The Origin and Type of Inoculum Determine the Effect of Arbuscular Mycorrhizal Fungi on Tomato under Different Irrigation Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Experiment and Experimental Conditions
2.2. Estimation of Mycorrhizal Variables
2.3. Evaluation of Fruit Quality
2.4. Evaluation of Growth, Development, and Yield
2.5. Statistical Analysis
3. Results
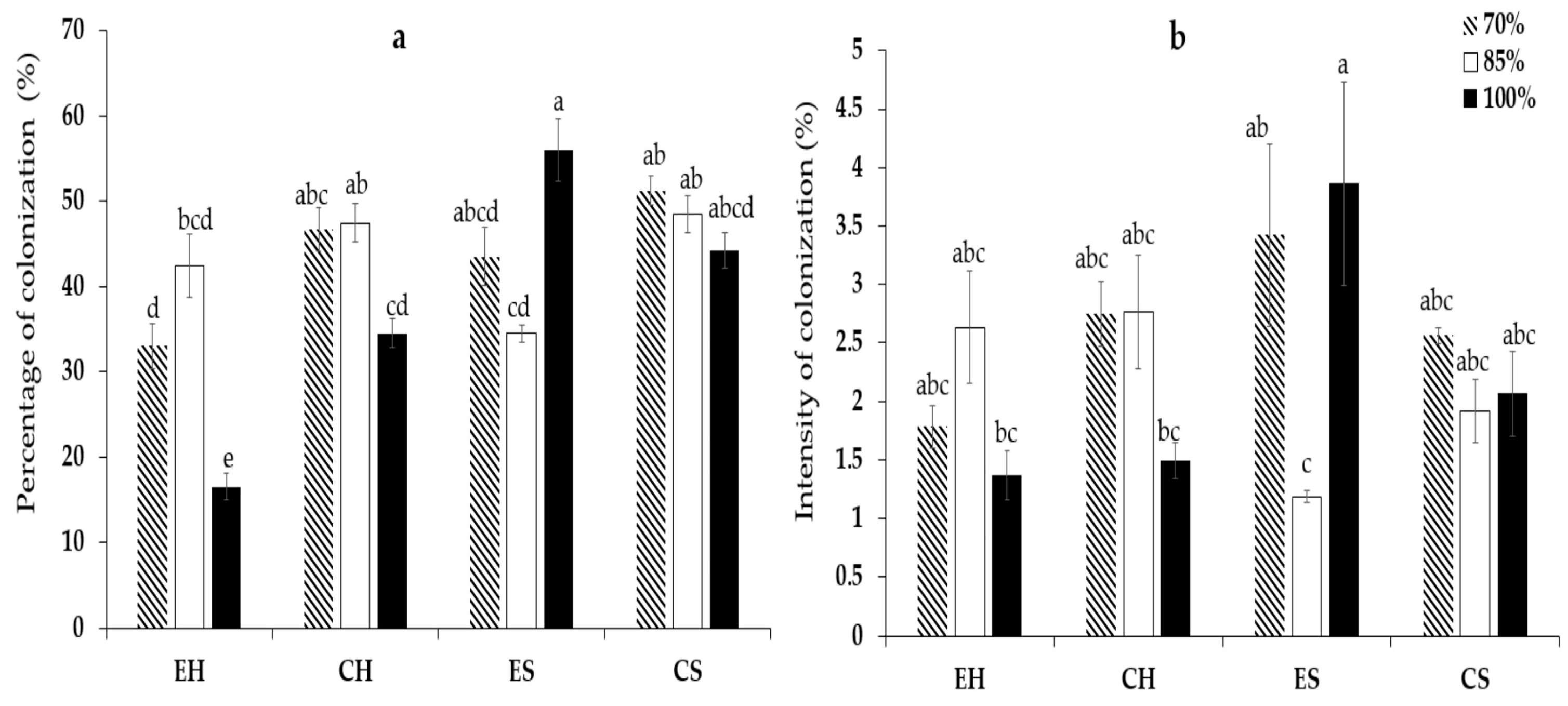
3.1. Mycorrhizal Variables
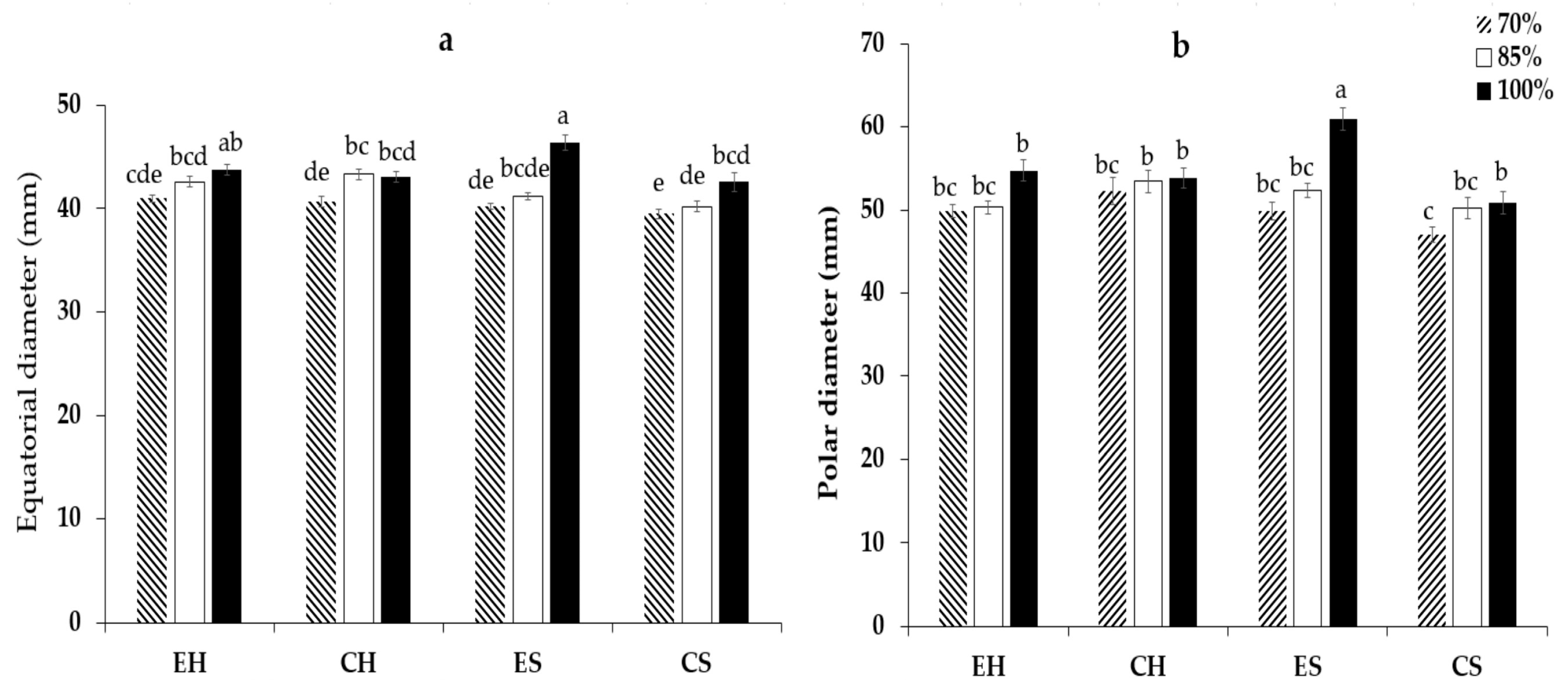
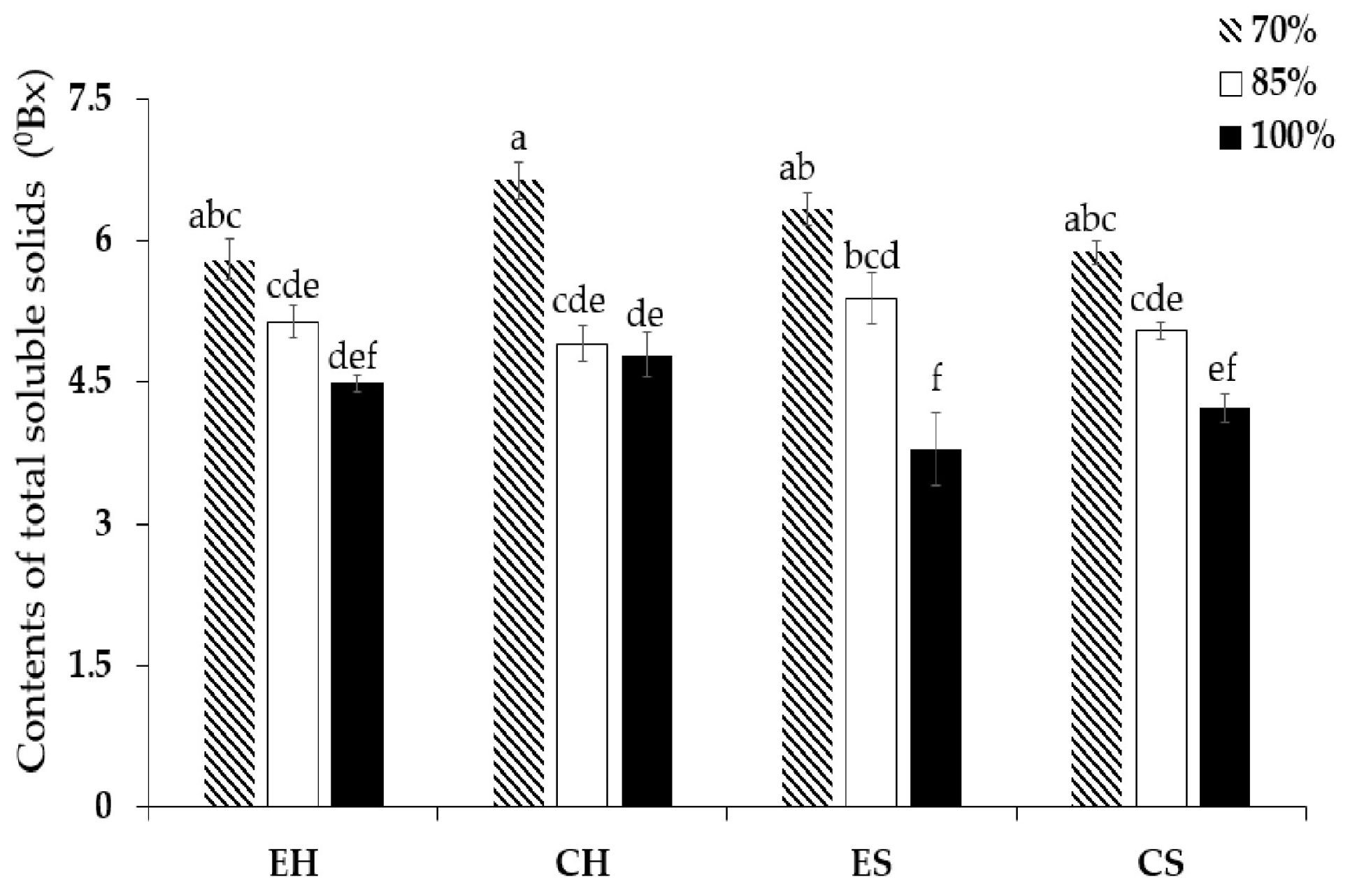
3.2. Fruit Quality
3.3. Growth and Yield
4. Discussion
4.1. Mycorrhizal Variables
4.2. Fruit Quality
4.3. Growth and Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walder, F.; van der Heijden, M.G.A. Regulation of Resource Exchange in the Arbuscular Mycorrhizal Symbiosis. Nat. Plants 2015, 1, 15159. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Anjum, N.A.; Muthukumar, T.; Sridevi, G.; Vasudhevan, P.; Maruthupandian, A. Arbuscular Mycorrhizae: Natural Modulators of Plant-Nutrient Relation and Growth in Stressful Environments. Arch. Microbiol. 2022, 204, 264. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Del Gallo, M.; Mahmoud, K.; Barik, D.; et al. Arbuscular Mycorrhizal Symbiosis: Plant Growth Improvement and Induction of Resistance under Stressful Conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.; Jajoo, A. Arbuscular Mycorrhizal Fungi (AMF) Protects Photosynthetic Apparatus of Wheat under Drought Stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Wu, S.; Shi, Z.; Chen, X.; Gao, J.; Wang, X. Arbuscular Mycorrhizal Fungi Increase Crop Yields by Improving Biomass under Rainfed Condition: A Meta-Analysis. PeerJ 2022, 10, e12861. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. How Harmonious Are Arbuscular Mycorrhizal Symbioses? Inconsistent Concepts Reflect Different Mindsets as Well as Results. New Phytol. 2015, 205, 1381–1384. [Google Scholar] [CrossRef]
