Monitoring of Wheat Stripe Rust Using Red SIF Modified by Pseudokurtosis
Abstract
:1. Introduction
2. Materials and Methods
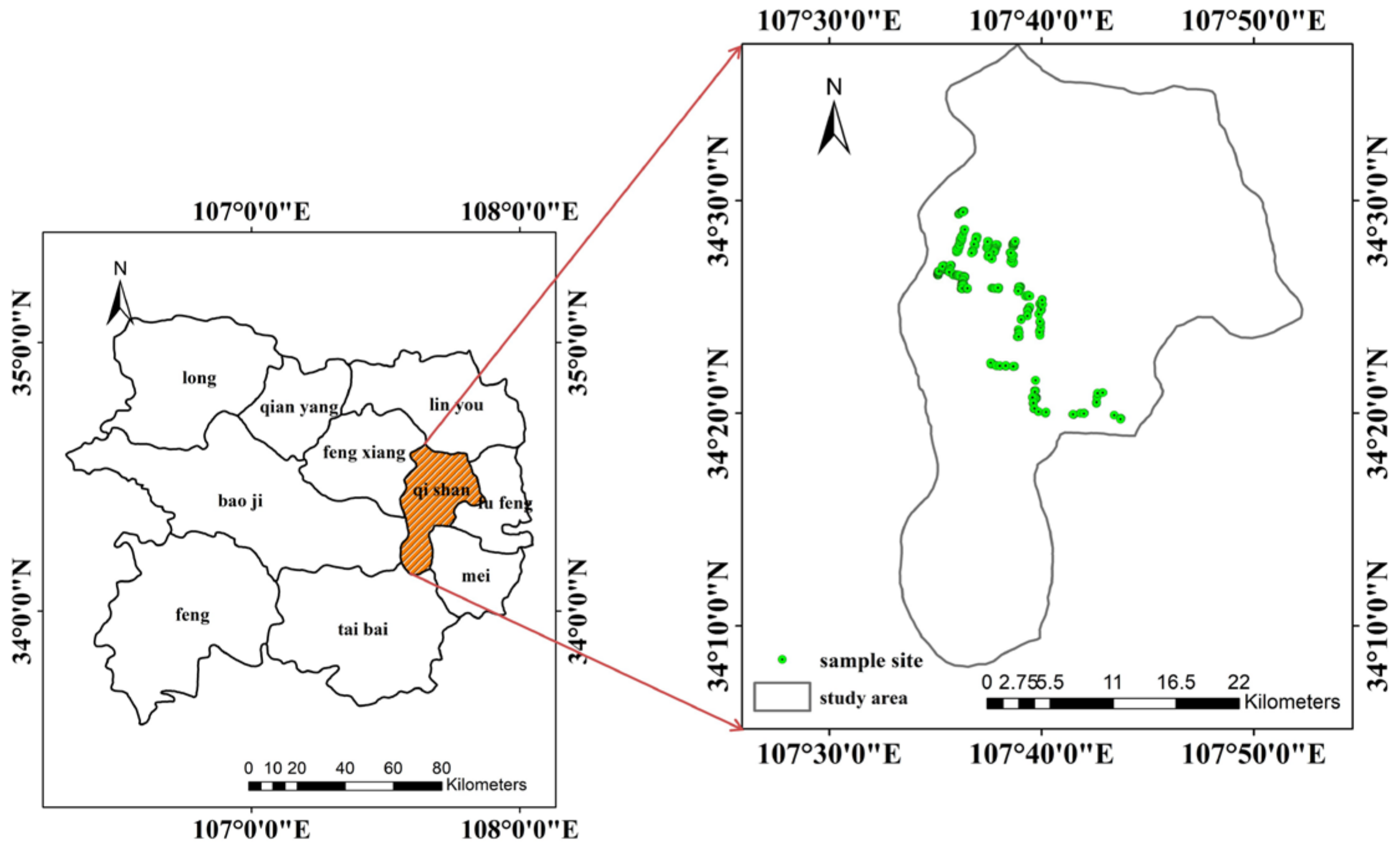
2.1. Overview of the Study Area and Data Acquisition
2.1.1. Plot Disease Field Experiment
2.1.2. Natural Disease Field Experiment
2.1.3. Disease Severity and Field Canopy Spectral Measurement
2.2. Simulation Data
2.2.1. Fluspect Model
2.2.2. SCOPE 2.0 Model
2.3. Canopy Spectral Processing
2.3.1. Full-Spectrum SIF Spectral Retrieval
2.3.2. Full-Spectrum SIF Spectral Preprocessing
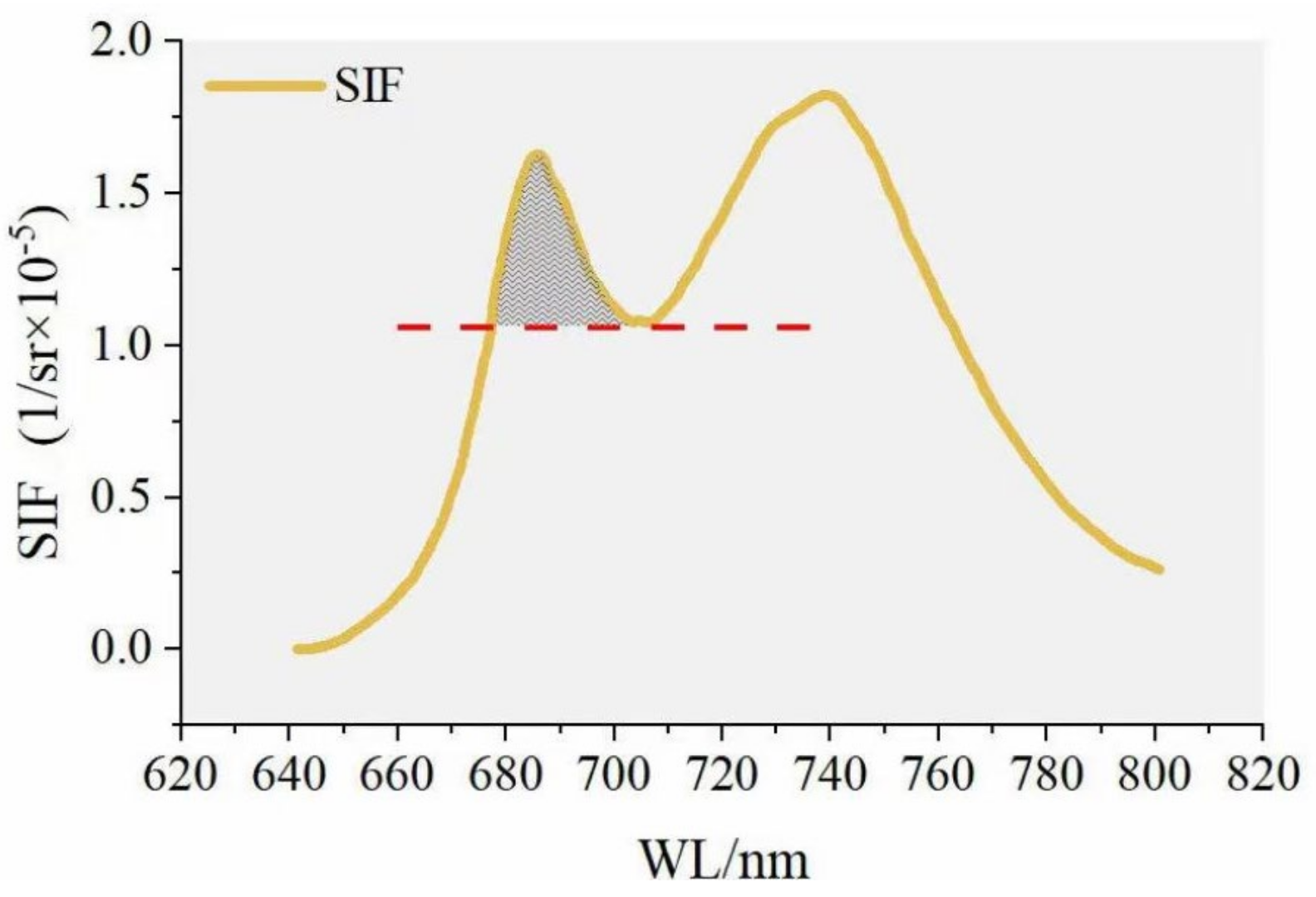
2.4. The Influence of Canopy on the Shape of SIF Spectrum
2.5. Modification of SIFB
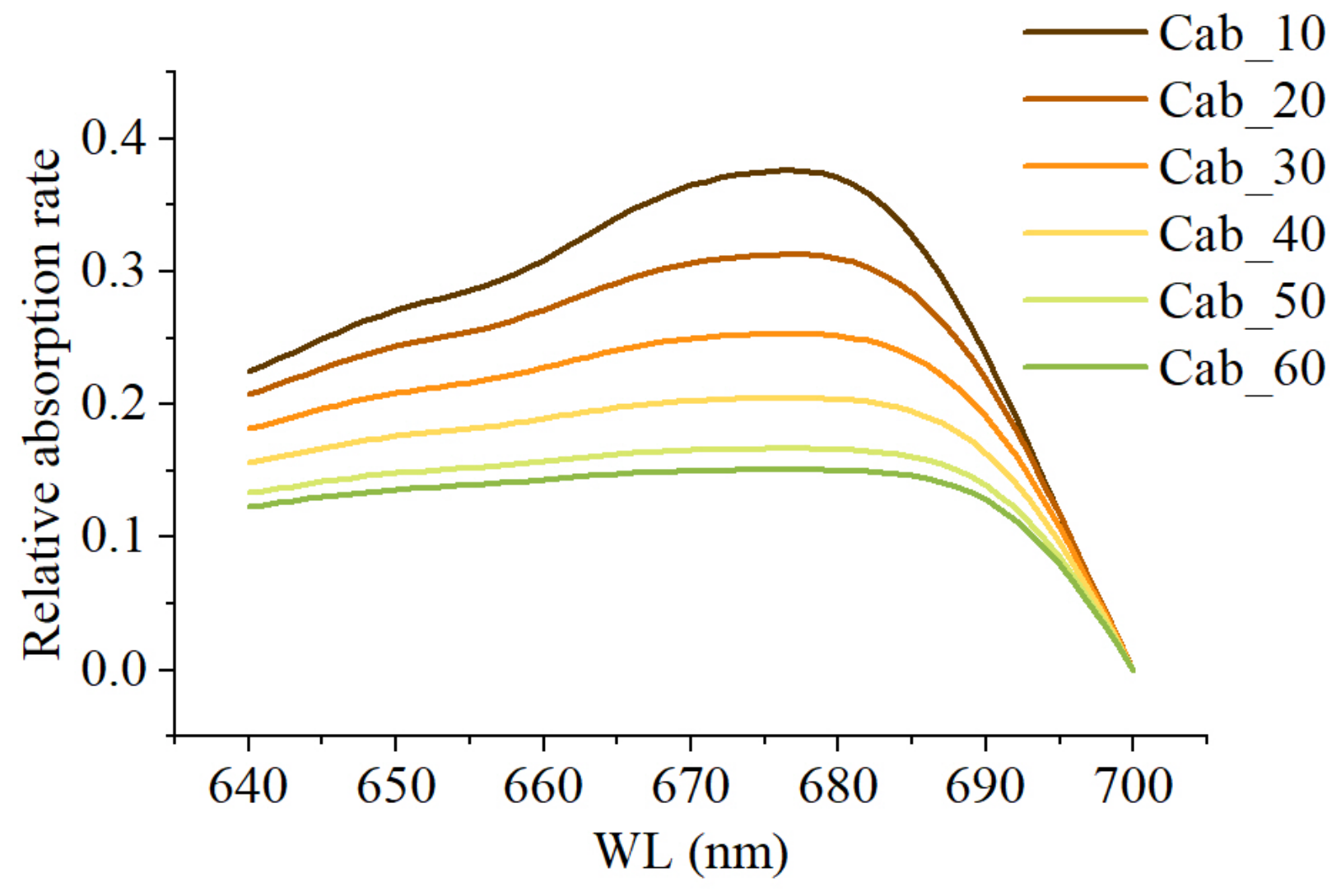
2.5.1. Photosynthetic Physiological Basis of PKB
2.5.2. Modifying SIFB by Means of PKB
2.5.3. Fusing MSIFB with Vegetation Indices (VIs)
3. Results
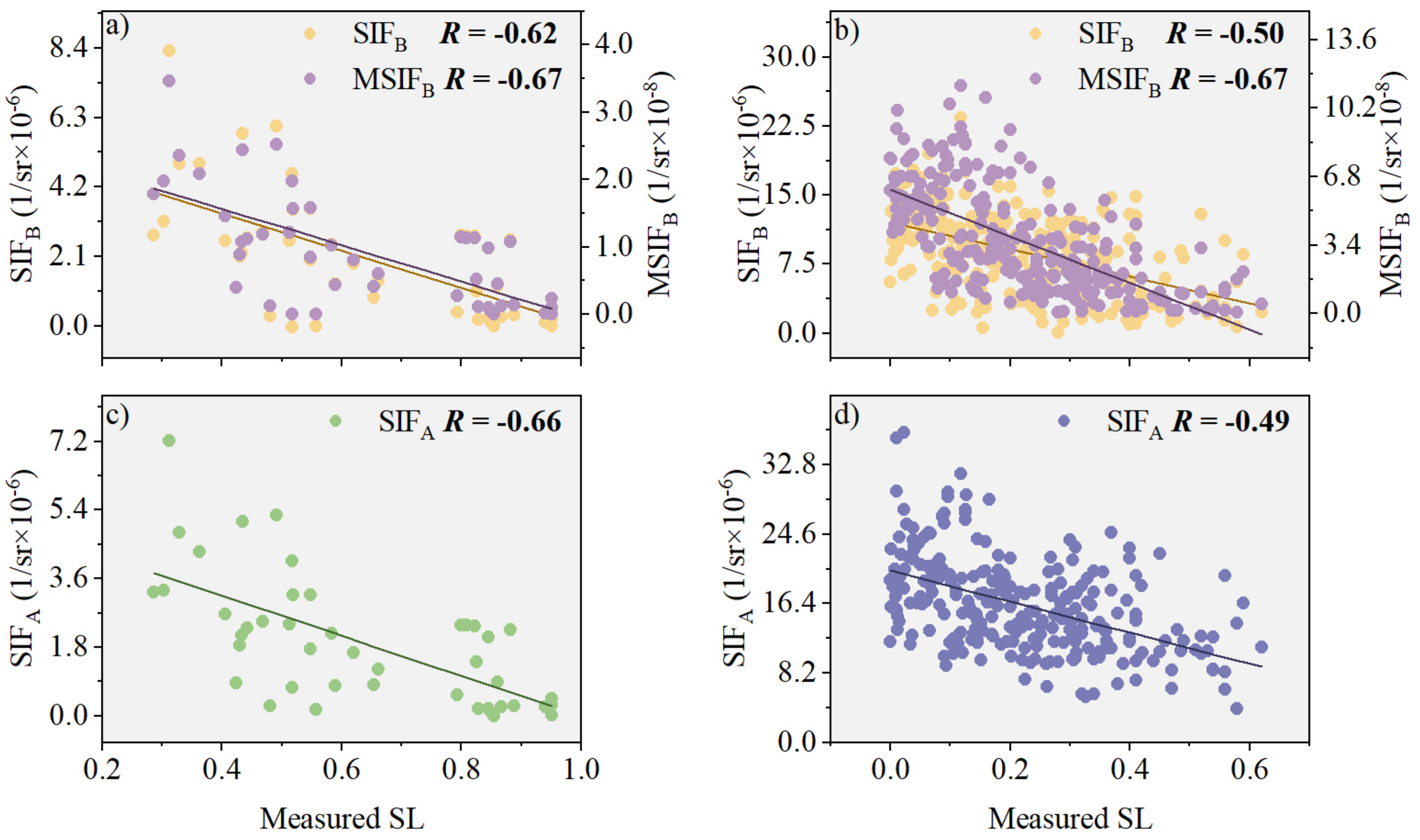
3.1. Performance Evaluation of PKB Modifying SIFB
3.2. Performance Evaluation of PKB Modifying SIFB-VIs
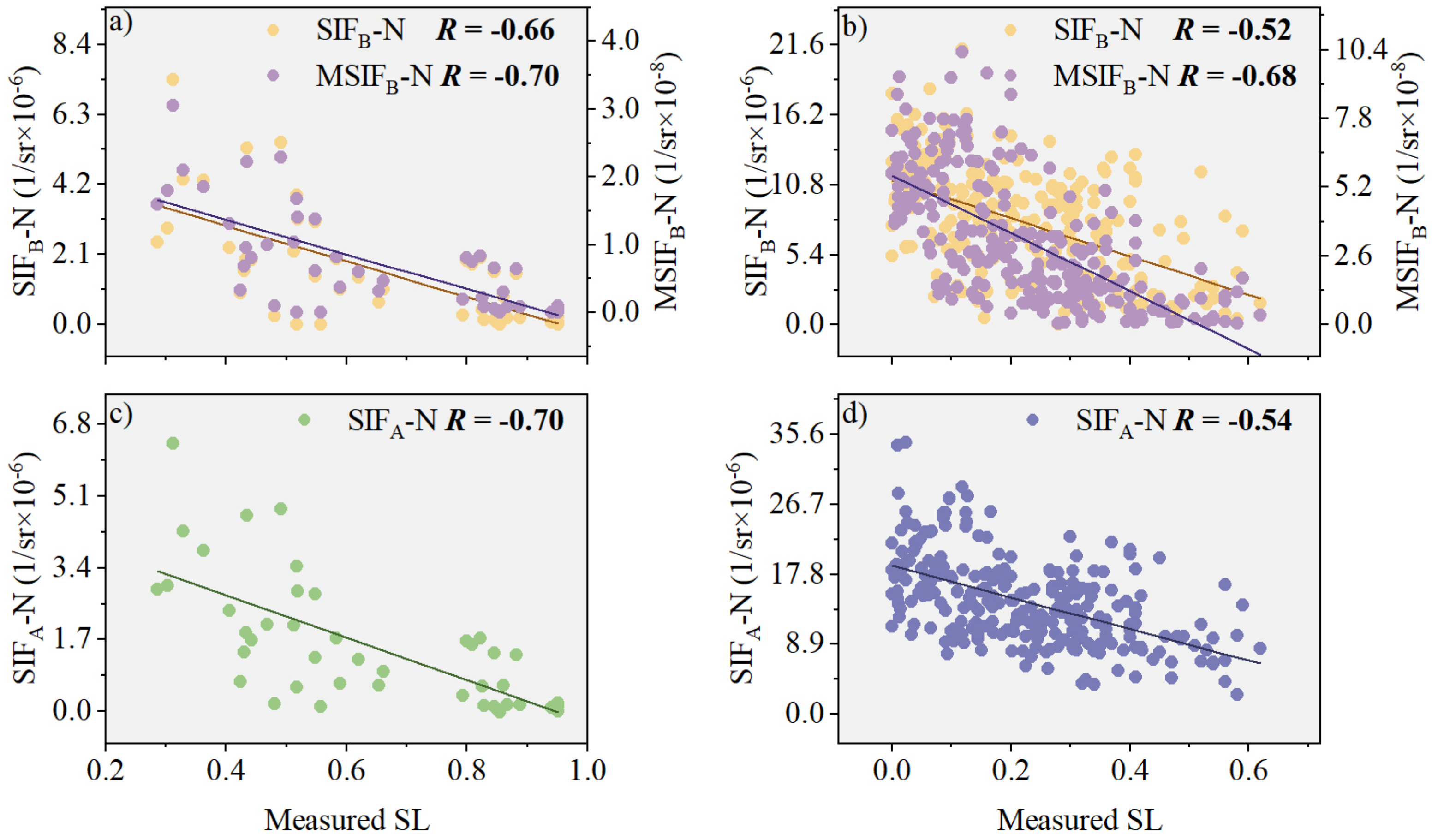
3.2.1. Performance Analysis of MSIFB-N in Monitoring Wheat Stripe Rust SL
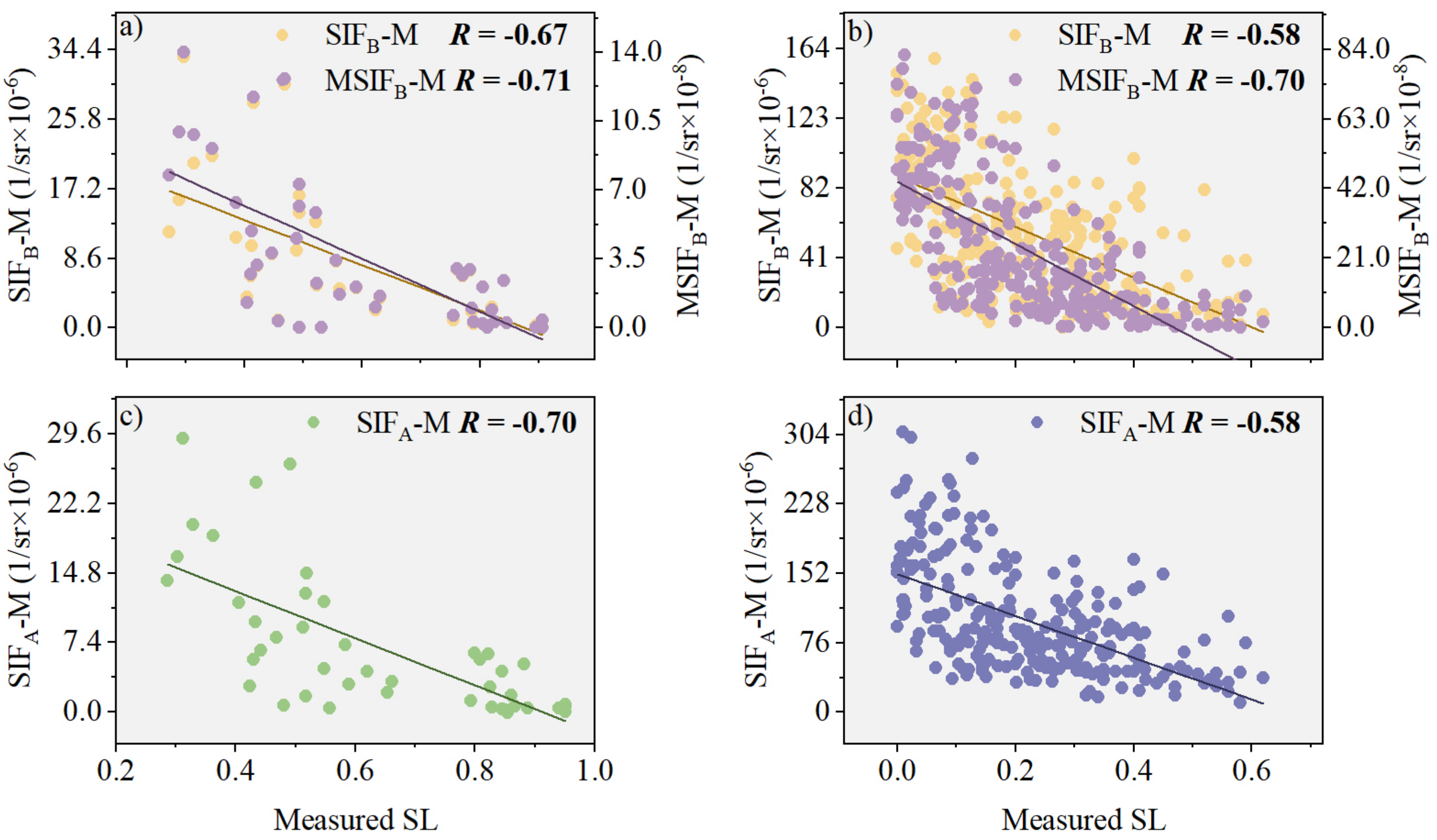
3.2.2. Performance Analysis of MSIFB-M in Monitoring Wheat Stripe Rust SL
