Comprehensive Dissection of Metabolites in Response to Low Phosphorus Stress in Different Root-Type Alfalfa at Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sand Culture
2.2. Stress Treatments and Sampling
2.3. Evaluation of Growth Indices
2.4. Determination of Morphological Indicators
2.5. Measurement of Phytohormones
2.6. Measurement of Organic Acids
2.7. Metabolite Extraction and Determination
2.8. Statistical Analysis
3. Results
3.1. Effects of P Stress on the Growth of Different Root-Type Alfalfa
3.2. Metabolomics Analysis
3.2.1. The Establishment of the Mass Spectrometry Analysis System
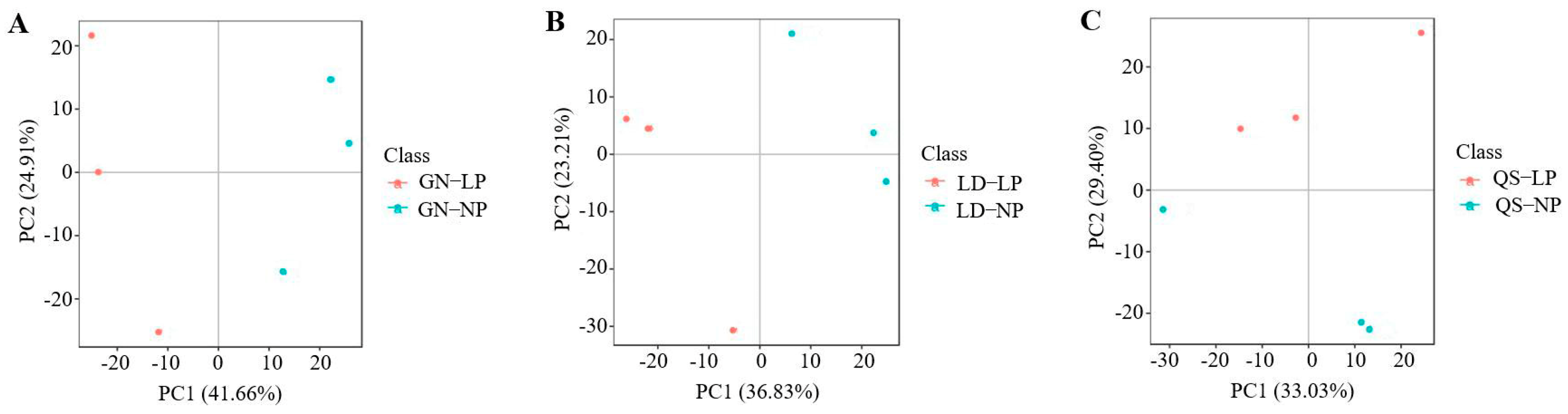
3.2.2. Principal Component Analysis
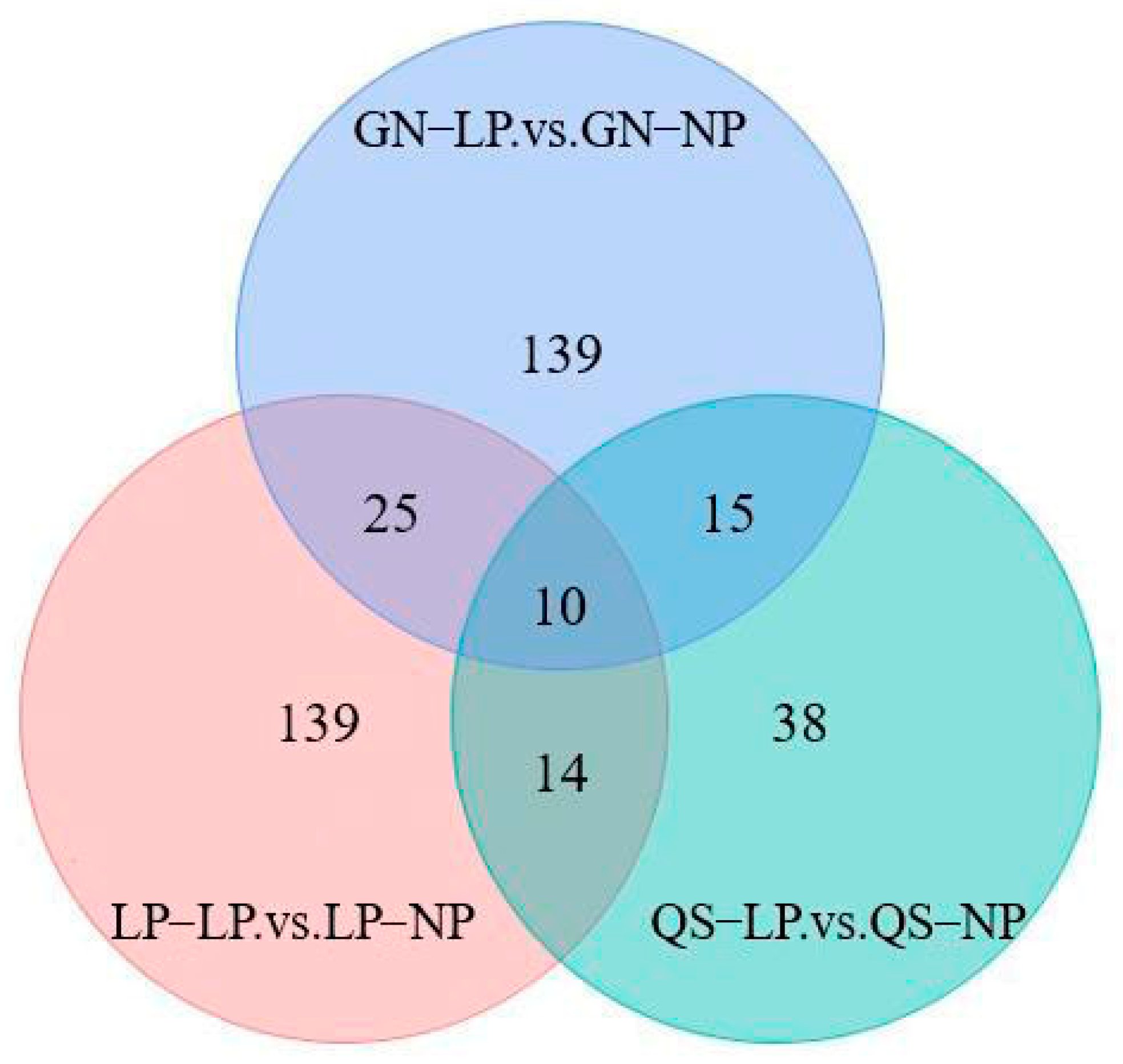
3.2.3. Differential Metabolite Screening
3.2.4. Analysis of Differentially Metabolized Substances
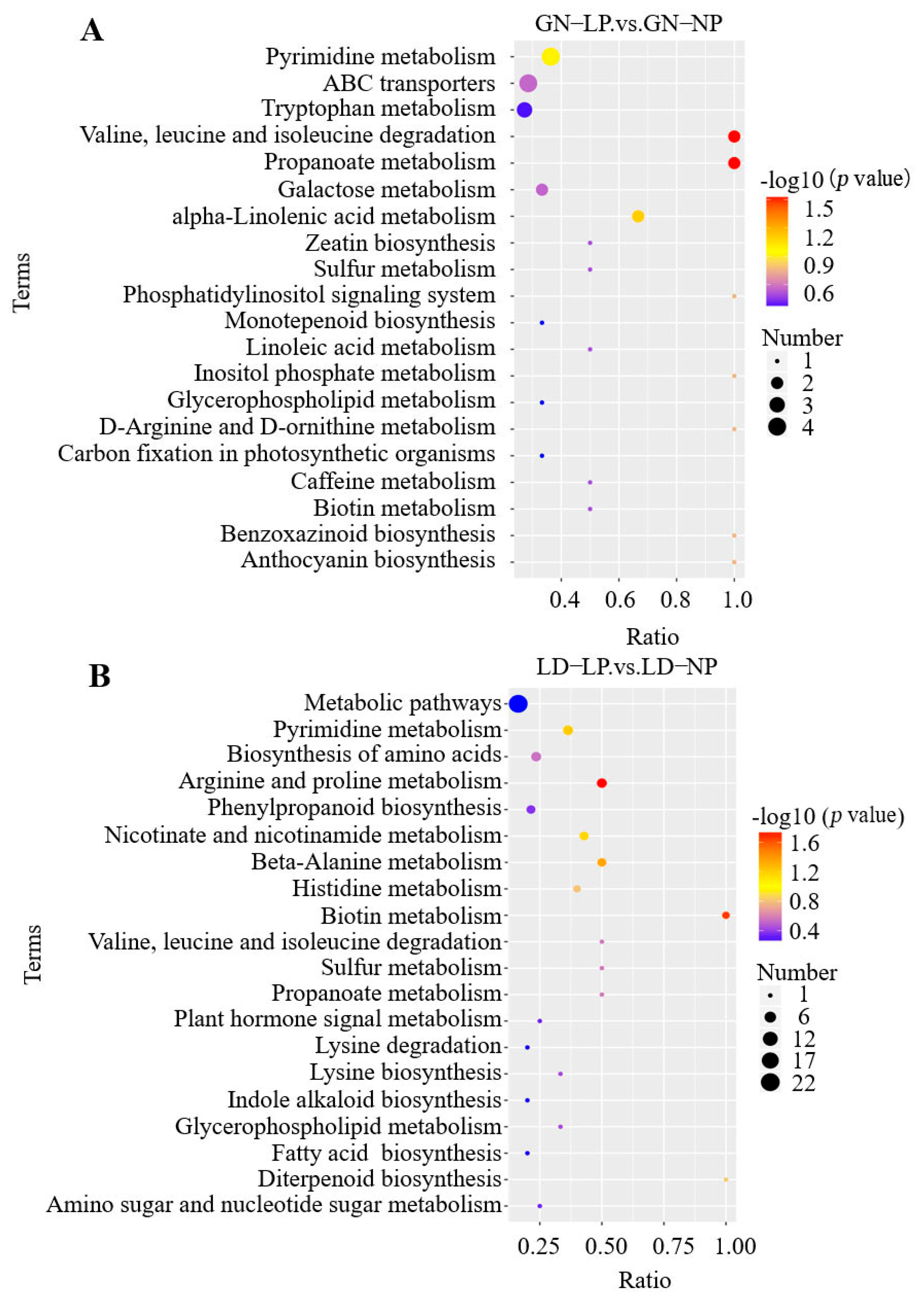
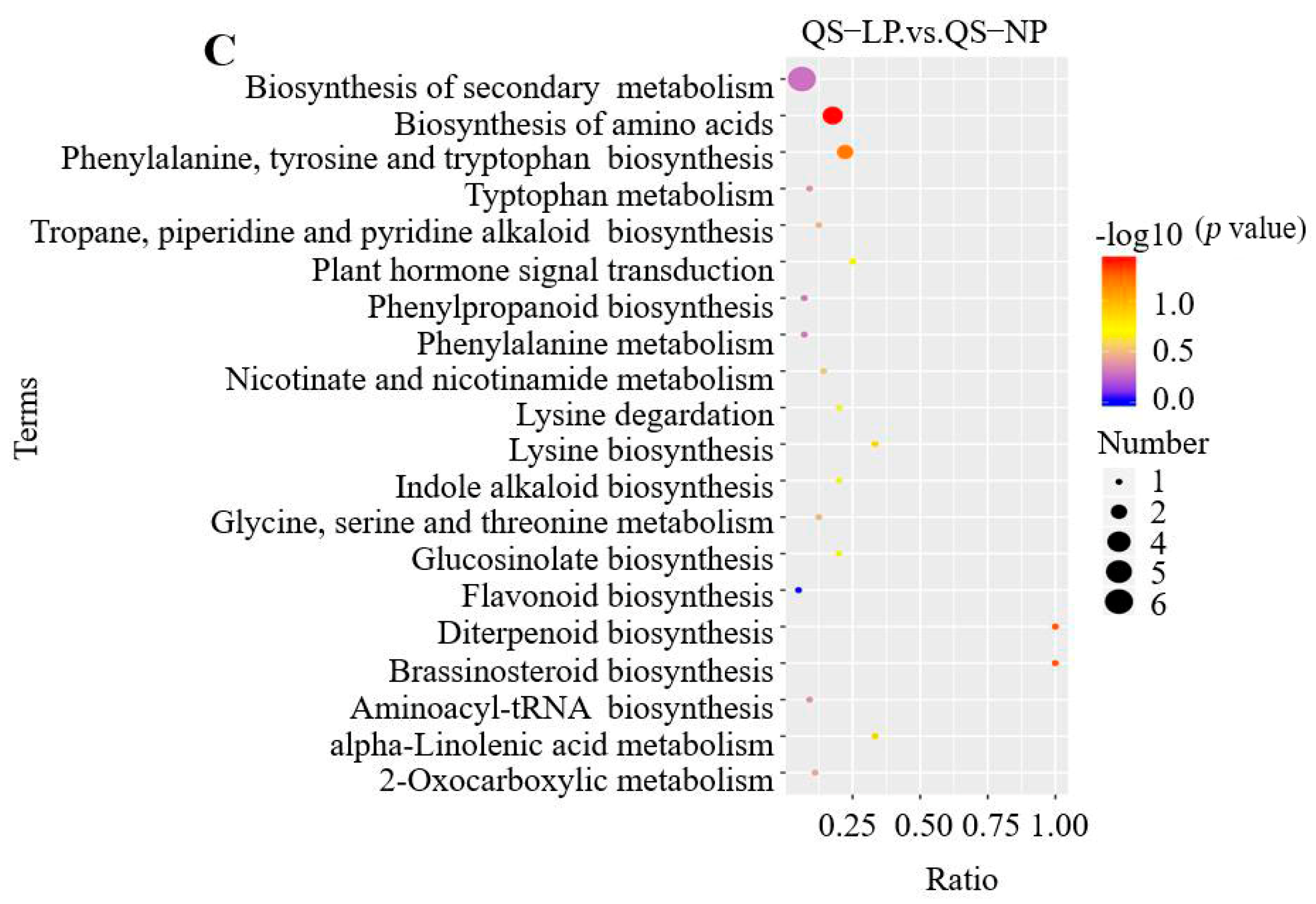
3.2.5. KEGG Pathway Enrichment Analysis
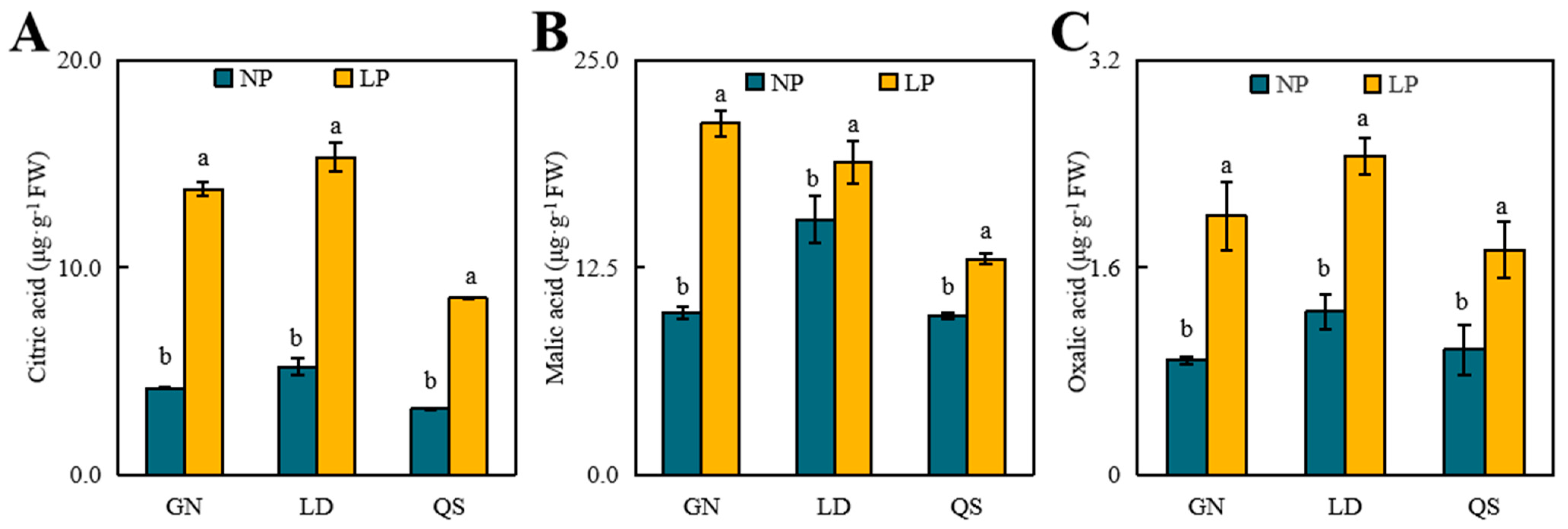
3.3. Effects of P Stress on Levels of Organic Acid Content
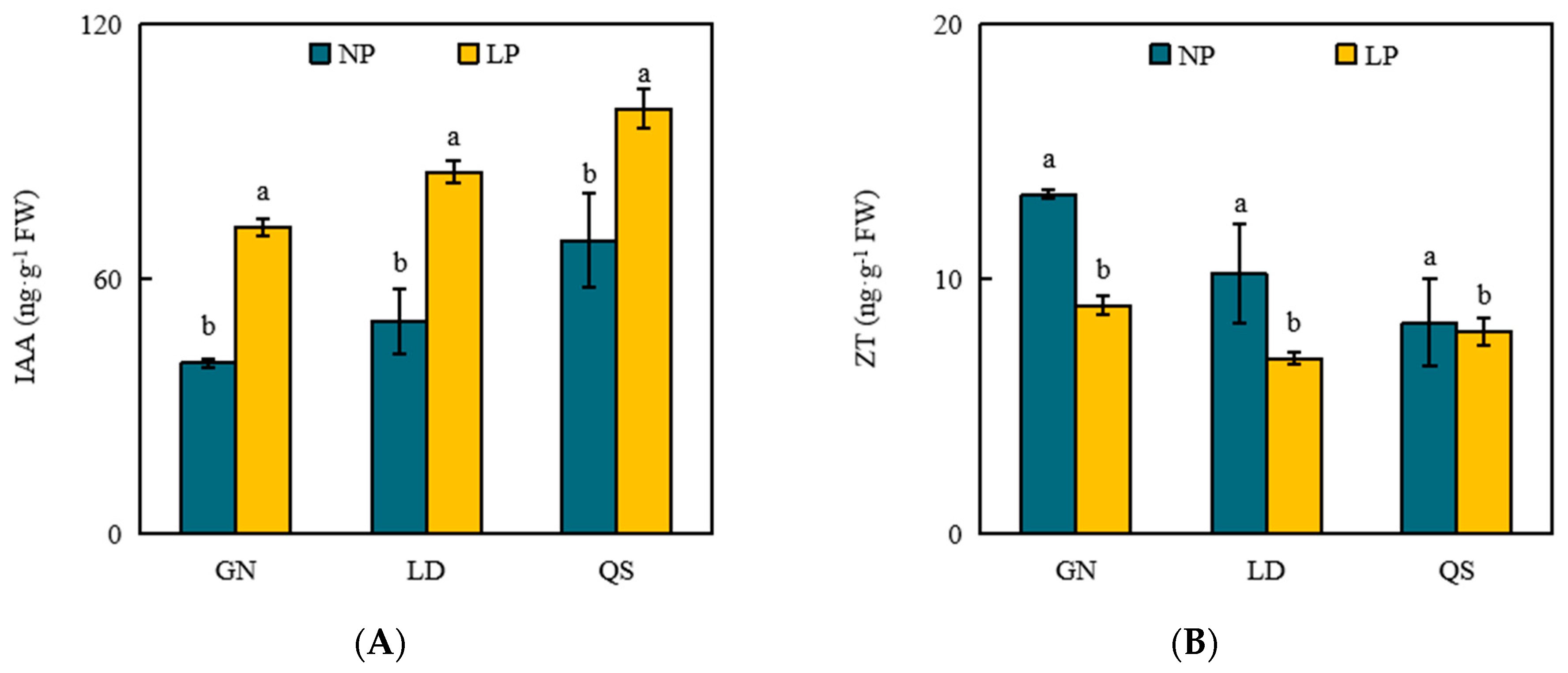
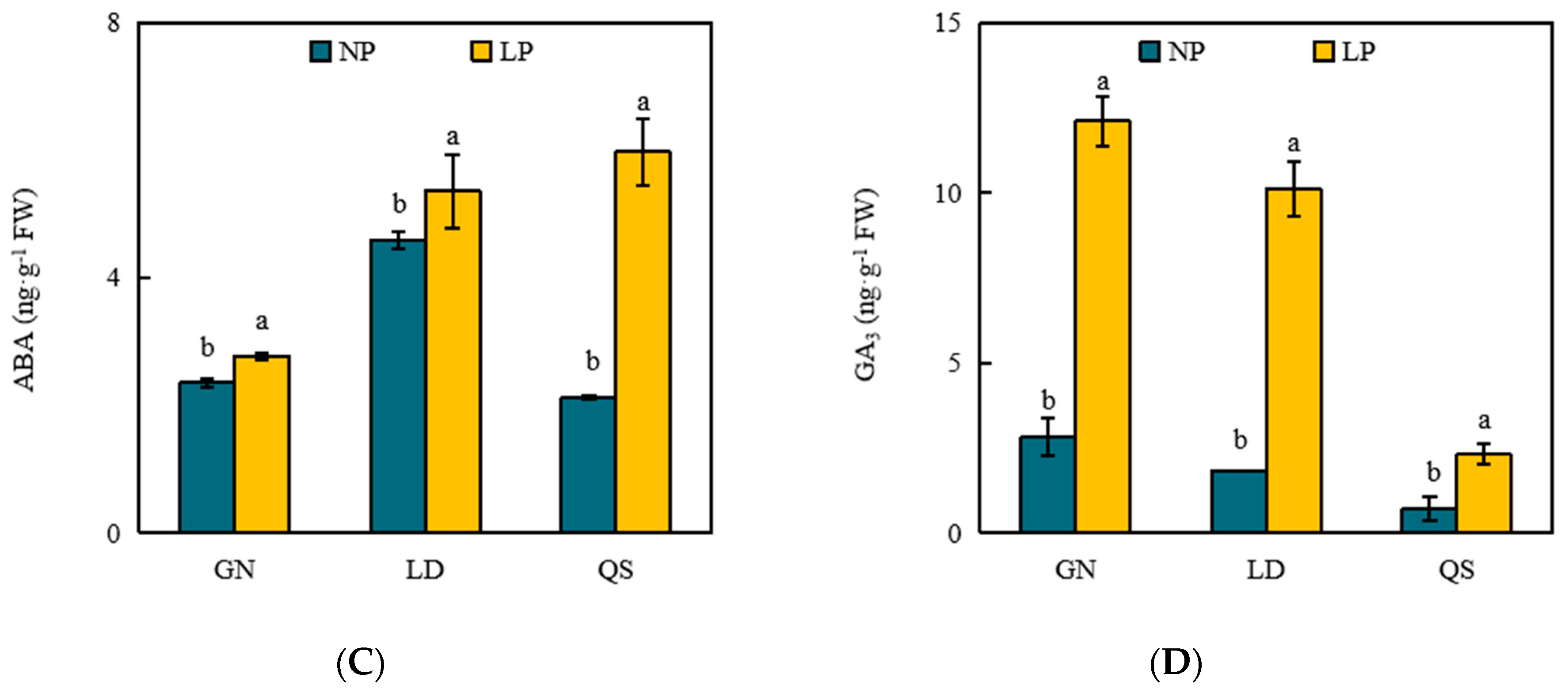
3.4. Effects of P Stress on Levels of Phytohormones
4. Discussion
4.1. Effects of LP Stress on the Growth of Different Root-Type Alfalfa
4.2. Effects of LP Stress on Differential Metabolites in Roots
4.3. Effects of LP Stress on Organic Acids in Roots
4.4. Effects of LP Stress on Phytohormones in Roots
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nan, L.L.; Nie, Z.N.; Zollinger, R.; Guo, Q.E. Evaluation of morphological and production characteristics and nutritive value of 47 lucerne cultivars/lines in temperate Australia. Plant Prod. Sci. 2019, 22, 490–500. [Google Scholar] [CrossRef]
