Optimization of Harvesting and Drying Techniques for Quality Seed Production in Specialty Crops: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methodology
2.1. Systematic Search
2.2. Publication Selection
- Unrelated studies: articles not pertinent to seed quality such as those focusing on plant genetic improvements, nutrition and fertilization, diseases and pathogens, and the effects of herbicides and desiccants.
- Studies on field crops: research that did not pertain to specialty crops, including soy, cotton, the common bean, peanut, quinoa, rice, and corn.
- Studies on tree species: articles addressing tree species not classified as specialty crops; for example, star fruit and palm trees.
- Studies excluding harvest or drying methods: research evaluating seed quality without directly incorporating harvest or drying methods such as those examining the maturation and physiological maturity of seeds as well as storage and seed conditioning (priming).
- Non-extractable data: Studies that precluded data extraction due to illegible graphical representations such as overlapping points in regression graphs.
- Yield-focused studies: research presenting yield indicators, including the number of pods per plant, number of seeds per plant, or quantity of seeds per fruit.
2.3. Data Collection
2.4. Statistical Data Analysis
3. Results
3.1. Literature Characterization
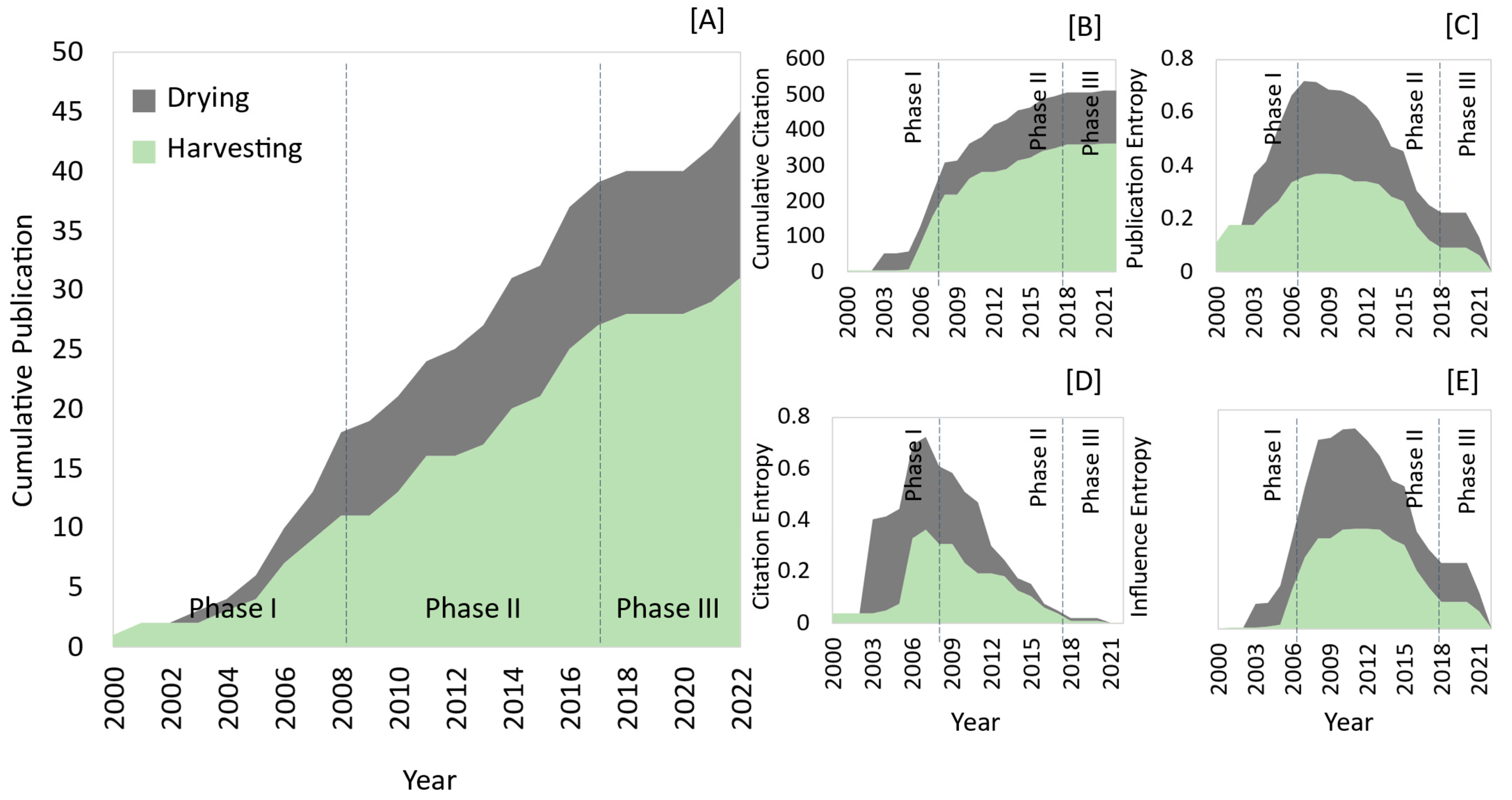
3.1.1. Dynamic Timeline and Engagement
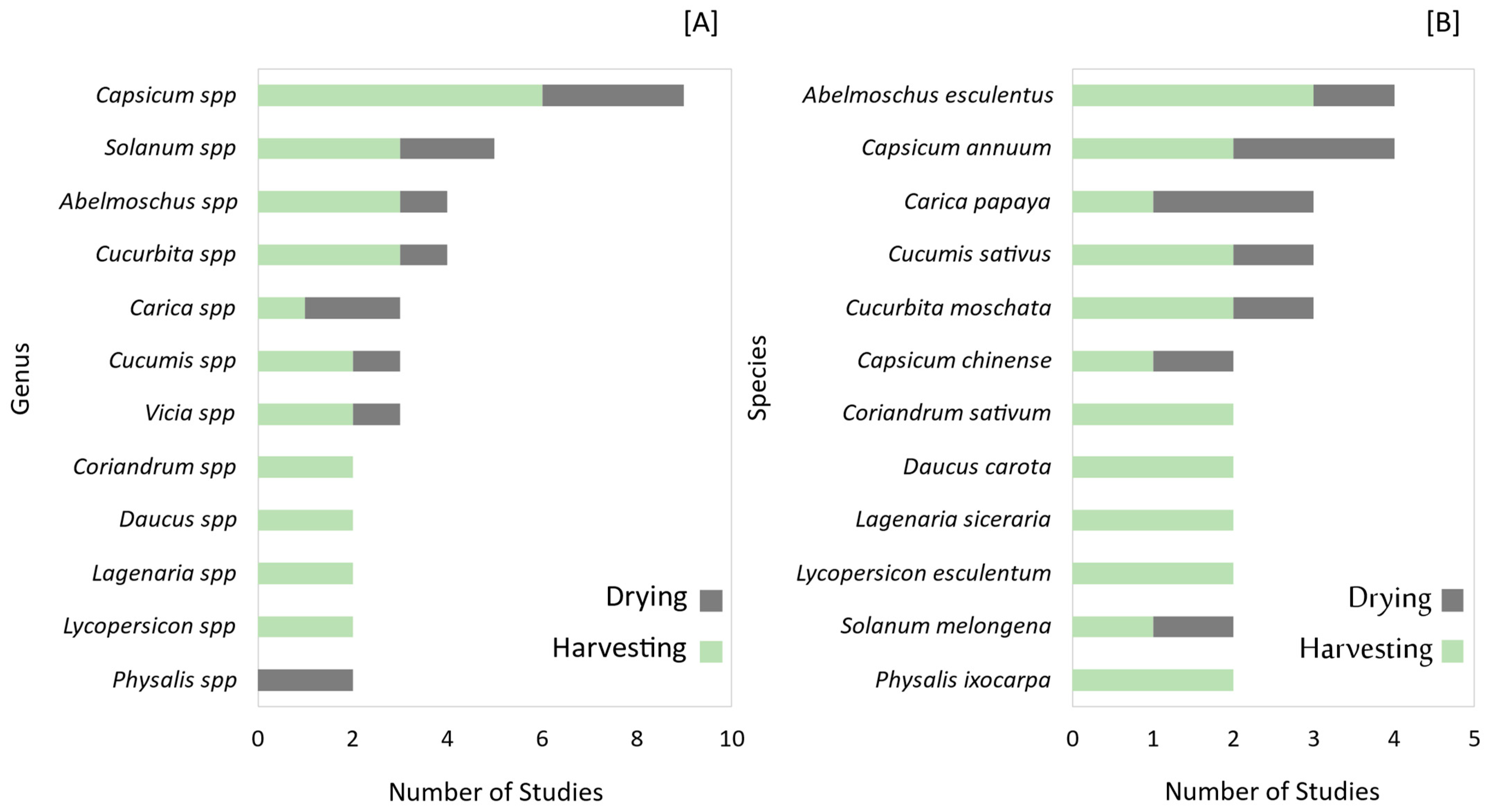
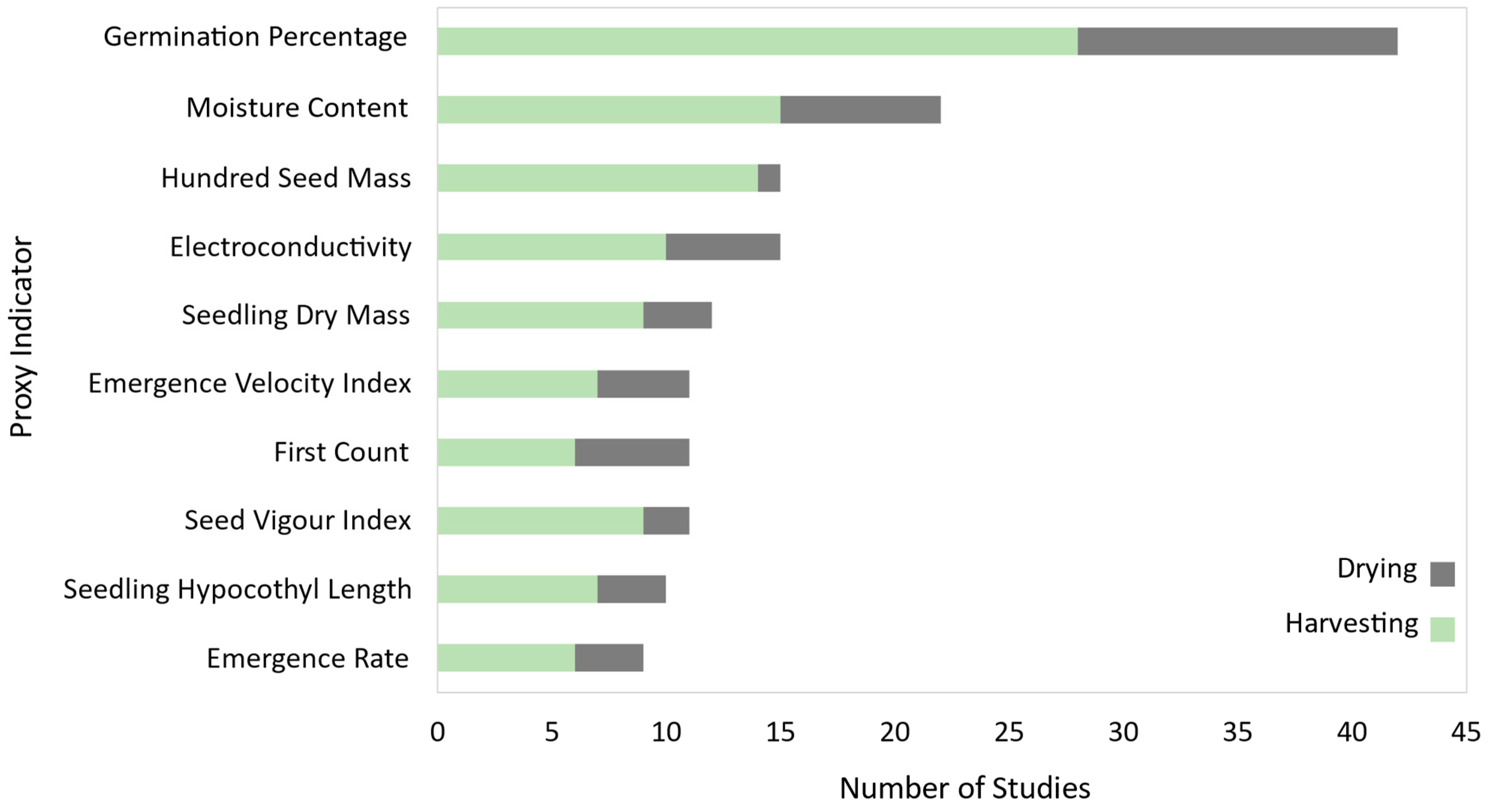
3.1.2. Most Influential Genres, Species, and Physiological Indicators
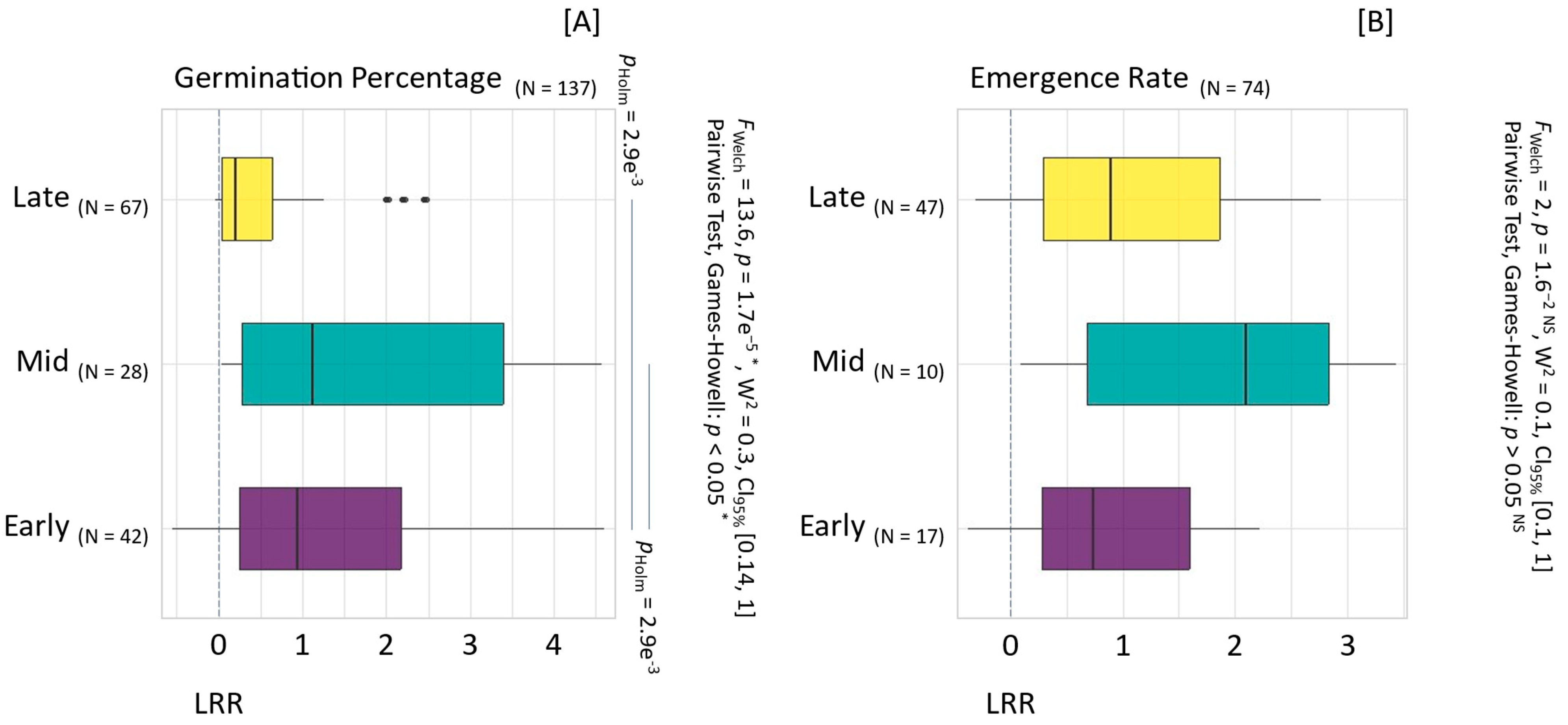
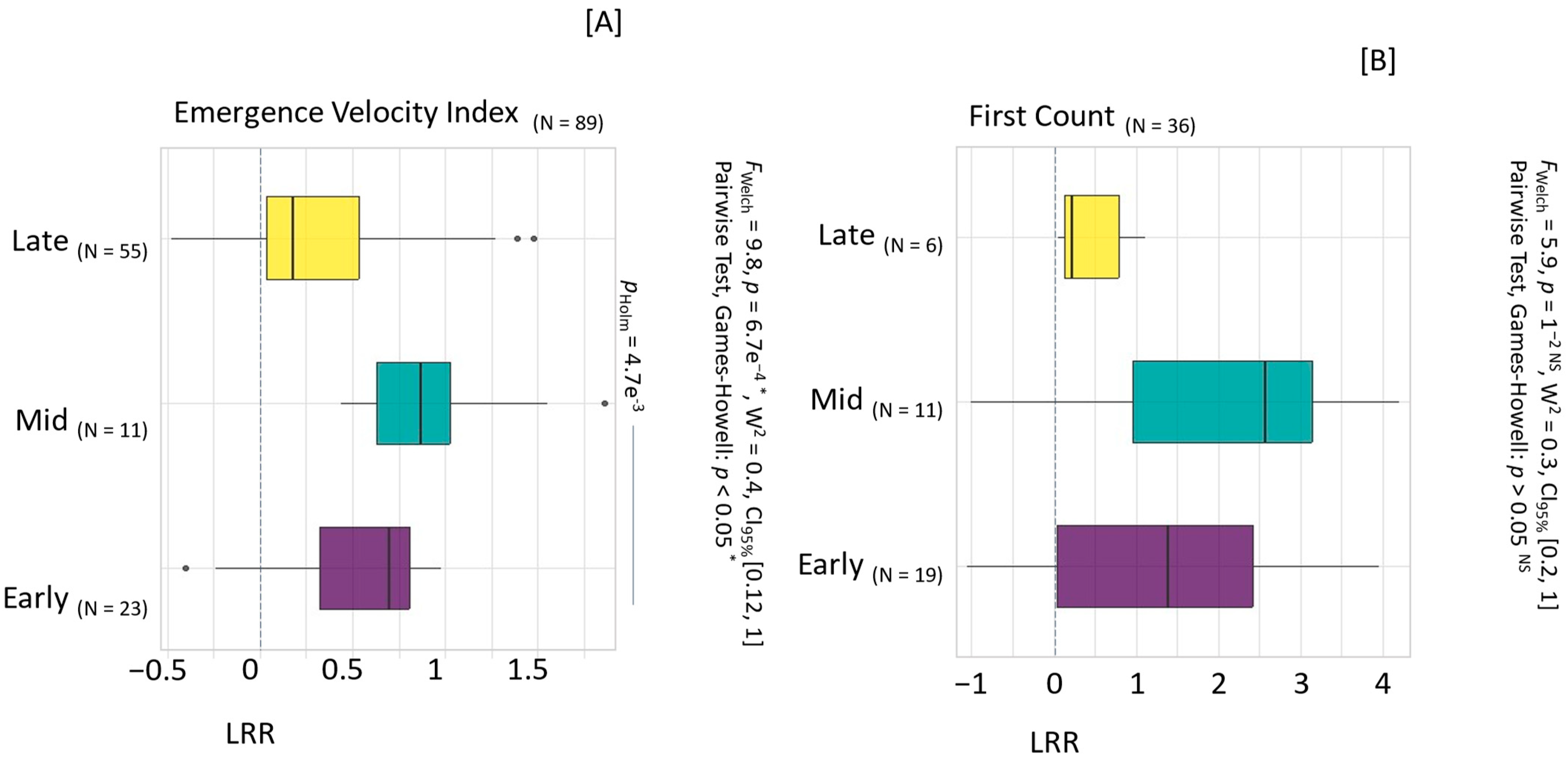
3.2. Impact of Harvesting on Seed Quality
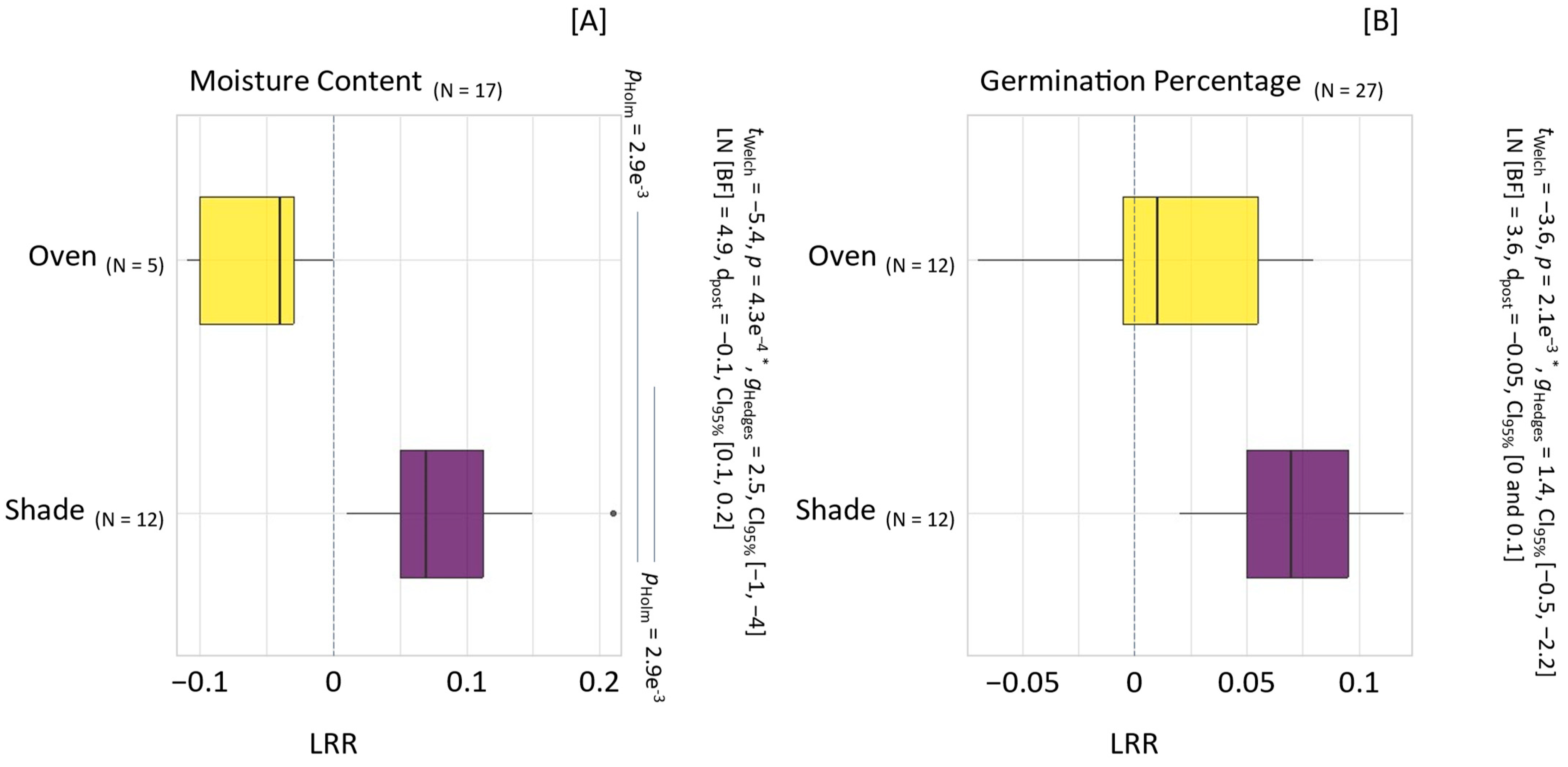
3.3. Impact of Drying on Seed Quality
4. Discussion
4.1. Key Learning
4.1.1. Harvesting and Drying Techniques Validated to Optimize Seed Quality
4.1.2. Declining Trends in Publication Entropy
4.1.3. Uneven Representation of Genres and Species
4.1.4. Inadequacy of Traditional Indicators to Describe Physiological Quality
4.1.5. Effectiveness of Shade- and Oven-Drying
4.1.6. Impact of Drying Temperatures on Seed Viability
4.2. Main Gaps Identified
4.2.1. Insufficient Species-Specific Data
4.2.2. Lack of Detailed Experimental Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, Z.A.; Khan, A.; Lone, A.A.; Bahar, F.A.; Baghel, S.; Lone, B.A.; Mahdi, S.S.; Jan, R.; Wani, O.A.; Nissar, R.; et al. Specialty Food Crops: An Alternate Way for Increasing Farmers’ Income. In Secondary Agriculture: Sustainability and Livelihood in India; Bahar, F.A., Anwar Bhat, M., Mahdi, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 59–68. ISBN 978-3-031-09218-3. [Google Scholar]
