Microplastics Can Inhibit Organic Carbon Mineralization by Influencing Soil Aggregate Distribution and Microbial Community Structure in Cultivated Soil: Evidence from a One-Year Pot Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Material Preparation and Experimental Design
2.3. Soil Sample Collection
2.4. Measurement Methods
2.4.1. Soil Chemical Properties
2.4.2. Composition and Stability Indices of Soil Aggregates
2.4.3. Soil Microbial Community Composition and Enzyme Activities
2.4.4. Soil Organic Carbon Mineralization
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
3.2. Particle Size Distribution and Stability Characteristic Indicators of Soil Aggregate
3.3. Soil Microbial Communities and Enzyme Activities
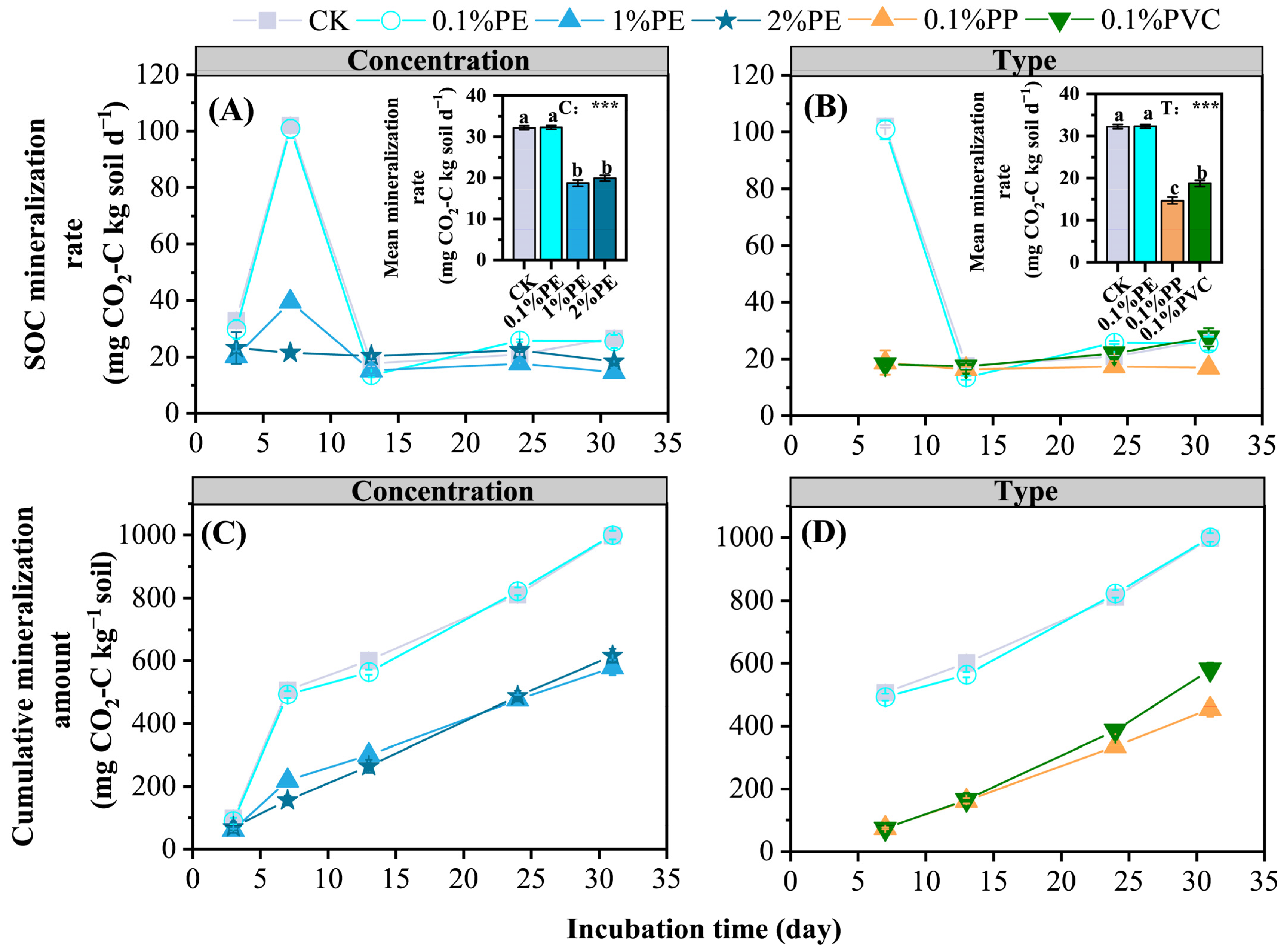
3.4. SOC Transformations
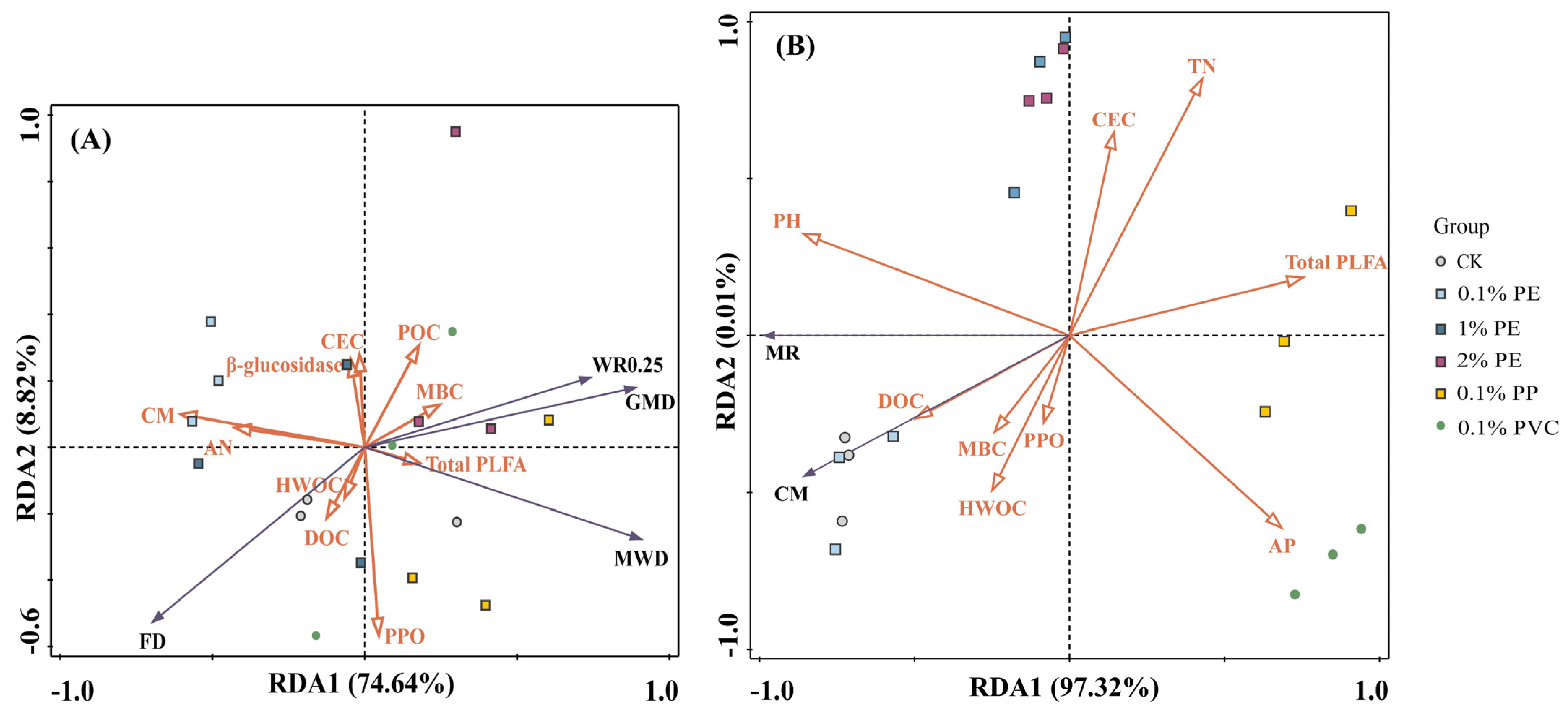
3.5. RDA Analysis
4. Discussion
4.1. Effects of MP Addition on Soil Aggregate Distribution and Stability in Vegetable Soils
4.2. Effects of MP Addition on Soil Carbon Fractions in Vegetable Soils
4.3. Effects of MP Addition on Soil Organic Carbon Mineralization in Vegetable Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLeo, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Ren, S.; Kai, W.; Jinrui, Z.; Jingjing, L.; Hanyue, Z.; Ruimin, Q.; Wen, X.; Changrong, Y.; Xuejun, L.; Fusuo, Z.; et al. Potential sources and occurrence of macro-plastics and microplastics pollution in farmland soils: A typical case of China. Crit. Rev. Environ. Sci. Technol. 2024, 54, 533–556. [Google Scholar] [CrossRef]
- Li, W.; Wufuer, R.; Duo, J.; Wang, S.; Luo, Y.; Zhang, D.; Pan, X. Microplastics in agricultural soils: Extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total Environ. 2020, 749, 141420. [Google Scholar] [CrossRef]
- Yu, Y.; Battu, A.K.; Varga, T.; Denny, A.C.; Zahid, T.M.; Chowdhury, I.; Flury, M. Minimal Impacts of Microplastics on Soil Physical Properties under Environmentally Relevant Concentrations. Environ. Sci. Technol. 2023, 57, 5296–5304. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, J.; Xue, J. Polyethylene microplastic and soil nitrogen dynamics: Unraveling the links between functional genes, microbial communities, and transformation processes. J. Hazard. Mater. 2023, 458, 131857. [Google Scholar] [CrossRef]
- Balabane, M.; Plante, A.F. Aggregation and carbon storage in silty soil using physical fractionation techniques. Eur. J. Soil Sci. 2004, 55, 415–427. [Google Scholar] [CrossRef]