- Dey, M.; Ghosh, S. Arbuscular Mycorrhizae in Plant Immunity and Crop Pathogen Control. Rhizosphere 2022, 22, 100524. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- FAO, UN Water. Progress on the Level of Water Stress: Global Status and Acceleration Needs for SDG Indicator 6.4.2; FAO, United Nations Water (UN Water): Rome, Italy, 2021; 95p. [Google Scholar]
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 2020, 101, e02978. [Google Scholar] [CrossRef] [PubMed]
- Biel, C.; Camprubí, A.; Lovato, P.E.; Calvet, C. On-Farm Reduced Irrigation and Fertilizer Doses, and Arbuscular Mycorrhizal Fungal Inoculation Improve Water Productivity in Tomato Production. Sci. Hortic. 2021, 288, 110337. [Google Scholar] [CrossRef]
- Candido, V.; Campanelli, G.; D’Addabbo, T.; Castronuovo, D.; Perniola, M.; Camele, I. Growth and Yield Promoting Effect of Artificial Mycorrhization on Field Tomato at Different Irrigation Regimes. Sci. Hortic. 2015, 187, 35–43. [Google Scholar] [CrossRef]
- Michałojć, Z.; Jarosz, Z.; Pitura, K.; Dzida, K. Effect of Mycorrhizal Colonization and Nutrient Solutions Concentration on the Yielding and Chemical Composition of Tomato Grown in Rockwool and Straw Medium. Acta Sci. Pol. Hortorum Cultus 2015, 14, 15–27. [Google Scholar]
- Bakr, J.; Daood, H.; Pék, Z.; Helyes, L.; Posta, K. Yield and Quality of Mycorrhized Processing Tomato under Water Scarcity. Appl. Ecol. Environ. Res. 2017, 15, 401–413. [Google Scholar] [CrossRef]
- Wahb-allah, M.A.; Abdel-Razzak, H.S.; Alsadon, A.A.; Ibrahim, A.A. Growth, Yield, Fruit Quality and Water Use Efficiency of Tomato under Arbuscular Mycorrhizal Inoculation and Irrigation Level Treatments. Life Sci. J. 2014, 11, 109–117. [Google Scholar]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights On the Impact of Arbuscular Mycorrhizal Symbiosis On Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The Association With Two Different Arbuscular Mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guevara, D.; Rodríguez-Larramendi, L.; Dell’Amico-Rodríguez, J.; Jerez-Mompie, E.; Estrada-Prado, W. Efecto de dos bioestimulantes y hongos micorrízico en plantas de tomate sembradas a altas temperaturas. Cult. Trop. 2018, 39, 41–48. [Google Scholar]