- Avijit, G.; Ranjan, D.B.; Ranjan, B.; Shrila, D. Rice residue promotes mobilisation and plant acquisition of soil phosphorus under wheat (Triticum aestivum)-rice (Oryza sativa) cropping sequence in a semi-arid Inceptisol. Sci. Rep. 2023, 13, 17545. [Google Scholar]
- Chao, Y.; Kang, J.; Sun, Y.; Yang, Q.; Wang, P.; Wu, M.; Li, Y.; Long, R.; Qin, Z. Molecular cloning and characterization of a novel gene encoding zinc finger protein from Medicago sativa L. Mol. Biol. Rep. 2009, 36, 2315–2321. [Google Scholar]
- Jia, X.; Wang, L.; Nussaume, L.; Yi, K. Cracking the code of plant central phosphate signaling. Trends Plant Sci. 2023, 28, 267–270. [Google Scholar]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar]
- Priyam, A.; Das, R.K.; Schultz, A.; Singh, P.P. A new method for biological synthesis of agriculturally relevant nanohydroxyapatite with elucidated effects on soil bacteria. Sci. Rep. 2019, 9, 15083. [Google Scholar] [CrossRef]
- Dissanayaka, S.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus deprived plants. J. Exp. Bot. 2020, 72, 199–223. [Google Scholar]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Duan, X.; Zhang, J.C. Effects of different phosphorus supply levels on organic acid secretion in hydrilla verticillata roots in plateau wetland. Int. J. Agric. Biol. 2020, 23, 1033–1043. [Google Scholar]
- Wei, L.J.; Zhang, J.; Wei, S.H.; Hu, D.L.; Liu, Y.Y.; Feng, L.; Li, C.; Qi, N.; Wang, C.; Liao, W. Nitric oxide enhanced salt stress tolerance in tomato seedlings, involving phytohormone equilibrium and photosynthesis. Int. J. Mol. Sci. 2022, 23, 4539. [Google Scholar] [CrossRef]