- Ojuederie, O.B.; Igwe, D.O.; Ludidi, N.N.; Ikhajiagbe, B. Editorial: Neglected and Underutilized Crop Species for Sustainable Food and Nutritional Security: Prospects and Hidden Potential. Front. Plant Sci. 2024, 14, 1358220. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.P.S.; Adhikari, R.; Bhatta, D.; Poudel, A.; Subedi, S.; Shrestha, S.; Shrestha, J. Initiatives for Biodiversity Conservation and Utilization in Crop Protection: A Strategy for Sustainable Crop Production. Biodivers. Conserv. 2023, 32, 4573–4595. [Google Scholar] [CrossRef]
- Kim, H.-J. Opportunities and Challenges of Alternative Specialty Crops: The Global Picture. HortScience 2016, 51, 1316–1319. [Google Scholar] [CrossRef]
- Alan, O.; Eser, B. Pepper Seed Yield and Quality in Relation to Fruit Position on the Mother Plant. Pak. J. Biol. Sci. 2007, 10, 4251–4255. [Google Scholar] [CrossRef] [PubMed]
- Hedau, N.K.; Singh, G.; Mahajan, V.; Singh, S.R.K.; Gahlain, A. Seed Quality and Vigour in Relation to Nodal Position and Harvesting Stage of Okra under Mid Hills of North-Western Himalayas. Indian J. Hortic. 2010, 67, 251–253. [Google Scholar]
- Dwivedi, S.L.; Spillane, C.; Lopez, F.; Ayele, B.T.; Ortiz, R. First the Seed: Genomic Advances in Seed Science for Improved Crop Productivity and Food Security. Crop Sci. 2021, 61, 1501–1526. [Google Scholar] [CrossRef]
- Ligterink, W.; Joosen, R.V.L.; Hilhorst, H.W.M. Unravelling the Complex Trait of Seed Quality: Using Natural Variation through a Combination of Physiology, Genetics and -Omics Technologies. Seed Sci. Res. 2012, 22, S45–S52. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y. Research on Multilateral Collaboration Strategies in Agricultural Seed Quality Assurance. Sci. Rep. 2024, 14, 11310. [Google Scholar] [CrossRef] [PubMed]
- Alkimim, E.R.; David, A.M.S.d.S.; Sousa, T.V.; Rodrigues, C.G.; Amaro, H.T.R. Different Harvest Times and Physiological Quality of Coriander Seeds. Rev. Bras. Eng. Agríc. Ambient. 2016, 20, 133–137. [Google Scholar] [CrossRef]
- Vinod, K.; Tomar, B.S.; Kaddi, G.; Kumar, S. Effect of Stage of Harvest and Post-Harvest Ripening of Fruits on Hybrid Seed Yield and Quality in Pumpkin (Cucurbita moschata). Indian J. Agric. Sci. 2014, 84, 737–741. [Google Scholar] [CrossRef]
