Effect of Irrigation and Cultivation Modes on Growth, Physiology, Rice Yield Parameters and Water Footprints
Abstract
1. Introduction
2. Materials and Methods
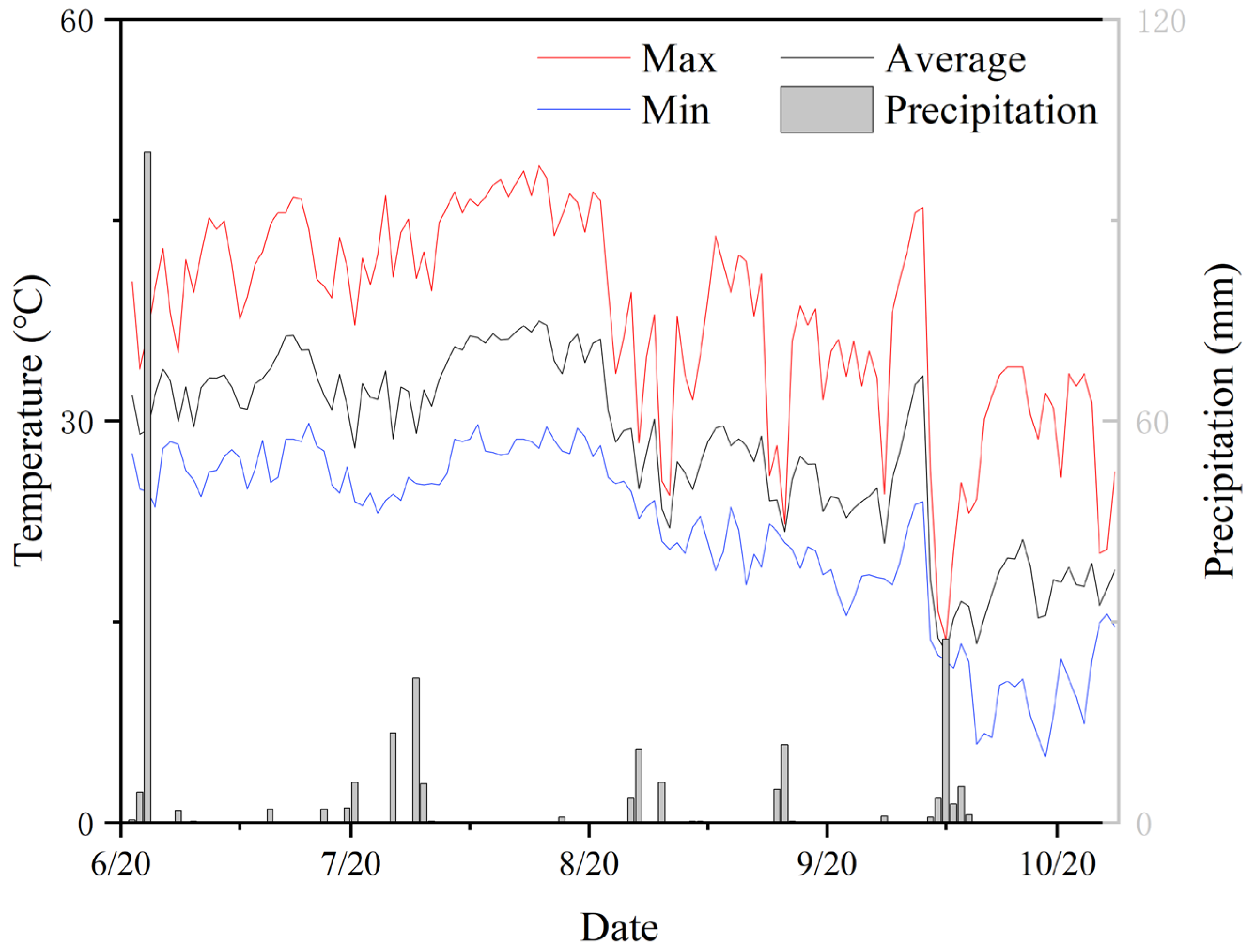
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurements
2.3.1. Crop Water Requirement
2.3.2. Nitrogen and Phosphorus Concentration
2.3.3. Rice Growth Indicators
2.3.4. Gas Exchange Parameters
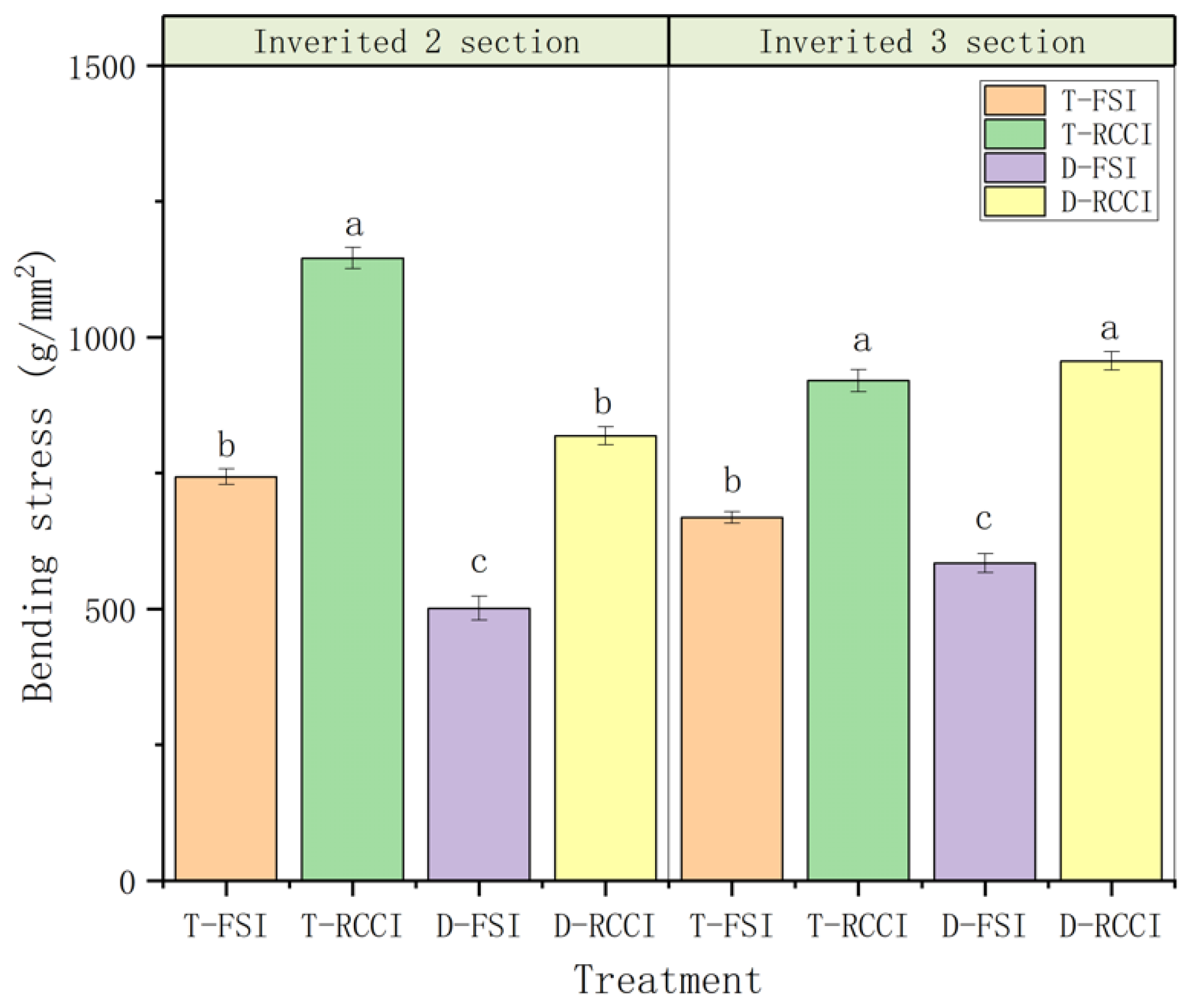
2.3.5. Lodging Coefficient and Stem Bending Resistance
2.3.6. Yield
2.3.7. Water Footprints
2.3.8. Water Use Efficiency
2.4. Statistical Analysis
3. Results
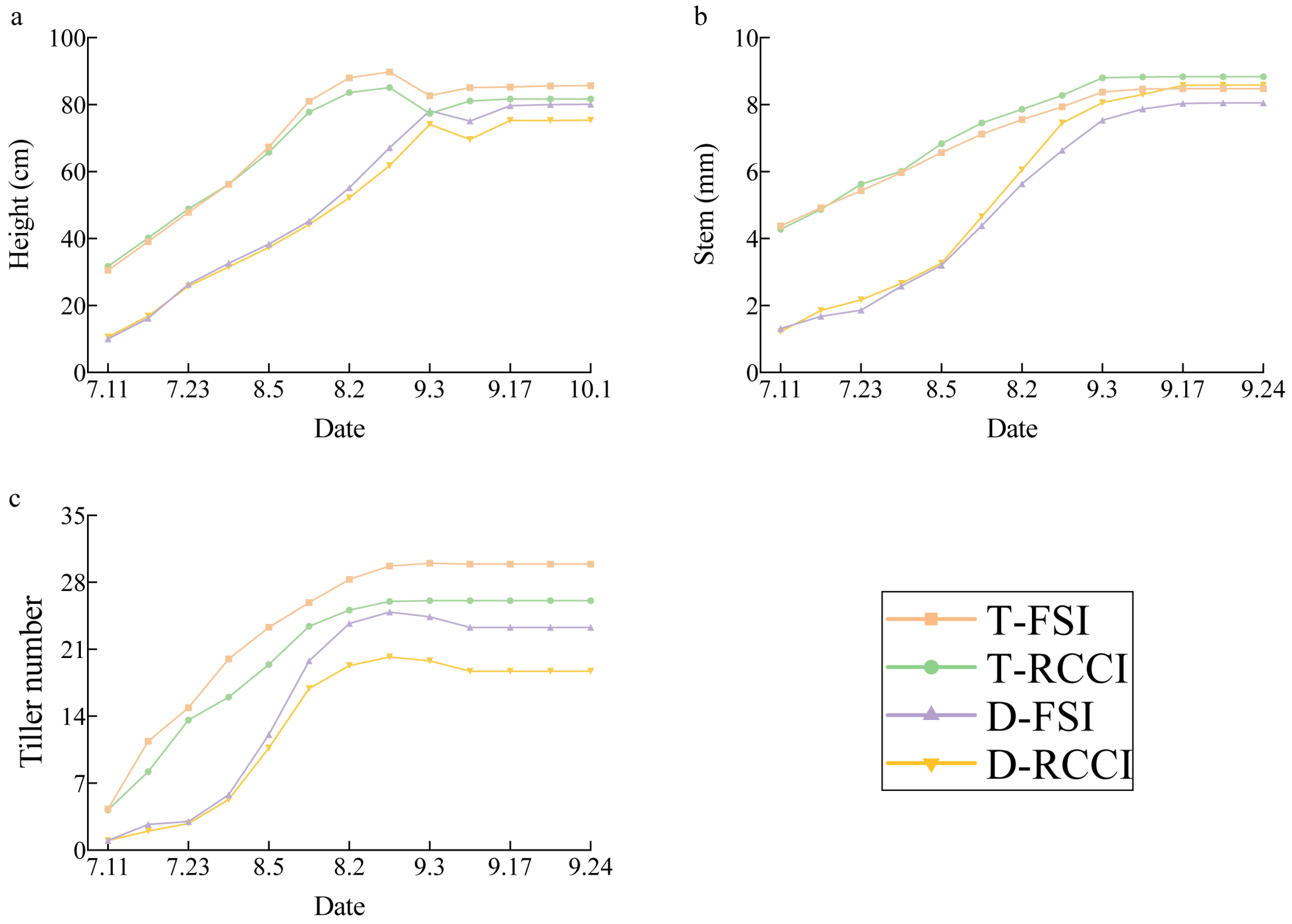
3.1. Effect of Treatments on Plant Growth Parameters