- Wang, B.; Si, W.; Wu, Y.; Zhang, X.; Wang, S.; Wu, C.; Lin, H.; Yin, L. Research progress in biosynthesis and metabolism regulation of gibberellins in Gibberella fujikuroi. Sheng Wu Gong Cheng Xue Bao 2020, 36, 189–200. [Google Scholar]
- Márquez-López, R.E.; Quintana-Escobar, A.O.; Loyola-Vargas, V.M. Cytokinins, the cinderella of plant growth regulators. Phytochem. Rev. 2019, 18, 1387–1408. [Google Scholar] [CrossRef]
- Katam, R.; Lin, C.W.; Grant, K.; Katam, C.S.; Chen, S.X. Advances in plant metabolomics and its applications in stress and single-cell biology. Int. J. Mol. Sci. 2022, 23, 6985. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhang, D.; Liu, A.; Zhu, W.; Zhang, J.; Yang, B. Integrative omic analysis reveals the dynamic change in phenylpropanoid metabolism in Morus alba under different stress. Plants 2023, 12, 3265. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics response for drought stress tolerance in Chinese wheat genotypes (Triticum aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef]
- Cardoso, L.L.; Freire, F.B.S.; Daloso, D.M. Plant metabolic networks under stress: A multi-species/stress condition Meta-analysis. J. Soil Sci. Plant Nut. 2023, 23, 4–21. [Google Scholar] [CrossRef]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef]
- Ye, T.T.; Shi, H.T.; Wang, Y.P.; Yang, F.; Chan, Z.L. Contrasting proteomic and metabolomic responses of bermudagrass to drought and salt stresses. Front. Plant Sci. 2016, 7, 1694. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Molero, G.; Erice, G.; Avice, J.C.; Nogués, S. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J. Exp. Bot. 2011, 62, 111–123. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Jin, J.; Christensen, M.J.; Gu, L.; Cheng, C.; Wang, J. Epichloëgansuensis increases the tolerance of Achnatherum inebrians to low-P stress by modulating amino acids metabolism and phosphorus utilization efficiency. J. Fungi 2021, 7, 390. [Google Scholar] [CrossRef]