- Kumar, V.; Tomar, B.S.; Singh, B.; Kumar, S. Effect of Post-Harvest Ripening and Drying Methods on Seed Quality and Storability in Pumpkin Cv Pusa Hybrid 1. Indian J. Agric. Sci. 2014, 84, 1144–1148. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.B. Seed Drying. In Principles of Seed Science and Technology; Springer: Boston, MA, USA, 2001; pp. 268–276. ISBN 978-1-4615-1619-4. [Google Scholar]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed Storage: Maintaining Seed Viability and Vigor for Restoration Use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Jangde, P.K.; Singh, A.; Arjunan, T.V. Efficient Solar Drying Techniques: A Review. Environ. Sci. Pollut. Res. 2022, 29, 50970–50983. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, A.M.; Sinniah, U.R.; Misran, A. Seed Quality of Lablab Beans (Lablab purpureus L.) as Influenced by Drying Methods and Storage Temperature. Agronomy 2022, 12, 699. [Google Scholar] [CrossRef]
- Singh, N.; Bhuker, A.; Jeevanadam, J. Effects of Metal Nanoparticle-Mediated Treatment on Seed Quality Parameters of Different Crops. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 1067–1089. [Google Scholar] [CrossRef] [PubMed]
- Eevera, T.; Kumaran, S.; Djanaguiraman, M.; Thirumaran, T.; Le, Q.H.; Pugazhendhi, A. Unleashing the Potential of Nanoparticles on Seed Treatment and Enhancement for Sustainable Farming. Environ. Res. 2023, 236, 116849. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shahnaz, T.; Zehab-ud-Din; Masoodi, J.H.; Nazir, S.; Qurashi, A.; Ahmed, G.H. Application of Nanotechnology in the Agricultural and Food Processing Industries: A Review. Sustain. Mater. Technol. 2024, 39, e00809. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Puchalski, C. Raman Method in Identification of Species and Varieties, Assessment of Plant Maturity and Crop Quality—A Review. Molecules 2022, 27, 4454. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, E. Nuclear DNA Replication and Seed Quality. Seed Sci. Res. 2009, 19, 15–25. [Google Scholar] [CrossRef]
- Pedreschi, R.; Lurie, S.; Hertog, M.; Nicolaï, B.; Mes, J.; Woltering, E. Post-Harvest Proteomics and Food Security. Proteomics 2013, 13, 1772–1783. [Google Scholar] [CrossRef]
- Franca-Neto, J.D.B.; Krzyzanowski, F.C. Use of the Tetrazolium Test for Estimating the Physiological Quality of Seeds. Seed Sci. Technol. 2022, 50, 31–44. [Google Scholar] [CrossRef]
- Barrozo, M.A.S.; Mujumdar, A.; Freire, J.T. Air-Drying of Seeds: A Review. Dry. Technol. 2014, 32, 1127–1141. [Google Scholar] [CrossRef]
