Biochar as an Alternative Litter Additive to Mitigate Gaseous Emissions from Broiler Housing and Subsequent Storage
Abstract
1. Introduction
2. Materials and Methods
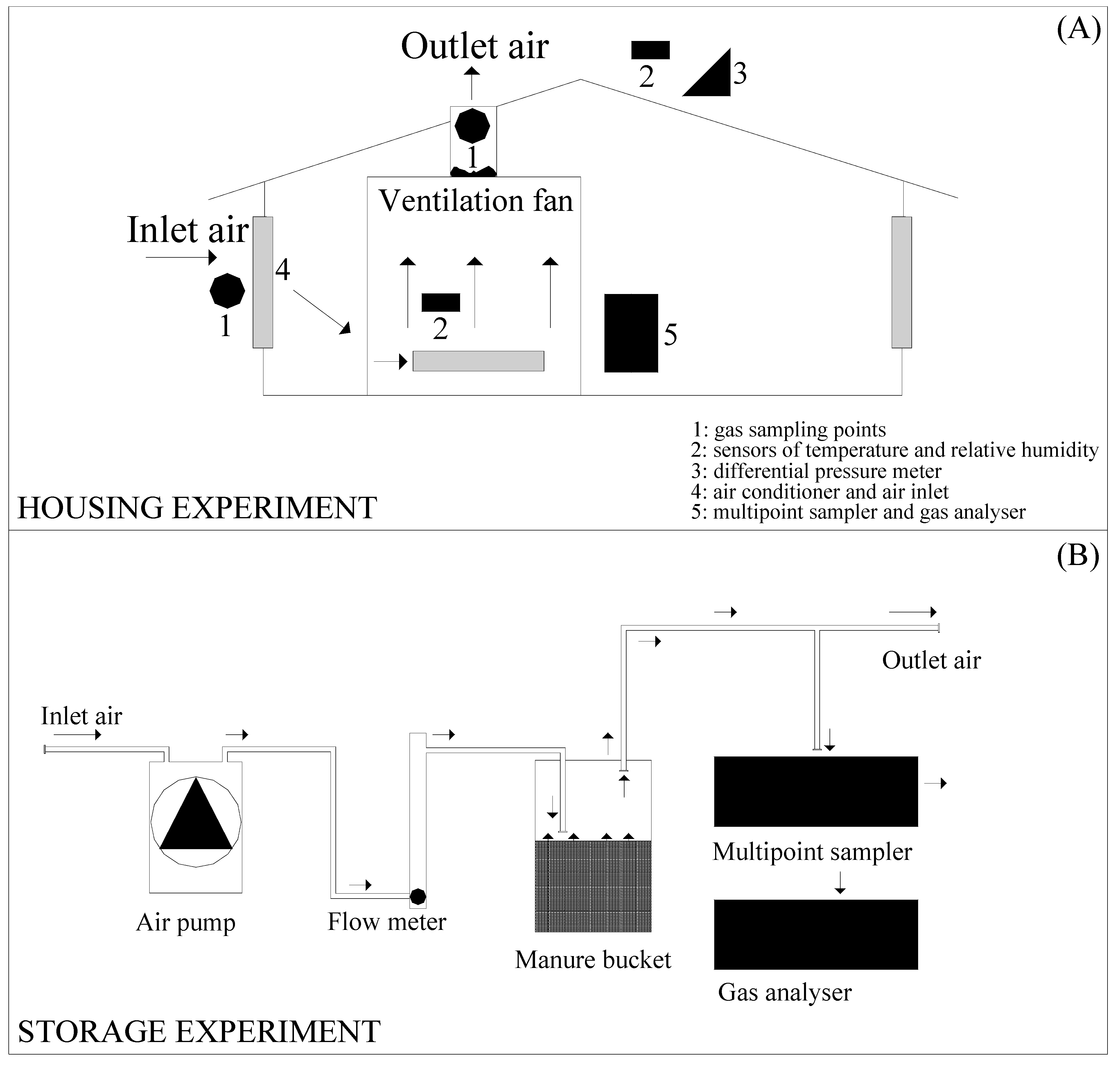
2.1. Housing Experiment
2.1.1. Treatments
2.1.2. Measurements
2.2. Storage Experiment
2.2.1. Treatments
2.2.2. Measurements
2.3. Data Analysis
3. Results and Discussion
3.1. Gaseous Emissions in Housing
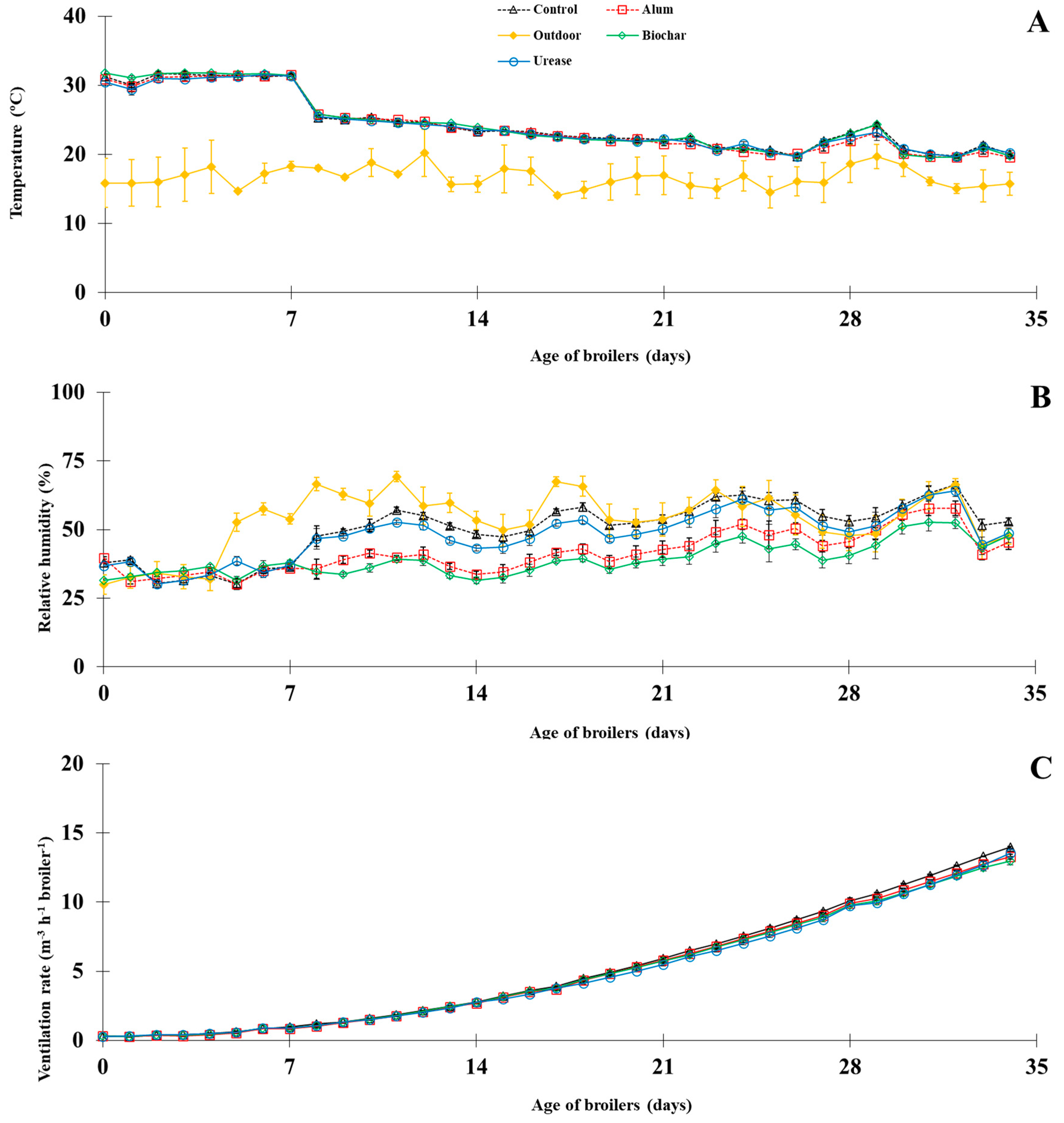
3.1.1. Experimental Condition
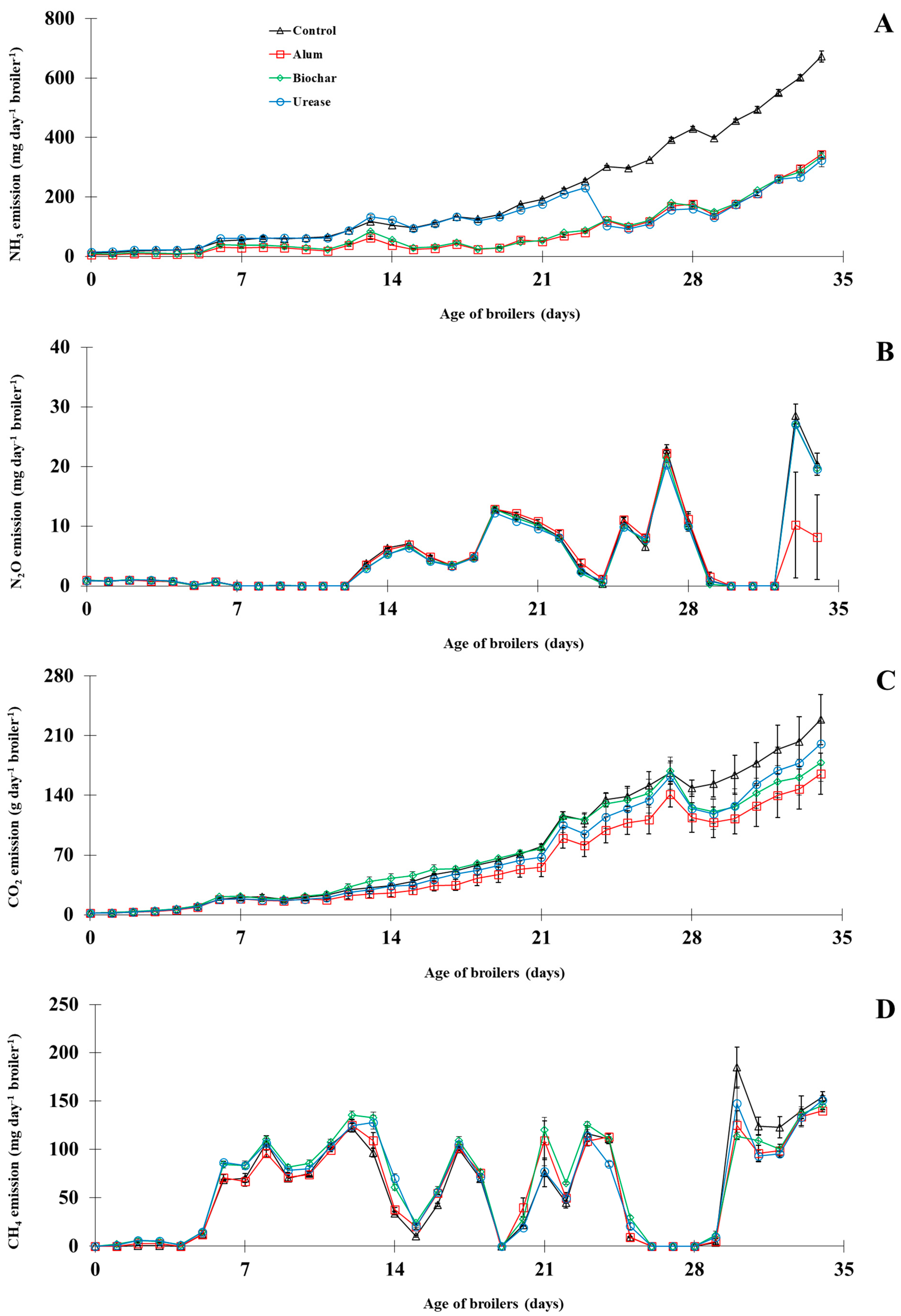
3.1.2. Ammonia and Greenhouse Gases
3.2. Gaseous Emissions in Storage
3.2.1. Composition of the Manures
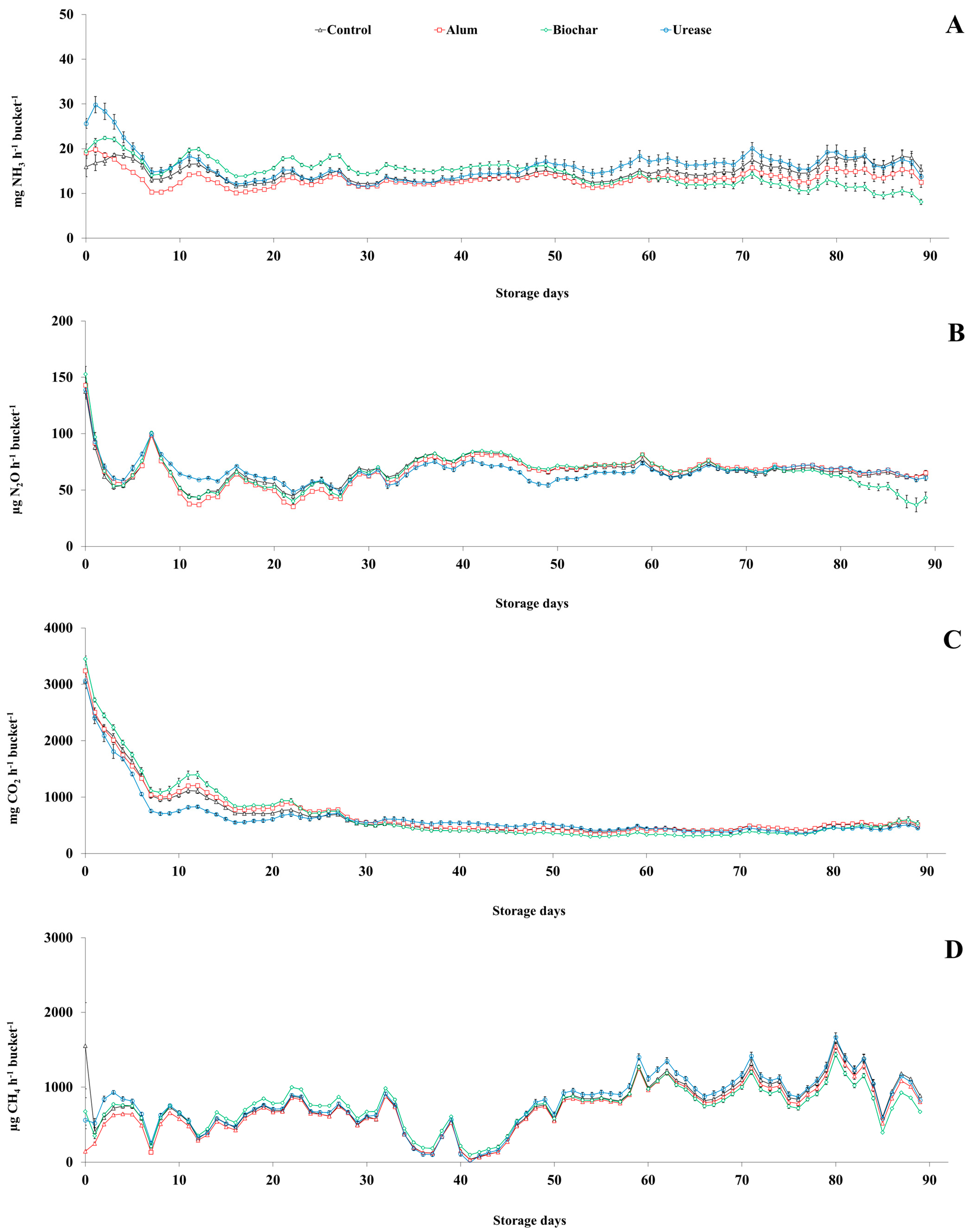
3.2.2. Ammonia and Greenhouse Gases
3.3. Implications and Recommendations
4. Conclusions
- At the housing stage, the results indicated that the NH3 emissions were significantly reduced by 40–60% by litter additives (Alum, Biochar, and Urease), while GWP emissions were significantly reduced by 31% by Alum. Furthermore, the addition of Biochar (a 58% reduction) had the same significant effect as Alum (a 60% reduction) to mitigate these losses. The reapplication of Urease (a 41% reduction) may be required to reach an equal or higher reduction.
- At the storage stage, the NH3 and GWP emissions were not significantly affected by the litter additives.
- At the combined housing and subsequent manure storage stages, the NH3 emissions were significantly reduced by 22–41% by litter additives, whereas GWP emissions were not significantly reduced.
