Genome-Wide Identification and Expression Analysis of Heat Shock Protein 20 (HSP20) Gene Family in Response to High-Temperature Stress in Chickpeas (Cicer arietinum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the HSP20 Gene Family Members in Chickpeas
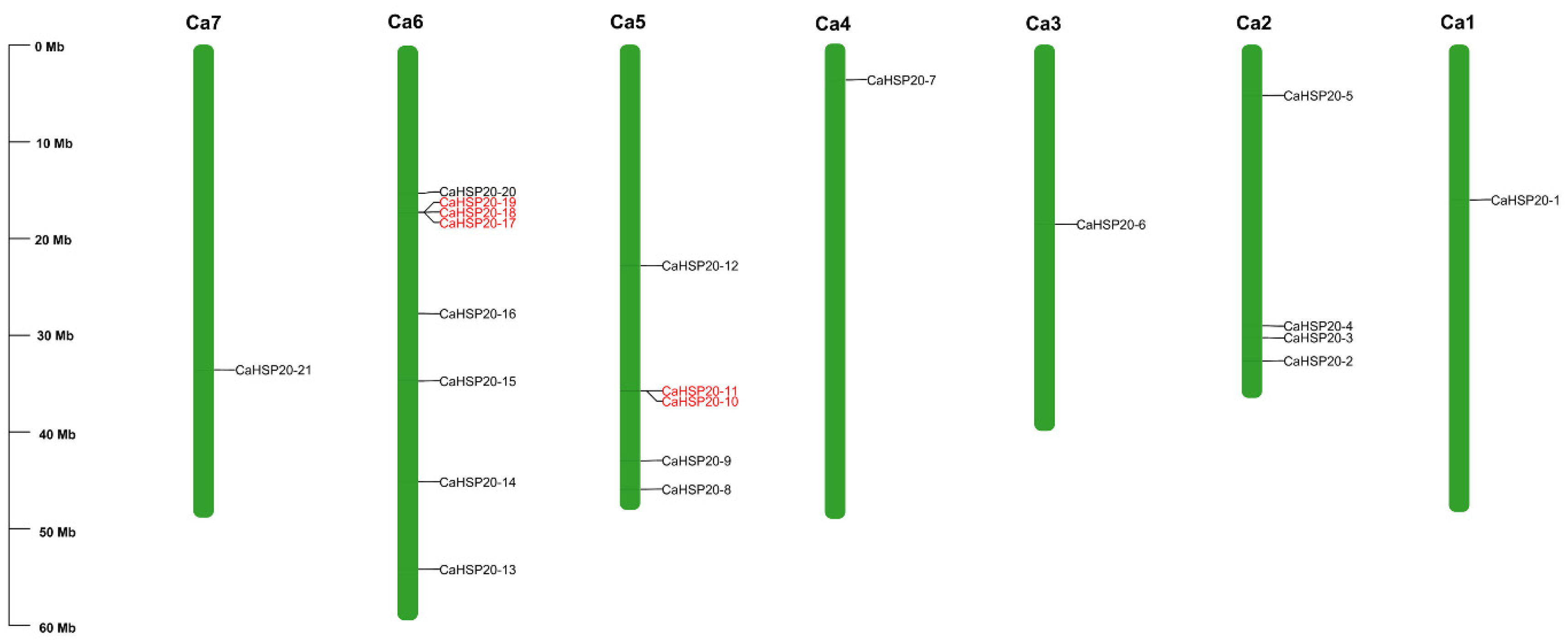
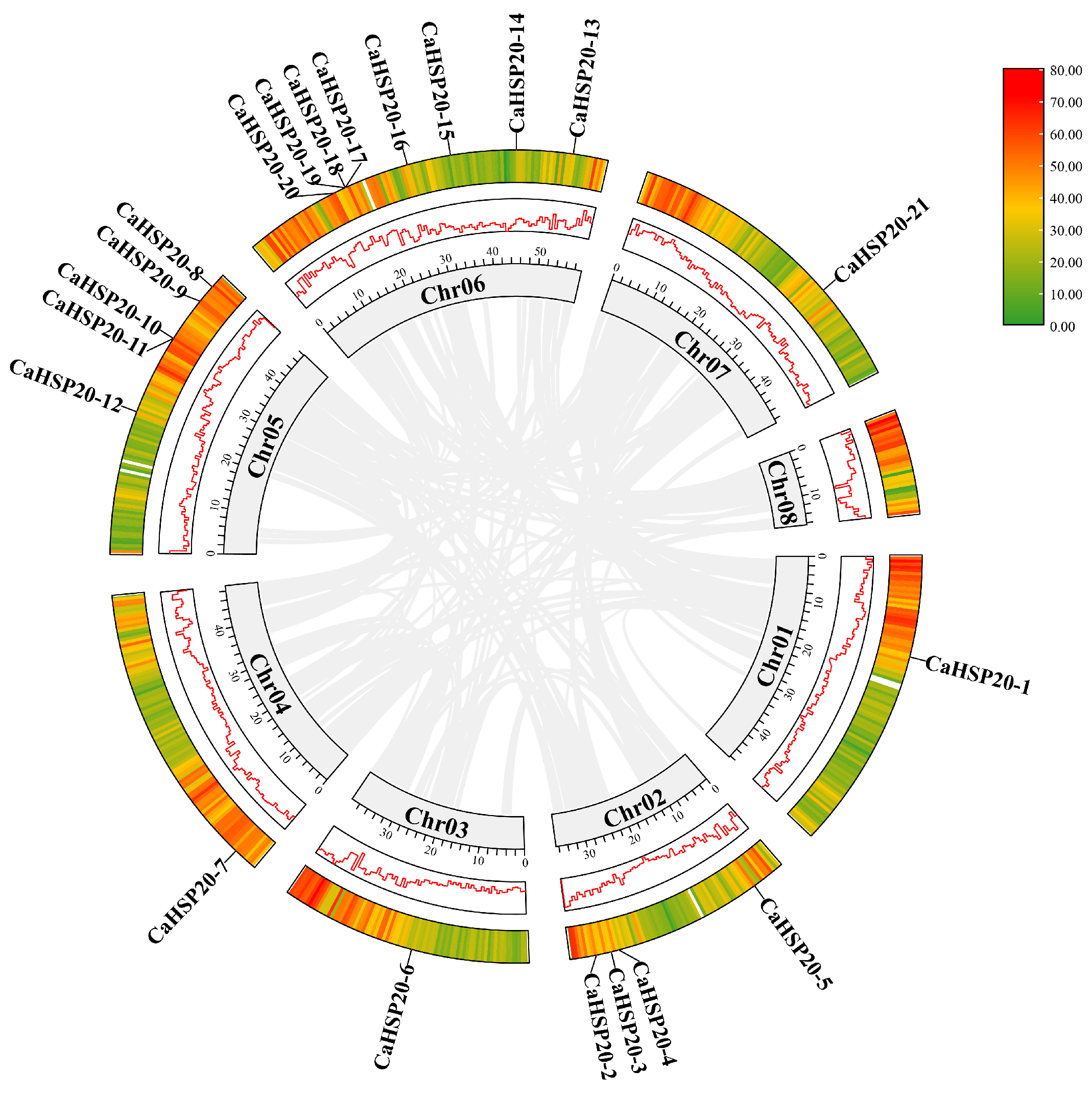
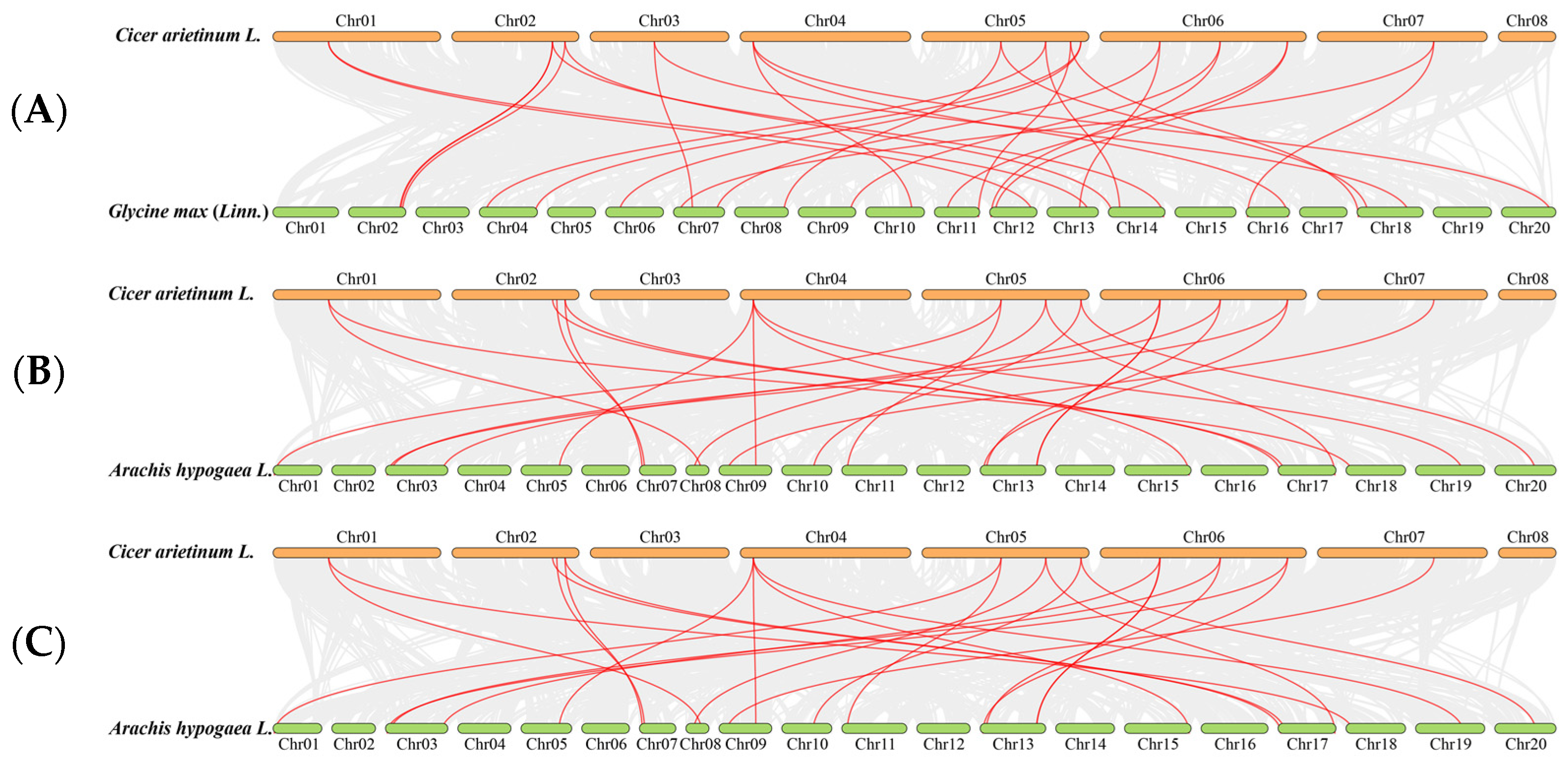
2.2. Chromosomal Distribution, Gene Duplication Event, Synteny Analyses, Gene Structure, and the Conserved Domain of CaHSP20s
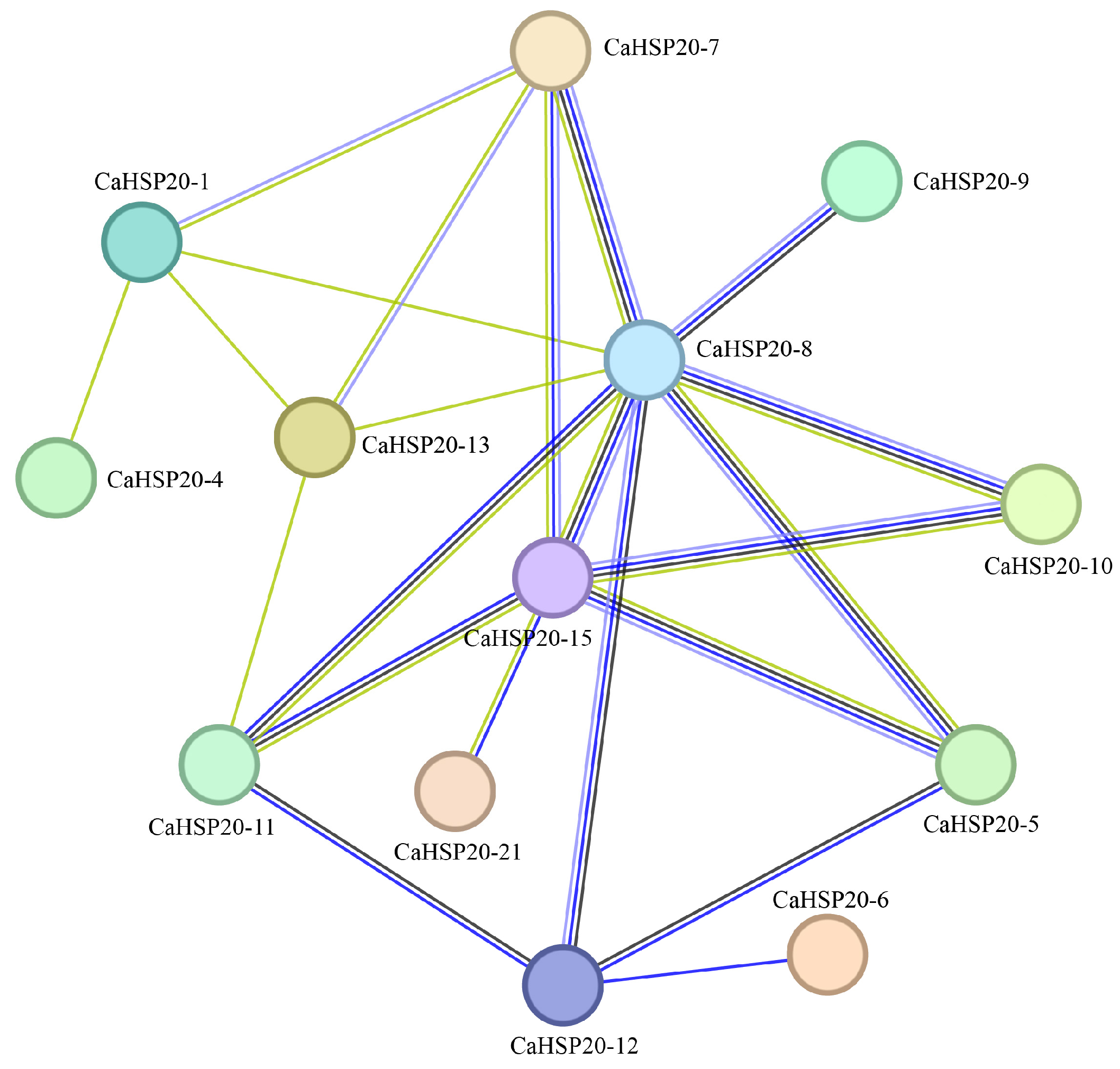
2.3. Protein–Protein Interaction Network of CaHSP20s
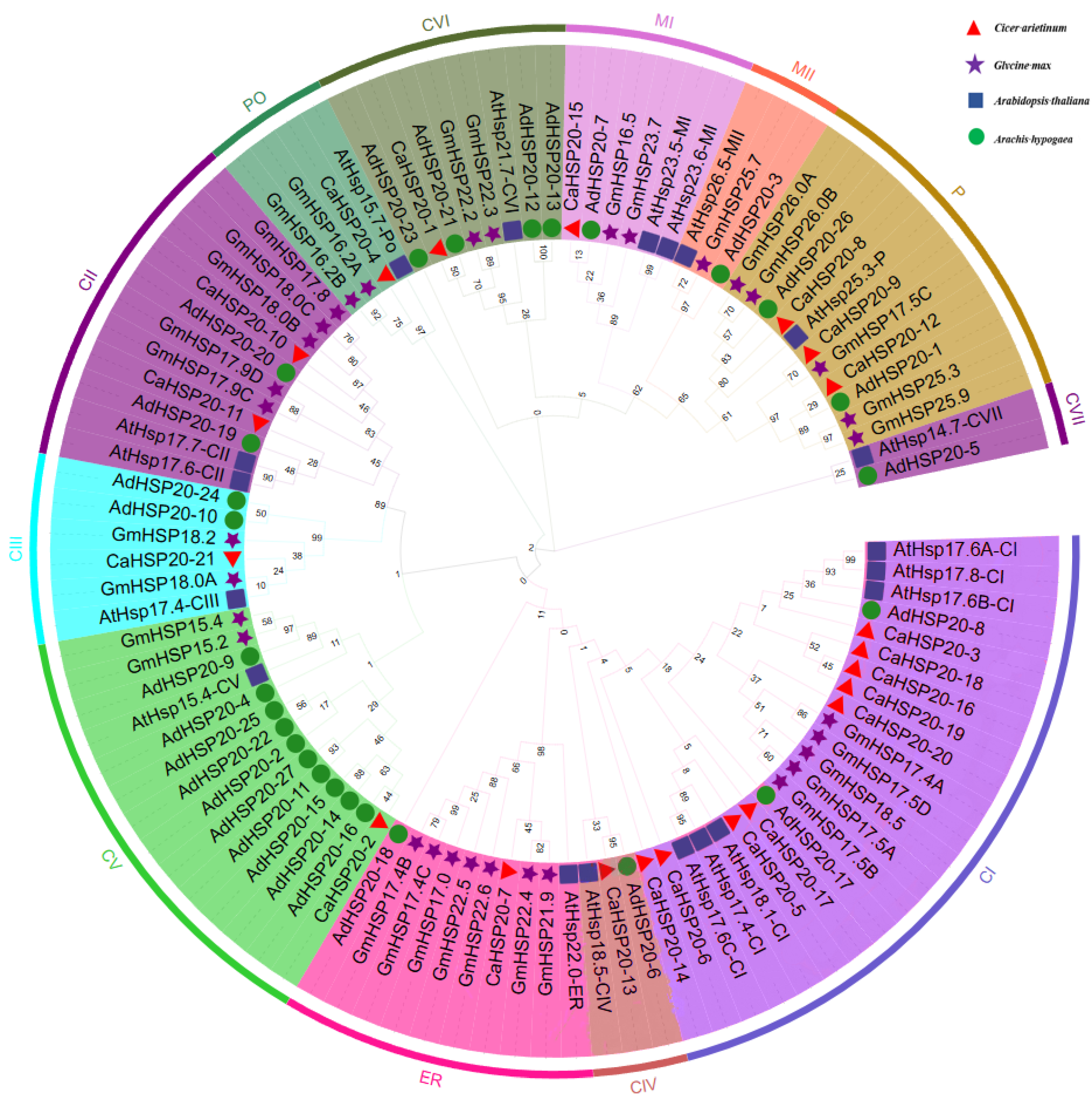
2.4. Phylogenetic Analysis of HSP20s
2.5. Prediction of Promoter Cis-Elements of CaHSP20s
2.6. The Analysis of Tissue Expression Profile for CaHSP20s Genes
2.7. Plant Materials and Growth Conditions
2.8. RNA Isolation and Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Identification and Characterization of CaHSP20s Gene Family
3.2. Chromosome Location, Gene Duplication Event, and Synteny Analyses of CaHSP20s Family
3.3. Construction of the Protein Interaction Networks of the CaHSP20s Family
3.4. Phylogenetic Analysis of the CaHSP20s Gene Family in Chickpeas
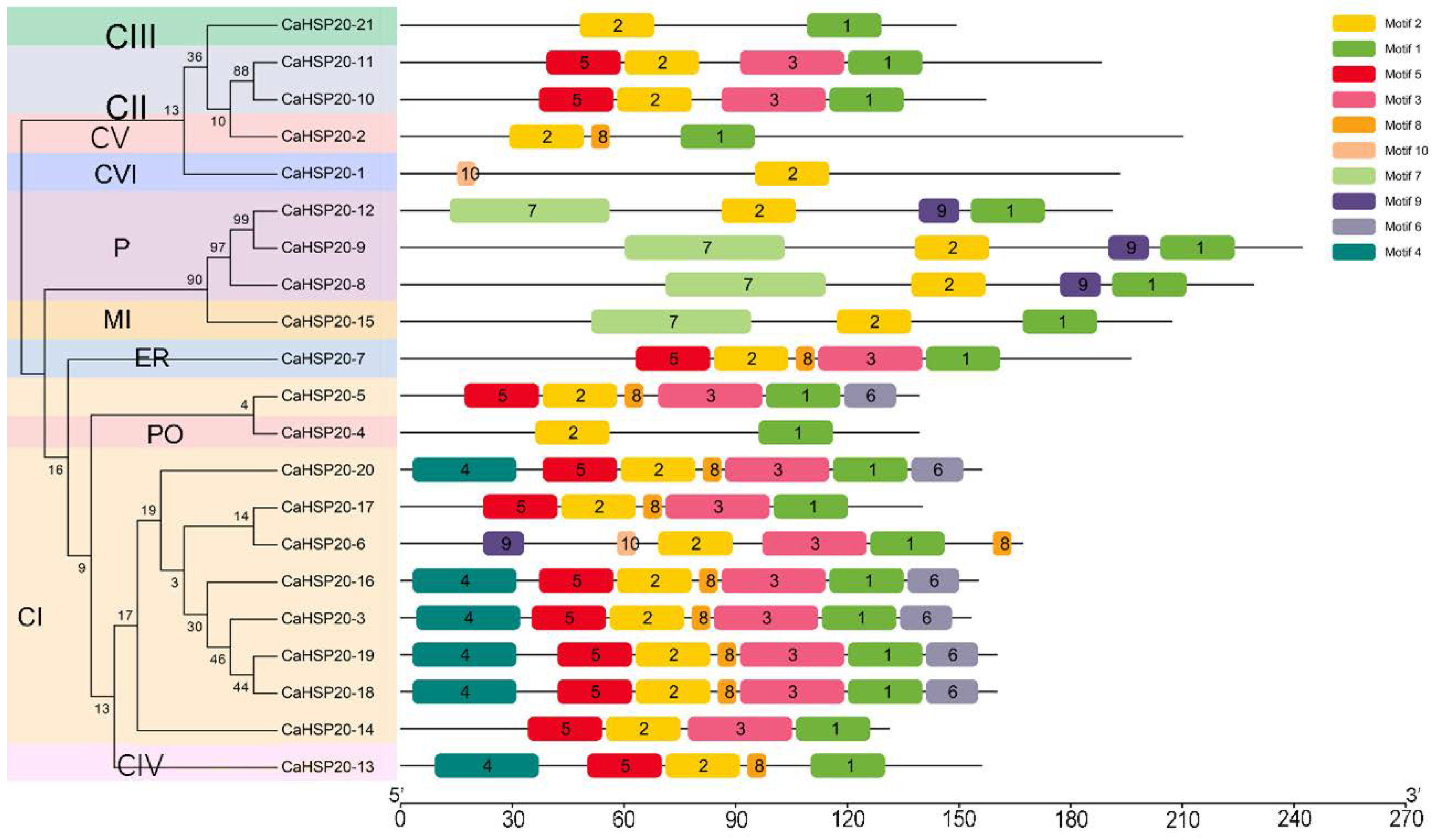
3.5. Gene Structure, Conserved Motifs, and Sequence Analysis of CaHSP20s Family
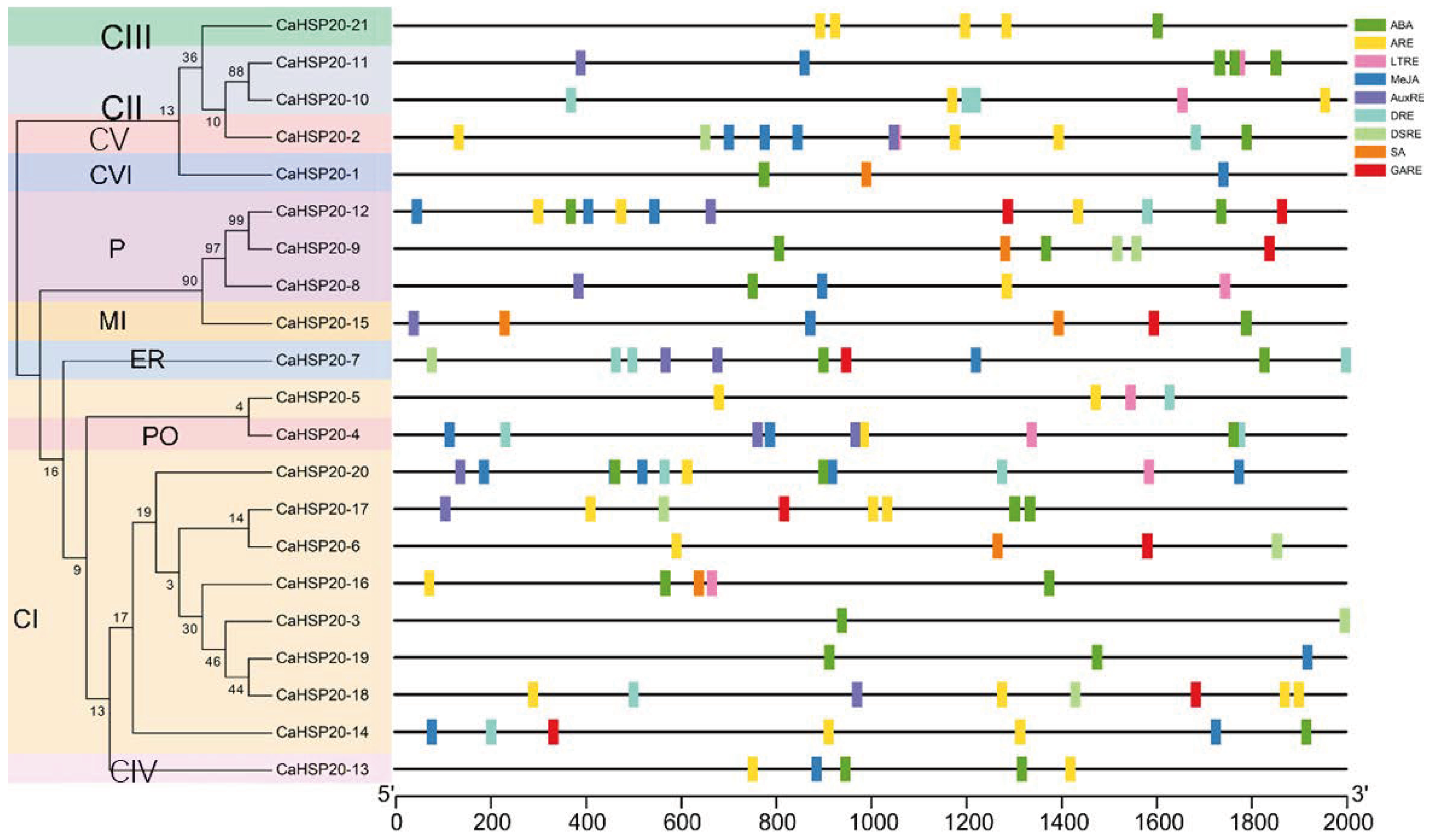
3.6. Promoter Analysis of CaHSP20s Genes
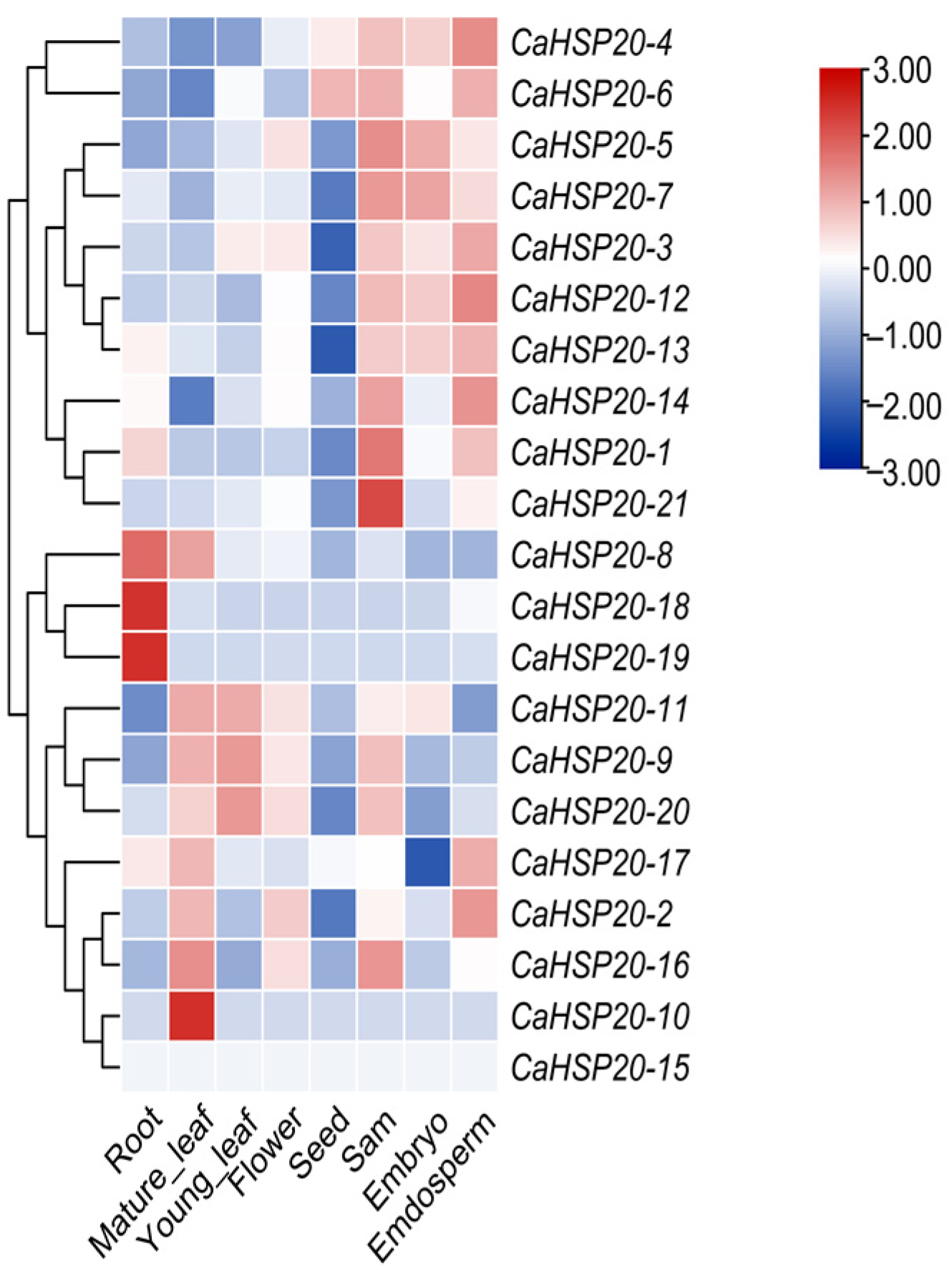
3.7. Tissue-Specific Expression Patterns of CaHSP20s Genes
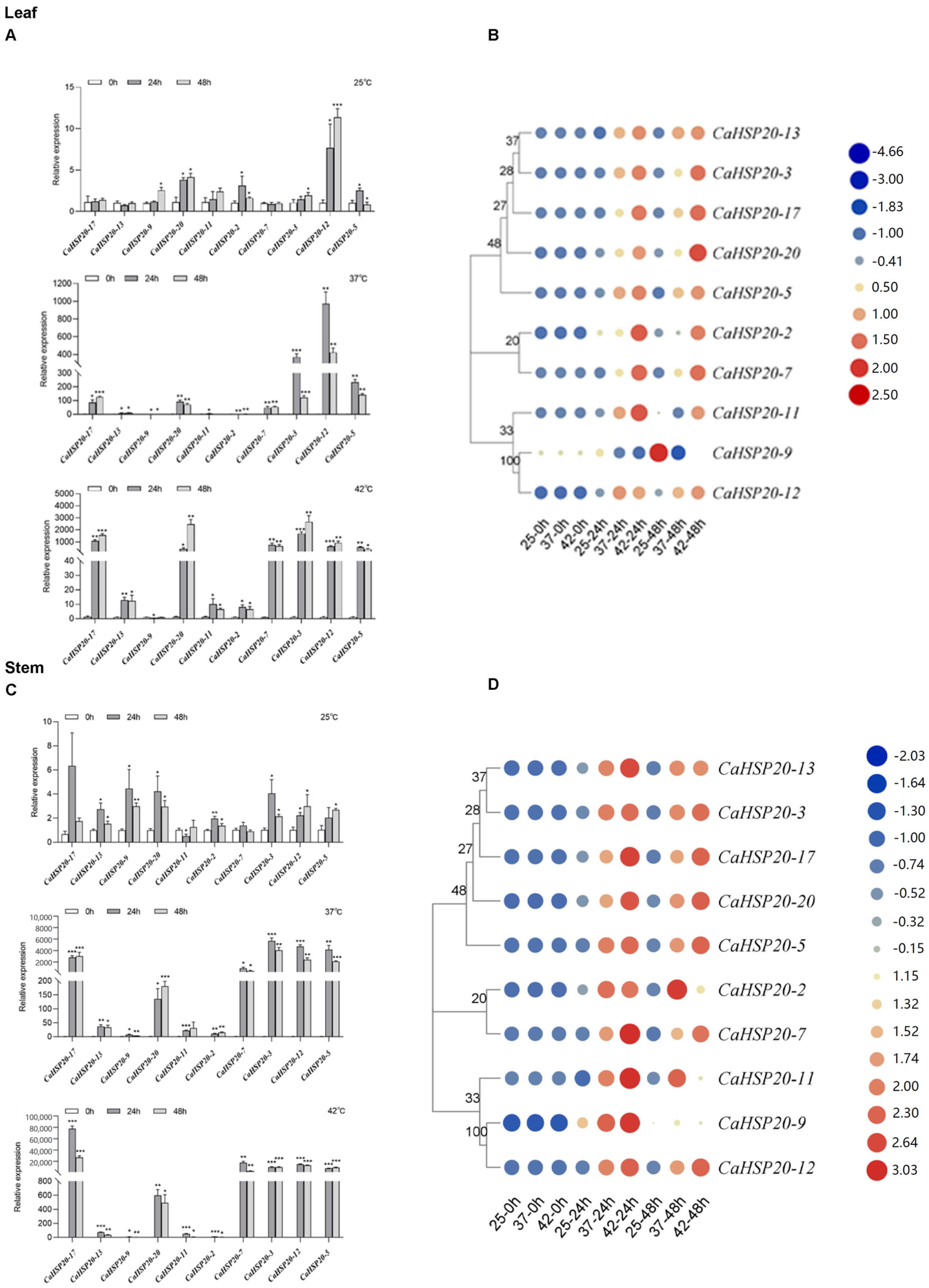
3.8. Expression Profiling of CaHSP20s Genes under Heat Stress Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell. 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Guo, Q.; Li, Q.; Liu, P.; Liang, G.; Lu, W.; Liu, Z.; Wu, H.; Lin, J.; Gu, C.; et al. Comparative transcriptomics analysis and functional study reveal important role of high-temperature stress response gene GmHSFA2 during flower bud development of CMS-based F1 in soybean. Front. Plant Sci. 2020, 11, 600217. [Google Scholar] [CrossRef]
- Celik, E.N.Y.; Baloglu, M.C.; Ayan, S. Gene expression profiles of Hsp family members in different poplar taxa under cadmium stress. Turk. J. Agric. For. 2021, 45, 102–110. [Google Scholar]
