Phenotypic and Gene Expression Analysis of Fruit Development of ‘Rojo Brillante’ and ‘Fuyu’ Persimmon (Diospyros kaki L.) Cultivars in Two Different Locations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Meteorological Data Collection
2.3. Phenotypic Evaluation and Sample Collection
2.4. RNA Extraction and Gene Expression Measurement
2.5. Data Analysis
3. Results
3.1. Phenological and Meteorological Differences between Locations
3.2. Differential Growth of Persimmon Cultivars in SP and JP Locations
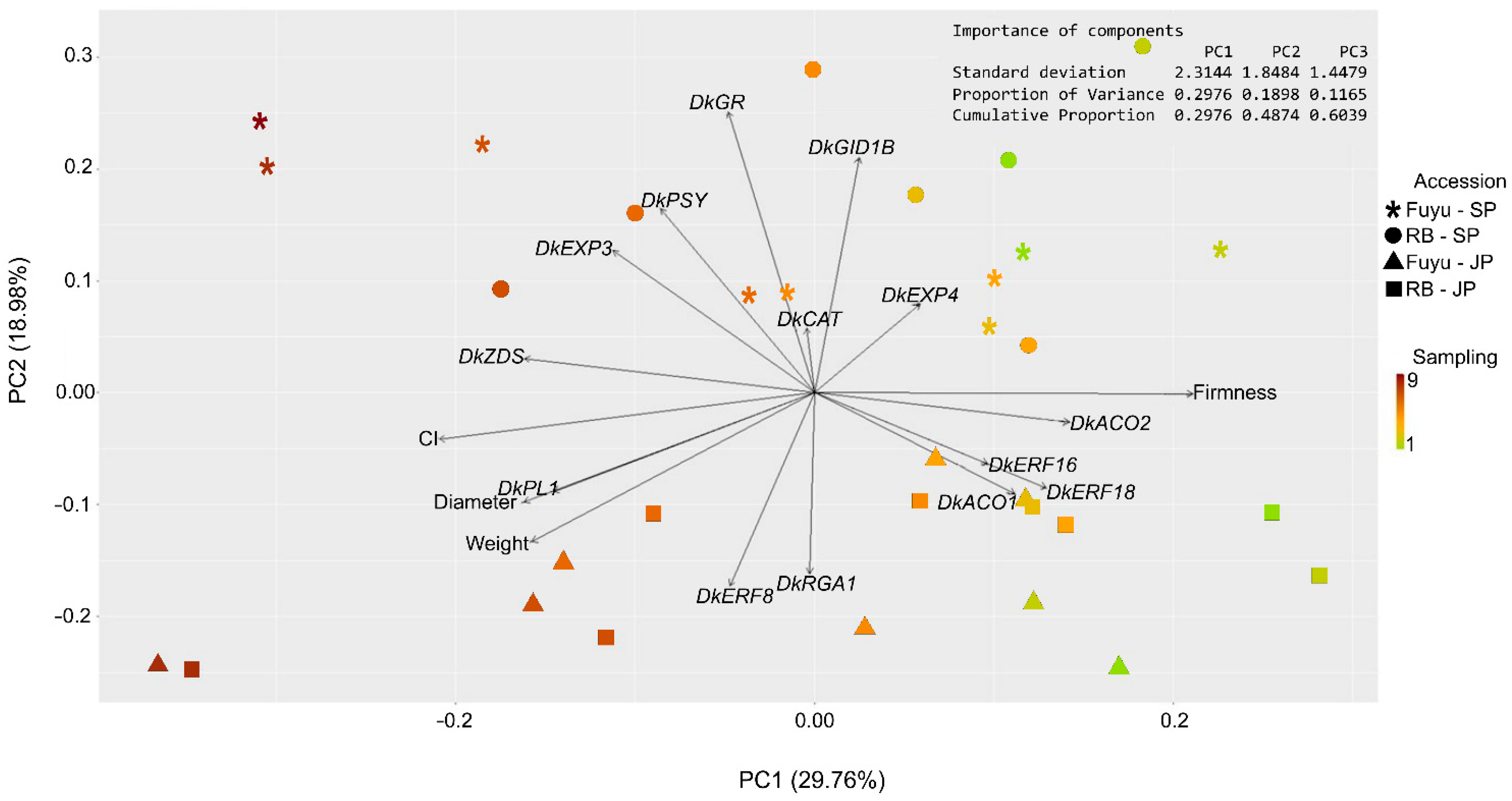
3.3. Gene Expression Measurements Associate with Phenotypic Data
3.4. Fruit Maturation Genes Are Similarly Expressed in Both Locations
3.5. GA and Oxidative Stress Gene Expression Patterns Differ between Locations
4. Discussion
4.1. Fruit Maturation and Ripening Genes Show Common Expression Patterns in JP and sp.
4.2. GA and Oxidative Stress-Related Genes Are Divergently Expressed in JP and sp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llácer, G.; Badenes, M.L. Persimmon production and market. In Options Méditerranéennes: Série A, Séminaires Méditerranéens, Proceedings of First Mediterranean Symposium on Persimmon, Faenza, Italy, 23–24 November 2001; Nº 51.1; Bellini, E., Giordani, E., Eds.; CIHEAM: Zaragoza, Spain, 2002; pp. 9–21. [Google Scholar]
- Conesa, C.; Laguarda-Miró, N.; Fito, P.; Seguí, L. Evaluation of Persimmon (Diospyros kaki Thunb. cv. Rojo Brillante) industrial residue as a source for value added products. Waste Biomass Valorization 2020, 11, 3749–3760. [Google Scholar] [CrossRef]
- Spanish Ministry of Agriculture, Fisheries and Food (MAPA). Available online: https://www.mapa.gob.es/es/estadistica/temas/estadistica-digital/powerbi-cultivos.aspx (accessed on 21 May 2024).
- Food and Agriculture Organization of the United Nations Statistics (FAOSTAT). Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 1 July 2024).
- Lucas-González, R.; Viuda-Martos, M.; Álvarez, J.A.P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Yakushiji, H.; Sugiura, H.; Yamasaki, A.; Azuma, A.; Koshita, Y. Tree growth, productivity, and fruit quality of ‘Fuyu’ persimmon trees onto different dwarfing rootstocks. Sci. Hortic. 2021, 278, 109869. [Google Scholar] [CrossRef]
- Sugiura, A.; Zheng, G.H.; Yonemori, K. Growth and ripening of persimmon fruit at controlled temperatures during growth stage III. HortScience 1991, 26, 574–576. [Google Scholar] [CrossRef]
- Glucina, P.G.; Toye, J.D. Flower bud and fruit development of persimmons. In Proceedings of the Ruakura Horticultural Conference: Persimmon; Ministry of Agriculture and Fisheries: Hamilton, New Zealand, 1985; pp. 11–14. [Google Scholar]