- Overall, it can be concluded that Biochar appears to be a good alternative to Alum due to its equal effectiveness in mitigating NH3 losses, without increasing the GWP potential in the housing stage and avoiding pollution swapping. However, Biochar seems to be more expensive, although it has additional advantages related to the circular economy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INE. Estatísticas Agrícolas 2020; Instituto Nacional de Estatística (INE): Lisbon, Portugal, 2020; p. 181.
- IPCC. Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019. Available online: http://www.ipcc-nggip.iges.or.jp (accessed on 23 June 2024).
- Barnes, A.P.; Stockdale, E.; Norton, L.; Eory, V.; Macleod, M.; Buys, G. Achieving cleaner growth in agriculture: Establishing feasible mitigation through a bottom-up approach. J. Clean. Prod. 2024, 454, 142287. [Google Scholar] [CrossRef]
- EC. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); European Commission (EC): Seville, Spain, 2017. JRC107189. p. 858. Available online: https://data.europa.eu/doi/10.2760/020485 (accessed on 23 June 2024).
- Naseem, S.; King, A.J. Ammonia production in poultry houses can affect health of humans, birds, and the environment-techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 2018, 25, 15269–15293. [Google Scholar] [CrossRef] [PubMed]
- Méda, B.; Hassouna, M.; Aubert, C.; Robin, P.; Dourmad, J.Y. Influence of rearing conditions and manure management practices on ammonia and greenhouse gas emissions from poultry houses. World’s Poult. Sci. J. 2011, 67, 441–456. [Google Scholar] [CrossRef]