- Wang, K.; Nan, L.L.; Guo, Q.E.; Yao, Y.H.; He, H.P.; Xia, J.; Ma, B. Effects of drought stress on root architecture of different root-type alfalfa. Acta Ecol. Sin. 2022, 42, 8365–8373. [Google Scholar]
- Nan, L.L.; Shi, S.L.; Zhang, J.H. Study on root system development ability of different root-type alfalfa. Acta Prataculturae Sin. 2014, 23, 117–124. [Google Scholar]
- Wang, K.; Nan, L.L.; Xia, J.; Wu, S.W.; Yang, L.L. Metabolomics reveal root differential metabolites of different root-type alfalfa under drought stress. Front. Plant Sci. 2024, 15, 1341826. [Google Scholar] [CrossRef]
- Nan, L.L.; Shi, S.L.; Chen, J.G.; Zhu, X.Q.; Guo, Q.E.; Zhao, W.H. Field evaluation of the response and resistance to low temperature of alfalfa root with different root types during over-wintering. Chin. J. Eco-Agric. 2011, 19, 619–625. [Google Scholar] [CrossRef]
- Nan, L.L.; Shi, S.L.; Guo, Q.E.; Tian, F.; Fan, J.J. Analysis of dynamic variations in crown characteristics of different root-type alfalfa plants. Chin. J. Eco-Agric. 2012, 20, 914–920. [Google Scholar] [CrossRef]
- Wang, K.; Nan, L.L.; Guo, Q.E. Changes in root endogenous hormone levels and rhizosphere fungi diversity in alfalfa under drought stress. Plant Growth Regul. 2023, 10, 874–894. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Open Access Libr. J. 1950, 347, 32. [Google Scholar]