- Xiang, J.; Chen, X.; Hu, W.; Xiang, Y.; Yan, W.; Zhang, S.; Guo, Z. Overexpressing heat-shock protein OsHSP50.2 improves drought tolerance in rice. Plant Cell Rep. 2018, 37, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Lu, Q.; Wen, X.; Lu, C. The combined effect of salt stress and heat shock on proteome profiling in Suaeda salsa. J. Plant Physiol. 2011, 168, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.T.; Oguchi, T.; Matsunaga, E.; Tanaka, S.; Shimizu, S.; Ueda, M.; Kawamoto, H.; Sato, H.; Sano, Y.; Matsumoto, K. Transcriptional enhancement of a bacterial choline oxidase A gene by an HSP terminator improves the glycine betaine production and salinity stress tolerance of Eucalyptus camaldulensis trees. Plant Biotechnol. 2018, 35, 215–224. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins–evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef]
- Mahmood, T.; Safdar, W.; Abbasi, B.H.; Ahmad, T.; Abbasi, R. An overview on the small heat shock proteins. Afr. J. Biotechnol. 2010, 9, 927–939. [Google Scholar]
- Carra, S.; Alberti, S.; Benesch, J.L.P.; Boelens, W.; Buchner, J.; Carver, J.A.; Ecroyd, H.; Gusev, N.; Hohfeld, J.; Kampinga, H.H.; et al. Small heat shock proteins: Multifaceted proteins with important implications for life. Cell Stress Chaperones. 2019, 24, 295–308. [Google Scholar] [CrossRef]
- Pandey, B.; Kaur, A.; Gupta, O.P.; Shukla, R.; Sharma, I. Identification of HSP20 gene family in wheat and barley and their differential expression profiling under heat stress. Appl. Biochem. Biotechnol. 2015, 175, 2427–2446. [Google Scholar] [CrossRef]
- Ji, X.R.; Yu, Y.H.; Ni, P.Y.; Zhang, G.H.; Xu, G.H.; Zhang, Y.; Wang, X.F.; He, L.L.; Li, X.G. Genome-wide identification of small heat-shock protein (Hsp20) gene family in grape and expression profile during berry development. BMC Plant Biol. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; Palma, J.M.; Corpas, F.J. Small Heat Shock Protein (sHSP) Gene Family from Sweet Pepper (Capsicum annuum L.) Fruits: Involvement in Ripening and Modulation by Nitric Oxide (NO). Plants 2023, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, G.; Singh, A.; Kaur, P.; Pareek, A.; Singh, P.; Sharma, S.; Yadav, G.; Sharma, N.K.; Pareek, A. Genome wide identification and characterization of small heat shock protein gene family in pigeonpea and their expression profiling during abiotic stress conditions. Int. J. Biol. Macromol. 