- Pereira, J.L.S.; Ferreira, S.; Pinheiro, V.; Trindade, H. Effect of magnesium sulphate addition to broiler litter on the ammonia, nitrous oxide, carbon dioxide and methane emissions from housing. Atmos. Pollut. Res. 2019, 10, 1284–1290. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2021; p. 368. [Google Scholar]
- Hassouna, M.; van der Weerden, T.J.; Beltran, I.; Amon, B.; Alfaro, M.A.; Anestis, V.; Cinar, G.; Dragoni, F.; Hutchings, N.J.; Leytem, A.; et al. DATAMAN: A global database of methane, nitrous oxide, and ammonia emission factors for livestock housing and outdoor storage of manure. J. Environ. Qual. 2023, 52, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.S.; Garcia, C.; Trindade, H. Review of measures to control airborne pollutants in broiler housing. In Air Pollution—Latest Status and Current Developments [Working Title]; Eyvaz, M., Albahnasawi, A., Alazaiza, M.Y.D., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Bittman, S.; Dedina, M.; Howard, C.M.; Oenema, O.; Sutton, M.A. Options for Ammonia Mitigation: Guidance from the UNECE Task Force on Reactive Nitrogen; Project Reference: CEH Project no. C04910; NERC/Centre for Ecology & Hydrology: Edinburgh, UK, 2014; p. 83. [Google Scholar]
- Sutton, M.A.; Howard, C.M.; Mason, K.E.; Brownlie, W.J.; Cordovil, C.M.d.S. (Eds.) Nitrogen Opportunities for Agriculture, Food & Environment. UNECE Guidance Document on Integrated Sustainable Nitrogen Management; UK Centre for Ecology & Hydrology: Edinburgh, UK, 2022; p. 157. ISBN 978-1-906698-78-2. [Google Scholar]
