Physiological and Biochemical Responses of Maize to Elevated CO2 Concentrations: Implications for Growth and Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Elevated-CO2 Treatment
2.3. Open-Top Chamber Design
2.4. Growth and Harvesting of Maize
2.5. Total Soluble Sugar, Starch, and Total Non-Structural Carbohydrate Measurement
2.6. Total Soluble Protein Measurement
2.7. Lignin Contents and Composition Measurements
2.8. Measurement of Chlorophyll and Malondialdehyde (MDA) Contents
2.9. Statistical Analysis
3. Results
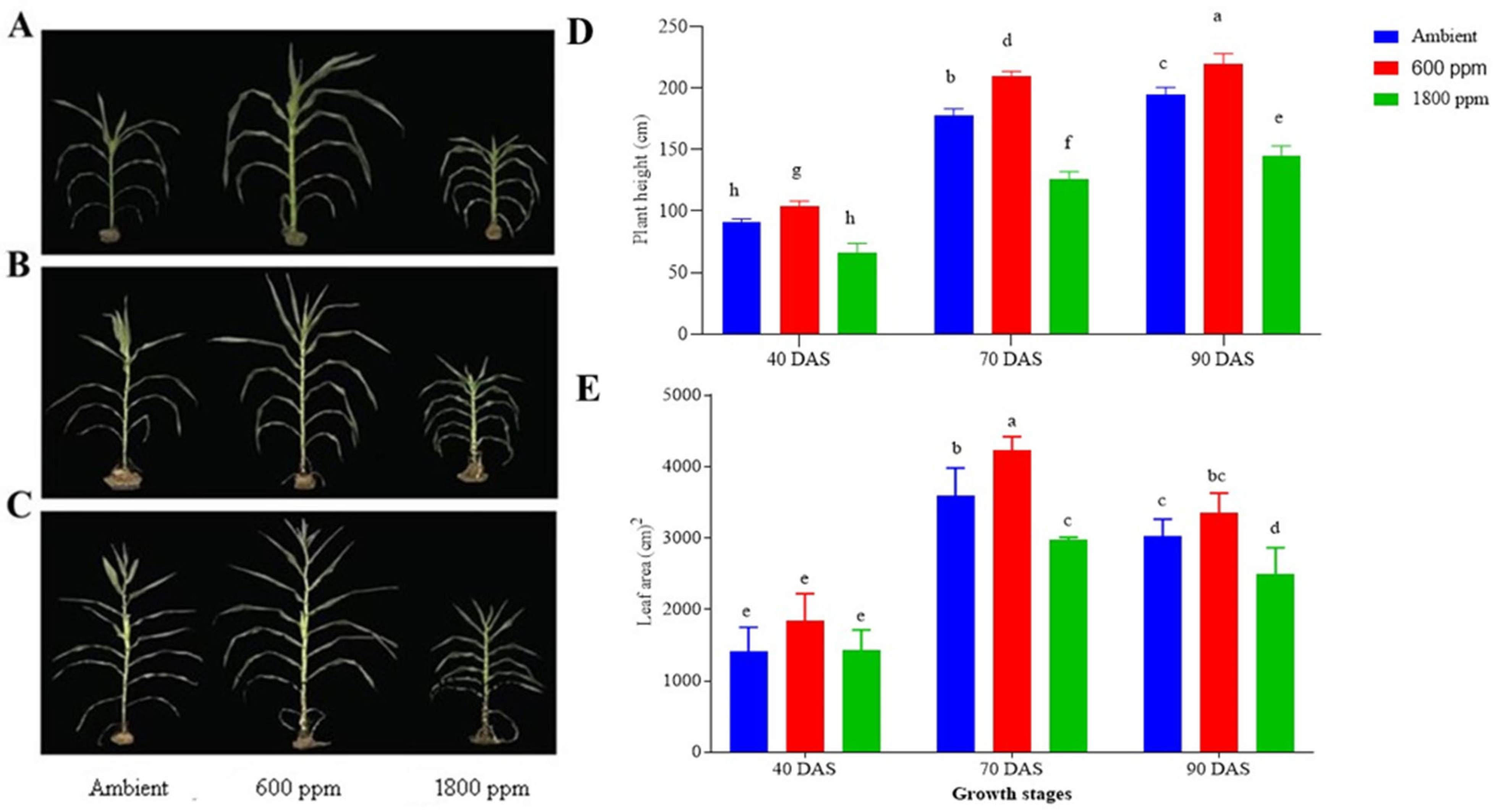
3.1. CO2 Causes Morphological Changes in Maize Plants
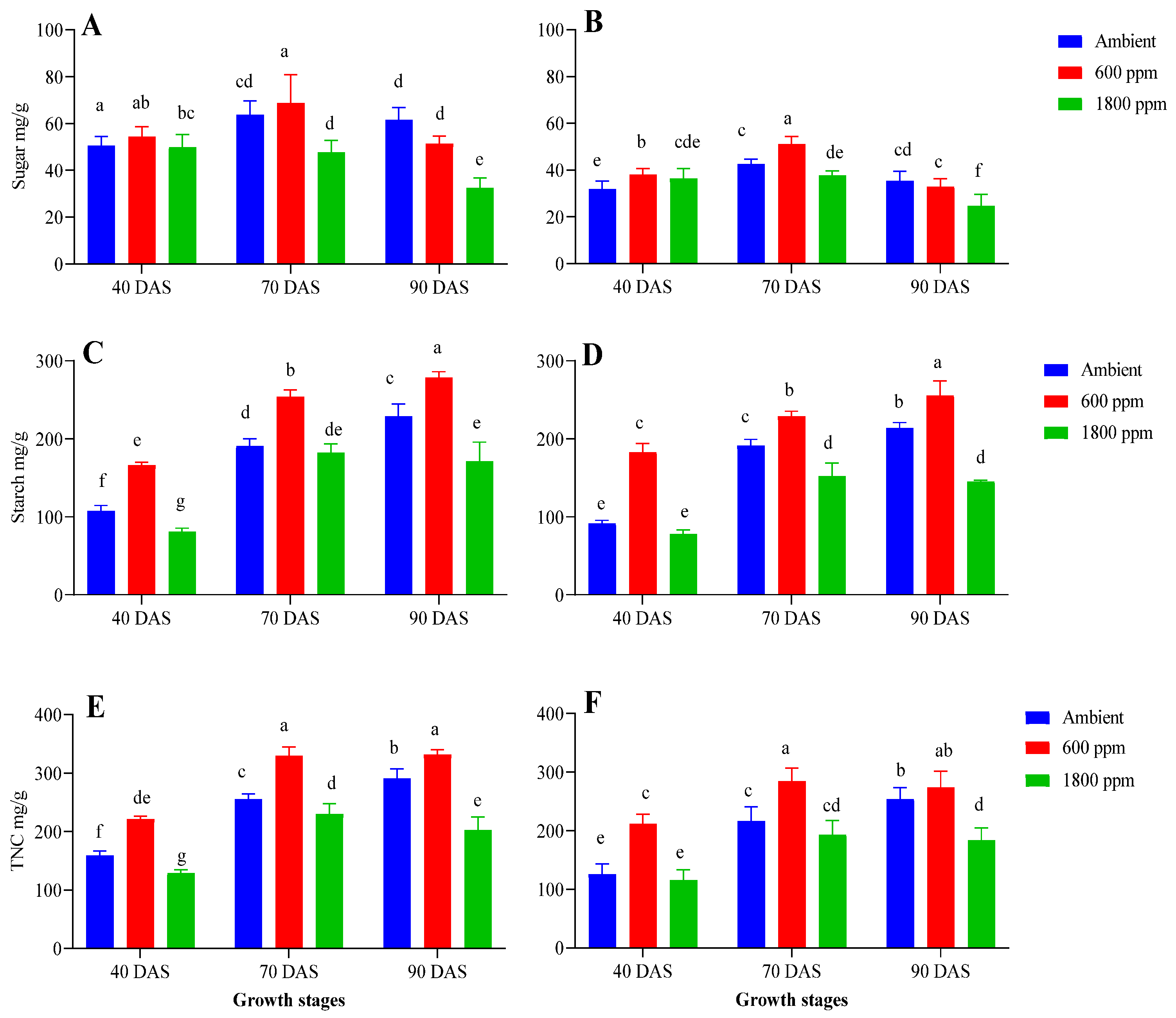
3.2. Elevated CO2 Leads to More Accumulation of Sugar and Starch in Maize Plants
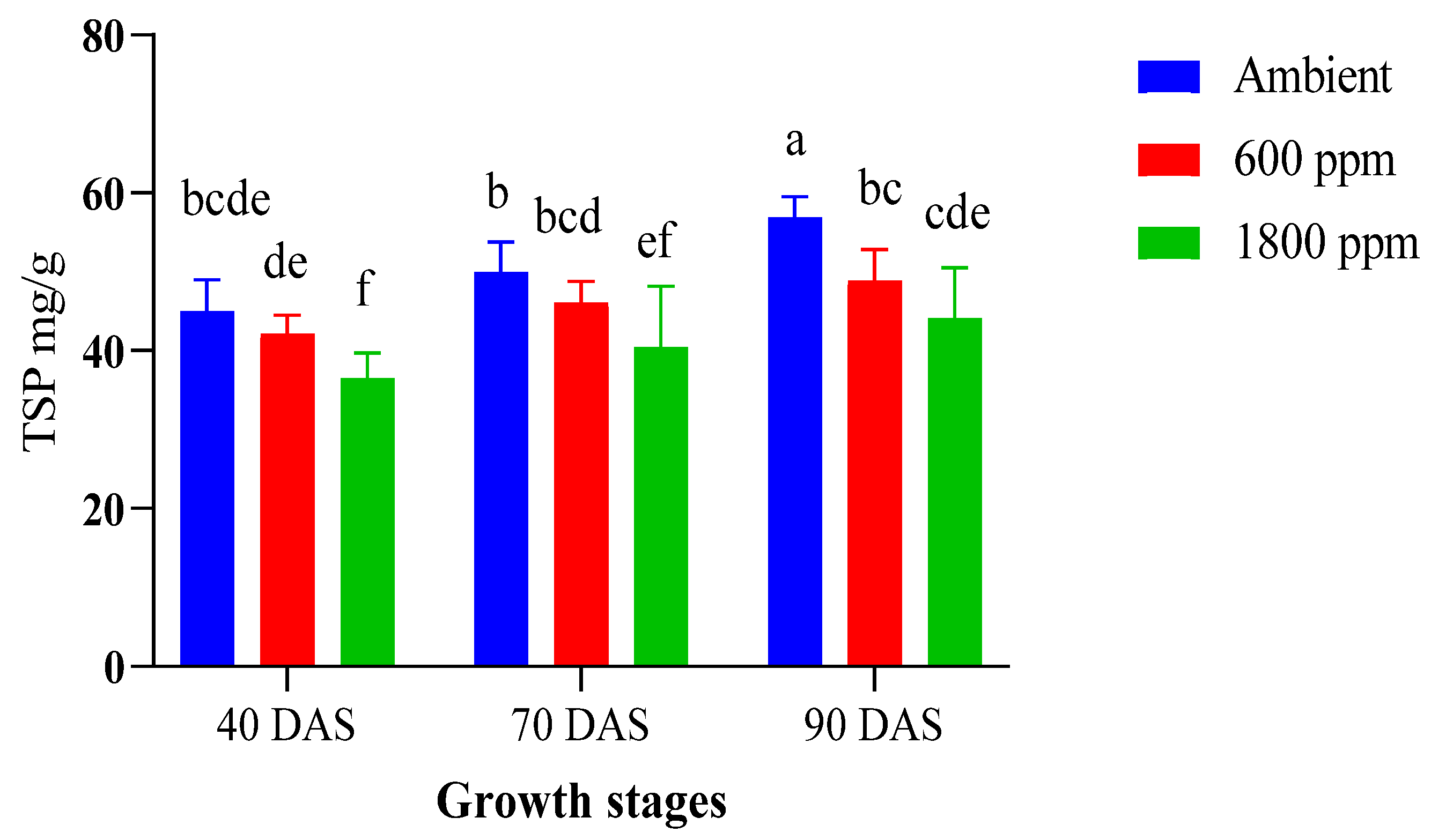
3.3. High CO2 Reduces Protein Accumulation in Maize Plant
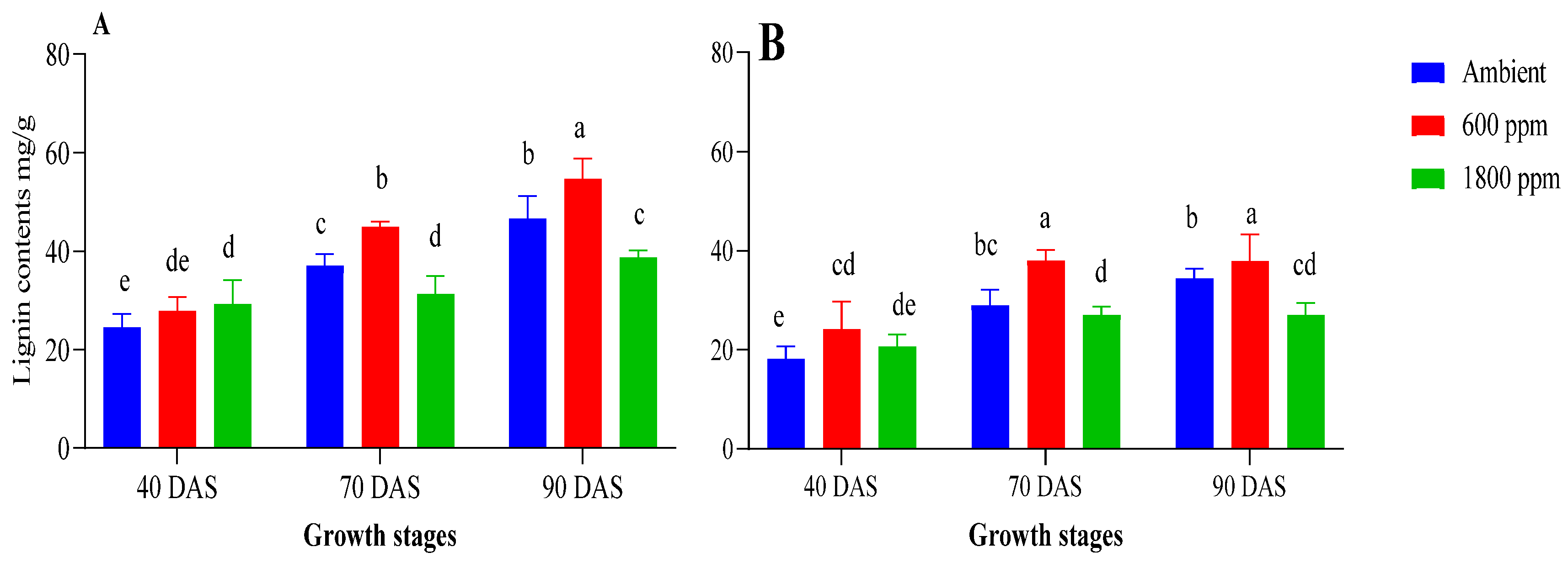
3.4. Lignin Accumulation and Composition Is Significantly Affected by CO2 Treatment
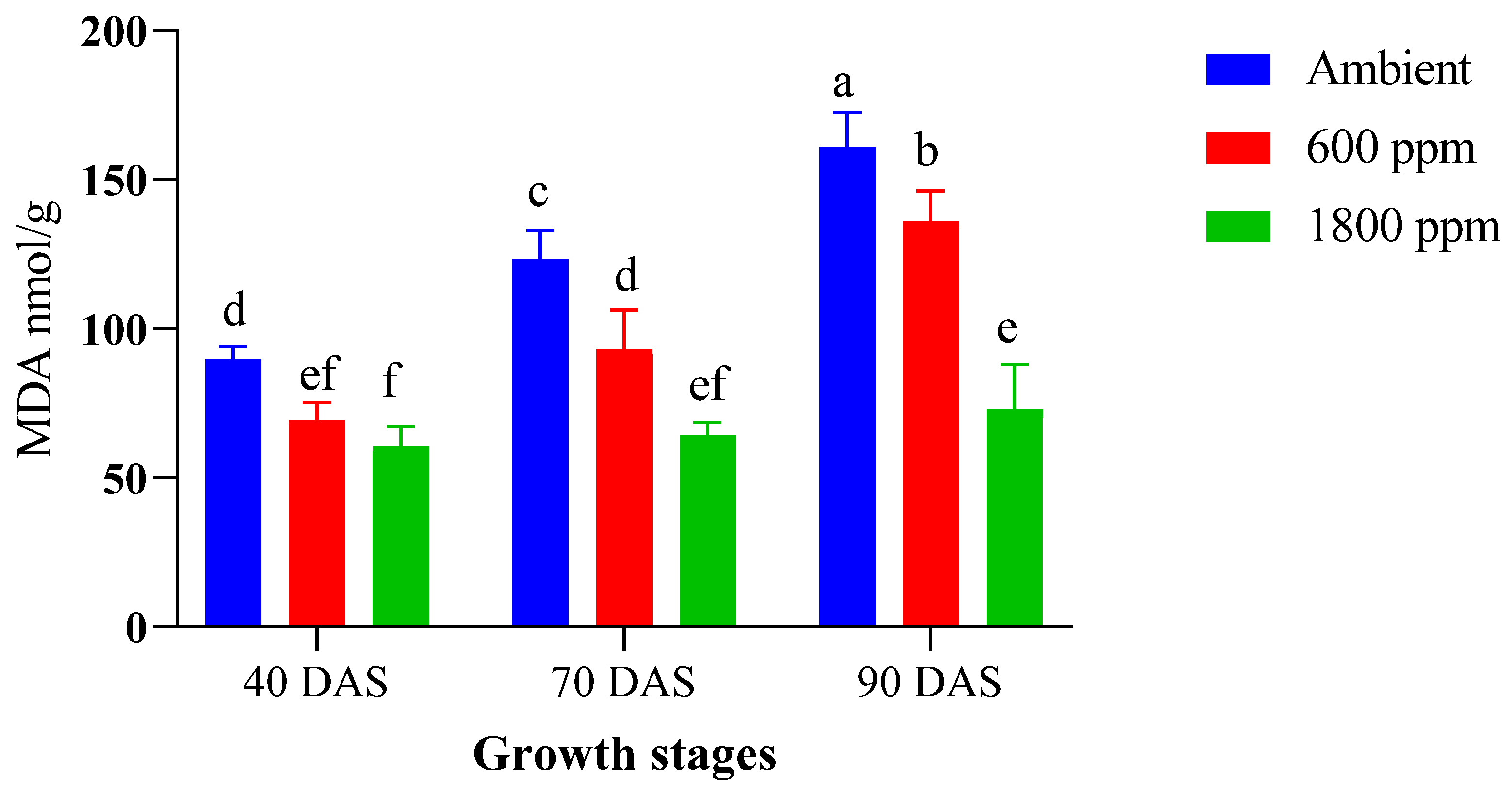
3.5. Biosynthesis of Chlorophylls, Carotenoids, and MDA in CO2-Treated Maize Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walker, A.P.; De Kauwe, M.G.; Bastos, A.; Belmecheri, S.; Georgiou, K.; Keeling, R.F.; McMahon, S.M.; Medlyn, B.E.; Moore, D.J.P.; Norby, R.J.; et al. Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. New Phytol. 2021, 229, 2413–2445. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Davis, S.J.; Creutzig, F.; Fuss, S.; Minx, J.; Gabrielle, B.; Kato, E.; Jackson, R.B.; Cowie, A.; Kriegler, E. Biophysical and economic limits to negative CO2 emissions. Nat. Clim. Chang. 2016, 6, 42–50. [Google Scholar] [CrossRef]
- Jobe, T.O.; Rahimzadeh Karvansara, P.; Zenzen, I.; Kopriva, S. Ensuring Nutritious Food under Elevated CO2 Conditions: A Case for Improved C4 Crops. Front. Plant Sci. 2020, 11, 1267. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Rome. 2018, p. 403. Available online: http://faostat.fao.org (accessed on 21 March 2024).
