q.; Wu, Y.H.; Zhao, X.X.; An, M.N.; Chen, J.G. Study on the TaqMan Real-time PCR to the Detection and Quantification of Rhizoctonia solani AG-3 of Tobacco Target Spot. Adv. Mater. Res. 2014, 1010, 80–83. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and Challenges in Legume Breeding for Pest and Disease Resistance. Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef]
- Tollenaere, C.; Susi, H.; Laine, A.-L. Evolutionary and Epidemiological Implications of Multiple Infection in Plants. Trends Plant Sci. 2016, 21, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Joshi, A.; Sahoo, S.; Prasad, B. Resistance breeding and exploitation of wild relatives for new resistance sources. Emerg. Trends Plant Pathol. 2021, 211–247. [Google Scholar]
- Makeshkumar, T.; Divya, K.; Asha, S. Transgenic Technology for Disease Resistance in Crop Plants. In Emerging Trends in Plant Pathology; Singh, K.P., Jahagirdar, S., Sarma, B.K., Eds.; Springer: Singapore, 2021; pp. 499–560. [Google Scholar]
- Gupta, S.; Kumar, A.; Patel, R.; Kumar, V. Genetically modified crop regulations: Scope and opportunity using the CRISPR-Cas9 genome editing approach. Mol. Biol. Rep. 2021, 48, 4851–4863. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, C.; Han, X.; Wang, Z.Y.; Ma, L.; Yuan, P.; Wu, J.N.; Zhu, X.F.; Liu, J.M.; Li, D.P.; et al. Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease. Mol. Plant Pathol. 2018, 19, 2149–2161. [Google Scholar] [CrossRef]
- Gui, Z.; Yuan, Q.-h. Studies on RAPD polymorphic in alfalfa resistant to brown blot. Acta Agrestia Sin. 2002, 10, 274. [Google Scholar]
- Liu, Y.; Hassan, S.; Kidd, B.N.; Garg, G.; Mathesius, U.; Singh, K.B.; Anderson, J.P. Ethylene Signaling Is Important for Isoflavonoid-Mediated Resistance to Rhizoctonia solani in Roots of Medicago truncatula. Mol. Plant-Microbe Interact. 2017, 30, 691–700. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, M.; Lee, H.K.; Tadege, M.; Ratet, P.; Udvardi, M.; Mysore, K.S.; Wen, J. An efficient reverse genetics platform in the model legume Medicago truncatula. New Phytol. 2014, 201, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Lichtenzveig, J.; Gleason, C.; Oliver, R.P.; Singh, K.B. The B-3 ethylene response factor MtERF1-1 mediates resistance to a subset of root pathogens in Medicago truncatula without adversely affecting symbiosis with rhizobia. Plant Physiol. 2010, 154, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Varma Penmetsa, R.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.-C.; Nam, Y.W.; Engstrom, E.; Xu, K.; Sckisel, G.; Pereira, M.; et al. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008, 55, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soil-borne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- You, M.P.; Barbetti, M.J. Environmental factors determine severity of Rhizoctonia damping-off and root rot in subterranean clover. Australas. Plant Pathol. 2017, 46, 357–368. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Singh, V.K.; Singh, O. Green Manuring for Sustainable Agriculture. In Encyclopedia of Green Materials; Baskar, C., Ramakrishna, S., Daniela La Rosa, A., Eds.; Springer: Singapore, 2022; pp. 1–6. [Google Scholar]
- Larkin, R.P.; Honeycutt, C.W. Effects of Different 3-Year Cropping Systems on Soil Microbial Communities and Rhizoctonia Diseases of Potato. Phytopathology 2006, 96, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P. Soil Health Paradigms and Implications for Disease Management. Annu. Rev. Phytopathol. 2015, 53, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Windels, C.E.; Brantner, J.R. Rhizoctonia inoculum and rotation crop effects on a following sugarbeet crop. Sugarbeet Res. Ext. Rep. 2007, 37, 182–194. [Google Scholar]