- Bist, R.B.; Subedi, S.; Chai, L.; Yang, X. Ammonia emissions, impacts, and mitigation strategies for poultry production: A critical review. J. Environ. Manag. 2023, 328, 116919. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.S.; Perdigão, A.; Marques, F.; Wessel, D.F.; Trindade, H.; Fangueiro, D. Mitigating Ammonia and Greenhouse Gas Emissions from Stored Pig Slurry Using Chemical Additives and Biochars. Agronomy 2022, 12, 2744. [Google Scholar] [CrossRef]
- Cockerill, S.A.; Gerber, P.F.; Walkden-Brown, S.W.; Dunlop, M.W. Suitability of litter amendments for the Australian chicken meat industry. Anim. Prod. Sci. 2020, 60, 1469–1481. [Google Scholar] [CrossRef]
- Ni, J.Q.; Erasmus, M.A.; Croney, C.C.; Li, C.; Li, Y. A critical review of advancement in scientific research on food animal welfare-related air pollution. J. Hazard. Mater. 2021, 408, 124468. [Google Scholar] [CrossRef]
- EN 13040; Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density. European standards (ES) 13040. European Committee for Standardization: Brussels, Belgium, 1999.
- EN 13654-1; Soil Improvers and Growing Media—Determination of Nitrogen—Part 1: Modified Kjeldahl Method. European Standards (ES) 13654-1. European Committee for Standardization: Brussels, Belgium, 2002.
- EN 13652; Soil Improvers and Growing Media—Extraction of Water Soluble Nutrients and Elements. European Standards (ES) 13652. European Committee for Standardization: Brussels, Belgium, 2002.