2022, 197, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Guo, W.L.; Li, B.Y.; Zhang, Y.; Wang, X.J.; Guo, J.K.; Huang, J.Z.; Zhang, X.L. Genome-wide identification, classification, and expression analysis of sHSP genes in Chinese cabbage (Brassica rapa ssp pekinensis). Genet. Mol. Res. 2015, 14, 11975–11993. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Yang, J.; Guo, C.; Chen, X.; Zhang, M.; Zhang, H.; Liu, X.; Xiao, Y.; Wang, Y. Genome-wide identification and classification of the Hsf and sHsp gene families in Prunus mume, and transcriptional analysis under heat stress. Peer J. 2019, 7, e7312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Jiang, C.; Liu, Y.; Zhang, C.; Li, L.; Qian, Y.; Xue, Y. Isolation of the Chinese rose sHSP gene promoter and its differential regulation analysis in transgenic Arabidopsis plants. Mol. Biol. Rep. 2012, 39, 1145–1151. [Google Scholar] [CrossRef]
- Khaskheli, G.B.; Zuo, F.; Yu, R.; Chen, S. Overexpression of small heat shock protein enhances heat-and salt-stress tolerance of bifidobacterium longum NCC2705. Curr. Microbiol. 2015, 71, 8–15. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Aranda, M.A.; Peng, B.; Gu, Q. Expression and localization patterns of a small heat shock protein that interacts with the helicase domain of cucumber green mottle mosaic virus. Phytopathology 2019, 109, 1648–1657. [Google Scholar] [CrossRef]
- Jiang, C.; Bi, Y.; Li, M.; Zhang, R.; Feng, S.; Ming, F. A small heat shock protein gene (RcHSP17.8) from Chinese rose confers resistance to various abiotic stresses in transgenic tobacco. Plant Cell Tissue Organ Cult. 2020, 141, 407–415. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, H.; Wang, H.; Wei, T.; Che, S.; Zhang, L.; Hu, B.; Long, H.; Song, W.; et al. Identification of MsHsp20 gene family in Malus sieversii and functional characterization of MsHsp16.9 in heat tolerance. Front. Plant Sci. 2017, 8, 1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Dong, J.; Tian, X.; Wang, J.; Palta, J.A.; Xu, S.; Fang, Y.; Wang, Z. Over-expression of the heat-responsive wheat gene TaHSP23.9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Front. Plant Sci. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Sari, H.; Uhdre, R.; Wallace, L.; Abraham, R.; Nguyen, T.; Sharma, S.; Kumar, A.; Bansal, S.; Singh, R.; Gupta, A. Genome-wide association study in Chickpea (Cicer arietinum L.) for yield and nutritional components. Euphytica 2024, 220, 84. [Google Scholar] [CrossRef]
- Saget, S.; Costa, M.; Barilli, E.; Barros, A.; Benn, T.; Amaral, L.; Ulbricht, C.; Coussa, E.; MacDonald, B. Substituting wheat with chickpea flour in pasta production delivers more nutrition at a lower environmental cost. Sustain. Prod. Consum. 2020, 24, 26–38. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Christodoulou, V. Chickpeas (Cicer arietinum L.) in animal nutrition: A review. Anim. Feed Sci. Technol. 2011, 168, 1–20. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Tokachichu, R.N.; Trethowan, R.M.; Tan, D.K.Y. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Yadav, S.; Singh, M.P. Bioregulators application improved heat tolerance and yield in chickpea (Cicer arietinum L.) by modulating zeaxanthin cycle. Plant Physiol. Rep. 2020, 25, 677–688. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, Y.; Feng, K.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Yang, Y.; et al. Genome-Wide Identification and Expression Profiling of Tomato Hsp20 Gene Family in Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 1215. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.; Gernhard, S.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. The plant sHSP superfamily: Five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones 2008, 13, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.D.; Siddique, M.; Vierling, E. The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins). Cell Stress Chaperones 2001, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, H.T.; Liu, H.R.; Lin, Z.Y.; Liu, Y.M.; Wang, Z.B.; Liu, S.S. Bioinformatics analysis of soybean Hsp20 gene family and analysis of response to high temperature and high humidity stress. Soybean Sci. 2023, 5, 565–578. (In Chinese) [Google Scholar]