- West, R.M. Best Practice in Statistics: Use the Welch t-Test When Testing the Difference between Two Groups. Ann. Clin. Biochem. 2021, 58, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Avalos-Pacheco, A.; Russo, M.; De Vito, R. A Variational Bayes Approach to Factor Analysis. In Proceedings of the Bayesian Statistics, New Generations New Approaches; Avalos-Pacheco, A., De Vito, R., Maire, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 15–21. [Google Scholar]
- Chen, R.X. A Brief Introduction to Shannon’s Information Theory. arXiv 2021, arXiv:161209316. [Google Scholar]
- Schwitter, P.; Detterbeck, A.; Herforth-Rahme, J. Effect of Harvest Frequency, Seed Extraction Time Point and Post-Harvest Cooling on Organic Tomato Seed Production. Sustainability 2022, 14, 11575. [Google Scholar] [CrossRef]
- Pathania, K.; Kaur, N.; Kaur, D.; Singh, R. Mobilization of Fruit Pulp Reserves towards Seeds in Cucumber (Cucumis sativus L.) Fruits Harvested at Variable Developmental Stages. Plant Physiol. Rep. 2022, 27, 268–274. [Google Scholar] [CrossRef]
- Tetteh, R.; Aboagye, L.M.; Boateng, S.K.; Darko, R. Seed Quality of Six Eggplant Cultivars as Influenced by Harvesting Time. J. Appl. Hortic. 2021, 23, 24–27. [Google Scholar] [CrossRef]
- García-Ruiz, R.F.; Castañeda-Garzón, S.L.; Valdéz-Hernández, E.F. Quality of Rocoto Pepper (Capsicum Pubescens Ruiz & Pav.) Seeds in Relation to Extraction Timing. Acta Agron. 2017, 67, 246–251. [Google Scholar] [CrossRef]
- Ruiz, M.B.; Parera, C.A. Effect of Harvesting Time on Seed Quality of Two Bell Pepper Cultivars (Capsicum annuum). Rev. Fac. Cienc. Agrar. 2017, 49, 67–77. [Google Scholar]
- Sharma, R.K.; Tomar, B.S.; Jain, S.K.; Nehra, M. Seed Yield and Quality Attributes in Bottle Gourd (Lagenaria Siceraria) as Influenced by Position of Seed in Fruit. Indian J. Agric. Sci. 2017, 87, 1320–1323. [Google Scholar] [CrossRef]
- Khan, H.; Bedi, S.; Singh, R. Seed Quality in Pumpkin (Cucurbita Pepo L) Cv. Punjab Samrat as Affected by Fruit Harvesting and Storage Duration. Agric. Res. J. 2016, 53, 220–223. [Google Scholar] [CrossRef]
- Bortey, H.M.; Dzomeku, B.M. Fruit and Seed Quality of Okra [Abelmoschus esculentus (L.) Moench] as Influenced by Harvesting Stage and Drying Method. Indian J. Agric. Res. 2016, 50, 330–334. [Google Scholar] [CrossRef]
- Mengarda, L.H.G.; Lopes, J.C. Seed Quality and Initial Seedling Growth of Malagueta Pepper and Their Relationship to the Position of Fruit Collection. Rev. Bras. Sementes 2012, 34, 644–650. [Google Scholar] [CrossRef]