3.2.3. Performance Analysis of MSIFB-NM in the Monitoring of the Wheat Stripe Rust SL
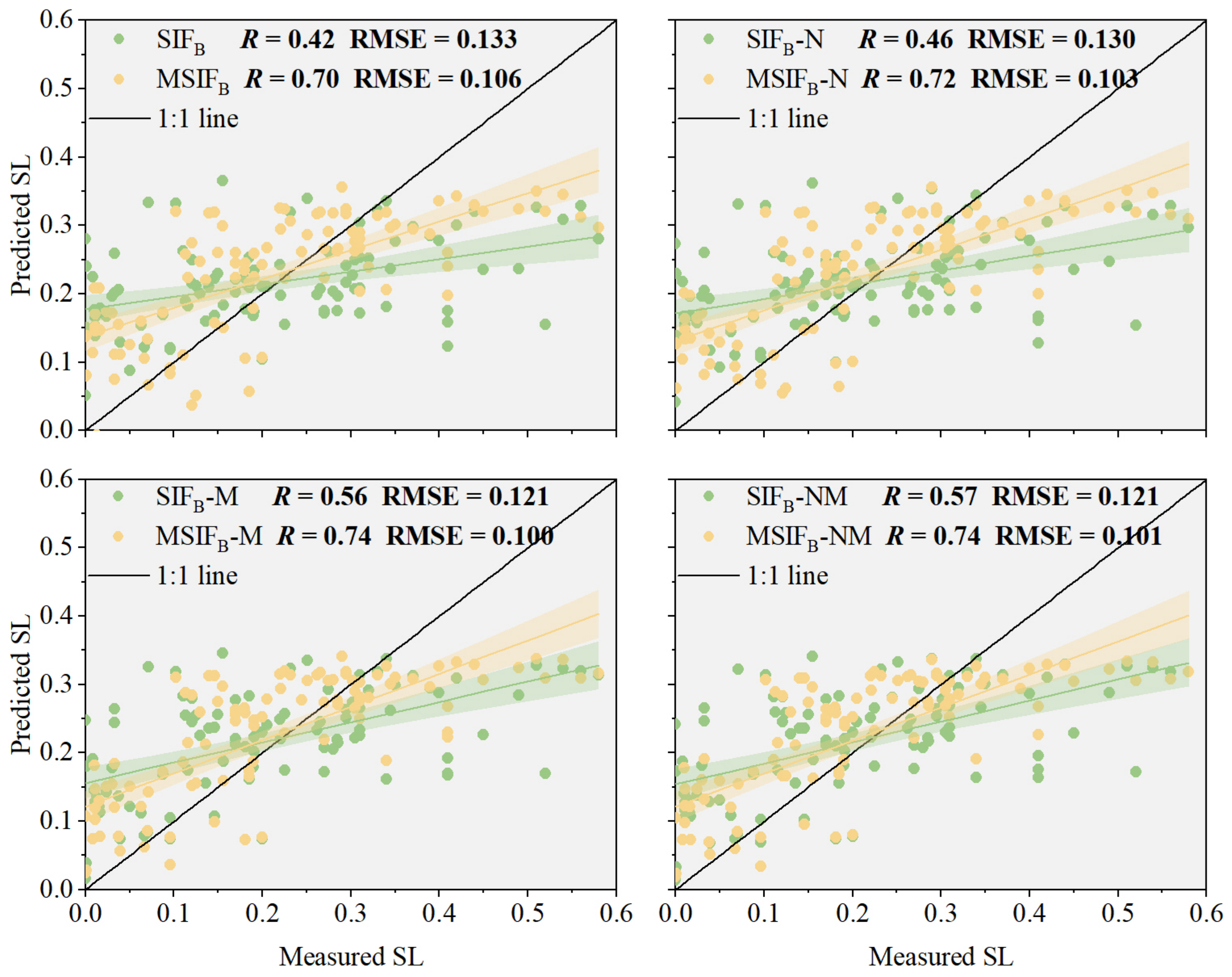
3.3. Model Evaluation of PKB for Improved Performance of SIFB
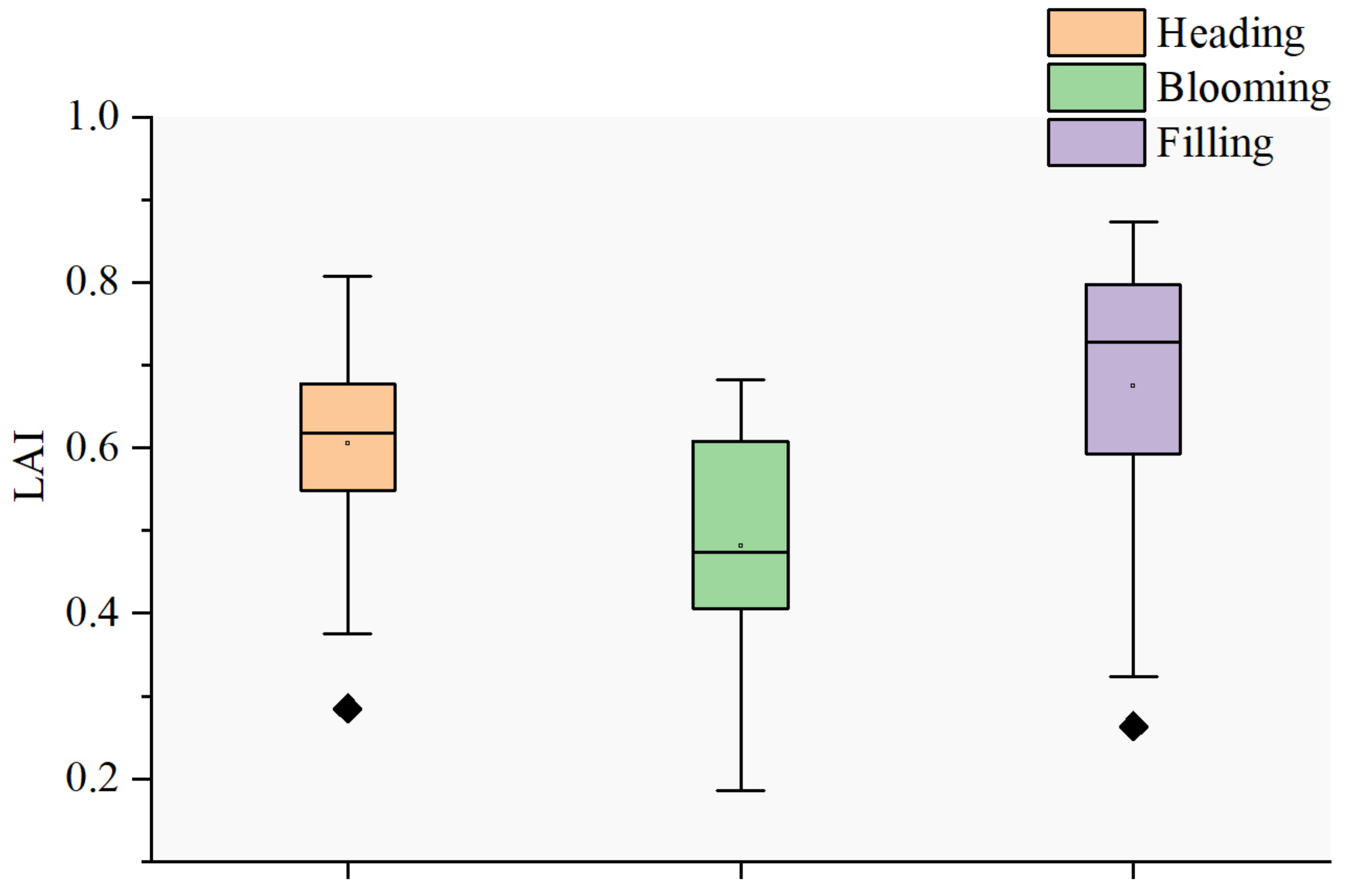
3.4. Changes in SL, SIFB, MSIFB, and Cab with Growth Period
4. Discussion
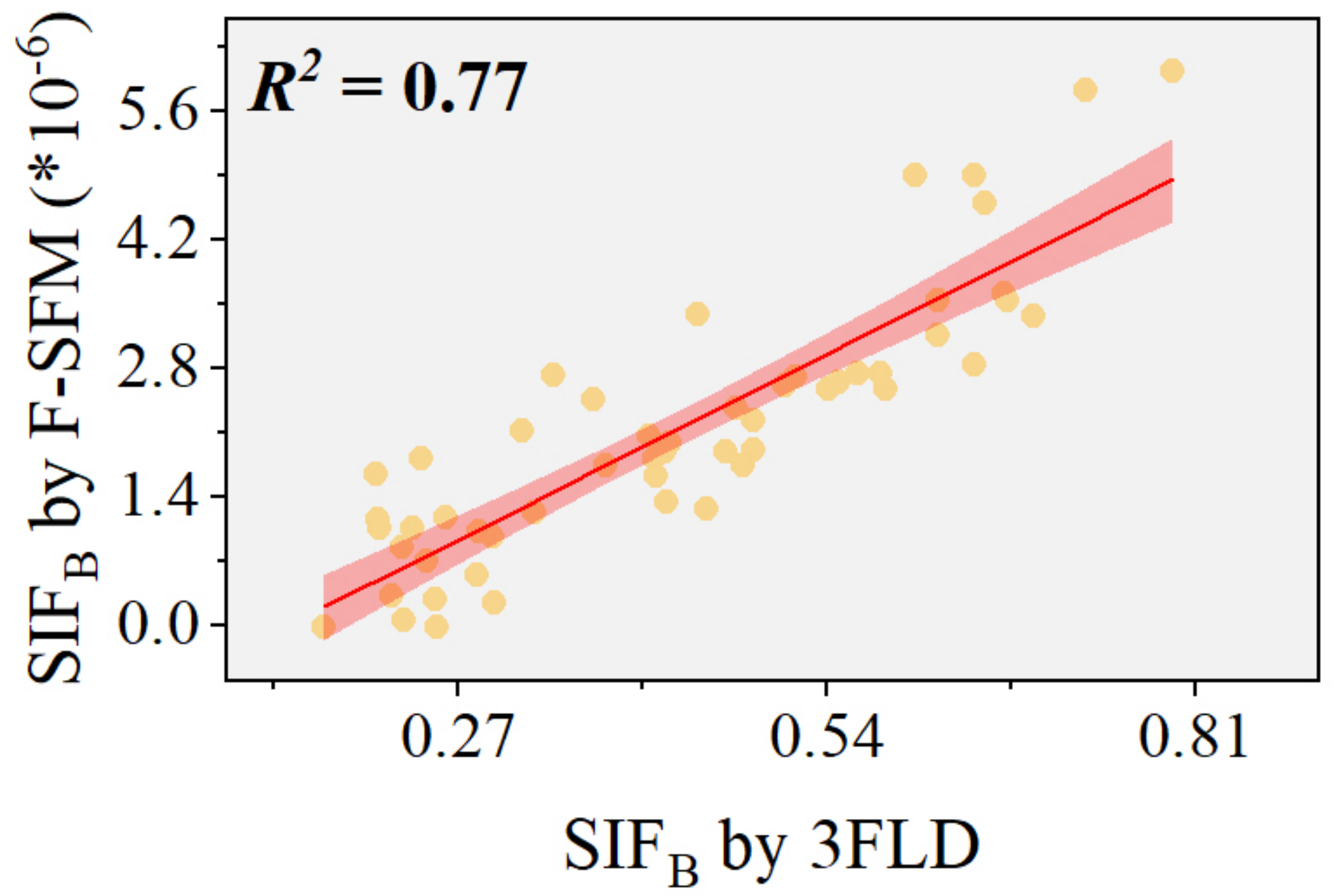
4.1. The Accuracy of F-SFM in Calculating PKB
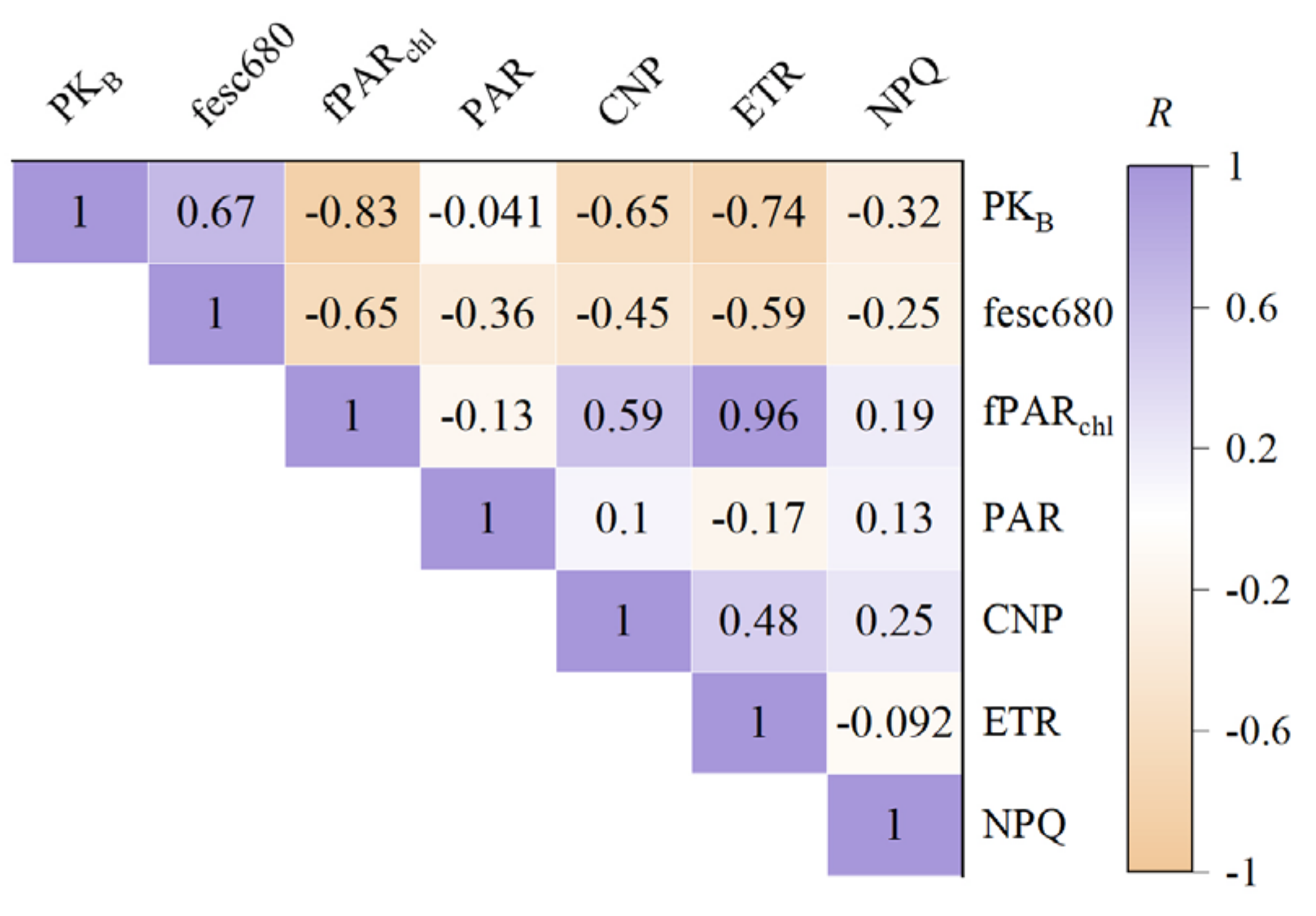
4.2. Physiological Mechanism of MSIFB Improving Remote Sensing Monitoring of Wheat Stripe Rust
4.3. Analysis of the Correlation between MSIFB and the SL Improved by VIs
4.4. Effect of Growth Period
4.5. Prospect
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soomro, S.; Samoo, M.S.; Jamali, A.R.; Channa, G.S. Assessing leaf rust resistance in Pakistani wheat landraces and its impact on grain yield. Plant Prot. 2024, 8, 269–274. [Google Scholar]
- Hussain, S.; Shah, S.J.A.; Leconte, M.; de Vallavieille-Pope, C. Assessment of genetic variability for wheat yellow rust resistance and Puccinia striiformis f. sp. tritici pathotypes from Pakistan. Plant Prot. 2024, 8, 239–255. [Google Scholar]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, 1327–1333. [Google Scholar] [CrossRef]