- Jiang, H.H.; Zhang, J.M.; He, H.H.; Li, L.M.; Wu, Q.Y.; Huang, X.; Wang, T. Identification and expression characteristics analysis of HSP 20 gene family in peanut. J. Zhaoqing Univ. 2022, 5, 6–14. (In Chinese) [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Jain, M.; Bansal, J.; Rajkumar, M.S.; Garg, R. An integrated transcriptome mapping the regulatory network of coding and long non-coding RNAs provides a genomics resource in chickpea. Commun. Biol. 2022, 5, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, P.; Mishra, D.; Dey, S.; Malviya, R.; Gayen, D. Genome-wide identification of the fibrillin gene family in chickpea (Cicer arietinum L.) and its response to drought stress. Int. J. Biol. Macromol. 2023, 234, 123757. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, W.; Wang, Y.; Guan, J. Protein Function Prediction Based on PPI Networks: Network Reconstruction vs Edge Enrichment. Front. Genet. 2021, 12, 758131. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Song, C.; Wang, H.; Song, S.; Jiao, J.; Wang, M.; Zheng, X.; Bai, T. Genome-wide characterization of the HSP20 gene family identifies potential members involved in temperature stress response in Apple. Front. Genet. 2020, 11, 609184. [Google Scholar] [CrossRef]
- Waters, E.R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 2013, 64, 391–403. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef]
- Gao, T.; Mo, Z.; Tang, L.; Yu, X.; Du, G.; Mao, Y. Heat Shock Protein 20 Gene Superfamilies in Red Algae: Evolutionary and Functional Diversities. Front. Plant Sci. 2022, 13, 817852. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Caitar, V.S.; de Carvalho, M.C.; Darben, L.M.; Kuwahara, M.K.; Nepomuceno, A.L.; Dias, W.P.; Abdelnoor, R.V.; Marcelino-Guimarães, F.C. Genome-wide analysis of the Hsp20 gene family in soybean: Comprehensive sequence, genomic organization and expression profile analysis under abiotic and biotic stresses. BMC Genom. 2013, 14, 577. [Google Scholar] [CrossRef]
- Jeffares, D.C.; Penkett, C.J.; Bähler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef]
- Su, L.; Wan, S.; Zhou, J.; Shao, Q.S.; Xing, B. Transcriptional regulation of plant seed development. Physiol. Plant. 2021, 173, 2013–2025. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhang, M.J.; Dong, C.M. Effects of exogenous MeJA on the antioxidant system and stress genes of Pinellia ternata under high temperature stress. Bull. Bot. Res. 2021, 1, 67–73. (In Chinese) [Google Scholar]