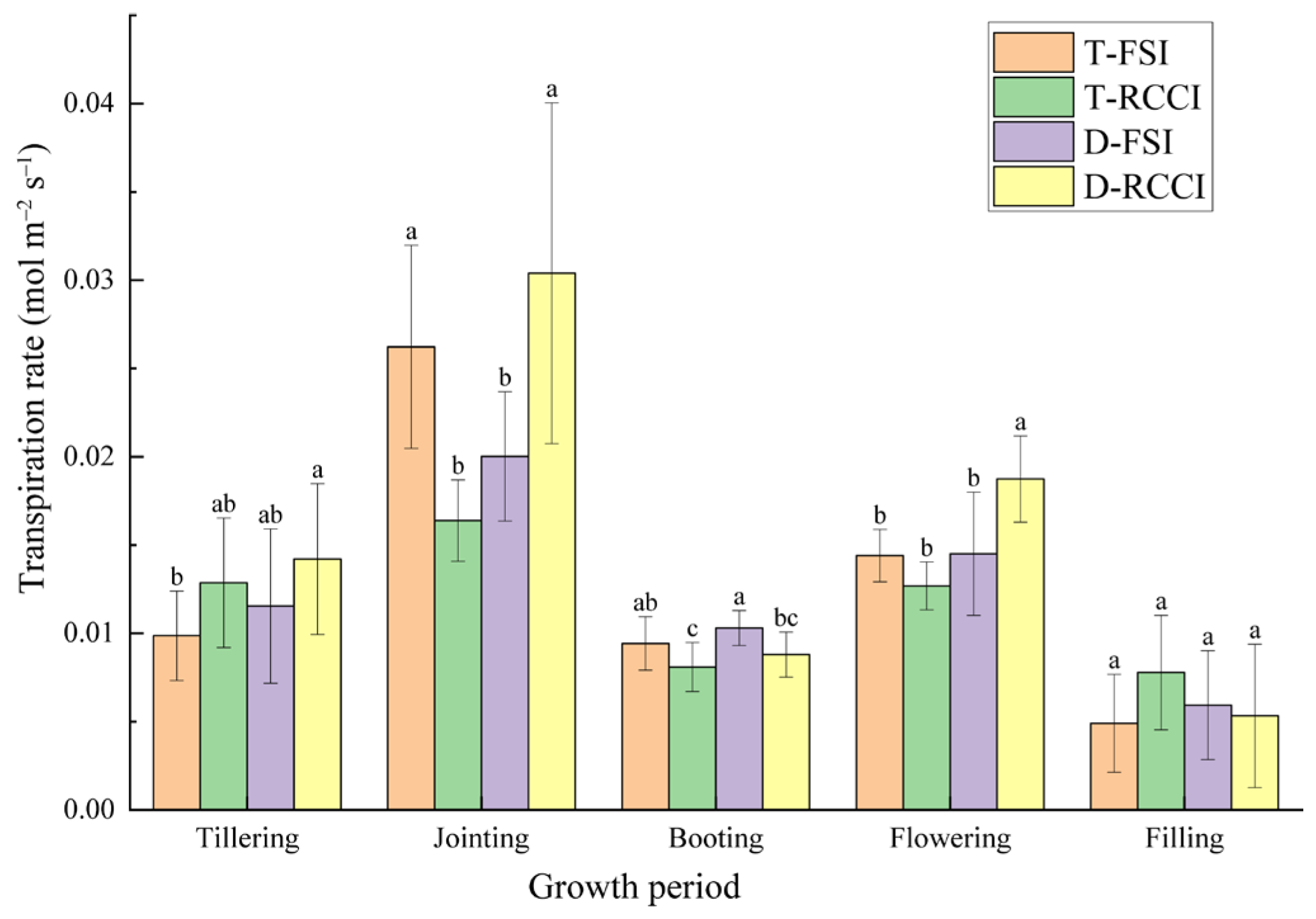
3.2. Effects of Treatments on Gas Exchange
3.2.1. Intercellular CO2 Concentrations
3.2.2. Stomatal Conductance
3.2.3. Net Photosynthetic Rate
3.3. Effect of Treatments on Production Parameters
3.4. Impact of Different Irrigation and Cultivation Patterns on the Water Footprint Per Unit of Rice Production
3.4.1. Graywater Footprint Per Unit Yield
3.4.2. Water Footprint Per Unit of Crop Production
4. Discussion
4.1. Effect of Treatments on Plant Growth Parameters
4.2. Gas Exchange Parameters
4.3. Effect of Treatments on Production Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srivastav, A.L.; Dhyani, R.; Ranjan, M.; Madhav, S.; Sillanpää, M. Climate-resilient strategies for sustainable management of water resources and agriculture. Environ. Sci. Pollut. Res. 2021, 28, 41576–41595. [Google Scholar] [CrossRef] [PubMed]
- Mojeed, A.; Misiekaba-Kia, P.; dos Anjos, V.A.A.P. Climate change, extreme weather & conflict exacerbate global food crisis. Premium Times, 19 February 2024. [Google Scholar]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Ling, T. A global study about water crisis. In Proceedings of the 2021 International Conference on Social Development and Media Communication (SDMC 2021), Sanya, China, 26–28 November 2021; pp. 809–814. [Google Scholar]
- Suzdaleva, A.; Goryunova, S.; Marchuk, A.; Borovkov, V. Mobilization strategy to overcome global crisis of water consumption. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012201. [Google Scholar]
- Huang, M. The decreasing area of hybrid rice production in China: Causes and potential effects on Chinese rice self-sufficiency. Food Secur. 2022, 14, 267–272. [Google Scholar] [CrossRef]
- Tang, L.; Risalat, H.; Cao, R.; Hu, Q.; Pan, X.; Hu, Y.; Zhang, G. Food security in China: A brief view of rice production in recent 20 years. Foods 2022, 11, 3324. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistics Publishing House: Beijing, China, 2022.