- Ashourloo, D.; Mobasheri, M.; Huete, A. Developing Two Spectral Disease Indices for Detection of Wheat Leaf Rust (Pucciniatriticina). Remote Sens. 2014, 6, 4723–4740. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.M.; Guanter, L.; He, L.; Zhang, Y. From Canopy-Leaving to Total Canopy Far-Red Fluorescence Emission for Remote Sensing of Photosynthesis: First Results From TROPOMI. Geophys. Res. Lett. 2019, 46, 12030–12040. [Google Scholar] [CrossRef]
- Wieneke, S.; Ahrends, H.; Damm, A.; Pinto, F.; Stadler, A.; Rossini, M.R.U. Airborne based spectroscopy of red and far-red sun-induced chlorophyll fluorescence: Implications for improved estimates of gross primary productivity. Remote Sens. Environ. 2016, 184, 654–667. [Google Scholar] [CrossRef]
- Somkuti, P.; Bösch, H.; Feng, L.; Palmer, P.I.; Parker, R.J.; Quaife, T. A new space-borne perspective of crop productivity variations over the US Corn Belt. Agric. For. Meteorol. 2020, 281, 107826. [Google Scholar] [CrossRef]
- Duan, W.; Jing, X.; Liu, L. Monitoring of Wheat Stripe Rust Based on Integration of SIF and Reflectance Spectrum. Spectrosc. Spectr. Anal. 2022, 42, 859–865. [Google Scholar]