- Wang, R.; Ma, J.; Zhang, Q.; Wu, C.; Zhao, H.; Wu, Y.; Yang, G.; He, G. Genome-wide identification and expression profiling of glutathione transferase gene family under multiple stresses and hormone treatments in wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Ri, H.C.; An, Z.; Wang, X.; Zhou, J.N.; Zheng, D.; Wu, H.; Wang, P.; Yang, J.; et al. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata. Nat. Commun. 2022, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.H.; Liu, J.P.; Zhai, Y.F.; Wang, H.; Gong, Z.H.; Wang, S.B.; Lu, M.H. Genome-wide analysis of the CaHsp20 gene family in pepper: Comprehensive sequence and expression profile analysis under heat stress. Front. Plant Sci. 2015, 6, 806. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhu, W.C.; Feng, X.H.; Jin, J.H.; Wei, A.M.; Gong, Z.H. Transcription Factor CaSBP12 Negatively Regulates Salt Stress Tolerance in Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2020, 21, 444. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, T.; Li, J.; Liu, B.; Fang, L.; Hu, Y.; Zhang, T. Identification and characterization of the GhHsp20 gene family in Gossypium hirsutum. Sci. Rep. 2016, 6, 32517. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, H.; Yan, Z.; Liao, D.; Li, C.; Zhou, J.; Liao, H. Full-length transcriptome survey and expression analysis of Cassia obtusifolia to discover putative genes related to aurantio-obtusin biosynthesis, seed formation and development, and stress response. Int. J. Mol. Sci. 2018, 19, 2476. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate change 2021: The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Zhou, Y.; Wang, Y.; Xu, F.; Song, C.; Yang, X.; Zhang, Z.; Yi, M.; Ma, N.; Zhou, X.; He, J. Small HSPs play an important role in crosstalk between HSF-HSP and ROS pathways in heat stress response through transcriptomic analysis in lilies (Lilium longiflorum). BMC Plant Biol. 2022, 22, 202. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, D.; Xue, P.; Wan, X. Identification of the DcHsp20 gene family in carnation (Dianthus caryophyllus) and functional characterization of DcHsp17.8 in heat tolerance. Planta 2022, 256, 2. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Vorst, O.; Fiers, M.W.; Stiekema, W.J.; Nap, J.P. In plants, highly expressed genes are the least compact. Trends Genet. 2006, 22, 528–532. [Google Scholar] [CrossRef] [PubMed]