- Eugene, B.; Moore, P.A., Jr.; Li, H.; Miles, D.M.; Trabue, S.; Burns, R.; Buser, M. Effect of alum additions to poultry litter on in-house ammonia and greenhouse gas concentrations and emissions. J. Environ. Qual. 2015, 44, 1530–1540. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar]
- Hagenkamp-Korth, F.; Haeussermann, A.; Hartung, E. Effect of urease inhibitor application on urease activity in three different cubicle housing systems under practical conditions. Agric. Ecosyst. Environ. 2015, 202, 168–177. [Google Scholar] [CrossRef]
- CR. Commission Regulation No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control. Off. J. Eur. Union 2008, 250, 1–84. Available online: http://data.europa.eu/eli/reg/2008/889/oj (accessed on 23 June 2024).
- EN 13037; Soil Improvers and Growing Media—Determination of pH. European Standards (ES) 13037. European Committee for Standardization: Brussels, Belgium, 2011.
- Pereira, J.L.S.; Figueiredo, V.; Pinto, A.F.M.A.; Silva, M.E.F.; Brás, I.; Perdigão, A.; Wessel, D.F. Effects of biochar and clinoptilolite on composition and gaseous emissions during the storage of separated liquid fraction of pig slurry. Appl. Sci. 2020, 10, 5652. [Google Scholar] [CrossRef]
- NREAP. Decree number 81/2013 of 14 June regarding new regime to perform the livestock activity. In Diário da República; 113; Portugal, 2013; pp. 3304–3329. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/81-2013-496729 (accessed on 23 June 2024).