- Amézketa, E. Soil Aggregate Stability: A Review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, X.; Wang, J.; Shao, J.; Fu, Y.; Liang, C.; Yan, E.; Chen, X.; Wang, X.; Bai, S.H. Differential magnitude of rhizosphere effects on soil aggregation at three stages of subtropical secondary forest successions. Plant Soil. 2019, 436, 365–380. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Ju, T.; Yang, K.; Chang, L.; Zhang, K.; Wang, X.; Zhang, J.; Xu, B.; Li, Y. Microplastics sequestered in the soil affect the turnover and stability of soil aggregates: A review. Sci. Total Environ. 2023, 904, 166776. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Wang, P.-Y.; Wang, Y.-B.; Zhou, R.; Koskei, K.; Munyasya, A.N.; Liu, S.-T.; Wang, W.; Su, Y.-Z.; Xiong, Y.-C. Fate of plastic film residues in agro-ecosystem and its effects on aggregate-associated soil carbon and nitrogen stocks. J. Hazard. Mater. 2021, 416, 125954. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Xiang, Y.; Rillig, M.C.; Peñuelas, J.; Sardans, J.; Liu, Y.; Yao, B.; Li, Y. Global Responses of Soil Carbon Dynamics to Microplastic Exposure: A Data Synthesis of Laboratory Studies. Environ. Sci. Technol. 2024, 58, 5821–5831. [Google Scholar] [CrossRef]
- Machado, A.A.d.S.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- Han, L.; Chen, L.; Feng, Y.; Kuzyakov, Y.; Chen, Q.a.; Zhang, S.; Chao, L.; Cai, Y.; Ma, C.; Sun, K.; et al. Microplastics alter soil structure and microbial community composition. Environ. Int. 2024, 185, 108508. [Google Scholar] [CrossRef]
- Machado, A.A.d.S.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Lu, Y.; Wu, J.; Zhang, L.; Wang, J.; Wu, W. Profile and nano-scale distribution of soil organic carbon for upland and paddy soils from an alluvial plain in South China. Chem. Geol. 2023, 640, 121740. [Google Scholar] [CrossRef]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta-analysis. Global Change Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef]
- Cao, L.; Wu, D.; Liu, P.; Hu, W.; Xu, L.; Sun, Y.; Wu, Q.; Tian, K.; Huang, B.; Yoon, S.J.; et al. Occurrence, distribution and affecting factors of microplastics in agricultural soils along the lower reaches of Yangtze River, China. Sci. Total Environ. 2021, 794, 148694. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic Disguising As Soil Carbon Storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef]