- Erenstein, O.; Jaleta, M.; Prasanna, B.; Mottaleb, K.; Sonder, K. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Leakey, A.D.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, J.; Yong, J.W.H.; Wang, Q.; Ma, J.; He, X. Higher Atmospheric CO2 Levels Favor C3 Plants over C4 Plants in Utilizing Ammonium as a Nitrogen Source. Front. Plant Sci. 2020, 11, 537443. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Bhargava, S.; Mitra, S. Elevated atmospheric CO2 and the future of crop plants. Plant Breed. 2021, 140, 1–11. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Atmospheric CO2 enrichment affects potatoes: 1. Aboveground biomass production and tuber yield. Eur. J. Agron. 2009, 30, 78–84. [Google Scholar] [CrossRef]
- Erbs, M.; Manderscheid, R.; Hüther, L.; Schenderlein, A.; Wieser, H.; Dänicke, S.; Weigel, H.-J. Free-air CO2 enrichment modifies maize quality only under drought stress. Agron. Sustain. Dev. 2015, 35, 203–212. [Google Scholar] [CrossRef][Green Version]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheung, M.; Rasooli, L.; Amirsadeghi, S.; Vanlerberghe, G.C. Plant respiration in a high CO2 world: How will alternative oxidase respond to future atmospheric and climatic conditions? Can. J. Plant Sci. 2014, 94, 1091–1101. [Google Scholar] [CrossRef]
- Vermerris, W.; Saballos, A.; Ejeta, G.; Mosier, N.S.; Ladisch, M.R.; Carpita, N.C. Molecular Breeding to Enhance Ethanol Production from Corn and Sorghum Stover. Crop Sci. 2007, 47, S-142–S-153. [Google Scholar] [CrossRef]
- Novaes, E.; Kirst, M.; Chiang, V.; Winter-Sederoff, H.; Sederoff, R. Lignin and biomass: A negative correlation for wood formation and lignin content in trees. Plant Physiol. 2010, 154, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Sato, S.; Arima, H.; Heeley, E.; Delcourt, C.; Cao, Y.; Chalmers, J.; Anderson, C.S. Estimated GFR and the Effect of Intensive Blood Pressure Lowering after Acute Intracerebral Hemorrhage. Am. J. Kidney Dis. 2016, 68, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X. Research on corn production efficiency and influencing factors of typical farms: Based on data from 12 corn-producing countries from 2012 to 2019. PLoS ONE 2021, 16, e0254423. [Google Scholar] [CrossRef]
- Jin, Z.; Zhuang, Q.; Wang, J.; Archontoulis, S.V.; Zobel, Z.; Kotamarthi, V.R. The combined and separate impacts of climate extremes on the current and future US rainfed maize and soybean production under elevated CO2. Glob. Chang. Biol. 2017, 23, 2687–2704. [Google Scholar] [CrossRef]
- Reddy, A.R.; Reddy, K.R.; Padjung, R.; Hodges, H.F. Nitrogen nutrition and photosynthesis in leaves of Pima cotton. J. Plant Nutr. 1996, 19, 755–770. [Google Scholar] [CrossRef]
- Leakey, A.D.B. Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proc. R. Soc. B Biol. Sci. 2009, 276, 2333–2343. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Plant height and evolutionary games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Z.-H.; Yang, B.; Chen, H.; Zhang, H.; Hou, D.-B. The contribution of photosynthesis traits and plant height components to plant height in wheat at the individual quantitative trait locus level. Sci. Rep. 2020, 10, 12261. [Google Scholar] [CrossRef] [PubMed]
- Vico, G.; Porporato, A. From rainfed agriculture to stress-avoidance irrigation: I. A generalized irrigation scheme with stochastic soil moisture. Adv. Water Resour. 2011, 34, 263–271. [Google Scholar] [CrossRef]
- Leadley, P.W.; Drake, B.G. Open top chambers for exposing plant canopies to elevated CO2 concentration and for measuring net gas exchange. In CO2 and Biosphere; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Ziska, L.; Clements, D.J.W.R. Observed changes in soyabean growth and seed yield from Abutilon theophrasti competition as a function of carbon dioxide concentration. Weed Res. 2013, 53, 140–145. [Google Scholar] [CrossRef]