- Jing, X.; Li, B.; Ye, Q.; Zou, Q.; Yan, J.; Du, K. Integrate the Canopy SIF and Its Derived Structural and Physiological Components for Wheat Stripe Rust Stress Monitoring. Remote Sens. 2022, 14, 3427. [Google Scholar] [CrossRef]
- Knyazikhin, Y.; Schull, M.A.; Stenberg, P.; Moettus, M.; Rautiainen, M.; Yang, Y.; Marshak, A.; Carmona, P.L.; Kaufmann, R.K.; Lewis, P. Hyperspectral remote sensing of foliar nitrogen content. Proc. Natl. Acad. Sci. USA 2012, 110, E185–E192. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.; Yoshida, Y.; Guanter, L.; Middleton, E.M. New methods for the retrieval of chlorophyll red fluorescence from hyperspectral satellite instruments: Simulations and application to GOME-2 and SCIAMACHY. Atmos. Meas. Tech. 2016, 9, 3939–3967. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjrvi, E.; Atherton, J.; Tol, C.V.D.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Wittenberghe, S.V.; Alonso, L.; Verrelst, J.; Moreno, J.; Samson, R. Bidirectional sun-induced chlorophyll fluorescence emission is influenced by leaf structure and light scattering properties—A bottom-up approach. Remote Sens. Environ. 2015, 158, 169–179. [Google Scholar] [CrossRef]
- Huang, C. Study on Remote Sensing Inversion Method of Vegetation Chlorophyll Fluorescence Induced by Sunlight; University of Chinese Academy of Sciences: Beijing, China, 2013. [Google Scholar]