| Name | Gene ID | Chr | Gene Start | Gene End | Strand | mRNA (bp) | CDS (bp) | Protein (aa) | pI | MW (kDa) | Instability Index | Hydropathy Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaHSP20-1 | Ca_06952 | Chr01 | 15999417 | 16000315 | reverse | 899 | 582 | 193 | 5.73 | 22.43 | 34.09 | −0.69 |
| CaHSP20-2 | Ca_10257 | Chr02 | 32641982 | 32642736 | forward | 755 | 633 | 210 | 9.16 | 24.44 | 37.68 | −0.83 |
| CaHSP20-3 | Ca_12525 | Chr02 | 30242080 | 30242541 | reverse | 462 | 462 | 153 | 6.85 | 17.44 | 41.85 | −0.66 |
| CaHSP20-4 | Ca_14336 | Chr02 | 29023413 | 29024198 | reverse | 786 | 420 | 139 | 7.03 | 15.48 | 46.12 | −0.34 |
| CaHSP20-5 | Ca_14692 | Chr02 | 5192005 | 5192424 | forward | 420 | 420 | 139 | 5.96 | 15.86 | 30.45 | −0.55 |
| CaHSP20-6 | Ca_19633 | Chr03 | 18515549 | 18516052 | reverse | 504 | 504 | 167 | 7.27 | 19.44 | 48.30 | −0.81 |
| CaHSP20-7 | Ca_12168 | Chr04 | 3702122 | 3702712 | reverse | 591 | 591 | 196 | 6.45 | 22.71 | 41.12 | −0.57 |
| CaHSP20-8 | Ca_04038 | Chr05 | 45931029 | 45931899 | forward | 871 | 690 | 229 | 7.67 | 26.03 | 39.09 | −0.66 |
| CaHSP20-9 | Ca_11311 | Chr05 | 42969085 | 42970157 | forward | 1073 | 729 | 242 | 9.35 | 27.46 | 57.14 | −0.72 |
| CaHSP20-10 | Ca_01653 | Chr05 | 35740491 | 35740964 | forward | 474 | 474 | 157 | 5.79 | 17.63 | 42.36 | −0.45 |
| CaHSP20-11 | Ca_01654 | Chr05 | 35738083 | 35738649 | forward | 567 | 567 | 188 | 5.46 | 21.37 | 40.02 | −0.84 |
| CaHSP20-12 | Ca_20062 | Chr05 | 22777083 | 22778273 | forward | 1191 | 576 | 191 | 6.32 | 22.08 | 43.37 | −0.77 |
| CaHSP20-13 | Ca_16264 | Chr06 | 54104152 | 54104622 | forward | 471 | 471 | 156 | 5.90 | 17.62 | 43.35 | −0.25 |
| CaHSP20-14 | Ca_13815 | Chr06 | 45062577 | 45062972 | forward | 396 | 396 | 131 | 5.49 | 15.15 | 41.72 | −0.75 |
| CaHSP20-15 | Ca_15814 | Chr06 | 34622371 | 34623153 | forward | 783 | 624 | 207 | 5.67 | 23.30 | 61.30 | −0.60 |
| CaHSP20-16 | Ca_14594 | Chr06 | 27635845 | 27636312 | forward | 468 | 468 | 155 | 5.56 | 17.87 | 63.28 | −0.73 |