- Maroco, J.P.; Edwards, G.E.; Ku, M.S. Photosynthetic acclimation of maize to growth under elevated levels of carbon dioxide. Planta 1999, 210, 115–125. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A.; Vodkin, L.O.; Walter, A.; Schurr, U. The Effects of Elevated CO2 Concentration on Soybean Gene Expression. An Analysis of Growing and Mature Leaves. Plant Physiol. 2006, 142, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Dermody, O.; Long, S.P.; DeLucia, E.H. How does elevated CO2 or ozone affect the leaf-area index of soybean when applied independently? New Phytol. 2006, 169, 145–155. [Google Scholar] [CrossRef]
- Hirel, B.; Andrieu, B.; Valadier, M.H.; Renard, S.; Quilleré, I.; Chelle, M.; Pommel, B.; Fournier, C.; Drouet, J.L. Physiology of maize II: Identification of physiological markers representative of the nitrogen status of maize (Zea mays) leaves during grain filling. Physiol. Plant. 2005, 124, 178–188. [Google Scholar] [CrossRef]
- Palit, P.; Ghosh, R.; Tolani, P.; Tarafdar, A.; Chitikineni, A.; Bajaj, P.; Sharma, M.; Kudapa, H.; Varshney, R.K. Molecular and Physiological Alterations in Chickpea under Elevated CO2 Concentrations. Plant Cell Physiol. 2020, 61, 1449–1463. [Google Scholar] [CrossRef]
- Kant, S.; Seneweera, S.; Rodin, J.; Materne, M.; Burch, D.; Rothstein, S.J.; Spangenberg, G. Improving yield potential in crops under elevated CO2: Integrating the photosynthetic and nitrogen utilization efficiencies. Front. Plant Sci. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Bunce, J.A.; Caulfield, F.A. Rising atmospheric carbon dioxide and seed yield of soybean genotypes. Crop Sci. 2001, 41, 385–391. [Google Scholar] [CrossRef]
- Wildman, S.J. The organization of grana-containing chloroplasts in relation to location of some enzymatic systems concerned with photosynthesis, protein synthesis, and ribonucleic acid synthesis. Biochem. Chloroplasts 1967, 2, 295–319. [Google Scholar]
- Yelle, S.; Beeson, R.; Trudel, M.; Gosselin, A. Acclimation of Two Tomato Species to High Atmospheric CO2 I. Sugar and Starch Concentrations. Plant Physiol. 1989, 90, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Delucia, E.H.; Sasek, T.W.; Strain, B.R. Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosynth. Res. 1985, 7, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.; Hocking, P. Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiol. Plant. 1993, 89, 570–576. [Google Scholar] [CrossRef]
- Driscoll, S.P.; Prins, A.; Olmos, E.; Kunert, K.J.; Foyer, C.H. Specification of adaxial and abaxial stomata, epidermal structure and photosynthesis to CO2 enrichment in maize leaves. J. Exp. Bot. 2006, 57, 381–390. [Google Scholar] [CrossRef]
- Lavola, A.; Julkunen-Tiitto, R. The effect of elevated carbon dioxide and fertilization on primary and secondary metabolites in birch, Betula pendula (Roth). Oecologia 1994, 99, 315–321. [Google Scholar] [CrossRef]
- Matros, A.; Amme, S.; Kettig, B.; Buck-Sorlin, G.H.; Sonnewald, U.; Mock, H.P. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 2006, 29, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Nakano, H.; Mae, T. Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994, 105, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Richet, N.; Afif, D.; Tozo, K.; Pollet, B.; Maillard, P.; Huber, F.; Priault, P.; Banvoy, J.; Gross, P.; Dizengremel, P. Elevated CO2 and/or ozone modify lignification in the wood of poplars (Populus tremula x alba). J. Exp. Bot. 2012, 63, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Estiarte, M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends Ecol. Evol. 1998, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Estiarte, M.; Llusia, J. Carbon-based secondary compounds at elevated CO2. Photosynthetica 1997, 33, 313–319. [Google Scholar] [CrossRef]
- Schlimme, M.; Blaschke, L.; Lagrimini, L.M.; Polle, A. Growth performance and lignification in tobacco with suppressed apoplastic anionic peroxidase activity under ambient and elevated CO2 concentrations. Int. J. Plant Sci. 2002, 163, 749–754. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Rahmat, A.; Rahman, Z.A. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2010, 16, 162–174. [Google Scholar] [CrossRef]