- Liu, X.; Liu, L.; Hu, J.; Guo, J.; Du, S. Improving the potential of red SIF for estimating GPP by downscaling from the canopy level to the photosystem level. Agric. For. Meteorol. 2020, 281, 107846. [Google Scholar] [CrossRef]
- Yang, P.; van der Tol, C. Linking canopy scattering of far-red sun-induced chlorophyll fluorescence with reflectance. Remote Sens. Environ. 2018, 209, 456–467. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, X.; An, S.; Heskel, M.; Tang, J. Advantage of multi-band solar-induced chlorophyll fluorescence to derive canopy photosynthesis in a temperate forest. Agric. For. Meteorol. 2019, 279, 107691. [Google Scholar] [CrossRef]
- Ren, K.; Dong, Y.; Huang, W.; Guo, A.; Jing, X. Monitoring of winter wheat stripe rust by collaborating canopy SIF with wavelet energy coefficients. Comput. Electron. Agric. 2023, 215, 108366. [Google Scholar] [CrossRef]
- Du, K.; Jing, X.; Zeng, Y.; Ye, Q.; Li, B.; Huang, J. An Improved Approach to Monitoring Wheat Stripe Rust with Sun-Induced Chlorophyll Fluorescence. Remote Sens. 2023, 15, 693. [Google Scholar] [CrossRef]