- Wei, H.; Wu, L.; Liu, Z.; Saleem, M.; Chen, X.; Xie, J.; Zhang, J. Meta-analysis reveals differential impacts of microplastics on soil biota. Ecotoxicol. Environ. Saf. 2022, 230, 113150. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Lu, R. Soil Agricultural Chemistry Analytical Method; Chinese Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Li, K.-w.; Lu, H.-l.; Nkoh, J.N.; Hong, Z.-n.; Xu, R.-k. Aluminum mobilization as influenced by soil organic matter during soil and mineral acidification: A constant pH study. Geoderma 2022, 418, 115853. [Google Scholar] [CrossRef]
- Mitran, T.; Mani, P.K.; Basak, N.; Mazumder, D.; Roy, M. Long-term manuring and fertilization influence soil inorganic phosphorus transformation vis-a-vis rice yield in a rice–wheat cropping system. Arch. Agron. Soil Sci. 2016, 62, 1–18. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Vance, E. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar]
- Tang, X.; Huang, W.; Lu, Y.; Li, B.; Wang, C.; Dong, Y. Characteristics and Stability of Organic Carbon and Ferric Oxidein in Soil Aggregates and Aggregate Stability in Lateritic Red Soil Region, Guangdong Province. J. Soil Water Conserv. 2021, 35, 200–209. (In Chinese) [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Phospholipid Fatty Acid Composition, Biomass, and Activity of Microbial Communities from Two Soil Types Experimentally Exposed to Different Heavy Metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, M.; Chen, Z.; Tang, Z.; Tan, J.; Tian, J.; Elsgaard, L.; Lu, Y.; Li, B. Nonlinear effects of biochar on greenhouse gas emissions and soil microbial community composition under intensive vegetable production in South China. Appl. Soil Ecol. 2023, 189, 104975. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Xiao, M.R.; Ma, Y.; Niu, H.; Zhang, G.S. Polyester microfiber and natural organic matter impact microbial communities, carbon-degraded enzymes, and carbon accumulation in a clayey soil. J. Hazard. Mater. 2021, 405, 124701. [Google Scholar] [CrossRef]
- Núñez, S.; Martínez-Yrízar, A.; Búrquez, A.; García-Oliva, F. Carbon mineralization in the southern Sonoran Desert. Acta Oecologica. 2001, 22, 269–276. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, L.; Degen, A.A.; Rafiq, M.K.; Kuzyakov, Y.; Zhao, J.; Zhang, R.; Zhang, T.; Wang, W.; Li, X.; et al. Long-term active restoration of extremely degraded alpine grassland accelerated turnover and increased stability of soil carbon. Global Change Biol. 2020, 26, 7217–7228. [Google Scholar] [CrossRef]