- He, Y. The Value of Corn Stover for Chinese Dairy Farms. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2019. [Google Scholar]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M.J.P.P. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiol. Plant. 2006, 126, 458–468. [Google Scholar] [CrossRef]
- Wheeler, R.; Mackowiak, C.; Siegriest, L.; Sager, J.C. Supraoptimal carbon dioxide effects on growth of soybean [Glycine max (L.) Merr.]. J. Plant Physiol. 1993, 142, 173–178. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; He, C.; Duan, A. Genes responsive to elevated CO2 concentrations in triploid white poplar and integrated gene network analysis. PLoS ONE 2014, 9, e98300. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Poorter, H.; Van Berkel, Y.; Baxter, R.; Den Hertog, J.; Dijkstra, P.; Gifford, R.; Griffin, K.; Roumet, C.; Roy, J.; Wong, S. The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell Environ. 1997, 20, 472–482. [Google Scholar] [CrossRef]
- Lavola, A.; Nybakken, L.; Rousi, M.; Pusenius, J.; Petrelius, M.; Kellomäki, S.; Julkunen-Tiitto, R. Combination treatment of elevated UVB radiation, CO2 and temperature has little effect on silver birch (Betula pendula) growth and phytochemistry. Physiol. Plant. 2013, 149, 499–514. [Google Scholar] [CrossRef]
- Liu, J.; Hochberg, U.; Ding, R.; Xiong, D.; Dai, Z.; Zhao, Q.; Chen, J.; Ji, S.; Kang, S. Elevated CO2 concentration increases maize growth under water deficit or soil salinity but with a higher risk of hydraulic failure. J. Exp. Bot. 2024, 75, 422–437. [Google Scholar] [CrossRef]
- Liu, J.X.; Feng, K.; Wang, G.L.; Xu, Z.S.; Wang, F.; Xiong, A.S. Elevated CO2 induces alteration in lignin accumulation in celery (Apium graveolens L.). Plant Physiol. Biochem. 2018, 127, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Koricheva, J.; Larsson, S.; Haukioja, E.; Keinänen, M. Regulation of woody plant secondary metabolism by resource availability: Hypothesis testing by means of meta-analysis. Oikos 1998, 83, 212–226. [Google Scholar] [CrossRef]
- Knops, J.M.; Naeem, S.; Reich, P. The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Glob. Chang. Biol. 2007, 13, 1960–1971. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; De Angelis, P.; Polle, A. Leaf litter production and decomposition in a poplar short-rotation coppice exposed to free air CO2 enrichment (POPFACE). Glob. Chang. Biol. 2005, 11, 971–982. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin, F.S., III; Klein, D.R. Carbon/Nutrient Balance of Boreal Plants in Relation to Vertebrate Herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Wang, Y. Combined effects of elevated CO2 and soil drought on carbon and nitrogen allocation of the desert shrub Caragana intermedia. Plant Soil 2007, 301, 87–97. [Google Scholar] [CrossRef]
- Blaschke, L.; Forstreuter, M.; Sheppard, L.J.; Leith, I.K.; Murray, M.B.; Polle, A. Lignification in beech (Fagus sylvatica) grown at elevated CO2 concentrations: Interaction with nutrient availability and leaf maturation. Tree Physiol. 2002, 22, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gwak, K.S.; Treasure, T.; Jameel, H.; Chang, H.M.; Park, S. Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem 2014, 7, 1942–1950. [Google Scholar] [CrossRef] [PubMed]

| Treatment No. | Name | Description |
|---|---|---|
| 1 | C0 | Ambient (380 ± 20 ppm) |
| 2 | C1 | Elevated CO2 (600 ± 20 ppm) |
| 3 | C2 | Elevated CO2 (1800 ± 20 ppm) |
| Growth Stages | Plant Organs | CO2 Concentration | ||
|---|---|---|---|---|
| Ambient | 600 ppm | 1800 ppm | ||
| 40 DAS | Leaf (g) | 4.43 (0.17) d | 4.93 (.084) d | 3.53 (0.18) d |
| Stem (g) | 1.833 (0.40) f | 2.33 (0.33) f | 1.16 (0.16) f | |
| Total | 6.263 (0.57) g | 7.26 (1.17) g | 4.69 (0.34) g | |
| 70 DAS | Leaf (g) | 11.61 (0.68) b | 14.22 (0.66) a | 8.94 (0.85) c |
| Stem (g) | 28.83 (2.32) c | 47.66 (1.64) a | 20.66 (2.5) d | |
| Total | 40.44 (3) d | 61.88 (2.3) a | 29.6 (3.35) e | |
| 90 DAS | Leaf (g) | 13.5 (0.88) a | 14.33 (1.25) a | 10.16 (0.60) bc |
| Stem (g) | 19.33 (1.62) d | 30.83 (0.90) c | 12.66 (1.52) e | |
| Total | 32. (2.5) e | 45.16 (2.15) c | 22.82 (2.12) f | |