- Chen, T.; Qin, G.; Tian, S. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Osorio, S.; Scossa, F.; Fernie, A.R. Molecular regulation of fruit ripening. Front. Plant Sci. 2013, 4, 198. [Google Scholar] [CrossRef]
- Pech, J.C.; Purgatto, E.; Bouzayen, M.; Latché, A. Ethylene and fruit ripening. Annu. Plant Rev. 2012, 44, 275–304. [Google Scholar]
- Giovannoni, J.J. Molecular Biology of Fruit Maturation and Ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef]
- Van de Poel, B.; Smet, D.; Van Der Straeten, D. Ethylene and Hormonal Cross Talk in Vegetative Growth and Development. Plant Physiol. 2015, 169, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Gapper, N.E.; McQuinn, R.P.; Giovannoni, J.J. Molecular and genetic regulation of fruit ripening. Plant Mol. Biol. 2013, 82, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Li, Z.; Liu, M. Role of ethylene response factors (ERFs) in fruit ripening. Food Qual. Saf. 2020, 4, 15–20. [Google Scholar] [CrossRef]
- Kou, J.; Zhao, Z.; Wang, W.; Wei, C.; Guan, J.; Ference, C. Comparative Study of Ripening Related Gene Expression and Postharvest Physiological Changes between Astringent and Nonastringent Persimmon Cultivars. J. Am. Soc. Hortic. Sci. 2020, 145, 203–212. [Google Scholar] [CrossRef]
- Liu, L.; Rao, J.P.; Chang, X.X.; Yi, S.C. Regulation of propylene and 1-methylcyclopropene on expressions of ACS and ACO genes in persimmon fruits. Agric. Sci. China 2009, 8, 1187–1192. [Google Scholar] [CrossRef]
- Yin, X.R.; Shi, Y.N.; Min, T.; Luo, Z.R.; Yao, Y.C.; Xu, Q.; Chen, K.S. Expression of ethylene response genes during persimmon fruit astringency removal. Planta 2012, 235, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Soqanloo, S.S. Effect of different regional climates on persimmon quality. J Civ. Eng. Environ. Sci. 2015, 1, 008–012. [Google Scholar] [CrossRef]
- Halevy, A.; Kessler, B. Increased tolerance of bean plants to soil drought by means of growth-retarding substances. Nature 1963, 197, 310–311. [Google Scholar] [CrossRef]
- Sun, T.P. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. Arab. Book 2008, 6, e0103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, T.P.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Nakajima, M.; Motoyuki, A.; Matsuoka, M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 2007, 58, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Cheng, Q.; Li, R.; He, S.; Li, B. Changes of reactive oxygen species and scavenging enzymes of persimmon fruit treated with CO2 deastringency and the effect of hydroxyl radicals on breakdown of cell wall polysaccharides in vitro. Sci. Hortic. 2016, 199, 81–87. [Google Scholar] [CrossRef]