- Information Office of the State Council of the People’s Republic of China. The Grain Issue in China; White Paper of Chinese Government; Information Office of the State Council of the People’s Republic of China: Beijing, China, 2019.
- Pathak, H.; Tewari, A.; Sankhyan, S.; Dubey, D.; Mina, U.; Singh, V.K.; Jain, N. Direct-seeded rice: Potential, performance and problems—A review. Curr. Adv. Agric. Sci. (Int. J.) 2011, 3, 77–88. [Google Scholar]
- Kumar, V.; Ladha, J.K. Direct seeding of rice: Recent developments and future research needs. Adv. Agron. 2011, 111, 297–413. [Google Scholar]
- Chen, X.; Tang, Y.; Xie, Y.; Li, S.; Chu, J.; Ao, F.; Peng, W.; Li, H.; Wan, Y. Research advances of rice mechanical direct-seeding technology in China. China Rice 2018, 24, 9–15. [Google Scholar]
- Espe, M.; Cassman, K.; Yang, H.; Guilpart, N.; Grassini, P.; Van Wart, J.; Anders, M.; Beighley, D.; Harrell, D.; Linscombe, S. Yield gap analysis of US rice production systems shows opportunities for improvement. Field Crops Res. 2016, 196, 276–283. [Google Scholar] [CrossRef]
- Ali, M.A.; Ladha, J.; Rickman, J.; Laies, J. Comparison of different methods of rice establishment and nitrogen management strategies for lowland rice. J. Crop Improv. 2006, 16, 173–189. [Google Scholar] [CrossRef]
- Mallareddy, M.; Thirumalaikumar, R.; Balasubramanian, P.; Naseeruddin, R.; Nithya, N.; Mariadoss, A.; Eazhilkrishna, N.; Choudhary, A.K.; Deiveegan, M.; Subramanian, E. Maximizing water use efficiency in rice farming: A comprehensive review of innovative irrigation management technologies. Water 2023, 15, 1802. [Google Scholar] [CrossRef]
- Shekhawat, K.; Rathore, S.S.; Chauhan, B.S. Weed management in dry direct-seeded rice: A review on challenges and opportunities for sustainable rice production. Agronomy 2020, 10, 1264. [Google Scholar] [CrossRef]
- Wenxia, W.; Yanzhi, Z.; Yongjun, Z.; Ziming, W.; Xueming, T.; Xiaohua, P.; Qinghua, S.; Yanhua, Z. Effects of different mechanical direct seeding patterns on yield and lodging resistance of high-quality late indica rice in south China. Chin. J. Rice Sci. 2020, 34, 46. [Google Scholar]
- Liu, K.; Xue, Y.; Lan, Y.; Fu, Y. Agricultural Water Utilization Efficiency in China: Evaluation, Spatial Differences, and Related Factors. Water 2022, 14, 684. [Google Scholar] [CrossRef]
- Wang, X.-C.; Klemeš, J.J.; Wang, Y.; Dong, X.; Wei, H.; Xu, Z.; Varbanov, P.S. Water-Energy-Carbon Emissions nexus analysis of China: An environmental input-output model-based approach. Appl. Energy 2020, 261, 114431. [Google Scholar] [CrossRef]
- Han, S.; Tian, F.; Gao, L. Current status and recent trend of irrigation water use in China. Irrig. Drain. 2020, 69, 25–35. [Google Scholar] [CrossRef]