- GB/T15795-2011; Technical Specifications for Forecasting Wheat Stripe Rust. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China; China National Standardization Management Committee: Beijing, China, 2011. [Google Scholar]
- Camacho, F.; Lacaze, R.; Weiss, M. GEOV1: LAI, FAPAR essential climate variables and FCOVER global time series capitalizing over existing products. Part 2: Validation and intercomparison with reference products. Remote Sens. Environ. 2013, 137, 310–329. [Google Scholar] [CrossRef]
- Vilfan, N.; Van der Tol, C.; Muller, O.; Rascher, U.; Verhoef, W. Fluspect-B: A model for leaf fluorescence, reflectance and transmittance spectra. Remote Sens. Environ. Interdiscip. J. 2016, 186, 596–615. [Google Scholar] [CrossRef]
- Vander Tol, C.; Verhoef, W.; Timmermans, J.; Verhoef, A.; Su, Z. An integrated model of soil-canopy spectral radiances, photosynthesis, fluorescence, temperature and energy balance. Biogeosciences 2009, 6, 3109–3129. [Google Scholar] [CrossRef]
- Liu, X.; Guanter, L.; Liu, L.; Damm, A.; Zbyněk, M.; Rascher, U.; Peng, D.; Du, S.; Gastellu-Etchegorry, J.P. Downscaling of solar-induced chlorophyll fluorescence from canopy level to photosystem level using a random forest model. Remote Sens. Environ. 2019, 231, 110772. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; He, Q.; Zhou, H. Quantitative Response of Maize Vcmax25 to Persistent Drought Stress at Different Growth Stages. Water 2021, 13, 1971. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Zhang, S.; Zhou, X. New Spectral Fitting Method for Full-Spectrum Solar-Induced Chlorophyll Fluorescence Retrieval Based on Principal Components Analysis. Remote Sens. 2015, 7, 10626. [Google Scholar] [CrossRef]
- Du, S.; Liu, L.; Liu, X.; Hu, J. Response of Canopy Solar-Induced Chlorophyll Fluorescence to the Absorbed Photosynthetically Active Radiation Absorbed by Chlorophyll. Remote Sens. 2017, 9, 911. [Google Scholar] [CrossRef]
- Goulas, Y.; Fournier, A.; Daumard, F.; Champagne, S.; Ounis, A.; Marloie, O.; Moya, I. Gross Primary Production of a Wheat Canopy Relates Stronger to Far Red Than to Red Solar-Induced Chlorophyll Fluorescence. Remote Sens. 2017, 9, 97. [Google Scholar] [CrossRef]
- Radoti, K.; Mel, T.B. Using optical fibers to measure absorption in intact conifer leaves, relative numbers of chloroplasts, and pigment content. J. Biol. Phys. 2020, 46, 33–43. [Google Scholar] [CrossRef]
- Bump, W. The Normal Curve Takes Many Forms: A Review of Skewness and Kurtosis. 1991. Available online: https://files.eric.ed.gov/fulltext/ED342790.pdf (accessed on 2 July 2023).