- Kluth, C.; Varrelmann, M. Maize genotype susceptibility to Rhizoctonia solani and its effect on sugar beet crop rotations. Crop Protect. 2010, 29, 230–238. [Google Scholar] [CrossRef]
- Anees, M.; Edel-Hermann, V.; Steinberg, C. Build up of patches caused by Rhizoctonia solani. Soil Biol. Biochem. 2010, 42, 1661–1672. [Google Scholar] [CrossRef]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia solani in sugar beet and effect of fungicide application and plant cultivar on inoculum potential in the soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P.; Brewer, M.T. Effects of crop rotation and biocontrol amendments on rhizoctonia disease of potato and soil microbial communities. Agriculture 2020, 10, 128. [Google Scholar] [CrossRef]
- Wiggins, B.E.; Kinkel, L.L. Green manures and crop sequences influence alfalfa root rot and pathogen inhibitory activity among soil-borne streptomycetes. Plant Soil 2005, 268, 271–283. [Google Scholar] [CrossRef]
- Mazzola, M. Mechanisms of natural soil suppressiveness to soil-borne diseases. Antonie van leeuwenhoek 2002, 81, 557–564. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Koocheki, A.; Leifert, C. Soil management for sustainable crop disease control: A review. Environ. Chem. Lett. 2008, 6, 149–162. [Google Scholar] [CrossRef]
- Fang, X.; You, M.P.; Barbetti, M.J. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. Eur. J. Plant Pathol. 2012, 134, 619–629. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsui, M.; Honjo, H.; Becker, J.O.; Fukui, R. Effects of soil pH on rhizoctonia damping-off of sugar beet and disease suppression induced by soil amendment with crop residues. Plant Soil 2011, 347, 255–268. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Macnish, G.C. Effects of cultivation and cultural practice on root rot of subterranean clover. Aust. J. Exp. Agric. 1984, 24, 550–554. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Yang, H.; Chang, Z. Effect of biofumigation and chemical fumigation on soil microbial community structure and control of pepper Phytophthora blight. World J. Microbiol. Biotechnol. 2014, 30, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Gossen, B.; Turnbull, G.D.; Chang, K.-F.; Howard, R. Seedbed preparation, timing of seeding, fertility and root pathogens affect establishment and yield of alfalfa. Can. J. Plant Sci. 2002, 82, 371–381. [Google Scholar] [CrossRef]
- Hawn, E.J. Studies on The Epidemiology of Crown Bud Rot of Alfalfa in Southern Alberta. Botany 1958, 36, 239–250. [Google Scholar] [CrossRef]
- Tesar, M.; Jackobs, J. Establishing the stand. Alfalfa Sci. Technol. 1972, 15, 415–435. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Akhtar, M.; Nosheen, A.; Keyani, R.; Yasmin, H.; Naz, R.; Mumtaz, S.; Hassan, M.N. Biocontrol of Rhizoctonia solani in Basmati Rice by the Application of Lactobacillus rhamnosus and Weissella confusa. Sci. Rep. 2022, 13, 13855. [Google Scholar] [CrossRef] [PubMed]
- El-Khash, M.N. Some Rhizoctonia solani (Kuhn)-Alfalfa (Medicago sativa L.) Relationships. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 1969. [Google Scholar]
- Asiry, K.; Ali, I. Effect of Some Biocontrol Agents Against Rhizoctonia solani in vitro. J. King Abdulaziz Univ. 2021, 30, 31–38. [Google Scholar]
- Alsohim, A.S. Influence of Pseudomonas fluorescens mutants produced by transposon mutagenesis on in vitro and in vivo biocontrol and plant growth promotion. Egypt. J. Biol. Pest Control 2020, 30, 19. [Google Scholar] [CrossRef]