- Mowat, A.D. Fruit development patterns of persimmon (Diospyros kaki L.) grown under a cool climate. Acta Hortic. 2000, 601, 113–119. [Google Scholar] [CrossRef]
- Beppu, K.; Ikeda, T.; Kataoka, I. Effect of high temperature exposure time during flower bud formation on the occurrence of double pistils in “Satohnishiki” sweet cherry. Sci. Hortic. 2001, 87, 77–84. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Bonet, L.; Ferrer, P.; Besada, C.; Salvador, A. Short-term effects of regulated deficit irrigation of ‘Rojo Brillante’ persimmon (Diospyros kaki) yield, fruit quality and post-harvest performance. Acta Hortic. 2011, 922, 113–120. [Google Scholar] [CrossRef]
- Naval, M.; Zuriaga, E.; Pecchioli, S.; Llácer, G.; Giordani, E.; Badenes, M.L. Analysis of genetic diversity among persimmon cultivars using microsatellite markers. Tree Genet. Genomes 2010, 6, 77–687. [Google Scholar] [CrossRef][Green Version]
- Meteorological Data from Moncada-IVIA Climatic Station. Available online: http://riegos.ivia.es/datos-meteorologicos (accessed on 15 October 2023).
- Jimenez-Cuesta, M.; Cuquerella, J.; Martinez-Javaga, J.M. Determination of a color index for citrus fruit degreening. Proc. Int. Soc. Citric. 1982, 2, 750–753. [Google Scholar]
- Dorta, T.; Gil-Muñoz, F.; Carrasco, F.; Zuriaga, E.; Ríos, G.; Blasco, M. Physiological Changes and Transcriptomic Analysis throughout On-Tree Fruit Ripening Process in Persimmon (Diospyros kaki L.). Plants 2023, 12, 2895. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.Y.; Wilkins, T.A. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 1994, 223, 7–12. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, A.; Gao, Z.; Yu, X.; Li, M.; Hou, Y.; Sun, C.; Qu, S. Selection of suitable reference genes for RTqPCR normalization under abiotic stresses and hormone stimulation in persimmon (Diospyros kaki Thunb. ) PLoS ONE 2016, 11, e0160885. [Google Scholar]
- Persimmon Genome Database (PersimmonDB). Available online: https://persimmon.kazusa.or.jp/ (accessed on 20 May 2023).
- Horiuchi, A.; Masuda, K.; Shirasawa, K.; Onoue, N.; Fujita, N.; Ushijima, K.; Akagi, T. Ongoing rapid evolution of a post-Y region revealed by chromosome-scale genome assembly of a hexaploid monoecious persimmon (Diospyros kaki). Mol. Biol. Evol. 2023, 40, msad151. [Google Scholar] [CrossRef] [PubMed]
- The Arabidopsis Information Resource (TAIR). Available online: https://www.arabidopsis.org/ (accessed on 20 May 2023).
- National Center for Biotechnology Information (NCBI). Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 20 May 2023).
- Primer3 Web Service Designer. Available online: https://primer3.ut.ee/ (accessed on 20 May 2023).
