Gibberellins Regulate Expression of Cyclins to Control Leaf Width in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Materials
2.2. Plant Growth Conditions and Hormones Treatment
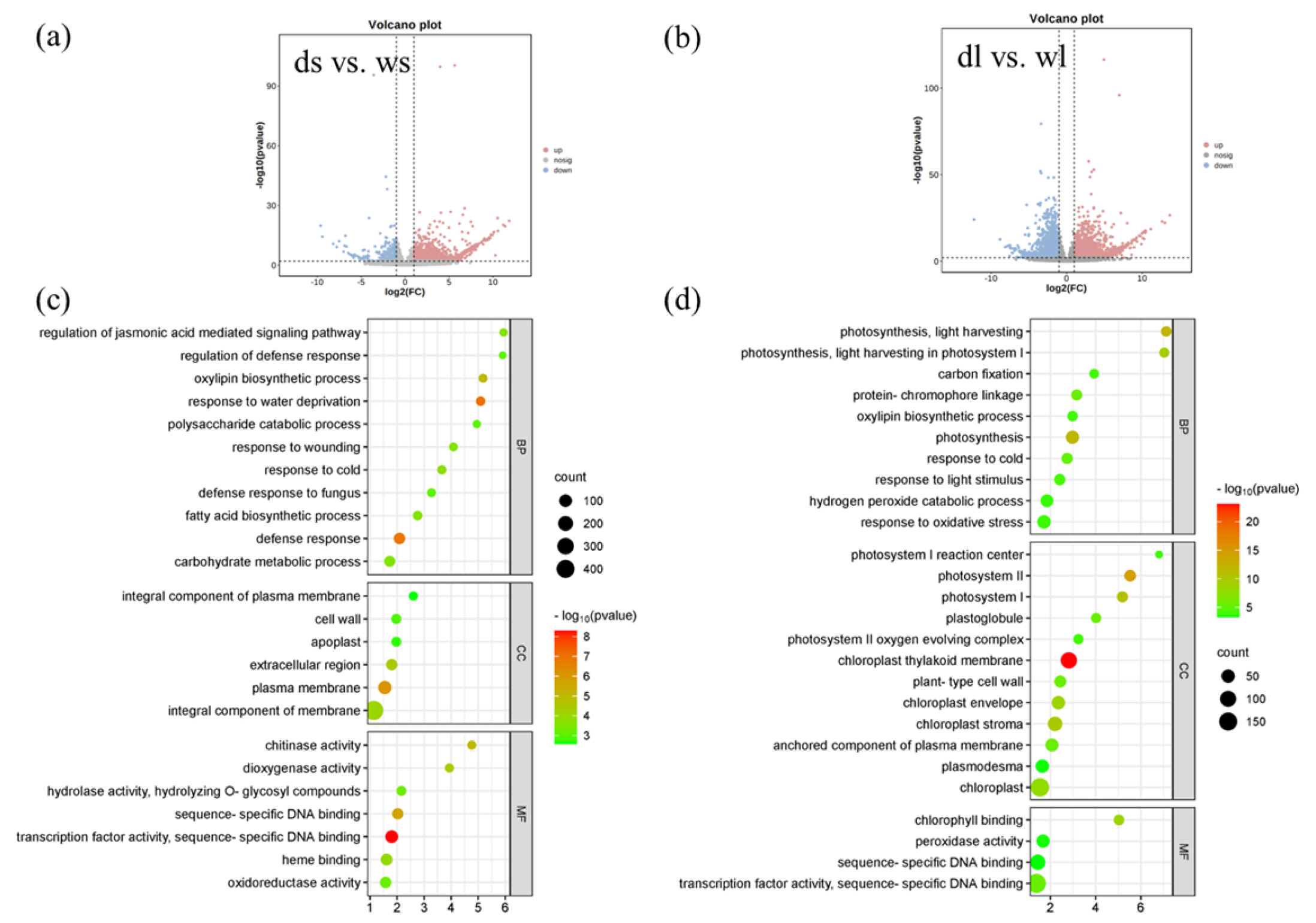
2.3. RNA Seq Analysis
2.4. Sectioning of Rice Tissues
2.5. In Situ Hybridization
2.6. Phylogenetic Tree and Sequences Analysis
3. Results
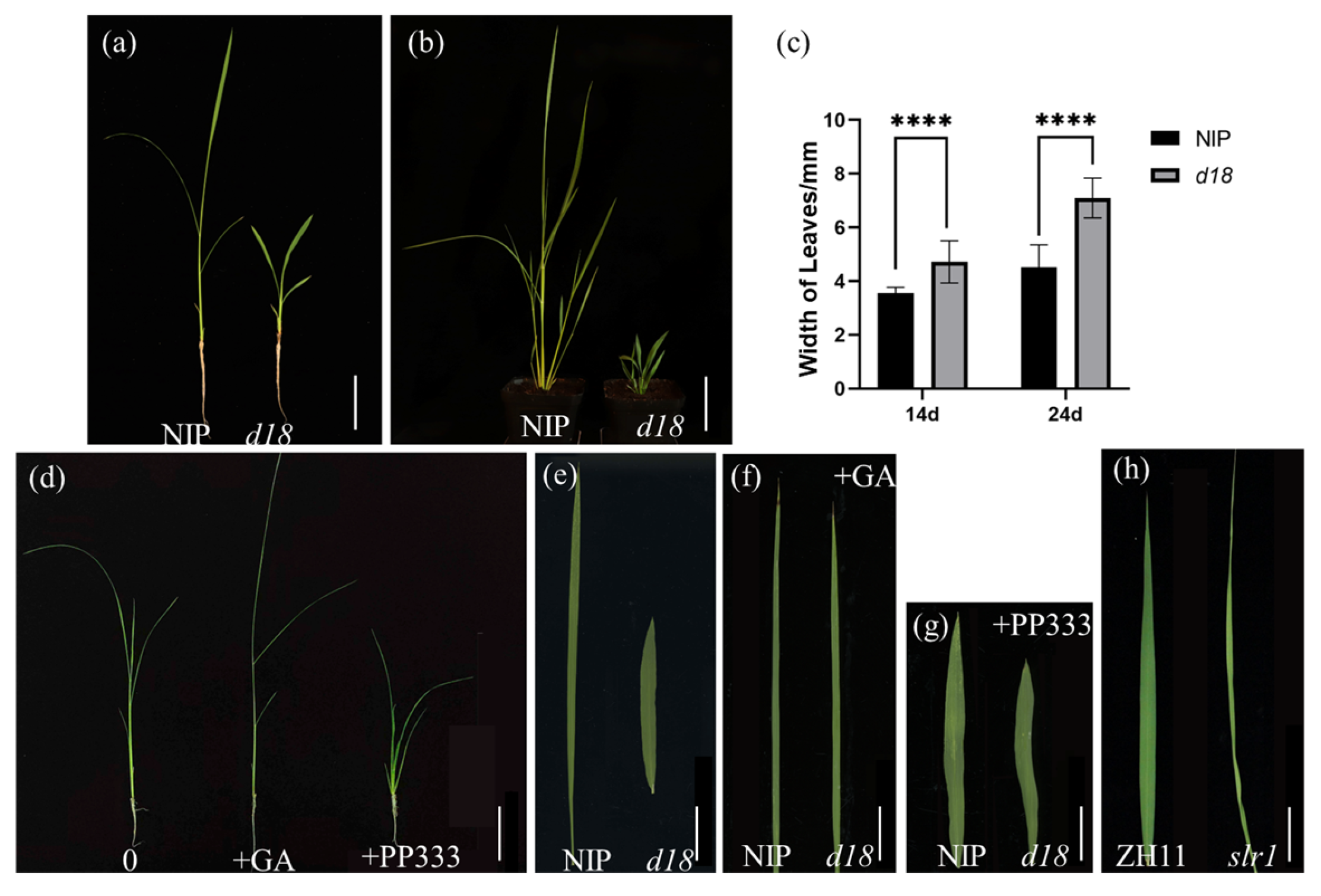
3.1. d18 Has Wider Blades Than NIP
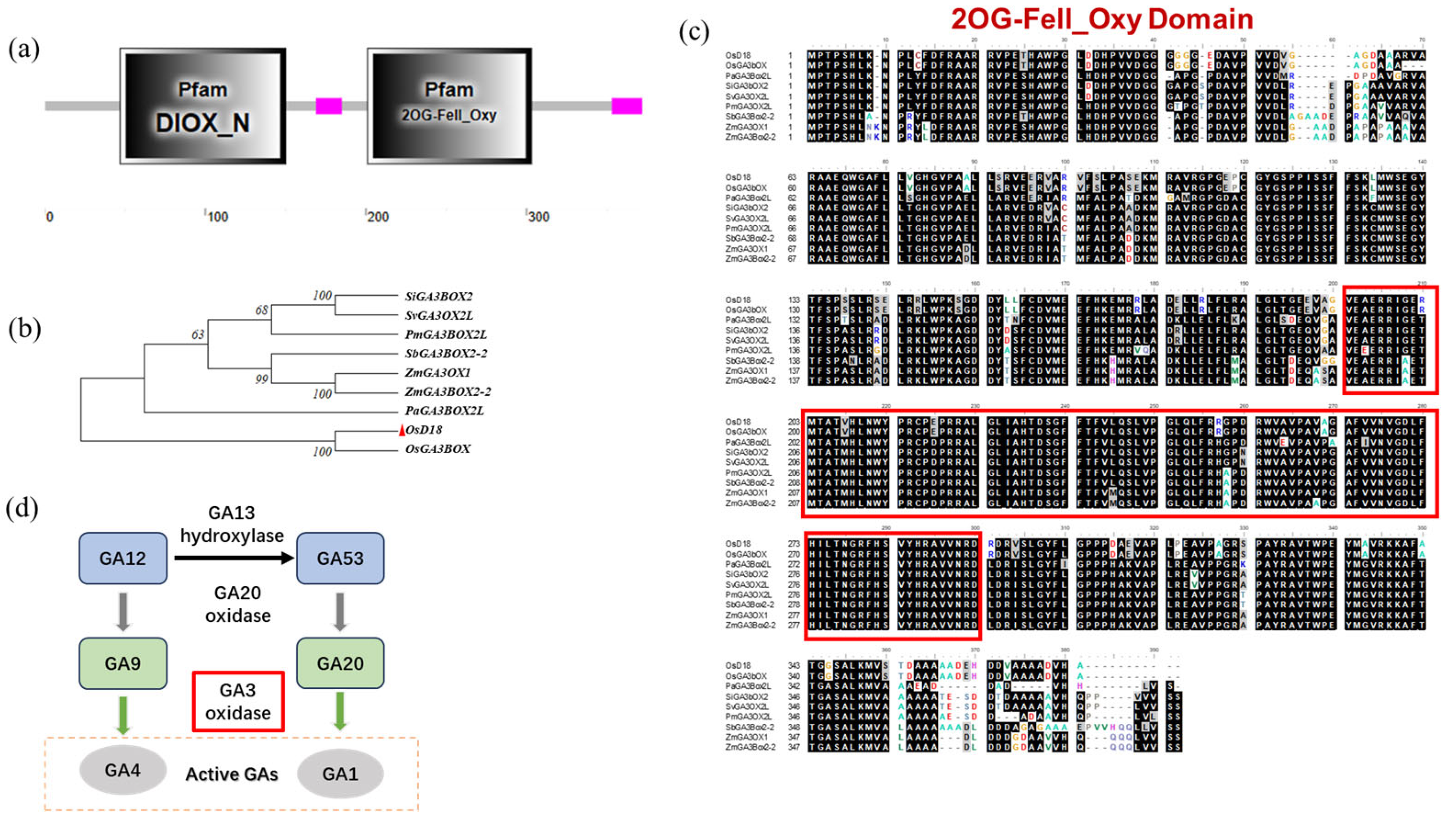
3.2. OsD18 Encodes GA3OX and Participates in the GA Synthesis Process
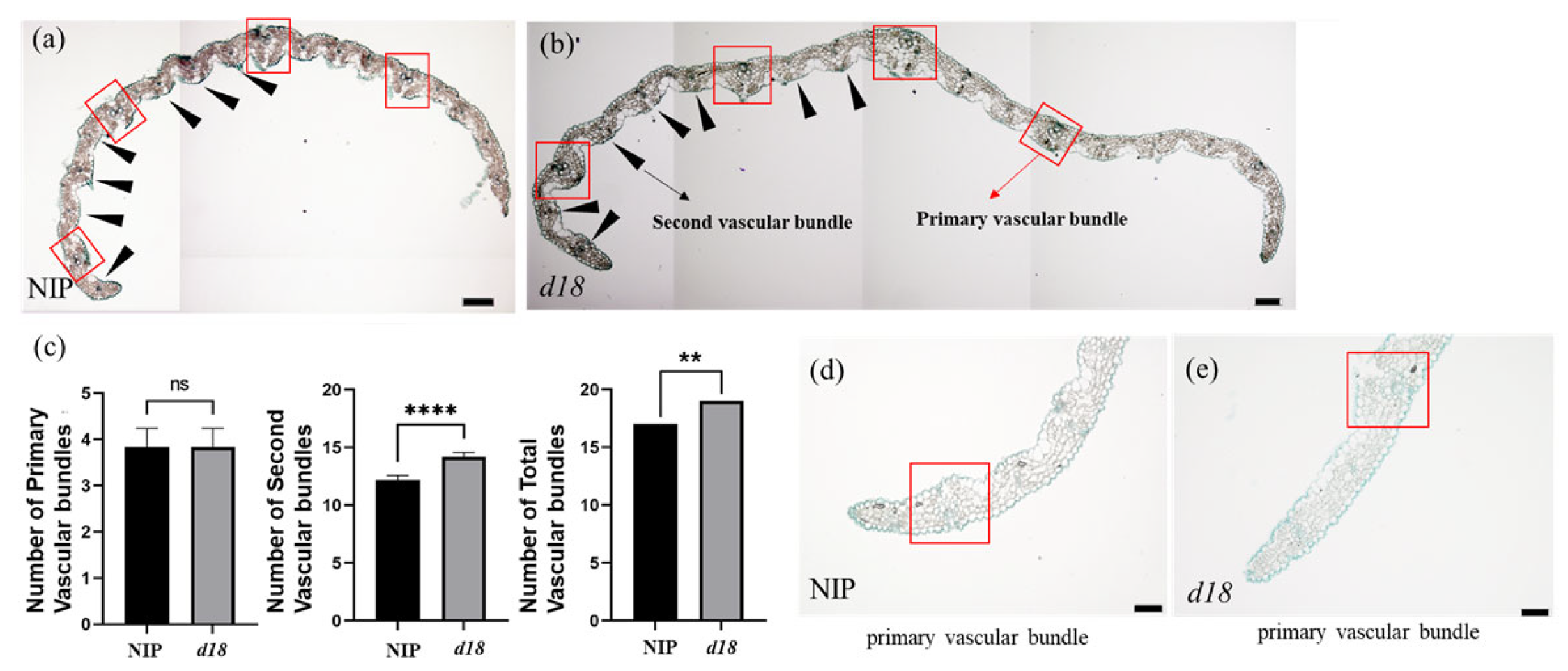
3.3. Cell Morphology Showed That OsD18 Had More Vascular Bundles
3.4. OsD18 Is Highly Expressed at the Margin of Rice Leaves
3.5. RNA-Seq Analysis Showed That Expression of Several Leaf Development Genes and Cell-Cycle-Related Genes Was Altered in d18
3.6. Several Cell-Cycle Related Genes Are More Active at the Margin of d18 Leaves
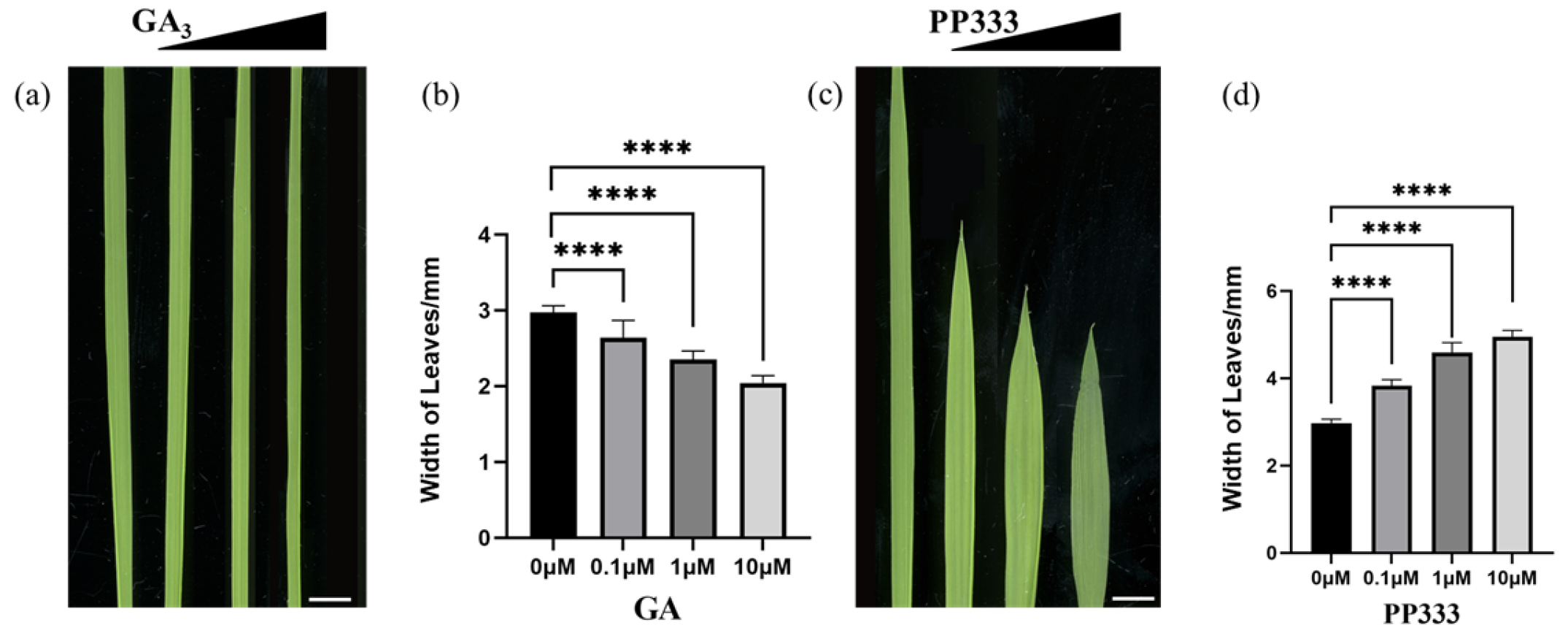
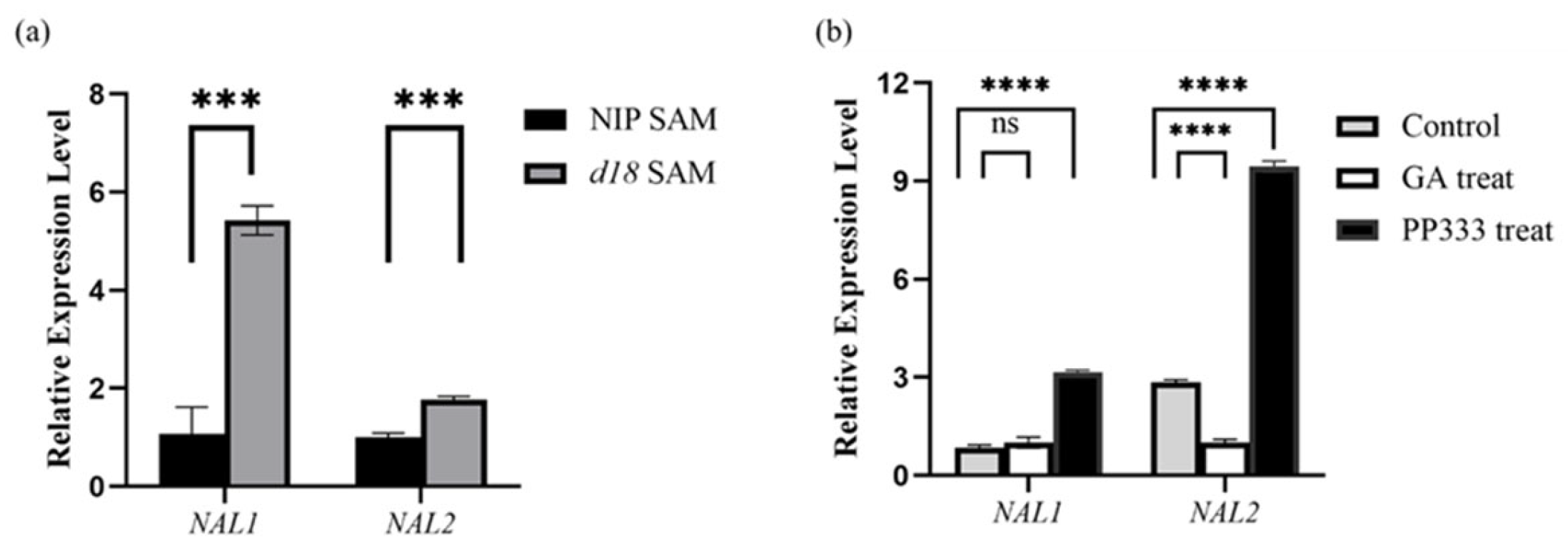
3.7. Leaf Development Marker Gene Expression Regulated by GA
4. Discussion
4.1. Effects of Gibberellins on the Development of Mid-Leaf Rachis
4.2. GAs Regulate Active of Cell Division to Control Leaf Development
4.3. Loss of Gibberellin Changes Genes with Leaf Development Expression Level