- Yan, J.; Wu, Q.; Qi, D.; Zhu, J. Rice yield, water productivity, and nitrogen use efficiency responses to nitrogen management strategies under supplementary irrigation for rain-fed rice cultivation. Agric. Water Manag. 2022, 263, 107486. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, L.; Li, S.; Liu, H.; Zhai, L.; Zhou, F.; Ye, Y.; Ruan, S.; Wen, W. Effects and potential of water-saving irrigation for rice production in China. Agric. Water Manag. 2019, 217, 374–382. [Google Scholar] [CrossRef]
- Raimondi, A.; Quinn, R.; Abhijith, G.R.; Becciu, G.; Ostfeld, A. Rainwater harvesting and treatment: State of the art and perspectives. Water 2023, 15, 1518. [Google Scholar] [CrossRef]
- Zhang, S.; Rasool, G.; Guo, X.; Sen, L.; Cao, K. Effects of different irrigation methods on environmental factors, rice production, and water use efficiency. Water 2020, 12, 2239. [Google Scholar] [CrossRef]
- Duan, S.-M.; Huang, Y.-D.; Yang, A.-Z.; Wu, W.-G.; Xin, X.; Xu, Y.-Z.; Gang, C. Effect of water stress on growth and yield of rice. Adv. J. Food Sci. Technol. 2016, 11, 537–544. [Google Scholar]
- Huang, J.; Hu, T.; Yasir, M.; Gao, Y.; Chen, C.; Zhu, R.; Wang, X.; Yuan, H.; Yang, J. Root growth dynamics and yield responses of rice (Oryza sativa L.) under drought—Flood abrupt alternating conditions. Environ. Exp. Bot. 2019, 157, 11–25. [Google Scholar] [CrossRef]
- Gao, S.; Yu, S.; Wang, M.; Cao, R.; Guo, R. Effect of controlled irrigation and drainage on saving water and reducing nitrogen and phosphorus loss in paddy field under alternate drought and flooding condition. Trans. Chin. Soc. Agric. Eng. 2017, 33, 122–128. [Google Scholar]
- Guo, X.; Li, X.; Lu, H. Study on dry matter accumulation and nitrogen uptake under alternating drought and flooding stress in rice tillering stage. J. Irrig. Drain. 2015, 34, 20–24. [Google Scholar]
- Machekposhti, M.F.; Shahnazari, A.; Yousefian, M.; Ahmadi, M.Z.; Sarjaz, M.R.; Arabzadeh, B.; Akbarzadeh, A.; Leib, B.G. The effect of alternate partial root-zone drying and deficit irrigation on the yield, quality, and physiochemical parameters of milled rice. Agric. Water Manag. 2023, 289, 108546. [Google Scholar] [CrossRef]
- Wu, D.-H.; Chen, C.-T.; Yang, M.-D.; Wu, Y.-C.; Lin, C.-Y.; Lai, M.-H.; Yang, C.-Y. Controlling the lodging risk of rice based on a plant height dynamic model. Bot. Stud. 2022, 63, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhong, X.; Tian, K.; Huang, N.; Pan, J.; He, T. Effect of nitrogen application on stem lodging resistance of rice and its morphological and mechanical mechanisms. Sci. Agric. Sin 2013, 46, 1323–1334. [Google Scholar]
- Chapagain, A.K.; Hoekstra, A.Y. Water Footprints of Nations; Unesco-IHE Institute for Water Education: Delft, The Netherlands, 2004. [Google Scholar]
- Zimmer, D. Virtual Water Trade: Proceedings of the International Expert Meeting on Virtual Water Trade. 2003, p. 93. Available online: https://ihedelftrepository.contentdm.oclc.org/digital/collection/p21063coll3/id/10351 (accessed on 5 August 2024).