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Pearson, K. On Lines and Planes of Closest Fit to Systems of Points in Space. Philos. Mag. 1901, 2, 14. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Ruduś, I.; Sasiak, M.; Kępczyński, J. Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol. Plant 2013, 35, 295–307. [Google Scholar] [CrossRef]
- He, Y.; Xue, J.; Li, H.; Han, S.; Jiao, J.; Rao, J. Ethylene response factors regulate ethylene biosynthesis and cell wall modification in persimmon (Diospyros kaki L.) fruit during ripening. Postharvest Biol. Technol. 2020, 168, 111255. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Quan, R.; Wang, X.C.; Huang, R. Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol. 2009, 150, 365–377. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Chen, J.Y.; Kuang, J.F.; Shan, W.; Xie, H.; Jiang, Y.M.; Lu, W.J. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J. Exp. Bot. 2013, 64, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Diretto, G.; Pirrello, J.; Roustan, J.P.; Li, Z.; Giuliano, G.; Bouzayen, M. The chimeric repressor version of an Ethylene Response Factor (ERF) family member, Sl-ERF. B3, shows contrasting effects on tomato fruit ripening. New Phytol. 2014, 203, 206–218. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhu, Q.G.; Deng, C.L.; Luo, Z.R.; Sun, N.J.; Grierson, D.; Yin, X.R.; Chen, K.S. Hypoxia-responsive ERFs involved in post deastringency softening of persimmon fruit. Plant Biotechnol. J. 2017, 15, 1409–1419. [Google Scholar] [CrossRef]
- Nakano, R.; Ogura, E.; Kubo, Y.; Inaba, A. Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiol. 2003, 131, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T. Studies on the effects of temperature on the growth and quality of fruits of Fuyu kaki. In Memoirs of the Faculty of Agriculture; No. 37; Kagawa University: Kagawa, Japan, 1982; pp. 1–63, (In Japanese with English Summary). [Google Scholar]
- Tomana, T.; Utsunomiya, N.; Kataoka, I. The effect of environmental temperature on fruit ripening on the tree II. The effect of temperatures around whole vines and clusters on the coloration of ‘Kyoho’ grapes. J. Jpn. Soc. Hortic. Sci. 1979, 48, 261–266. [Google Scholar] [CrossRef]
- Utsunomiya, N.; Yamada, H.; Kataoka, I.; Tomana, T. The effect of fruit temperatures on the maturation of satsuma mandarin (Citrus unshiu Marc.) fruits. J. Jpn. Soc. Hortic. Sci. 1982, 51, 135–141. [Google Scholar] [CrossRef][Green Version]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.; Gilroy, S. ROS in plant development. Physiol. Plant. 2010, 138, 384–392. [Google Scholar] [CrossRef]