- Sarhan, E.; Shehata, H. Potential Plant Growth-promoting Activity of Pseudomonas spp. and Bacillus spp. as Biocontrol Agents Against Damping-off in Alfalfa. Plant Pathol. J. 2014, 13, 8–17. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [PubMed]
- Bickel, S.; Or, D. The chosen few—Variations in common and rare soil bacteria across biomes. ISME J. 2021, 15, 3315–3325. [Google Scholar] [CrossRef]
- Swidergall, M.; Ernst, J.F. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot. Cell 2014, 13, 950–957. [Google Scholar] [CrossRef]
- Nassimi, Z.; Taheri, P.; Kong, X.; Dong, W.; Tarighi, S. The antimicrobial peptide AsR416 can inhibit the growth, sclerotium formation and virulence of Rhizoctonia solani AG1-IA. Eur. J. Plant Pathol. 2021, 160, 469–485. [Google Scholar] [CrossRef]
- Oard, S.; Rush, M.; Oard, J. Characterization of antimicrobial peptides against a US strain of the rice pathogen Rhizoctonia solani. J. Appl. Microbiol. 2004, 97, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Datta, K.; Sarkar, S.N.; Datta, S.K. Expression of antimicrobial peptide snakin-1 confers effective protection in rice against sheath blight pathogen, Rhizoctonia solani. Plant Biotechnol. Rep. 2021, 15, 39–54. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, C.; Han, W.; Harris-Shultz, K.; Ji, P.; Li, Y.; Zhao, T. Identification of fungal pathogens and analysis of genetic diversity of Fusarium tricinctum causing root rots of alfalfa in north-east China. Plant Pathol. 2021, 70, 804–814. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Ning, Y.; He, Z.; Wang, G.-L. Exploiting broad-spectrum disease resistance in crops: From molecular dissection to breeding. Annu. Rev. Plant Biol. 2020, 71, 575–603. [Google Scholar] [CrossRef] [PubMed]
- Berg, L.E.; Miller, S.S.; Dornbusch, M.R.; Samac, D.A. Seed rot and damping-off of alfalfa in Minnesota caused by Pythium and Fusarium species. Plant Dis. 2017, 101, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, S.; Li, B.; Wang, A.; Li, H.; Li, X.; Luo, J.; Liu, F.; Mu, W. Comparative toxicity of multiple exposure routes of pyraclostrobin in adult zebrafish (Danio rerio). Sci. Total Environ. 2021, 777, 145957. [Google Scholar] [CrossRef]
- Hancock, J.G. Seedling diseases of alfalfa in California. Plant Dis. 1983, 67, 1203–1208. [Google Scholar] [CrossRef]
- Ahmad, S.M.F. Studies on damping-off disease of alfalfa in Saudi Arabia. In Proceedings of the First Conference on the Biological Aspects of Saudi Arabia, Riyadh, Saudi Arabia, 15–17 January 1977. [Google Scholar]
- Wei, X.; Xu, Z.; Zhang, N.; Yang, W.; Liu, D.; Ma, L. Synergistic Action of Commercially Available Fungicides for Protecting Wheat from Common Root Rot Caused by Bipolaris sorokiniana in China. Plant Dis. 2021, 105, 667–674. [Google Scholar] [CrossRef]
- Ayesha, M.S.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Shaanker, R.U. Seed Treatment with Systemic Fungicides: Time for Review. Front. Plant Sci. 2021, 12, 654512. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting Sustainability of Fungicide Seed Treatments for Field Crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, X.; Hua, H.; Han, C.; Wu, X. Sensitivity of Rhizoctonia spp. to flutolanil and characterization of the point mutation in succinate dehydrogenase conferring fungicide resistance. Eur. J. Plant Pathol. 2019, 155, 13–23. [Google Scholar] [CrossRef]