- Preite, L.; Solari, F.; Vignali, G. Technologies to Optimize the Water Consumption in Agriculture: A Systematic Review. Sustainability 2023, 15, 5975. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little Evidence That Farmers Should Consider Abundance or Diversity of Arbuscular Mycorrhizal Fungi When Managing Crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef]
- Sagadin, M.B.; Monteoliva, M.I.; Luna, C.M.; Cabello, M.N. Diversity and Infectivity of Native Arbuscular Mycorrhizal Fungi from Prosopis Alba Areas with Contrasting Edaphoclimatic Characteristics in the Argentinian Parque Chaqueño. AgriScientia 2018, 35, 19–33. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, F.; Sánchez-Gallen, I.; Cuevas, L.; Oro, L.; Meli, P. Diversidad, Abundancia y Variación Estacional En La Comunidad de Hongos Micorrizógenos Arbusculares En La Selva Lacandona, Chiapas, México. Sci. Fungorum 2017, 45, 37–51. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Ortas, I. Mycorrhizas in Fruit Nutrition: Important Breakthroughs; Elsevier: Amsterdam, The Netherlands, 2020; pp. 339–351. [Google Scholar] [CrossRef]
- Šmilauer, P.; Šmilauerová, M.; Kotilínek, M.; Košnar, J. Arbuscular Mycorrhizal Fungal Communities of Forbs and C3 Grasses Respond Differently to Cultivation and Elevated Nutrients. Mycorrhiza 2021, 31, 455–470. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, E.B.; Egerton-Warburton, L.M.; Hart, M.M.; Klironomos, J.N.; Maherali, H.; Tedersoo, L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait based predictive framework. J. Ecol. 2018, 106, 480–489. [Google Scholar] [CrossRef]