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poethig, R.S.; Sussex, I.M. The developmental morphology and growth dynamics of the tobacco leaf. Planta 1985, 165, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.; Nonomura, K.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Kitano, H.; Nagato, Y. Rice plant development: From zygote to spikelet. Plant Cell Physiol. 2005, 46, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Laux, T.; Jurgens, G. Embryogenesis: A New Start in Life. Plant Cell 1997, 9, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Lester, D.R.; Ross, J.J.; Davies, P.J.; Reid, J.B. Mendel’s stem length gene (Le) encodes a gibberellin 3 beta-hydroxylase. Plant Cell 1997, 9, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Ueguchi-Tanaka, M.; Sentoku, N.; Kitano, H.; Matsuoka, M.; Kobayashi, M. Cloning and functional analysis of two gibberellin 3 beta -hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 8909–8914. [Google Scholar] [CrossRef]
- Hsieh, K.T.; Wu, C.C.; Lee, S.J.; Chen, Y.-H.; Shiue, S.-Y.; Liao, Y.-C.; Liu, S.-H.; Wang, I.-W.; Tseng, C.-S.; Li, W.-H.; et al. Rice GA3ox1 modulates pollen starch granule accumulation and pollen wall development. PLoS ONE 2023, 18, e0292400. [Google Scholar] [CrossRef]
- Achard, P.; Genschik, P. Releasing the brakes of plant growth: How GAs shutdown DELLA proteins. J. Exp. Bot. 2009, 60, 1085–1092. [Google Scholar] [CrossRef]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 2005, 15, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Pandeya, D.; Koh, H.; Hwang, J.; Kim, G.; Paek, N. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef]