- Cao, X.; Zeng, W.; Wu, M.; Li, T.; Chen, S.; Wang, W. Water resources efficiency assessment in crop production from the perspective of water footprint. J. Clean. Prod. 2021, 309, 127371. [Google Scholar] [CrossRef]
- Fang, H.; Wu, N.; Adamowski, J.; Wu, M.; Cao, X. Crop water footprints and their driving mechanisms show regional differences. Sci. Total Environ. 2023, 904, 167549. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Y.; Zhuo, L.; Wang, W.; Li, M.; Feng, B.; Wu, P. Quantitative evaluation of spatial scale effects on regional water footprint in crop production. Resour. Conserv. Recycl. 2021, 173, 105709. [Google Scholar] [CrossRef]
- Ruisen, Z.; Xinguang, D.; Yingjie, M. Sustainable water saving: New concept of modern agricultural water saving, starting from development of Xinjiang’s agricultural irrigation over the last 50 years. Irrig. Drain. J. Int. Comm. Irrig. Drain. 2009, 58, 383–392. [Google Scholar] [CrossRef]
- Shu, R.; Cao, X.; Wu, M. Clarifying regional water scarcity in agriculture based on the theory of blue, green and grey water footprints. Water Resour. Manag. 2021, 35, 1101–1118. [Google Scholar] [CrossRef]
- GB 11894-89; Water Quality—Determination of Total Nitrogen—Alkaline Potassium Persulfate Digestion—UV Spectrophotometric Method. Standards Press of China: Beijing, China, 1986.
- HJ 535-2009; Water Quality-Determination of Ammonia Nitroge—Nessler’s Reagent Spectrophotometry. China Environmental Science Press: Beijing, China, 2010.
- HJ/T 346-2007; Water Quality-Determination of Nitrate-Nitrogen-Ultraviolet Spectrophotometry. China Environmental Science Press: Beijing, China, 2007.
- GB 11893-89; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. Standards Press of China: Beijing, China, 1990.
- Cao, R.; Gao, S. Influence of drought and flood alternation stress in different growth stages on rice yield and components. J. Drain. Irrig. Mach. Eng. 2019, 37, 639–644. [Google Scholar]
- Xue, W.; Otieno, D.; Ko, J.; Werner, C.; Tenhunen, J. Conditional variations in temperature response of photosynthesis, mesophyll and stomatal control of water use in rice and winter wheat. Field Crops Res. 2016, 199, 77–88. [Google Scholar] [CrossRef]
- Zhu, R.; Wu, F.; Zhou, S.; Hu, T.; Huang, J.; Gao, Y. Cumulative effects of drought–flood abrupt alternation on the photosynthetic characteristics of rice. Environ. Exp. Bot. 2020, 169, 103901. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef]
- Xiong, Z.; Dun, Z.; Wang, Y.; Yang, D.; Xiong, D.; Cui, K.; Peng, S.; Huang, J. Effect of stomatal morphology on leaf photosynthetic induction under fluctuating light in rice. Front. Plant Sci. 2022, 12, 754790. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Ali, B.; Hussain, H.A.; Qadir, T.; Hussain, S. Temperature extremes: Impact on rice growth and development. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Berlin/Heidelberg, Germany, 2019; pp. 153–171. [Google Scholar]
- Shrestha, S.; Mahat, J.; Shrestha, J.; Madhav, K.; Paudel, K. Influence of high temperature stress on rice growth and development. A review. Heliyon 2022, 8, e12651. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Fahad, S. Heat stress decreases rice grain weight: Evidence and physiological mechanisms of heat effects prior to flowering. Int. J. Mol. Sci. 2022, 23, 10922. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Vinci, G.; Ruggieri, R.; Ruggeri, M.; Prencipe, S.A. Rice production chain: Environmental and social impact assessment—A review. Agriculture 2023, 13, 340. [Google Scholar] [CrossRef]
- Masuda, K. Eco-efficiency assessment of intensive rice production in Japan: Joint application of life cycle assessment and data envelopment analysis. Sustainability 2019, 11, 5368. [Google Scholar] [CrossRef]