- Šmilauer, P.; Košnar, J.; Kotilínek, M.; Pecháčková, S.; Šmilauerová, M. Host Age and Surrounding Vegetation Affect the Community and Colonization Rates of Arbuscular Mycorrhizal Fungi in a Temperate Grassland. New Phytol. 2021, 232, 290–302. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; Alatalo, J.M.; Al-Quraishy, S.; Andriyanova, E.; Anslan, S.; et al. Temperature and pH Define the Realised Niche Space of Arbuscular Mycorrhizal Fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity, 1st ed.; Cornwall: Cornwall, UK, 2004. [Google Scholar]
- Anjos, L.; Gaistard, C.; Deckers, J.; Dondeyne, S.; Eberhardt, E.; Gerasimova, M.; Harms, B.; Jones, A.; Krasilnikov, P.; Reinsch, T.; et al. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; JRC91947; Schad, P., Van Huyssteen, C., Micheli, E., Eds.; FAO: Rome, Italy, 2015. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; CONABIO: Ciudad de México, Mexico, 2004; 97p.
- Rzedowski, J. Vegetation of Mexico, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, Mexico, 2006; 504p.
- Martínez, P.F.; Roca, D. Sustratos Para El Cultivo Sin Suelo. Materiales, Propiedades Y Manejo. In Sustratos, Manejo Del Clima, Automatización Y Control En Sistemas de Cultivo Sin Suelo; Victor, J., Flórez, R., Eds.; Editorial Universidad Nacional de Colombia: Bogotá, Colombia, 2011; pp. 37–77. [Google Scholar]
- Castellanos Ramos, J.Z. Manual de Producción de Tomate En Invernadero; Soluciones Impresas OCMA: Celaya, Mexico, 2009. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Estimation of VA Mycorrhizal Infection Levels. Research for Methods Having a Functional Significance. In Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France, 1–5 July 1985. [Google Scholar]
- Casierra-Posada, F.; Cardozo, M.; Julián, F. Cárdenas-Hernández. Análisis del crecimiento en frutos de tomate (Lycopersicon esculentum Mill.) cultivados bajo invernadero. Agron. Colomb. 2007, 25, 299–305. [Google Scholar]
- Barceló, M.; van Bodegom, P.M.; Tedersoo, L.; den Haan, N.; Veen, G.F.; Ostonen, I.; Trimbos, K.; Soudzilovskaia, N.A. The Abundance of Arbuscular Mycorrhiza in Soils Is Linked to the Total Length of Roots Colonized at Ecosystem Level. PLoS ONE 2020, 15, e0237256. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does Plant—Microbe Interaction Confer Stress Tolerance in Plants: A Review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Thanoon, A.H.; Skwarylo, B.; Patkowska, E.; Mielniczuk, E. Mycorrhizal Inoculation as an Alternative for the Ecological Production of Tomato (Lycopersicon esculentum Mill.). Int. Agrophys. 2020, 34, 253–264. [Google Scholar] [CrossRef]
- Kameoka, H.; Maeda, T.; Okuma, N.; Kawaguchi, M. Structure-Specific Regulation of Nutrient Transport and Metabolism in Arbuscular Mycorrhizal Fungi. Plant Cell Physiol. 2019, 60, 2272–2281. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Loeher, M.; Cavagnaro, T.R. Ecological Intensification and Arbuscular Mycorrhizas: A Meta-Analysis of Tillage and Cover Crop Effects. J. Appl. Ecol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Carrillo, M.; Franco, A.; Río, M. Productividad de Tomate Mediante Micorriza Arbuscular En Agricultura Protegida. Rev. Mex. Cienc. Agric. 2018, 5, 513–518. [Google Scholar] [CrossRef][Green Version]
- Stock, S.C.; Koester, M.; Boy, J.; Godoy, R.; Nájera, F.; Matus, F.; Merino, C.; Abdallah, K.; Leuschner, C.; Spielvogel, S.; et al. Plant Carbon Investment in Fine Roots and Arbuscular Mycorrhizal Fungi: A Cross-Biome Study on Nutrient Acquisition Strategies. Sci. Total Environ. 2021, 781, 146748. [Google Scholar] [CrossRef]
- Chafai, W.; Haddioui, K.; Caid, H.; Labazi, H.; AlZain, M.; Noman, O.; Parvez, M.; Addi, M.; Khalid, A. Impact of Arbuscular Mycorrhizal Fungal Strains Isolated from Soil on the Growth, Yield, and Fruit Quality of Tomato Plants under Different Fertilization Regimens. Horticulturae 2023, 9, 973. [Google Scholar] [CrossRef]
- Yang, H.; Du, T.; Qiu, R.; Chen, J.; Wang, F.; Li, Y.; Wang, C.; Gao, L.; Kang, S. Improved Water Use Efficiency and Fruit Quality of Greenhouse Crops under Regulated Deficit Irrigation in Northwest China. Agric. Water Manag. 2016, 179, 193–204. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit Irrigation and Sustainable Water-Resource Strategies in Agriculture for China’s Food Security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef] [PubMed]