- Calvet, S.; Cambra-Lopez, M.; Estelles, F.; Torres, A.G. Characterization of gas emissions from a Mediterranean broiler farm. Poult. Sci. 2011, 90, 534–542. [Google Scholar] [CrossRef]
- Anderson, K.; Moore, P.A., Jr.; Martin, J.; Ashworth, A.J. Evaluation of a novel poultry litter amendment on greenhouse gas emissions. Atmosphere 2021, 12, 563. [Google Scholar] [CrossRef]
- Ritz, C.W.; Tasistro, A.S.; Kissel, D.E.; Fairchild, B. Evaluation of surface-applied char on the reduction of ammonia volatilization from broiler litter. J. Appl. Poult. Res. 2011, 20, 240–245. [Google Scholar] [CrossRef]
- Linhoss, J.E.; Purswell, J.L.; Street, J.T.; Rowland, M.R. Evaluation of biochar as a litter amendment for commercial broiler production. J. Appl. Poult. Res. 2019, 28, 1089–1098. [Google Scholar] [CrossRef]
- Singh, A.; Casey, K.D.; King, W.D.; Pescatore, A.J.; Gates, R.S.; Ford, M.J. Efficacy of urease inhibitor to reduce ammonia emission from poultry houses. J. Appl. Poult. Res. 2009, 18, 34–42. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abd El-Hack, M.E.; Ghanima, M.M.A.; Shukry, M.; Alhotan, R.A.; Hussein, E.O.; Suliman, G.M.; Ba-Awadh, H.; Ammari, A.A. Ammonia emissions in poultry houses and microbial nitrification as a promising reduction strategy. Sci. Total Environ. 2021, 781, 146978. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.L.; Van Zwieten, L.; Singh, B.-P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kalus, K.; Konkol, D.; Korczyński, M.; Koziel, J.A.; Opaliński, S. Laying hens biochar diet supplementation—Effect on performance, excreta N content, NH3 and VOCs emissions, Egg Traits and Egg Consumers Acceptance. Agriculture 2020, 10, 237. [Google Scholar] [CrossRef]
- Calvet, S.; Arrufat, B.; Salaet, I.; Atares, S.; Sobreviela, A.; Herrero, C.; Romero, J.; Estellés, F. A urease inhibitor reduces ammonia emission in fattening pigs reared on slatted floor in summer conditions. Biosyst. Eng. 2022, 221, 43–53. [Google Scholar] [CrossRef]
- Rodhe, L.; Karlsson, S. Ammonia emissions from broiler manure—Influence of storage and spreading method. Biosyst. Eng. 2002, 82, 455–462. [Google Scholar] [CrossRef]
- Drozdz, D.; Wystalska, K.; Malinska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of poultry manure in Poland—Current state and future perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef] [PubMed]
- Kreidenweis, U.; Breier, J.; Herrmann, C.; Libra, J.; Prochnow, A. Greenhouse gas emissions from broiler manure treatment options are lowest in well-managed biogas production. J. Clean. Prod. 2021, 280, 124969. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.; Liang, Y.; Burns, R.T. Reduction of ammonia emissions from stored laying hen manure through topical application of zeolite, Al+ Clear, Ferix-3, or poultry litter treatment. J. Appl. Poult. Res. 2008, 17, 421–443. [Google Scholar] [CrossRef]
- Brouček, J.; Čermák, B. Emission of harmful gases from poultry farms and possibilities of their reduction. Ekológia 2015, 3, 89–100. [Google Scholar] [CrossRef]
- Pardo, G.; Moral, R.; Aguilera, E.; del Prado, A. Gaseous emissions from management of solid waste: A systematic review. Glob. Change Biol. 2015, 21, 1313–1327. [Google Scholar] [CrossRef]
| Parameters | Control | Alum | Biochar | Urease | SEM | p-Value |
|---|---|---|---|---|---|---|
| Number of broilers (0 days) | 22 | 22 | 22 | 22 | ||