- Aono, M.; Kubo, A.; Saji, H.; Tanaka, K.; Kondo, N. Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. 1993, 34, 129–135. [Google Scholar]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, N.S.; Malhotra, S.P.; Dhawan, S.K.; Singh, R. Antioxidant Systems in Ripening Tomato Fruits. Biol. Plant. 2004, 48, 49–53. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Modi, V.V. Ethylene and the ripening of mangoes. Plant Physiol. 1969, 44, 308–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Masia, A. Superoxide dismutase and catalase activities in apple fruit during ripening and post-harvest and with special reference to ethylene. Physiol. Plant. 1998, 104, 668–672. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016, 104, 294–303. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez, F.; Keller, C.; Monnet, F.; Sallanon, H. Acclimation to drought stress enhances oxidative stress tolerance in Solanum lycopersicum L. fruits. Plant Stress 2008, 2, 145–151. [Google Scholar]
- Sofo, A.; Cicco, N.; Paraggio, M.; Scopa, A. Regulation of the Ascorbate–Glutathione Cycle in Plants Under Drought Stress. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Anjum, N., Chan, M.T., Umar, S., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Leung, D.W.M. Studies of Catalase in Plants Under Abiotic Stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zuccarelli, R.; Freschi, L. Glutathione Reductase: Safeguarding Plant Cells Against Oxidative Damage. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shohat, H.; Eliaz, N.I.; Weiss, D. Gibberellin in tomato: Metabolism, signaling and role in drought responses. Mol. Hortic. 2021, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Wüthrich, S.; Blösch, R.; Rindisbacher, A.; Cannarozzi, G.; Tadele, Z. Gibberellin Deficiency Confers Both Lodging and Drought Tolerance in Small Cereals. Front. Plant Sci. 2016, 7, 643. [Google Scholar] [CrossRef]
- Pal, S.; Zhao, J.; Khan, A.; Yadav, N.S.; Batushansky, A.; Barak, S.; Rewald, B.; Fait, A.; Lazarovitch, N.; Rachmilevitch, S. Paclobutrazol induces tolerance in tomato to deficit irrigation through diversified effects on plant morphology, physiology and metabolism. Sci. Rep. 2016, 6, 39321. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
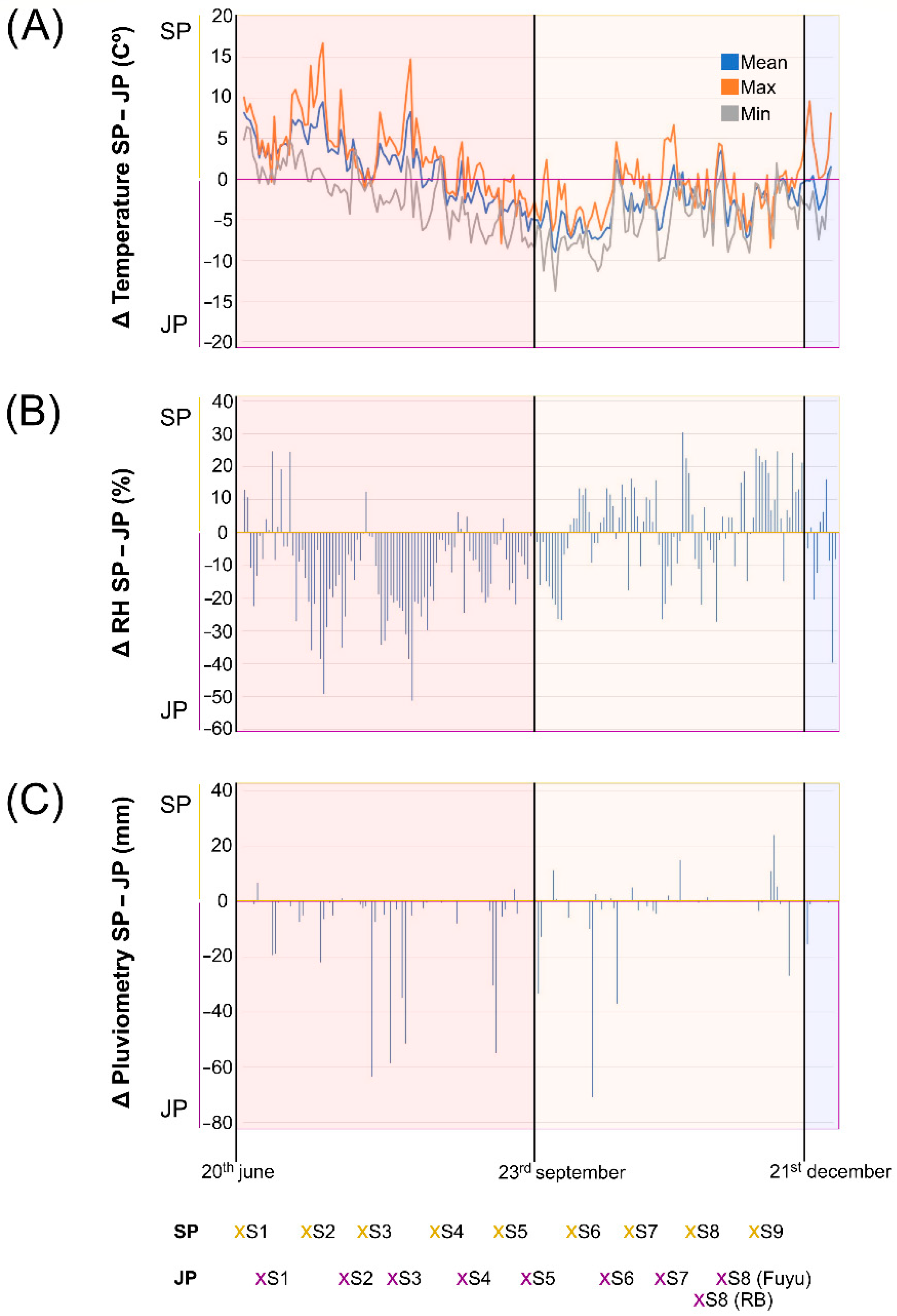




| Accession | Origin | Fruit Astringency | Location | +30D AFS |
|---|---|---|---|---|
| Rojo Brillante | Spain | PVA * | SP | 20 June |
| Rojo Brillante | Spain | PVA * | JP | 27 June |
| Fuyu | Japan | J-PCNA | SP | 20 June |
| Fuyu | Japan | J-PCNA | JP | 27 June |
| Sampling | SP | JP |
|---|---|---|
| S1 | 20 June | 27 June |
| S2 | 11 July | 23 July |
| S3 | 29 July | 8 August |
| S4 | 22 August | 31 August |
| S5 | 12 September | 20 September |
| S6 | 6 October | 17 October |
| S7 | 25 October | 4 November |
| S8 | 14 November (only Fuyu) | 16 November (RB)/24 November (Fuyu) |
| S9 | 5 December (only Fuyu) |
| Gene Name | Accession Number | Protein Name | Function |
|---|---|---|---|
| DkACO1 | AB073008 | 1-aminocyclopropane-1-carboxylate oxidase 1 | Key enzyme in ethylene biosynthesis. |