- Nazareno Saparrat, M.C.; Ruscitti, M.F.; Arango, M.C. Micorrizas Arbusculares: Biología y Aplicaciones En El Sector Agroforestal, 1st ed.; Editorial de la Universidad Nacional de La Plata (EDULP): Buenos Aires, Argentina, 2020; 135p. [Google Scholar]
- Florido, M.; Fundora, L. Tolerancia a estrés por déficit hídrico en tomate (Solanum lycopersicum L.). Cult. Trop. 2014, 35, 70–88. [Google Scholar]
| Source Ecosystem | Type of Inoculum | |
|---|---|---|
| Monospecific Inoculum (EH and ES) | Inoculum in Consortium (CH and CS) | |
| Humid ecosystem | Glomus sp. 1 | Claroideoglomus etunicatum (W.N. Becker and Gerd.) C. Walker and A. Schüssler Funneliformis mosseae (T.H. Nicolson and Gerd.) C. Walker and A. Schüssler Glomus sp. 1 Glomus rubiforme (Gerd. and Trappe) R.T. Almeida and N.C. Schenck |
| Semi-arid ecosystem | Claroideoglomus etunicatum | Acaulospora morrowiae Spain and N.C. Schenck Claroideoglomus etunicatum Glomus macrocarpum Tul. and C. Tul. |
| Irrigation Dose | Water Volume (mL/Day) |
|---|---|
| 100% | 830.2 ± 53 |
| 85% | 706 ± 45.1 |
| 70% | 581.8 ± 37.2 |
| Sources of Variation | ANOVA | |
|---|---|---|
| Colonization (%) | Intensity of Colonization (%) | |
| AMF inoculums | 0.001 *** | 0.142 ns |
| Dose of irrigation | 0.313 ns | 0.099 ns |
| Interaction | 0.001 *** | 0.001 *** |
| SE | 1.60 | 0.15 |
| Sources of Variation | ANOVA | ||
|---|---|---|---|
| Equatorial Diameter (mm) | Polar Diameter (mm) | TSS (°Bx) | |
| AMF inoculums | 0.001 *** | 0.001 *** | 0.117 ns |
| Dose of irrigation | 0.001 *** | 0.001 *** | 0.001 *** |
| Interaction | 0.001 *** | 0.001 ** | 0.004 *** |
| SE | 0.23 | 0.46 | 0.10 |
| ANOVA | ||||
|---|---|---|---|---|
| Sources of Variation | Plant Height (cm) | Stem Diameter (mm) | Yield (g/Plant) | Quantity (Fruits/Plant) |
| AMF inoculums | 0.009 ** | 0.593 ns | 0.822 ns | 0.892 ns |
| Dose of irrigation | 0.500 ns | 0.001 *** | 0.001 *** | 0.001 *** |