- Kalyanrao; Tomar, B.S.; Singh, B. Effect of Stage of Harvest and Post Harvest Ripening on Hybrid Seed Yield and Quality in Bottle Gourd. Indian J. Hortic. 2014, 71, 428–432. [Google Scholar]
- Pereira, F.E.C.B.; Torres, S.B.; Silva, M.I.d.L.; Grangeiro, L.C.; Benedito, C.P. Physiological Quality of Pepper Seeds in Relation to Age and Period of Post-Harvest Resting. Rev. Cienc. Agron. 2014, 45, 737–744. [Google Scholar] [CrossRef]
- Yogeesha, H.S.; Vasugi, C.; Somashekhar; Bhanuprakash, K.; Naik, L.B. Papaya (Carica papaya) Seed Quality as Influenced by Stage of Fruit Harvest, Postharvest Ripening and Seed Extraction. Indian J. Agric. Sci. 2013, 83, 928–932. [Google Scholar]
- Queiroz, L.A.F.; Pinho, É.V.d.R.V.; Oliveira, J.A.; Ferreira, V.d.F.; Carvalho, B.O.; Bueno, A.C.R. Influence of Maturation Stage and Drying on the Quality of’habanero Yellow’pepper Seeds. Rev. Bras. Sementes 2011, 33, 472–481. [Google Scholar] [CrossRef]
- K’Opondo, F.B. Influence of Drying Method and Fruit Position on the Mother Plant on Seed Quality of Spiderplant (Cleome gynandra L.) Morphotypes from Western Kenya. Adv. Appl. Sci. Res. 2011, 2, 74–83. [Google Scholar]
- Passam, H.C.; Theodoropoulou, S.; Karanissa, T.; Karapanos, I.C. Influence of Harvest Time and After-Ripening on the Seed Quality of Eggplant. Sci. Hortic. 2010, 125, 518–520. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, C.; Hou, Y.; Li, X. Optimum Harvest Time of Vicia Cracca in Relation to High Seed Quality during Pod Development. Crop Sci. 2008, 48, 709–715. [Google Scholar] [CrossRef]
- Castro, M.M.; Godoy, A.R.; Inacio Cardoso, A.I. Okra Seed Quality as a Function of Age and Fruit Post Harvest Rest. Ciênc. Agrotecnologia 2008, 32, 1491–1495. [Google Scholar] [CrossRef]
- Alan, O.; Eser, B. The Effect of Fruit Maturity and Post-Harvest Ripening on Seed Quality in Hot and Conic Pepper Cultivars. Seed Sci. Technol. 2008, 36, 467–474. [Google Scholar] [CrossRef]
- Kumar, V.; Tomar, B.S.; Dadlani, M.; Singh, B.; Kumar, S. Effect of Fruit Retention and Seed Position on the Seed Yield and Quality in Pumpkin (Cucurbita moschata) cv Pusa Hybrid 1. Indian J. Agric. Sci. 2015, 85, 1210–1213. [Google Scholar] [CrossRef]
- Sousa, T.V.; Alkimim, E.R.; David, A.M.S.S.; Sá, J.R.; Pereira, G.A.; Amaro, H.T.R.; Mota, W.F. Harvest Season and Physiological Quality of Coriander Seeds Produced in the North of Minas Gerais. Rev. Bras. Plantas Med. 2011, 591–597. [Google Scholar] [CrossRef]