| DkACO2 | AB073009 | 1-aminocyclopropane-1-carboxylate oxidase 2 | Key enzyme in ethylene biosynthesis. |
| DkERF16 | KJ170916 | Ethylene response factor 16 | Regulator of ethylene biosynthesis. Possible promoter of fruit ripening by cell wall modification. |
| DkERF18 | KJ170918 | Ethylene response factor 18 | Regulator of ethylene biosynthesis. |
| DkERF8 | JN256078 | Ethylene response factor 8 | Regulator of ethylene biosynthesis. Possible promoter of fruit ripening by cell wall modification. |
| DkEXP3 | XM_052336116 (D. lotus) | Expansin 3 | Cell wall modifying and loosening enzyme. |
| DkEXP4 | XM_052320777 (D. lotus) | Expansin 4 | Cell wall modifying and loosening enzyme. |
| DkPSY | FJ713744 | Phytoene synthase | Catalysis in the first committed step of the carotenoid biosynthesis pathway. |
| DkZDS | XM_052336035 (D. lotus) | Zeta-carotene desaturase | Involved in the biosynthesis of carotenes. |
| DkPL1 | XM_052333091 (D. lotus) | Pectate-lyase 1 | Cell wall degradation enzyme. |
| DkGID1B | XM_052314796 (D. lotus) | Gibberellin receptor GID1B-like | Triggers ubiquitination of DELLA proteins, enhancing transcription of gibberellin response genes. |
| DkRGA1 | XM_052332049 (D. lotus) | RGA-like protein | DELLA subfamily member that acts as a negative regulator of GA signalling. |
| DkCAT | XM_052346323 (D. lotus) | Catalase 1 | Catalyzes the reduction of hydrogen peroxide, protecting cells against oxidative stress. |
| DkGR | XM_052313957 (D. lotus) | Glutathione reductase | Protect cells against oxidative stress damage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorta, T.; Onoue, N.; Hsiang, T.-F.; Nishiyama, S.; Ríos, G.; Tao, R.; Blasco, M. Phenotypic and Gene Expression Analysis of Fruit Development of ‘Rojo Brillante’ and ‘Fuyu’ Persimmon (Diospyros kaki L.) Cultivars in Two Different Locations. Agronomy 2024, 14, 1555. https://doi.org/10.3390/agronomy14071555
Dorta T, Onoue N, Hsiang T-F, Nishiyama S, Ríos G, Tao R, Blasco M. Phenotypic and Gene Expression Analysis of Fruit Development of ‘Rojo Brillante’ and ‘Fuyu’ Persimmon (Diospyros kaki L.) Cultivars in Two Different Locations. Agronomy. 2024; 14(7):1555. https://doi.org/10.3390/agronomy14071555
Chicago/Turabian StyleDorta, Tania, Noriyuki Onoue, Tzu-Fan Hsiang, Soichiro Nishiyama, Gabino Ríos, Ryutaro Tao, and Manuel Blasco. 2024. "Phenotypic and Gene Expression Analysis of Fruit Development of ‘Rojo Brillante’ and ‘Fuyu’ Persimmon (Diospyros kaki L.) Cultivars in Two Different Locations" Agronomy 14, no. 7: 1555. https://doi.org/10.3390/agronomy14071555
APA StyleDorta, T., Onoue, N., Hsiang, T.-F., Nishiyama, S., Ríos, G., Tao, R., & Blasco, M. (2024). Phenotypic and Gene Expression Analysis of Fruit Development of ‘Rojo Brillante’ and ‘Fuyu’ Persimmon (Diospyros kaki L.) Cultivars in Two Different Locations. Agronomy, 14(7), 1555. https://doi.org/10.3390/agronomy14071555