- Al-Amri, S.M. Response of growth, essential oil composition, endogenous hormones and microbial activity of Mentha piperita to some organic and biofertilizers agents. Saudi J. Biol. Sci. 2021, 28, 5435–5441. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, P.M.; Cheng, L.L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.L.; Zeng, C.W.; Liu, S.Q. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Maruyama, H.; Nishida, S.; Tawaraya, K.; Wasaki, J. Landrace of japonica rice, Akamai exhibits enhanced root growth and efficient leaf phosphorus remobilization in response to limited phosphorus availability. Plant Soil. 2017, 414, 327–338. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Liu, Q.; Mao, L.; Li, H.; Li, S.; Guo, R. Dynamic variation of nutrient absorption, metabolomic and transcriptomic indexes of soybean (Glycine max) seedlings under phosphorus deficiency. AoB Plants 2023, 15, plad014. [Google Scholar] [CrossRef]
- Ingrisano, R.; Tosato, E.; Trost, P.; Gurrieri, L.; Sparla, F. Proline, cysteine and branched-chain amino acids in abiotic stress response of land plants and microalgae. Plants 2023, 12, 3410. [Google Scholar] [CrossRef]
- Widodo, J.H.P.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, sahara and clipper which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The significance of methionine, histidine and tryptophannbsp in plant responses and adaptation to cadmium stress. Plant Soil Environ. 2014, 60, 426–432. [Google Scholar] [CrossRef]
- Ge, C.R.; Georgiev, A.; Öhman, A.; Wieslander, A.; Kelly, A.A. Tryptophan residues promote membrane association for a plant lipid glycosyl transferase involved in phosphate stress. J. Biol. Chem. 2011, 286, 6669–6684. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, Y.; Tian, P.; Yu, X. Mathematical estimation of endogenous proline as a bioindicator to regulate the stress of trivalent chromium on rice plants grown in different nitrogenous conditions. Toxics 2023, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Bertazzini, M.; Cagnano, G. Stress-driven increase in proline levels, and not proline levels themselves, correlates with the ability to withstand excess salt in a group of 17 Italian rice genotypes. Plant Biol. 2018, 21, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Liang, Y.; Lu, T.; Liu, Z.; Jin, X.; Hou, L.; Xu, J.; Zhao, H.; Shi, Y.; et al. Comparative transcriptomic and metabolomic analyses reveal the protective effects of silicon against low phosphorus stress in tomato plants. Plant Physiol. Bioch. 2021, 166, 78–87. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lysoe, E.; Armarego-Marriott, T.; Erban, A.; Paruch, L.; Van Eerde, A.; Bock, R.; Liu-Clarke, J. Transcriptome and metabolome analyses provide insights into root and root-released organic anion responses to phosphorus deficiency in oat. J. Exp. Bot. 2018, 69, 3759–3771. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Exogenous citrate and malate alleviate cadmium stress in Oryza sativa L.: Probing role of cadmium localization and iron nutrition. Ecotoxicol. Environ. Saf. 2018, 166, 215–222. [Google Scholar] [CrossRef]
- Wen, T.; Dong, L.J.; Wang, L.; Ma, F.W.; Zou, Y.J.; Li, C.Y. Changes in root architecture and endogenous hormone levels in two rootstocks under alkali stress. Sci. Hortic. 2018, 235, 198–204. [Google Scholar] [CrossRef]
- Nacry, P.; Canivenc, G.; Muller, B.; Azmi, A.; Van, O.H.; Rossignol, M.; Doumas, P. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 2005, 138, 2061–2074. [Google Scholar] [CrossRef]