- Sun, K.; Lu, H.; Fan, F.; Zhang, P.; Liu, G.; Yu, X. Occurrence of Chenopodium quinoa mitovirus 1 in Chenopodium quinoa in China. Plant Dis. 2020, 103, 715. [Google Scholar] [CrossRef]
- Koyama, T.; Sato, F.; Ohme-Takagi, M. Roles of miR319 and TCP Transcription Factors in Leaf Development. Plant Physiol. 2017, 175, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, S.G.; Lee, M.; Lee, I.; Park, H.-Y.; Seo, P.J.; Jung, J.-H.; Kwon, E.-J.; Suh, S.W.; Paek, K.-H.; et al. HD-ZIP III activity is modulated by competitive inhibitors via a feedback loop in Arabidopsis shoot apical meristem development. Plant Cell 2008, 20, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Takahashi, H.; Iwakawa, H.; Nakagawa, A.; Ishikawa, T.; Tanaka, H.; Matsumura, Y.; Pekker, I.; Eshed, Y.; Vial-Pradel, S.; et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 2013, 140, 1958–1969. [Google Scholar] [CrossRef]
- Komaki, S.; Sugimoto, K. Control of the plant cell cycle by developmental and environmental cues. Plant Cell Physiol. 2012, 53, 953–964. [Google Scholar] [CrossRef]
- Wood, D.J.; Endicott, J.A. Structural insights into the functional diversity of the CDK-cyclin family. Open Biol. 2018, 8, 180112. [Google Scholar] [CrossRef]