- Samarah, N.H. Effect of Air-drying Immature Seeds in Harvested Pods on Seed Quality of Common Vetch (Vicia sativa L.). N. Z. J. Agric. Res. 2006, 49, 331–339. [Google Scholar] [CrossRef]
- Vidigal, D.D.S.; Dias, D.C.F.D.S.; Naveira, D.D.S.P.C.; Rocha, F.B.; Bhering, M.C. Physiological Quality of Tomato Seeds in Relation to Fruit Age and Post-Harvest Storage. Rev. Bras. Sementes 2006, 28, 87–93. [Google Scholar] [CrossRef]
- Dias, D.C.F.S.; Ribeiro, F.P.; Dias, L.A.S.; Silva, D.J.H.; Vidigal, D.S. Tomato Seed Quality Harvested from Different Trusses. Seed Sci. Technol. 2006, 34, 681–689. [Google Scholar] [CrossRef]
- Reghin, M.Y.; Dalla Pria, M.; Otto, R.F.; Vinne, J. van der Épocas de Colheita de Umbelas e Comprimento Da Haste Floral No Rendimento e No Potencial Fisiológico de Sementes de Cebola. Hortic. Bras. 2004, 22, 286–289. [Google Scholar] [CrossRef]
- Barbedo, A.; Camara, F.; Nakagawa, J.; Barbedo, C. Plant Population, Harvest Method and Seed Quality of Carrot, Brasilia Cultivar. Pesqui. Agropecuária Bras. 2000, 35, 1645–1652. [Google Scholar] [CrossRef]
- Jing, H.; Bergervoet, J.; Jalink, H.; Klooster, M.; Du, S.; Bino, R.; Hilhorst, H.; Groot, S. Cucumber (Cucumis sativus L.) Seed Performance as Influenced by Ovary and Ovule Position. Seed Sci. Res. 2000, 10, 435–445. [Google Scholar] [CrossRef]
- Murugesan, P.; Vanangamudi, K. Effect of Seed and Fruit Position on Seed Quality in Ash Gourd (Benincasa hispida (Thunb.) Cogn). Seed Res. 2005, 33, 156–159. [Google Scholar]
- Amjad, M.; Anjum, M.A. Effect of Root Size, Plant Spacing and Umbel Order on the Quality of Carrot Seed. Int. J. Agric. Biol. 2001, 3, 239–242. [Google Scholar]
- Siddique, A.; Wright, D. Effects of Different Drying Time and Temperature on Moisture Percentage and Seed Quality (Viability and Vigour) of Pea Seeds (Pisum sativum L.). Asian J. Plant Sci. 2003, 2, 978–982. [Google Scholar] [CrossRef]
- Aroucha, E.M.M.; da Silva, R.F.; de Oliveira, J.G.; Viana, A.P.; Gonzaga, M.P. Época de Colheita e Período de Repouso Dos Frutos de Mamão (Carica papaya L.) Cv Golden Na Qualidade Fisiológica Das Sementes. Ciênc. Rural 2005, 35, 537–543. [Google Scholar] [CrossRef]
- Martins, G.N.; da Silva, R.F.; Pereira, M.G.; Araújo, E.F.; Posse, S.C.P. Influência Do Repouso Pós-Colheita de Frutos Na Qualidade Fisiológica de Sementes de Mamão. Rev. Bras. Sementes 2006, 28, 142–146. [Google Scholar] [CrossRef]
- Hunje, R.; Vyakarnahal, B.; Jagadeesh, R. Influence of Drying Methods of Fruits on Seed Quality in Chilli (Capsicum annuum L.). Karnataka J. Agric. Sci. 2007, 20, 269–271. [Google Scholar]