- Cheng, X.; Man, X.; Wang, Z.; Liang, L.; Zhang, F.; Wang, Z.; Liu, P.; Lei, B.; Hao, J.; Liu, X. Fungicide SYP-14288 Inducing Multidrug Resistance in Rhizoctonia solani. Plant Dis. 2020, 104, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Dai, T.; Hu, Z.; Cui, T.; Wang, W.; Han, P.; Hu, M.; Hao, J.; Liu, P.; Liu, X. Cytochrome P450 and Glutathione S-Transferase Confer Metabolic Resistance to SYP-14288 and Multi-Drug Resistance in Rhizoctonia solani. Front. Microbiol. 2022, 13, 806339. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, Y.; Guo, Z. Research progress and prospect of alfalfa resistance to pathogens and pests. Plants 2022, 11, 2008. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, B.; Wang, H.; Li, X.; Yang, F.; Ding, X.; Yan, J.; Chu, Z. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat. Genet. 2019, 51, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Schie, C.V.; Takken, F. Susceptibility Genes 101: How to Be a Good Host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Büschges, R.; Hollricher, K.; Panstruga, R.; Simons, G.; Wolter, M.; Frijters, A.; Van, D.R.; Van, D.; Diergaarde, P.; Groenendijk, J. The barley Mlo gene: A novel control element of plant pathogen resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef]
- Ma, Z.; Song, T.; Zhu, L.; Ye, W.; Wang, Y.; Shao, Y.; Dong, S.; Zhang, Z.; Dou, D.; Zheng, X.; et al. A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 2015, 27, 2057–2072. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.W.; Niu, Y.; Jia, Y.; Ordon, J.; Copeland, C.; Emonet, A.; Geldner, N.; Guan, R.; Stolze, S.C.; Nakagami, H. Coordination of microbe-host homeostasis by crosstalk with plant innate immunity. Nat. Plants 2021, 7, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Chen, T.; Nan, Z. Creation of novel barley germplasm using an Epichloë endophyte. Chin. Sci. Bull. 2021, 66, 2608–2617. [Google Scholar] [CrossRef]
- Yang, B.; Yang, S.; Zheng, W.; Wang, Y. Plant immunity inducers: From discovery to agricultural application. Stress Biol. 2022, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.L.; Zhao, J.H.; Wang, S.; Jin, Y.; Chen, Z.Q.; Fang, Y.Y.; Hua, C.L.; Ding, S.W.; Guo, H.S. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]

| Disease Name | Country/Region | Disease Everity (%) | Yield Losses (%) | References |
|---|---|---|---|---|
| Damping-off | USA Turkey Canada Riyadh Egypt | 95.0 | 90.0 | [61] |
| 62.2 | __ | [27] | ||
| 87.2 | __ | [62] | ||
| 26.1 | __ | [28] | ||
| __ | 21.1 | [34] | ||
| 70.0 | __ | [28] | ||
| 85.0 | __ | [29] | ||
| Root rot | Canada | 68.0 | __ | [40] |
| 97.0 | 85.1 | [62] | ||
| Ryadh | 19.7 | __ | [28] | |
| Al-Qasim | 17.0 | 51.5 | [63] | |
| China | ≥60.0 | ≥60.0 | [6] | |
| 68.3 | 56.7 | [5] | ||
| Seed rot | USA | __ | __ | [64] |
| Riyadh | 31.6 | __ | [65] | |
| Egypt | 27.0 | __ | [28] | |
| Crown rot | Mexico | 82.5 | 24.3 | [66] |
| Crown bud rot | Canada | 80.6 | __ | [67] |
| Stem canker | USA | __ | __ | [43] |
| Blights | China | ≥60.0 | ≥60.0 | [6] |
| 80.0 | 55.5 | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akber, M.A.; Fang, X. Research Progress on Diseases Caused by the Soil-Borne Fungal Pathogen Rhizoctonia solani in Alfalfa. Agronomy 2024, 14, 1483. https://doi.org/10.3390/agronomy14071483
Akber MA, Fang X. Research Progress on Diseases Caused by the Soil-Borne Fungal Pathogen Rhizoctonia solani in Alfalfa. Agronomy. 2024; 14(7):1483. https://doi.org/10.3390/agronomy14071483
Chicago/Turabian StyleAkber, Muhammad Abdullah, and Xiangling Fang. 2024. "Research Progress on Diseases Caused by the Soil-Borne Fungal Pathogen Rhizoctonia solani in Alfalfa" Agronomy 14, no. 7: 1483. https://doi.org/10.3390/agronomy14071483
APA StyleAkber, M. A., & Fang, X. (2024). Research Progress on Diseases Caused by the Soil-Borne Fungal Pathogen Rhizoctonia solani in Alfalfa. Agronomy, 14(7), 1483. https://doi.org/10.3390/agronomy14071483