- Jiang, C.F.; Gao, X.H.; Liao, L.L.; Nicholas, P.H.; Fu, X.D. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Siao, W.; Zhang, N.; Lei, Z.; Lin, D.; Bhalerao, R.P.; Lu, C.; Xu, W. Katanin-dependent microtubule ordering in association with ABA is important for root hydrotropism. Int. J. Mol. Sci. 2022, 23, 3846. [Google Scholar] [CrossRef] [PubMed]

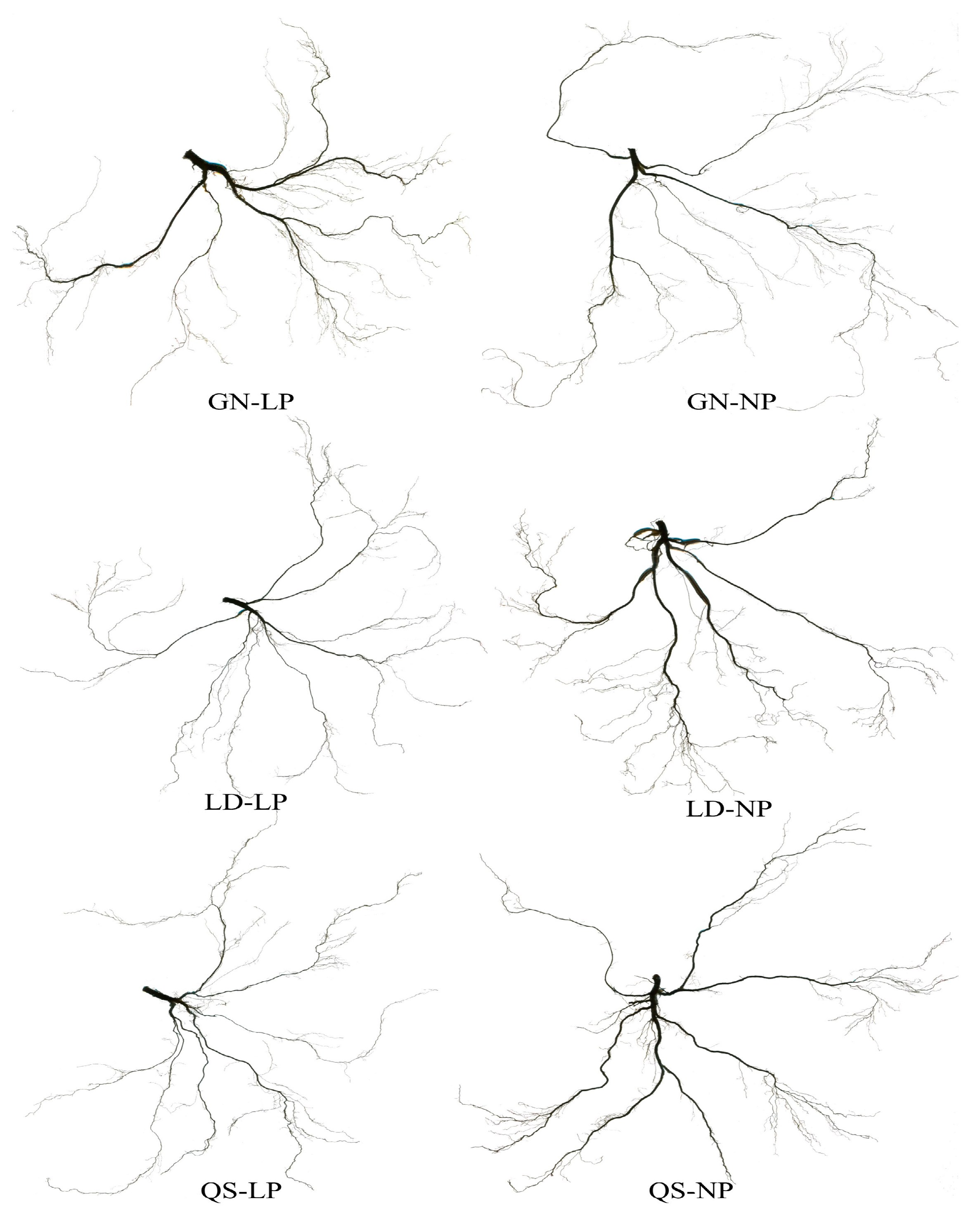




| Indices | GN | LD | QS | |||
|---|---|---|---|---|---|---|
| LP | NP | LP | NP | LP | NP | |
| Plant height/cm | 57.40 ± 10.11 b | 63.87 ± 8.25 a | 56.23 ± 10.11 b | 59.28 ± 10.27 a | 53.10 ± 4.99 b | 55.48 ± 5.48 a |
| Leaf length/cm | 1.73 ± 0.10 b | 1.94 ± 0.16 a | 1.34 ± 0.13 b | 1.57 ± 0.12 a | 1.23 ± 0.23 b | 1.45 ± 0.11 a |
| Leaf width/cm | 0.85 ± 0.11 b | 0.93 ± 0.04 a | 0.69 ± 0.09 b | 0.74 ± 0.05 a | 0.61 ± 0.05 b | 0.74 ± 0.05 a |
| Leaf area/cm2 | 0.49 ± 0.09 b | 0.60 ± 0.07 a | 0.31 ± 0.07 b | 0.39 ± 0.03 a | 0.25 ± 0.07 b | 0.36 ± 0.02 a |
| Total length/cm | 3408.56 ± 6.09 a | 1712.10 ± 2.61 b | 3491.61 ± 4.23 a | 1569.48 ± 6.70 b | 2843.40 ± 3.01 a | 1438.60 ± 6.84 b |
| Total surface area/cm2 | 46.03 ± 4.24 b | 57.74 ± 0.98 a | 44.88 ± 5.80 b | 66.98 ± 0.94 a | 33.07 ± 5.72 b | 53.10 ± 1.14 a |
| Total volume/cm3 | 1.36 ± 0.53 b | 4.09 ± 0.32 a | 1.30 ± 0.34 b | 4.09 ± 0.65 a | 0.81 ± 0.23 b | 3.45 ± 0.56 a |
| Ground biomass/g·10 plant−1 | 8.56 ± 1.24 b | 9.57 ± 0.75 a | 5.47 ± 1.40 b | 10.39 ± 0.69 a | 6.48 ± 1.12 b | 10.09 ± 0.23 a |
| Root biomass/g·10 plant−1 | 13.98 ± 1.91 a | 6.91 ± 0.95 b | 8.87 ± 0.98 a | 3.27 ± 0.22 b | 6.88 ± 0.23 a | 3.24 ± 0.18 b |
| Root/shoot ratio | 1.67 ± 0.41 a | 0.73 ± 0.11 b | 1.72 ± 0.62 a | 0.31 ± 0.02 b | 1.08 ± 0.19 a | 0.33 ± 0.08 b |
| Category | Metabolites | Fold Change (FC) | ||
|---|---|---|---|---|