- Camacho, I.P.; Hernández, V.A.G.; Moreno, J.C.M.; Garay, Ó.J.A.; Lomelí, A.P. Efecto de Desarrollo y Secado de Semillas de Physalis ixocarpa Brot En Germinación, Vigor y Contenido de Azúcares. Interciencia 2008, 33, 762–766. [Google Scholar]
- Demir, I.; Tekin, A.; Ökmen, Z.A.; Okcu, G.; Kenanoğlu, B.B. Seed Quality, and Fatty Acid and Sugar Contents of Pepper Seeds (Capsicum annuum L.) in Relation to Seed Development and Drying Temperatures. Turk. J. Agric. For. 2008, 32, 529–536. [Google Scholar]
- Hanapiah, N.F.H.; Sinniah, U.R.; Yusoff, M.M. Seed Quality of Lablab Bean (Lablab purpureus) as Influenced by Seed Maturity and Drying Methods. Agronomy 2022, 12, 363. [Google Scholar] [CrossRef]
- Asbrouck, J.V.; Taridno, P. Fast Field Drying as a Method to Maintain Quality, Increase Shelf Life and Prevent Post Harvest Infections on Cucumis sativum L. As. J. Food Ag-Ind. 2009, 133–137. [Google Scholar]
- Resende, O.; Almeida, D.P.; Costa, L.M.; Mendes, U.C.; Sales, J.d.F. Adzuki Beans (Vigna angularis) Seed Quality under Several Drying Conditions. Food Sci. Technol. 2012, 32, 151–155. [Google Scholar] [CrossRef]
- de França, L.V.; Croda, M.D.; Nascimento, W.M.; Freitas, R.A.d. Physiological Quality of Eggplant Seeds with Different Extraction and Drying Methods. J. Seed Sci. 2013, 35, 51–55. [Google Scholar] [CrossRef]
- Santos, H.O.; Dutra, S.M.F.; Pereira, R.W.; Pires, R.M.d.O.; Von Pinho, E.V.d.R.; Rosa, S.D.V.F.d.; Carvalho, L.M.d. Physiological Quality of Habanero Pepper (Capisicum chinense) Seeds Based on Development and Drying Process. Afr. J. Agric. Res. 2016, 11, 1102–1109. [Google Scholar] [CrossRef]
- Botey, H.M.; Ochuodho, J.O.; Ngode, L.; Danquah, E.O.; Agyare, R. Physiological Quality of African Eggplant Seeds as Influenced by Natural Fermentation and Drying Methods. J. Hortic. For. 2021, 13, 51–57. [Google Scholar]
- Balbuena-Mascada, S.; Peña-Lomelí, A.; Magaña-Lira, N.; Sahagún-Castellanos, J.; Martínez-Solís, J. Drying Temperatures of Tomatillo (Physalis ixocarpa Brot. Ex Horm.) Seeds. Rev. Chapingo Ser. Hortic. 2022, 28, 79–92. [Google Scholar] [CrossRef]
- Sundareswaran, S.; Ray Choudhury, P.; Vanitha, C.; Yadava, D.K. Seed Quality: Variety Development to Planting—An Overview. In Seed Science and Technology: Biology, Production, Quality; Dadlani, M., Yadava, D.K., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 1–16. ISBN 978-981-19588-8-5. [Google Scholar]