- Hu, S.; Hu, X.; Hu, J.; Shang, L.; Dong, G.; Zeng, D.; Guo, L.; Qian, Q. Xiaowei, a New Rice Germplasm for Large-Scale Indoor Research. Mol. Plant 2018, 11, 1418–1420. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, S.; Yin, H.; Zhuo, Z.; Meng, G. A comprehensive benchmarking of differential splicing tools for RNA-seq analysis at the event level. Brief Bioinform. 2023, 24, bbad121. [Google Scholar] [CrossRef]
- Mi, X.; Yue, Y.; Tang, M.; An, Y.; Xie, H.; Qiao, D.; Ma, Z.; Liu, S.; Wei, C. TeaAS: A comprehensive database for alternative splicing in tea plants (Camellia sinensis). BMC Plant Biol. 2021, 21, 280. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, A.; Li, Y.; Liu, X.; Jiang, W.; Hu, K. Molecular mechanism of oroxyli semen against triple-negative breast cancer verified by bioinformatics and in vitro experiments. Medicine 2023, 102, e34835. [Google Scholar] [CrossRef]
- Kiernan, J.A. Staining paraffin sections without prior removal of the wax. Biotech. Histochem. 1996, 71, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, T. Single Copy Oligonucleotide Fluorescence In Situ Hybridization Probe Design Platforms: Development, Application and Evaluation. Int. J. Mol. Sci. 2021, 22, 7124. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Ranjan, R.; Parida, S.K.; Agarwal, P.; Tyagi, A.K. Mediator subunit OsMED14_1 plays an important role in rice development. Plant J. 2020, 101, 1411–1429. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhai, L.; Zhu, W.; Qi, X.; Yu, Z.; Wang, H.; Chen, F.; Wang, L.; Chen, S. Characterization of the TCP Gene Family in Chrysanthemum nankingense and the Role of CnTCP4 in Cold Tolerance. Plants 2022, 11, 936. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasui, Y.; Ohmori, Y.; Takebayashi, Y.; Sakakibara, H.; Hirano, H.Y. WUSCHEL-RELATED HOMEOBOX4 acts as a key regulator in early leaf development in rice. PLoS Genet. 2018, 14, e1007365. [Google Scholar] [CrossRef]
- Hao, X.H.; Hu, S.; Zhao, D.; Tian, L.-F.; Xie, Z.-J.; Wu, S.; Hu, W.-L.; Lei, H.; Li, D.-P. OsGA3ox genes regulate rice fertility and plant height by synthesizing diverse active GA. Yi Chuan 2023, 45, 845–855. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, J.; Liu, Y.; Zhang, Q.; Gao, Y.; Fang, K.; Cao, Q.; Qin, L.; Xing, Y. Roles of the GA-mediated SPL Gene Family and miR156 in the Floral Development of Chinese Chestnut (Castanea mollissima). Int. J. Mol. Sci. 2019, 20, 1577. [Google Scholar] [CrossRef]
- Yang, C.; Ma, Y.; Li, J. The rice YABBY4 gene regulates plant growth and development through modulating the gibberellin pathway. J. Exp. Bot. 2016, 67, 5545–5556. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Qian, Q.; Bu, Q.; Li, S.; Chen, Q.; Sun, J.; Liang, W.; Zhou, Y.; Chu, C.; Li, X.; et al. Mutation of the rice Narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiol. 2008, 147, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Kim, J.H.; Lee, H.J.; Kim, D.H.; Yu, J.; Bae, S.; Cho, Y.-G.; Kang, K.K. Generation and Transcriptome Profiling of Slr1-d7 and Slr1-d8 Mutant Lines with a New Semi-Dominant Dwarf Allele of SLR1 Using the CRISPR/Cas9 System in Rice. Int. J. Mol. Sci. 2020, 21, 5492. [Google Scholar] [CrossRef]
- Zheng, T.; Dai, L.; Liu, Y.; Li, S.; Zheng, M.; Zhao, Z.; Qu, G.-Z. Overexpression Populus d-Type Cyclin Gene PsnCYCD1;1 Influences Cell Division and Produces Curved Leaf in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 5837. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yan, M.; Zou, J.; Jiang, M.; Yang, K.; Le, J. A2-type cyclin is required for the asymmetric entry division in rice stomatal development. J. Exp. Bot. 2018, 69, 3587–3599. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Das, A.; Yamaguchi, M.; Hashimoto, J.; Tsutsumi, N.; Uchimiya, H.; Umeda, M. Cell cycle function of a rice B2-type cyclin interacting with a B-type cyclin-dependent kinase. Plant J. 2003, 34, 417–425. [Google Scholar] [CrossRef]
- You, J.; Xiao, W.; Zhou, Y.; Shen, W.; Ye, L.; Yu, P.; Yu, G.; Duan, Q.; Zhang, X.; He, Z.; et al. The APC/CTAD1-WIDE LEAF 1-NARROW LEAF 1 pathway controls leaf width in rice. Plant Cell 2022, 34, 4313–4328. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, S.; Cai, M.; Cui, S.; Ren, Y.; Zhang, X.; Liu, T.; Zhou, C.; Jin, X.; Zhang, L.; et al. ESCRT-III component OsSNF7.2 modulates leaf rolling by trafficking and endosomal degradation of auxin biosynthetic enzyme OsYUC8 in rice. J. Integr. Plant Biol. 2023, 65, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Matsuda, Y.; Ozawa, K.; Nishimura, T.; Koshiba, T.; Fraaije, M.W.; Sekiguchi, H. NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Mol. Genet. Genomics 2008, 279, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.D.; Um, T.Y.; Yang, W.T.; Park, T.-H.; Hong, S.-Y.; Kim, K.-M.; Chung, Y.-S.; Yun, D.-J.; Kim, D.-H. Characterization of dwarf and narrow leaf (dnl-4) mutant in rice. Plant Signal. Behav. 2021, 16, 1849490. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sang, X.; Zhang, T.; Yu, Z.; Li, Y.; Zhao, F.; Wang, Z.; Wang, Y.; Yu, P.; Wang, N.; et al. ABNORMAL VASCULAR BUNDLES regulates cell proliferation and procambium cell establishment during aerial organ development in rice. New Phytol. 2017, 213, 275–286. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, L.; Zeng, D.; Gao, Z.; Guo, L.; Fang, Y.; Zhang, G.; Dong, G.; Yan, M.; Liu, J.; et al. Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol. Biol. 2010, 73, 283–292. [Google Scholar] [CrossRef]




| Primer Name | Type | Sequence (5′-3′) |
|---|---|---|