| GN_LP vs. GN_NP | LD_LP vs. LD_NP | QS_LP vs. QS_NP | ||
| Amino acids | L-histidine | - | 0.331 | - |
| 4-oxoproline | - | 0.675 | - | |
| D-proline | - | 0.510 | - | |
| L-saccharopine | - | - | 0.367 | |
| L-tryptophan | - | - | 7.918 | |
| Organic acids | Methylmalonic acid | 0.367 | - | - |
| Methylmalonate | 0.221 | - | - | |
| Malonic acid | - | 2.735 | - | |
| Shikimic acid | - | - | 0.575 | |
| Plant growth regulating substance | 5-Aminopentanoate | - | 0.621 | - |
| Gibberellic acid | - | - | 0.361 | |
| Brassinolide | - | - | 4.431 | |
| Biotinoids | Biotin | - | 3.439 | - |
| Desthiobiotin | - | 2.068 | - | |
| Polyamine substances | Agmatine | - | 0.207 | - |
| Nicotinic acids | Quinolinic acid | - | 0.538 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Nan, L.; Wang, K.; Yao, Y. Comprehensive Dissection of Metabolites in Response to Low Phosphorus Stress in Different Root-Type Alfalfa at Seedling Stage. Agronomy 2024, 14, 1697. https://doi.org/10.3390/agronomy14081697
Xia J, Nan L, Wang K, Yao Y. Comprehensive Dissection of Metabolites in Response to Low Phosphorus Stress in Different Root-Type Alfalfa at Seedling Stage. Agronomy. 2024; 14(8):1697. https://doi.org/10.3390/agronomy14081697
Chicago/Turabian StyleXia, Jing, Lili Nan, Kun Wang, and Yuheng Yao. 2024. "Comprehensive Dissection of Metabolites in Response to Low Phosphorus Stress in Different Root-Type Alfalfa at Seedling Stage" Agronomy 14, no. 8: 1697. https://doi.org/10.3390/agronomy14081697
APA StyleXia, J., Nan, L., Wang, K., & Yao, Y. (2024). Comprehensive Dissection of Metabolites in Response to Low Phosphorus Stress in Different Root-Type Alfalfa at Seedling Stage. Agronomy, 14(8), 1697. https://doi.org/10.3390/agronomy14081697