| Interaction | 0.985 ns | 0.547 ns | 0.453 ns | 0.201 ns |
| SE | 0.23 | 0.01 | 46.9 | 0.87 |
| Independent Factors | ANOVA | |||
|---|---|---|---|---|
| AMF Inoculums | Plant Height (cm) | Stem Diameter (mm) | Yield (g/Plant) | Quantity (Fruits/Plant) |
| EH | 65.2 b | 1.73 | 2213.4 | 64.8 |
| CH | 65.3 b | 1.74 | 2228.6 | 64.5 |
| ES | 68.9 a | 1.75 | 2243.7 | 63.44 |
| CS | 69.2 a | 1.71 | 2193.3 | 63.44 |
| Dose of irrigation | ||||
| 70% | 66.3 | 1.67 b | 1730.6 c | 61.2 c |
| 85% | 67.6 | 1.74 a | 2220.9 b | 63.4 b |
| 100% | 67.7 | 1.79 a | 2707.7 a | 67.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-Echevarría, A.; Ramírez-Tobias, H.M.; Méndez-Cortés, H.; Rojas-Velázquez, Á.N.; López-Palacios, C.; Hipólito-Piedras, R.P. The Origin and Type of Inoculum Determine the Effect of Arbuscular Mycorrhizal Fungi on Tomato under Different Irrigation Regimes. Agronomy 2024, 14, 1687. https://doi.org/10.3390/agronomy14081687
Mena-Echevarría A, Ramírez-Tobias HM, Méndez-Cortés H, Rojas-Velázquez ÁN, López-Palacios C, Hipólito-Piedras RP. The Origin and Type of Inoculum Determine the Effect of Arbuscular Mycorrhizal Fungi on Tomato under Different Irrigation Regimes. Agronomy. 2024; 14(8):1687. https://doi.org/10.3390/agronomy14081687
Chicago/Turabian StyleMena-Echevarría, Aracely, Hugo M. Ramírez-Tobias, Heriberto Méndez-Cortés, Ángel Natanael Rojas-Velázquez, Cristian López-Palacios, and Reyna P. Hipólito-Piedras. 2024. "The Origin and Type of Inoculum Determine the Effect of Arbuscular Mycorrhizal Fungi on Tomato under Different Irrigation Regimes" Agronomy 14, no. 8: 1687. https://doi.org/10.3390/agronomy14081687
APA StyleMena-Echevarría, A., Ramírez-Tobias, H. M., Méndez-Cortés, H., Rojas-Velázquez, Á. N., López-Palacios, C., & Hipólito-Piedras, R. P. (2024). The Origin and Type of Inoculum Determine the Effect of Arbuscular Mycorrhizal Fungi on Tomato under Different Irrigation Regimes. Agronomy, 14(8), 1687. https://doi.org/10.3390/agronomy14081687