| Histone H4-F | Forward Primer | ATGTCTGGGAGAGGCAAGGG |
| Histone H4-R | Reverse Primer | TAATACGACTCACTATAGGGAGACTAGCCGCCGAATCCGTAGA |
| CYCB2-F | Forward Primer | ATGGAGAACATGAGATCTGAG |
| CYCB2-R | Reverse Primer | TAATACGACTCACTATAGGGAGATTACAGTGCCACGCTCTT |
| D18-F | Forward Primer | GAGGATCGGCGAGAGGATGA |
| D18-R | Reverse Primer | TAATACGACTCACTATAGGGAGAGAGGAAGTAGCCGAGCGAGA |
| NAL1-qF | Forward Primer | GATGACTTTGACATTTCCACCGT |
| NAL1-qR | Reverse Primer | GAGTGATTCATTGGTAATGATA |
| NAL2-qF | Forward Primer | CCGGAGCAGCTGATGATCC |
| NAL2-qR | Reverse Primer | GGATCATCAGCTGCTCCGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, R.; Guo, X.; Shan, S.; Wang, Q. Gibberellins Regulate Expression of Cyclins to Control Leaf Width in Rice. Agronomy 2024, 14, 1597. https://doi.org/10.3390/agronomy14071597
Zou R, Guo X, Shan S, Wang Q. Gibberellins Regulate Expression of Cyclins to Control Leaf Width in Rice. Agronomy. 2024; 14(7):1597. https://doi.org/10.3390/agronomy14071597
Chicago/Turabian StyleZou, Ruifeng, Xiaoyuan Guo, Siyao Shan, and Quan Wang. 2024. "Gibberellins Regulate Expression of Cyclins to Control Leaf Width in Rice" Agronomy 14, no. 7: 1597. https://doi.org/10.3390/agronomy14071597
APA StyleZou, R., Guo, X., Shan, S., & Wang, Q. (2024). Gibberellins Regulate Expression of Cyclins to Control Leaf Width in Rice. Agronomy, 14(7), 1597. https://doi.org/10.3390/agronomy14071597