| Harvest Time | Lignin Monomers | CO2 Treatment | ||
|---|---|---|---|---|
| Ambient | 600 ppm | 1800 ppm | ||
| 40 DAS | p-Hydroxyphenyl (H) | 27.34 ± 0.55 c | 27.43 ± 0.48 c | 30.66 ± 0.62 b |
| Guaiacyl (G) | 94.75 ± 10.32 c | 160.48 ± 7.35 b | 73.90 ± 1.62 c | |
| Syringyl (S) | 25.88 ± 3.44 de | 37.52 ± 7.55 bc | 17.84 ± 1.10 e | |
| S/G | 0.27 ± 0.007 bc | 0.23 ± 0.039 cd | 0.24 ± 0.009 cd | |
| 70 DAS | p-Hydroxyphenyl (H) | 14.69 ± 0.53 e | 27.15 ± 0.34 c | 2.81 ± 0.29 h |
| Guaiacyl (G) | 186.93 ± 10.65 b | 221.03 ± 8.80 a | 67.94 ± 3.58 c | |
| Syringyl (S) | 36.79 ± 1.43 bcd | 42.66 ± 0.83 b | 18.72 ± 0.61 e | |
| S/G | 0.19 ± 0.007 de | 0.19 ± 0.07 de | 0.27 ± 0.08 abc | |
| 90 DAS | p-Hydroxyphenyl (H) | 15.89 ± 0.16 de | 13.46 ± 0.59 ef | 9.81 ± 0.58 g |
| Guaiacyl (G) | 182.32 ± 1.34 b | 182.13 ± 10.01 b | 70.07 ± 3.78 c | |
| Syringyl (S) | 28.65 ± 6.09 cde | 60.98 ± 2.036 a | 22.67 ± 1.55 e | |
| S/G | 0.15 ± 0.15 e | 0.33 ± 0.01 a | 0.32 ± 0.32 cde | |
| Harvest Time | Lignin Monomers | CO2 Treatment | ||
|---|---|---|---|---|
| Ambient | 600 ppm | 1800 ppm | ||
| 40 DAS | p-Hydroxyphenyl (H) | 9.02 ± 0.80 e | 25.08 ± 1.89 a | 6.81 ± 0.24 e |
| Guaiacil (G) | 143.56 ± 6.86 g | 207.47 ± 14.28 ef | 155.33 ± 12.23 g | |
| Syringe (S) | 144.75 ± 15.55 e | 148.4 ± 11.77 e | 127.79 ± 11 e | |
| S/G | 1.021 ± 0.15 cd | 0.71 ± e 0.007 | 0.82 ± de 0.005 | |
| 70 DAS | p-Hydroxyphenyl (H) | 21.78 ± 1.81 bc | 25.01 ± 0.47 ab | 13.59 ± 2.54 d |
| Guaiacil (G) | 372.77 ± 20.42 d | 420.03 ± 5.31 c | 158.76 ± 9.22 g | |
| Syringe (S) | 251.53 ± 19.97 d | 425.39 ± 6.28 c | 231.18 ± 19.28 d | |
| S/G | 0.67 ± 0.05 e | 1.012 ± 0.002 cd | 1.45 ± 0.03 b | |
| 90 DAS | p-Hydroxyphenyl (H) | 14.06 ± 0.38 d | 14.66 ± 0.50 d | 18.99 ± 0.48 c |
| Guaiacil (G) | 785.18 ± 12.27 a | 454.3 ± 14.56 b | 238.64 ± 13.04 e | |
| Syringe (S) | 827.64 ± 12.23 a | 458.12 ± 14.94 b | 546.24 ± 8.71 c | |
| S/G | 1.05 ± 0.03 c | 1.01 ± 0.04 cd | 2.29 ± 0.08 a | |
| Harvest | CO2 Levels | Chlorophyll | |||
|---|---|---|---|---|---|
| a | b | a + b | Carotenoid | ||
| I | Ambient | 4.62 ± 0.10 ab | 2.31 ± 0.17 b | 7.01 ± 0.03 b | 2.2 ± 0.17 ab |
| 600 ppm | 3.81 ± 0.65 bcd | 1.87 ± 0.64 bcd | 5.74 ± 0.29 bcd | 1.54 ± 0.64 cd | |
| 1800 ppm | 3.32 ± 0.98 cde | 1.05 ± 0.35 fgh | 4.26 ± 0.92 ef | 1.12 ± 0.35 def | |
| II | Ambient | 5.22 ± 0.18 a | 3.31 ± 0.12 a | 8.61 ± 0.59 a | 2.56 ± 0.12 a |
| 600 ppm | 4.87 ± 0.20 ab | 2.03 ± 0.02 bc | 6.97 ± 0.27 b | 1.84 ± 0.02 bc | |
| 1800 ppm | 4.33 ± 0.43 abcd | 1.27 ± 0.12 efg | 5.67 ± 0.68 cd | 1.3 ± 0.12 cde | |
| III | Ambient | 4.51 ± 0.05 abc | 1.74 ± 0.03 bcde | 6.32 ± 0.09 bc | 1.70 ± 0.03 bcd |
| 600 ppm | 4.18 ± 0.14 abcd | 1.50 ± 0.07 cdef | 5.74 ± 0.25 bcd | 1.54 ± 0.07 cd | |
| 1800 ppm | 2.35 ± 0.13 e | 0.45± 0 02 gh | 2.88 0.17 f | 0.77 ± 0.02 f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, P.; Safiul Azam, F.M.; Lian, T.; Abdelbacki, A.M.M.; Albaqami, M.; Jan, R.; Kim, K.-M.; Wang, W. Physiological and Biochemical Responses of Maize to Elevated CO2 Concentrations: Implications for Growth and Metabolism. Agronomy 2024, 14, 1751. https://doi.org/10.3390/agronomy14081751
Khan P, Safiul Azam FM, Lian T, Abdelbacki AMM, Albaqami M, Jan R, Kim K-M, Wang W. Physiological and Biochemical Responses of Maize to Elevated CO2 Concentrations: Implications for Growth and Metabolism. Agronomy. 2024; 14(8):1751. https://doi.org/10.3390/agronomy14081751
Chicago/Turabian StyleKhan, Pirzada, Fardous Mohammad Safiul Azam, Tong Lian, Ashraf M. M. Abdelbacki, Mohammed Albaqami, Rahmatullah Jan, Kyung-Min Kim, and Weixuan Wang. 2024. "Physiological and Biochemical Responses of Maize to Elevated CO2 Concentrations: Implications for Growth and Metabolism" Agronomy 14, no. 8: 1751. https://doi.org/10.3390/agronomy14081751
APA StyleKhan, P., Safiul Azam, F. M., Lian, T., Abdelbacki, A. M. M., Albaqami, M., Jan, R., Kim, K.-M., & Wang, W. (2024). Physiological and Biochemical Responses of Maize to Elevated CO2 Concentrations: Implications for Growth and Metabolism. Agronomy, 14(8), 1751. https://doi.org/10.3390/agronomy14081751