| Number of broilers (10–35 days) | 20 | 20 | 20 | 20 | ||
| Liveweight (g) | ||||||
| Day 0 | 45.0 ± 0.1 | 44.0 ± 0.1 | 44.7 ± 0.3 | 44.7 ± 0.3 | 0.360 | 0.138 |
| Day 3 | 77.0 ± 1.0 | 77.0 ± 1.3 | 79.0 ± 0.5 | 78.3 ± 0.8 | 1.763 | 0.615 |
| Day 7 | 172.7 ± 2.4 | 171.0 ± 3.6 | 176.0 ± 1.5 | 177.3 ± 2.5 | 4.634 | 0.540 |
| Day 10 | 261.3 ± 7.3 | 253.0 ± 2.5 | 263.0 ± 7.5 | 266.7 ± 2.3 | 10.295 | 0.622 |
| Day 15 | 551.7 ± 1.4 | 536.0 ± 5.6 | 546.7 ± 14.0 | 550.3 ± 4.2 | 14.023 | 0.688 |
| Day 18 | 781.7 ± 6.8 | 740.7 ± 12.2 | 755.0 ± 22.4 | 756.0 ± 6.0 | 23.282 | 0.428 |
| Day 23 | 1295.0 ± 18.9 | 1256.7 ± 15.9 | 1235.0 ± 21.4 | 1208.3 ± 12.6 | 31.921 | 0.145 |
| Day 29 | 2020.0 ± 36.8 | 1983.3 ± 29.0 | 1955.0 ± 48.8 | 1945.0 ± 9.0 | 61.283 | 0.637 |
| Day 35 | 2793.3 ± 13.8 | 2656.7 ± 32.2 | 2598.3 ± 57.2 | 2710.0 ± 49.2 | 77.582 | 0.179 |
| Daily weight gain (g day−1) | ||||||
| 0–35 days | 79.8 ± 0.4 | 75.9 ±0.9 | 74.2 ± 1.6 | 77.4 ± 1.4 | 2.216 | 0.179 |
| Daily feed intake (g day−1) | ||||||
| 0–35 days | 118.1 ± 2.4 | 114.0 ± 0.9 | 113.1 ± 0.1 | 113.5 ± 1.2 | 1.910 | 0.116 |
| Feed conversion ratio | ||||||
| 0–35 days | 1.48 ± 0.04 | 1.50 ± 0.02 | 1.53 ± 0.03 | 1.47 ± 0.03 | 0.055 | 0.734 |
| Parameters | Control | Alum | Biochar | Urease | SEM | p-Value |
|---|---|---|---|---|---|---|
| Climatic conditions | ||||||
| Outdoor T (°C) | 16.7 ± 2.0 | 16.7 ± 2.0 | 16.7 ± 2.0 | 16.7 ± 2.0 | 1.266 | 0.214 |
| Indoor T (°C) | 24.5 ± 2.0 | 24.2 ± 2.1 | 24.5 ± 2.1 | 24.3 ± 2.0 | 0.255 | 0.680 |
| Outdoor RH (%) | 53.4 ± 5.4 | 53.4 ± 5.4 | 53.4 ± 5.4 | 53.4 ± 5.4 | 6.099 | 0.404 |
| Indoor RH (%) | 50.4 ± 5.0 a | 41.7 ± 3.7 b | 39.3 ± 3.0 b | 47.9 ± 4.4 a | 1.382 | 0.000 |
| VR (m3 h−1 broiler−1) | 5.1 ± 2.2 | 5.0 ± 2.1 | 4.9 ± 2.0 | 4.9 ± 2.1 | 0.131 | 0.255 |
| Gas emissions | ||||||
| NH3 (mg day−1 broiler−1) | 204.5 ± 2.4 a | 81.5 ± 1.4 c | 85.9 ± 1.8 c | 119.7 ± 1.4 b | 3.405 | 0.000 |
| N2O (mg day−1 broiler−1) | 5.2 ± 0.3 | 4.4 ± 0.6 | 4.9 ± 0.1 | 4.9 ± 0.1 | 0.499 | 0.550 |
| CO2 (g day−1 broiler−1) | 78.5 ± 6.2 a | 58.4 ± 8.8 b | 71.8 ± 5.6 a | 68.4 ± 8.4 a | 7.641 | 0.047 |
| CH4 (mg day−1 broiler−1) | 59.9 ± 2.9 | 58.5 ± 0.5 | 65.0 ± 1.4 | 61.1 ± 1.3 | 3.247 | 0.311 |
| GWP (mg CO2-eq. day−1 broiler−1) | 4099.1 ± 990.3 | 2826.5 ± 153.8 | 3131.1 ± 39.7 | 3013.1 ± 3.8 | 781.720 | 0.434 |
| Parameters | Control | Alum | Biochar | Urease | SEM | p-Value |
|---|---|---|---|---|---|---|
| 0 days of experiment | ||||||
| pH (H2O) | 4.8 ± 0.1 b | 3.4 ± 0.1 c | 6.3 ± 0.1 a | 4.8 ± 0.1 b | 0.065 | 0.000 |
| 35 days of experiment | ||||||
| pH (H2O) | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.2 ± 0.1 | 0.188 | 0.560 |
| Dry matter (g kg−1) | 433.0 ± 12.7 | 475.3 ± 20.6 | 478.5 ± 34.5 | 457.8 ± 6.5 | 32.290 | 0.523 |
| Total C (g kg−1) | 219.8 ± 6.6 | 239.2 ± 11.1 | 246.5 ± 19.5 | 235.2 ± 4.3 | 18.514 | 0.565 |
| Total N (g kg−1) | 6.4 ± 0.2 | 6.7 ± 0.4 | 6.3 ± 0.3 | 6.3 ± 0.3 | 0.338 | 0.506 |
| NH4+-N (g N kg−1) | 1.8 ± 0.2 | 2.3 ± 0.4 | 2.1 ± 0.2 | 1.8 ± 0.2 | 0.472 | 0.666 |
| NO3−-N (mg N kg−1) | 6.8 ± 0.1 | 6.8 ± 0.1 | 6.8 ± 0.1 | 6.8 ± 0.1 | 0.188 | 0.514 |
| C:N ratio | 34.4 ± 0.2 | 36.1 ± 3.9 | 3.19 ± 1.1 | 37.9 ± 2.9 | 3.657 | 0.623 |
| Parameters | Control | Alum | Biochar | Urease | SEM | p-Value |
|---|---|---|---|---|---|---|
| NH3 (mg bucket−1) | 31,628 ± 561 | 28,857 ± 1049 | 31,690 ± 1062 | 34,955 ± 2054 | 3976.400 | 0.544 |