| pH | Soil Type | BD (g/cm3) | SWC (%) | WHC (%) | SOM (g/kg) | TN (mg/kg) | TP (mg/kg) |
|---|---|---|---|---|---|---|---|
| 7.11 | loam | 1.40 | 43.90 | 30.40 | 2.38 | 34.54 | 29.56 |
| Treatments | Irrigation Modes | Cultivation Modes |
|---|---|---|
| T-FSI | Shallow water and frequent irrigation | Transplanting |
| T-RCCI | Rain-catching and controlled irrigation | Transplanting |
| D-FSI | Shallow water and frequent irrigation | Direct-seeded |
| D-RCCI | Rain-catching and controlled irrigation | Direct-seeded |
| Modes | Water Limits | Irrigation Quantity at Different Growth Stages | |||||
|---|---|---|---|---|---|---|---|
| Recovery | Tillering | Jointing | Booting | Flowering | Filling | ||
| FSI | Upper (mm) | 30 | 30 | 50 | 40 | 40 | 0 |
| Lower (mm) | 10 | 10~60% * | 10 | 10 | 10 | 60~70% * | |
| Rain storage (mm) | 40 | 100 | 150 | 150 | 80 | 0 | |
| RCCI | Upper (%) | 30 | 100 | 100 | 100 | 100 | 80 |
| Lower (%) | 10 | 60~70 | 70~80 | 80 | 70 | / | |
| Rain storage (mm) | 80 | 120 | 200 | 200 | 100 | 0 | |
| Growth Period | Treatment | Yield (kg/ha) | IW (mm) | Effective Rainfall (mm) | IWP (kg/m3) | WPE (kg/m3) |
|---|---|---|---|---|---|---|
| Field growth Period | T-FSI | 5525 c | 1017 a | 124 b | 0.54 d | 0.49 c |
| T-RCCI | 4151 d | 614 c | 136 a | 0.68 c | 0.55 c | |
| D-FSI | 9031 a | 795 b | 116 c | 1.13 b | 0.99 b | |
| D-RCCI | 8088 b | 530 d | 136 a | 1.53 a | 1.22 a | |
| Whole growth period | T-FSI | 5525 c | 1036 a | 125 b | 0.53 d | 0.48 c |
| T-RCCI | 4151 d | 633 c | 137 a | 0.66 c | 0.54 c | |
| D-FSI | 9031 a | 795 b | 116 c | 1.13 b | 0.99 b | |
| D-RCCI | 8088 b | 530 d | 136 a | 1.53 a | 1.22 a |
| Treatments | Yield (kg/ha) | GWF (mm) | GWFg (m3/kg) | ||
|---|---|---|---|---|---|
| Field Growth Period | Whole Growth Period | Field Growth Period | Whole Growth Period | ||
| T-FSI | 5525 c | 36 a | 45 a | 0.066 a | 0.082 a |
| T-RCCI | 4151 d | 12 c | 20 c | 0.028 b | 0.049 b |
| D-FSI | 9031 a | 56 b | 56 b | 0.062 a | 0.062 b |
| D-RCCI | 8088 b | 13 c | 13 d | 0.016 b | 0.016 c |
| Treatments | Yield (kg/ha) | GWF (mm) | GWFg (m3/kg) | ||
|---|---|---|---|---|---|
| Field Growth Period | Whole Growth Period | Field Growth Period | Whole Growth Period | ||
| T-FSI | 5525 c | 923 a | 943 a | 1.67 a | 1.71 a |
| T-RCCI | 4151 d | 602 c | 623 c | 1.45 b | 1.50 b |
| D-FSI | 9031 a | 731 b | 731 b | 0.81 c | 0.81 c |
| D-RCCI | 8088 b | 511 d | 511 d | 0.63 d | 0.63 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Rasool, G.; Wang, S.; Guo, X.; Zhao, Z.; Zhang, Y.; Wei, Z.; Xia, Q. Effect of Irrigation and Cultivation Modes on Growth, Physiology, Rice Yield Parameters and Water Footprints. Agronomy 2024, 14, 1747. https://doi.org/10.3390/agronomy14081747
Zhang S, Rasool G, Wang S, Guo X, Zhao Z, Zhang Y, Wei Z, Xia Q. Effect of Irrigation and Cultivation Modes on Growth, Physiology, Rice Yield Parameters and Water Footprints. Agronomy. 2024; 14(8):1747. https://doi.org/10.3390/agronomy14081747
Chicago/Turabian StyleZhang, Shuxuan, Ghulam Rasool, Shou Wang, Xiangping Guo, Zhengfeng Zhao, Yiwen Zhang, Zhejun Wei, and Qibing Xia. 2024. "Effect of Irrigation and Cultivation Modes on Growth, Physiology, Rice Yield Parameters and Water Footprints" Agronomy 14, no. 8: 1747. https://doi.org/10.3390/agronomy14081747
APA StyleZhang, S., Rasool, G., Wang, S., Guo, X., Zhao, Z., Zhang, Y., Wei, Z., & Xia, Q. (2024). Effect of Irrigation and Cultivation Modes on Growth, Physiology, Rice Yield Parameters and Water Footprints. Agronomy, 14(8), 1747. https://doi.org/10.3390/agronomy14081747