| CaHSP20-17 | Ca_06336 | Chr06 | 17172499 | 17172921 | forward | 423 | 423 | 140 | 5.24 | 15.69 | 42.04 | −0.73 |
| CaHSP20-18 | Ca_06335 | Chr06 | 17171634 | 17172116 | forward | 483 | 483 | 160 | 5.57 | 18.41 | 62.45 | −0.71 |
| CaHSP20-19 | Ca_06334 | Chr06 | 17163907 | 17164389 | reverse | 483 | 483 | 160 | 5.82 | 18.38 | 58.18 | −0.68 |
| CaHSP20-20 | Ca_05301 | Chr06 | 15188993 | 15189463 | reverse | 471 | 471 | 156 | 6.19 | 17.86 | 52.39 | −0.71 |
| CaHSP20-21 | Ca_16176 | Chr07 | 33565963 | 33566633 | forward | 671 | 450 | 149 | 7.79 | 16.97 | 47.51 | −0.62 |
| Seq1 | Seq2 | Ka | Ks | Ka/Ks Ratio | Date (MY) | Duplication Type |
|---|---|---|---|---|---|---|
| CaHSP10 | CaHSP11 | 0.327 | 1.005 | 0.325 | 33.500 | Tandem |
| CaHSP19 | CaHSP18 | 0.008 | 0.117 | 0.068 | 3.898 | Tandem |
| CaHSP19 | CaHSP17 | 0.253 | 1.230 | 0.206 | 40.985 | Tandem |
| CaHSP18 | CaHSP17 | 0.247 | 1.576 | 0.157 | 52.539 | Tandem |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wu, Y.; Li, Y.; Zhang, Z.; He, D.; Yan, J.; Zou, H.; Liu, Y. Genome-Wide Identification and Expression Analysis of Heat Shock Protein 20 (HSP20) Gene Family in Response to High-Temperature Stress in Chickpeas (Cicer arietinum L.). Agronomy 2024, 14, 1696. https://doi.org/10.3390/agronomy14081696
Liu S, Wu Y, Li Y, Zhang Z, He D, Yan J, Zou H, Liu Y. Genome-Wide Identification and Expression Analysis of Heat Shock Protein 20 (HSP20) Gene Family in Response to High-Temperature Stress in Chickpeas (Cicer arietinum L.). Agronomy. 2024; 14(8):1696. https://doi.org/10.3390/agronomy14081696
Chicago/Turabian StyleLiu, Sushuang, Yizhou Wu, Yang Li, Zaibao Zhang, Dandan He, Jianguo Yan, Huasong Zou, and Yanmin Liu. 2024. "Genome-Wide Identification and Expression Analysis of Heat Shock Protein 20 (HSP20) Gene Family in Response to High-Temperature Stress in Chickpeas (Cicer arietinum L.)" Agronomy 14, no. 8: 1696. https://doi.org/10.3390/agronomy14081696
APA StyleLiu, S., Wu, Y., Li, Y., Zhang, Z., He, D., Yan, J., Zou, H., & Liu, Y. (2024). Genome-Wide Identification and Expression Analysis of Heat Shock Protein 20 (HSP20) Gene Family in Response to High-Temperature Stress in Chickpeas (Cicer arietinum L.). Agronomy, 14(8), 1696. https://doi.org/10.3390/agronomy14081696