| NH3 (mg broiler−1 day−1) | 46 ± 1 | 42 ± 2 | 46 ± 2 | 51 ± 3 | 5.797 | 0.544 |
| N2O (mg bucket−1) | 146 ± 2 | 145 ± 2 | 144 ± 1 | 145 ± 1 | 2.384 | 0.891 |
| N2O (µg broiler−1 day−1) | 213 ± 3 | 211 ± 2 | 211 ± 1 | 212 ± 2 | 0.011 | 0.891 |
| CO2 (g bucket−1) | 1480 ± 25 | 1527 ± 35 | 1508 ± 31 | 1397 ± 64 | 132.580 | 0.777 |
| CO2 (mg broiler−1 day−1) | 2157 ± 36 | 2225 ± 51 | 2199 ± 46 | 2037 ± 94 | 0.191 | 0.777 |
| CH4 (mg bucket−1) | 1639 ± 24 | 1502 ± 35 | 1582 ± 38 | 1686 ± 65 | 137.500 | 0.606 |
| CH4 (µg broiler−1 day−1) | 2389 ± 35 | 2190 ± 51 | 2307 ± 55 | 2458 ± 95 | 0.204 | 0.606 |
| GWP (g CO2-eq. bucket−1) | 85 ± 1 | 81 ± 1 | 83 ± 1 | 86 ± 2 | 3.547 | 0.503 |
| GWP (mg CO2-eq. broiler−1 day−1) | 124 ± 2 | 118 ± 2 | 121 ± 2 | 126 ± 3 | 0.010 | 0.503 |
| Parameters | Control | Alum | Biochar | Urease | SEM | p-Value |
|---|---|---|---|---|---|---|
| NH3 (mg broiler−1) | ||||||
| Housing | 7157 ± 85 a | 2854 ± 49 c | 3007 ± 64 c | 4190 ± 49 b | 119.180 | 0.000 |
| Storage | 4150 ± 74 | 3786 ± 138 | 4158 ± 139 | 4586 ± 270 | 521.700 | 0.547 |
| Total | 11,307 ± 54 a | 6640 ± 273 c | 7164 ± 242 c | 8776 ± 446 b | 503.690 | 0.000 |
| N2O (mg broiler−1) | ||||||
| Housing | 183 ± 10 | 157 ± 22 | 173 ± 1 | 172 ± 4 | 17.456 | 0.550 |
| Storage | 19 ± 1 | 19 ± 1 | 19 ± 1 | 19 ± 1 | 0.313 | 0.891 |
| Total | 202 ± 11 | 176 ± 22 | 192 ± 1 | 191 ± 5 | 17.601 | 0.551 |
| CO2 (g broiler−1) | ||||||
| Housing | 2746 ± 218 a | 2044 ± 308 b | 2513 ± 197 a | 2394 ± 292 a | 267.430 | 0.047 |
| Storage | 194 ± 3 | 200 ± 5 | 198 ± 4 | 183 ± 8 | 17.395 | 0.777 |
| Total | 2940 ± 224 a | 2244 ± 316 b | 2711 ± 193 a | 2578 ± 292 a | 0.497 | 0.047 |
| CH4 (mg broiler−1) | ||||||
| Housing | 2096 ± 100 | 2047 ± 16 | 2275 ± 48 | 2140 ± 45 | 113.640 | 0.311 |
| Storage | 215 ± 3 | 197 ± 5 | 208 ± 5 | 221 ± 9 | 18.040 | 0.606 |
| Total | 2311 ± 104 | 2244 ± 23 | 2482 ± 47 | 2361 ± 44 | 116.810 | 0.311 |
| GWP (g CO2-eq. broiler−1) | ||||||
| Housing | 107 ± 4 | 99 ± 5 | 110 ± 1 | 105 ± 1 | 4.931 | 0.260 |
| Storage | 11 ± 1 | 11 ± 1 | 11 ± 1 | 11 ± 1 | 0.465 | 0.503 |
| Total | 118 ± 4 | 110 ± 5 | 120 ± 1 | 117 ± 1 | 4.777 | 0.217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.L.S.; Martins, F.; Bonifácio, G.; Garcia, C.; Teixeira, J.; Trindade, H. Biochar as an Alternative Litter Additive to Mitigate Gaseous Emissions from Broiler Housing and Subsequent Storage. Agronomy 2024, 14, 1595. https://doi.org/10.3390/agronomy14071595
Pereira JLS, Martins F, Bonifácio G, Garcia C, Teixeira J, Trindade H. Biochar as an Alternative Litter Additive to Mitigate Gaseous Emissions from Broiler Housing and Subsequent Storage. Agronomy. 2024; 14(7):1595. https://doi.org/10.3390/agronomy14071595
Chicago/Turabian StylePereira, José L. S., Filipa Martins, Gabriel Bonifácio, Carla Garcia, José Teixeira, and Henrique Trindade. 2024. "Biochar as an Alternative Litter Additive to Mitigate Gaseous Emissions from Broiler Housing and Subsequent Storage" Agronomy 14, no. 7: 1595. https://doi.org/10.3390/agronomy14071595
APA StylePereira, J. L. S., Martins, F., Bonifácio, G., Garcia, C., Teixeira, J., & Trindade, H. (2024). Biochar as an Alternative Litter Additive to Mitigate Gaseous Emissions from Broiler Housing and Subsequent Storage. Agronomy, 14(7), 1595. https://doi.org/10.3390/agronomy14071595