- Rascher, U.; Alonso, L.; Burkart, A.; Cilia, C.; Zemek, F. Sun-induced fluorescence—A new probe of photosynthesis: First maps from the imaging spectrometer HyPlant. Glob. Chang. Biol. 2015, 21, 4673–4684. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L. The potential of the meris terrestrial chlorophyll index for crop yield prediction the potential of the meris terrestrial chlorophyll index for crop yield prediction. Remote Sens. Lett. 2014, 5, 733–742. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Hu, J.; Guan, L. Assessing the wavelength-dependent ability of solar-induced chlorophyll fluorescence to estimate the GPP of winter wheat at the canopy level. Int. J. Remote Sens. 2017, 38, 4396–4417. [Google Scholar] [CrossRef]
- Zhao, F.; Li, R.; Verhoef, W.; Cogliati, S.; Liu, X.; Huang, Y.; Guo, Y.; Huang, J. Reconstruction of the full spectrum of solar-induced chlorophyll fluorescence: Intercomparison study for a novel method. Remote Sens. Environ. 2018, 219, 233–246. [Google Scholar] [CrossRef]
- Scodellaro, R.; Cesana, I.; D’Alfonso, L.; Bouzin, M.; Collini, M.; Chirico, G.; Colombo, R.; Miglietta, F.; Celesti, M.; Schuettemeyer, D.; et al. A novel hybrid machine learning phasor-based approach to retrieve a full set of solar-induced fluorescence metrics and biophysical parameters. Remote Sens. Environ. 2022, 280, 113196. [Google Scholar] [CrossRef]
- Wang, C.; Guan, K.; Peng, B.; Chen, M.; Jiang, C.; Zeng, Y.; Wu, G.; Wang, S.; Wu, J.; Yang, X. Satellite footprint data from OCO-2 and TROPOMI reveal significant spatio-temporal and inter-vegetation type variabilities of solar-induced fluorescence yield in the US Midwest. Remote Sens. Environ. 2020, 241, 111728. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Malenovsk, Z.; Magney, T.; Wittenberghe, S.V.; Fernández-Marín, B.; Maignan, F.; Zhang, Y.; Maseyk, K.; Atherton, J.; Albert, L.P. Chlorophyll a fluorescence illuminates a path connecting plant molecular biology to Earth-system science. Nat. Plants 2021, 7, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Gladieux, P.; Leconte, M.; Gautier, A.; Justesen, A.F.; Hovmøller, M.S.; Enjalbert, J.M.; De Vallavieille-Pope, C.; Mcdonald, B.A. Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen Puccinia striiformis f.sp. tritici. PLoS Pathog. 2014, 10, e1003903. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, L.; Wang, S.; Chen, J.; Chen, B.; Zhu, K.; Amir, M.; Wang, X.; Liu, Y.; Wang, P.; et al. Analysis on the relationship between sun-induced chlorophyll fluorescence and gross primary productivity of winter wheat in northern China. Ecol. Indic. 2022, 139, 108905. [Google Scholar] [CrossRef]
- Dufranne, D.; Moureaux, C.; Vancutsem, F.; Bodson, B.; Aubinet, M. Comparison of carbon fluxes, growth and productivity of a winter wheat crop in three contrasting growing seasons. Agric. Ecosyst. 2011, 141, 133–142. [Google Scholar] [CrossRef]
- Kangasjrvi, S.; Tikkanen, M.; Durian, G.; Aro, E.M. Photosynthetic light reactions—An adjustable hub in basic production and plant immunity signaling. Plant Physiol. Biochem. 2014, 81, 128–134. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Bowling, S.A.; Guo, A.; Cao, H.; Gordon, A.S.; Dong, K.X. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell Online 1994, 6, 1845–1857. [Google Scholar]
- Hu, Y.; Zhong, S.; Zhang, M.; Liang, Y.; Gong, G.; Chang, X.; Tan, F.; Yang, H.; Qiu, X.; Luo, L. Potential Role of Photosynthesis in the Regulation of Reactive Oxygen Species and Defence Responses to Blumeria graminis f. sp. tritici in Wheat. Int. J. Mol. Sci. 2020, 21, 57–67. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Unit | Values | Unit Description |
|---|---|---|---|
| Cab | ug cm−2 | 5–80 interval 5 | Chlorophyll AB content |
| Cdm | g cm−2 | 0.002, 0.01, 0.02 | Dry matter content |
| Cw | cm | 0.005, 0.01, 0.02, 0.04 | Leaf water equivalent layer |
| N | - | 1, 2 | Leaf thickness parameters |
| qLs | - | 1, 0.5 | Fraction of functional reaction centers |
| Stressfactor | - | 1, 3, 5 | Stress factor to reduce Vcmax |
| LAI | m2 m−2 | 1, 2, 4, 6 | Leaf area index |
| SZA | Degree | 20, 60 | Solar zenith angle |
| VZA | Degree | 0, 30, 60 | Observation zenith angle |
| RAA | Degree | 90 | Relative azimuth angle |
| Vcmax25 | umol m−2 s−1 | 60 | Maximum carboxylation capacity |
| Parameter | Unit | Values | Unit Description |
|---|---|---|---|
| Cab | ug cm−2 | 5, 20, 40, 60, 80 | Chlorophyll AB content |
| Cdm | g cm−2 | 0.002,0.01, 0.02 | Dry matter content |
| Cw | cm | 0.005, 0.01, 0.02, 0.04 | Leaf water equivalent layer |
| N | - | 1, 2 | Leaf thickness parameters |
| qLs | - | 1, 0.5 | Fraction of functional reaction centers |
| stressfactor | - | 1, 3, 5 | Stress factor to reduce Vcmax |
| LAI | m2 m−2 | 1, 2, 4, 6 | Leaf area index |
| SZA | Degree | 20, 60 | Solar zenith angle |
| VZA | Degree | 0, 30, 60 | Oobservation zenith angle |
| RAA | Degree | 90 | Relative azimuth angle |
| FQE | - | 0.04 | Fluorescence quantum yield Efficiency at photosystem level |
| Vcmax25 | umol m−2 s−1 | 60 | Maximum carboxylation capacity |
| Independent Variable | R | RMSE |
|---|---|---|
| SIFB | 0.536 | 0.133 |
| MSIFB | 0.650 | 0.121 |
| SIFB-N | 0.554 | 0.131 |
| MSIFB-N | 0.664 | 0.118 |
| SIFB-M | 0.594 | 0.128 |
| MSIFB-M | 0.671 | 0.117 |
| SIFB-NM | 0.597 | 0.128 |
| MSIFB-NM | 0.667 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, X.; Ye, Q.; Chen, B.; Li, B.; Du, K.; Xue, Y. Monitoring of Wheat Stripe Rust Using Red SIF Modified by Pseudokurtosis. Agronomy 2024, 14, 1698. https://doi.org/10.3390/agronomy14081698
Jing X, Ye Q, Chen B, Li B, Du K, Xue Y. Monitoring of Wheat Stripe Rust Using Red SIF Modified by Pseudokurtosis. Agronomy. 2024; 14(8):1698. https://doi.org/10.3390/agronomy14081698
Chicago/Turabian StyleJing, Xia, Qixing Ye, Bing Chen, Bingyu Li, Kaiqi Du, and Yiyang Xue. 2024. "Monitoring of Wheat Stripe Rust Using Red SIF Modified by Pseudokurtosis" Agronomy 14, no. 8: 1698. https://doi.org/10.3390/agronomy14081698
APA StyleJing, X., Ye, Q., Chen, B., Li, B., Du, K., & Xue, Y. (2024). Monitoring of Wheat Stripe Rust Using Red SIF Modified by Pseudokurtosis. Agronomy, 14(8), 1698. https://doi.org/10.3390/agronomy14081698