- Marcos Filho, J. Seed Vigor Testing: An Overview of the Past, Present and Future Perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Kameswara Rao, N.; Dulloo, M.E.; Engels, J.M.M. A Review of Factors That Influence the Production of Quality Seed for Long-Term Conservation in Genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar] [CrossRef]
- Cromarty, A.; Ellis, R.H.; Roberts, E.H. The Design of Seed Storage Facilities for Genetic Conservation; Bioversity International: Rome, Italy, 1982. [Google Scholar]
- Tierney, J.F.; Fisher, D.J.; Burdett, S.; Stewart, L.A.; Parmar, M.K.B. Comparison of Aggregate and Individual Participant Data Approaches to Meta-Analysis of Randomised Trials: An Observational Study. PloS Med. 2020, 17, e1003019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Khan, B.; Negeri, Z.F. An Evaluation of Computational Methods for Aggregate Data Meta-Analyses of Diagnostic Test Accuracy Studies. BMC Med. Res. Methodol. 2024, 24, 111. [Google Scholar] [CrossRef] [PubMed]
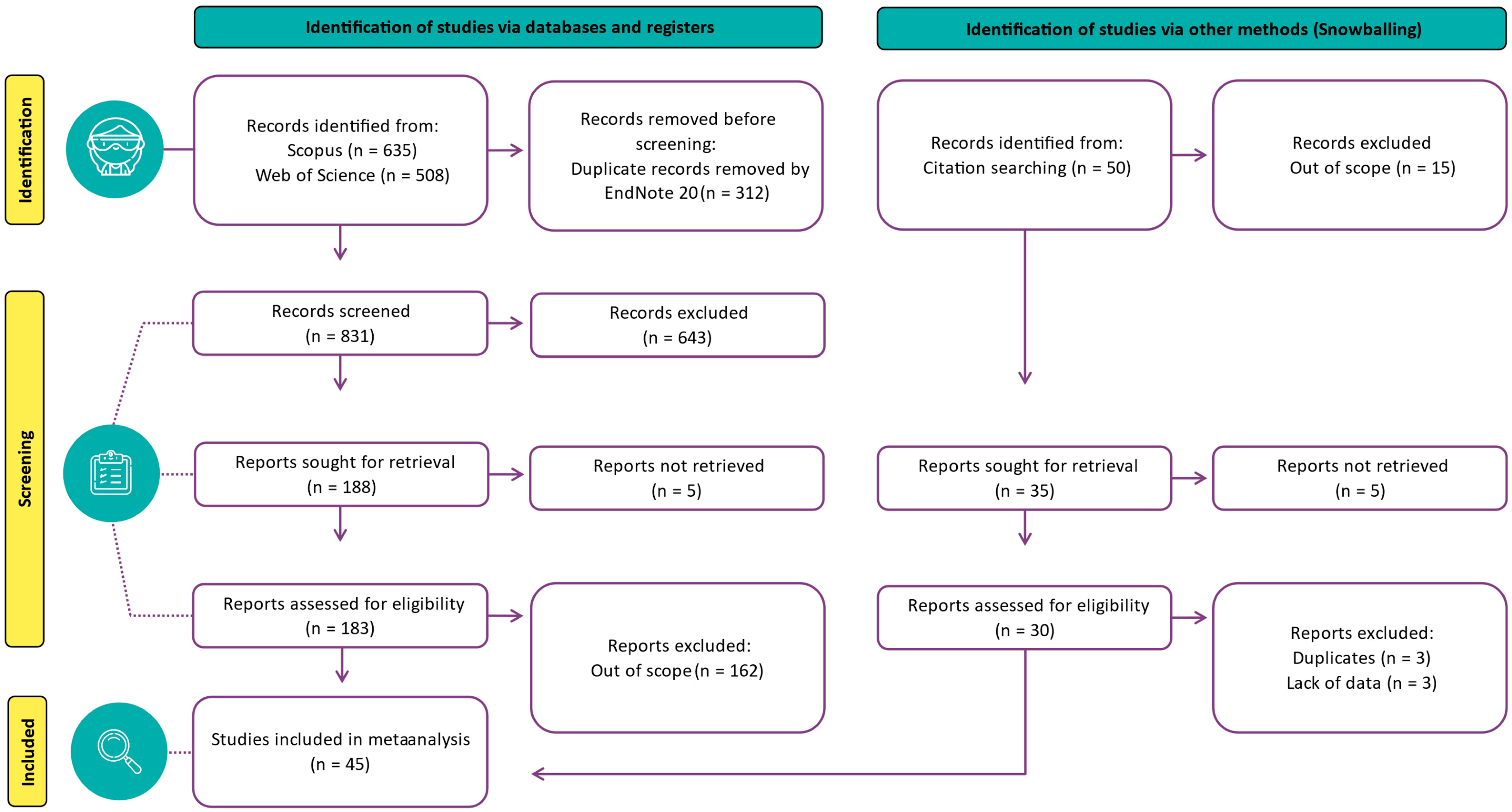


| Element | Description |
|---|---|
| Problem | Seed quality in specialty crops |
| Intervention | Harvesting and drying |
| Comparison | Harvest timings and drying methods |
| Outcome | Improvement in physiological traits |
| Database | Search String |
|---|---|
| Scopus | (“fruit*” OR “nut*” OR “vegetable*” OR “herb*” OR “spice*” OR “medicinal plant*” OR “nurser*” OR “flo*” OR “horticult*”) AND ((“harvest*” OR “dry*”) AND (“seed quality”)) |
| Web Of Science | (“fruit*” OR “nut*” OR “vegetable*” OR “herb*” OR “spice*” OR “medicinal plant*” OR “nurser*” OR “flo*” OR “horticult*”) AND ((“harvest*” OR “dry*”) AND (“seed quality”)) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, L.M.; de Almeida Moreira, B.R.; Martins, C.C. Optimization of Harvesting and Drying Techniques for Quality Seed Production in Specialty Crops: A Systematic Review and Meta-Analysis. Agronomy 2024, 14, 1705. https://doi.org/10.3390/agronomy14081705
Pedrosa LM, de Almeida Moreira BR, Martins CC. Optimization of Harvesting and Drying Techniques for Quality Seed Production in Specialty Crops: A Systematic Review and Meta-Analysis. Agronomy. 2024; 14(8):1705. https://doi.org/10.3390/agronomy14081705
Chicago/Turabian StylePedrosa, Laura Monteiro, Bruno Rafael de Almeida Moreira, and Cibele Chalita Martins. 2024. "Optimization of Harvesting and Drying Techniques for Quality Seed Production in Specialty Crops: A Systematic Review and Meta-Analysis" Agronomy 14, no. 8: 1705. https://doi.org/10.3390/agronomy14081705