- Fang, Z.; Sallach, J.B.; Hodson, M.E. Size- and concentration-dependent effects of microplastics on soil aggregate formation and properties. J. Hazard. Mater. 2024, 465, 133395. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape- and polymer-dependent effects on soil aggregation and organic matter loss—An experimental and meta-analytical approach. Microplast. Nanoplast. 2021, 1, 7. [Google Scholar] [CrossRef]
- Lozano Yudi, M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 714541. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Padbhushan, R.; Rakshit, R.; Das, A.; Sharma, R.P. Effects of various organic amendments on organic carbon pools and water stable aggregates under a scented rice-potato-onion cropping system. Paddy Water Environ. 2016, 14, 481–489. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.; Huang, Q.; Qin, J.; Zheng, Y.; Wang, J.; Li, X.; Wang, Q.; Guo, G.; Hu, S. Plastic mulch debris in rhizosphere: Interactions with soil-microbe-plant systems. Sci. Total Environ. 2022, 807, 151435. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, J.; Tao, J.; Yang, Y.; Wu, D.; Han, L.; Li, S.; Wang, J. Current advances in interactions between microplastics and dissolved organic matters in aquatic and terrestrial ecosystems. Trac-Trends Anal. Chem. 2023, 158, 116882. [Google Scholar] [CrossRef]
- Maie, N.; Watanabe, A.; Kimura, M. Chemical characteristics and potential source of fulvic acids leached from the plow layer of paddy soil. Geoderma 2004, 120, 309–323. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Shen, J.; Xu, F.; Hou, H.; Xie, C.; Wang, B.; An, S. Mechanism of polyethylene and biodegradable microplastic aging effects on soil organic carbon fractions in different land-use types. Sci. Total Environ. 2024, 912, 168961. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil. 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Y.; Zhang, X.; Yang, X.; Drury, C. Soil organic carbon in clay and silt sized particles in Chinese mollisols: Relationship to the predicted capacity. Geoderma 2006, 132, 315–323. [Google Scholar] [CrossRef]
- Shi, J.; Sun, Y.; Wang, X.; Wang, J. Microplastics reduce soil microbial network complexity and ecological deterministic selection. Environ. Microbiol. 2022, 24, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Z.; Zhang, Y.; Jia, H.; Li, Y.; Yao, H. Abundances of agricultural microplastics and their contribution to the soil organic carbon pool in plastic film mulching fields of Xinjiang, China. Chemosphere 2023, 316, 137837. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Krull, E.S.; Baldock, J.A.; Skjemstad, J.O. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct. Plant Biol. FPB 2003, 30, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zheng, Z.; Ying, Z.; Qidao, S.; Ping, F.; Beidou, X.; Wenbing, T. Effects of microplastics on soil organic carbon and greenhouse gas emissions in the context of straw incorporation: A comparison with different types of soil. Environ. Pollut. 2021, 288, 117733. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Peng, Y.; Fan, Z.; Zhang, Z.; Wang, X.; Zhu, K.; Shang, J.; Wang, J. Effects of Microplastics on Soil Carbon Mineralization: The Crucial Role of Oxygen Dynamics and Electron Transfer. Environ. Sci. Technol. 2023, 57, 13588–13600. [Google Scholar] [CrossRef]
- Gao, W.; Ma, T.; Shi, B.; Yang, Z.; Li, Y.; Zhu, J.; He, J.-S. Effects of nitrogen and phosphorus addition on the mineralization potential of soil organic carbon and the corresponding regulations in the Tibetan alpine grassland. Appl. Soil Ecol. 2024, 196, 105314. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Wang, G.; Liu, X.; Zhang, Q.; Chen, Y.; Song, W.; Qu, W.; Chu, X.; Li, P. Imbalanced nitrogen-phosphorus input alters soil organic carbon storage and mineralisation in a salt marsh. Catena 2022, 208, 105720. [Google Scholar] [CrossRef]
- Ya, H.; Xing, Y.; Zhang, T.; Lv, M.; Jiang, B. LDPE microplastics affect soil microbial community and form a unique plastisphere on microplastics. Appl. Soil Ecol. 2022, 180, 104623. [Google Scholar] [CrossRef]
- Crowther, T.W.; Van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Ma, Q.; Wu, X.; Zhang, Y.; Yu, W. Temperature sensitivity of a PLFA-distinguishable microbial community differs between varying and constant temperature regimes. Geoderma 2017, 308, 54–59. [Google Scholar] [CrossRef]
- Fraterrigo, J.M.; Balser, T.C.; Turner, M.G. Microbial community variation and its relationship with nitrogen mineralization in historically altered forests. Ecology 2006, 87, 570–579. [Google Scholar] [CrossRef]
- Turpeinen, R.; Kairesalo, T.; Häggblom, M.M. Microbial community structure and activity in arsenic-, chromium- and copper-contaminated soils. FEMS Microbiol. Ecol. 2004, 47, 39–50. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Stromberger, M.E. Relationships between microbial community structure and soil environmental conditions in a recently burned system. Soil Biol. Biochem. 2007, 39, 1703–1711. [Google Scholar] [CrossRef]
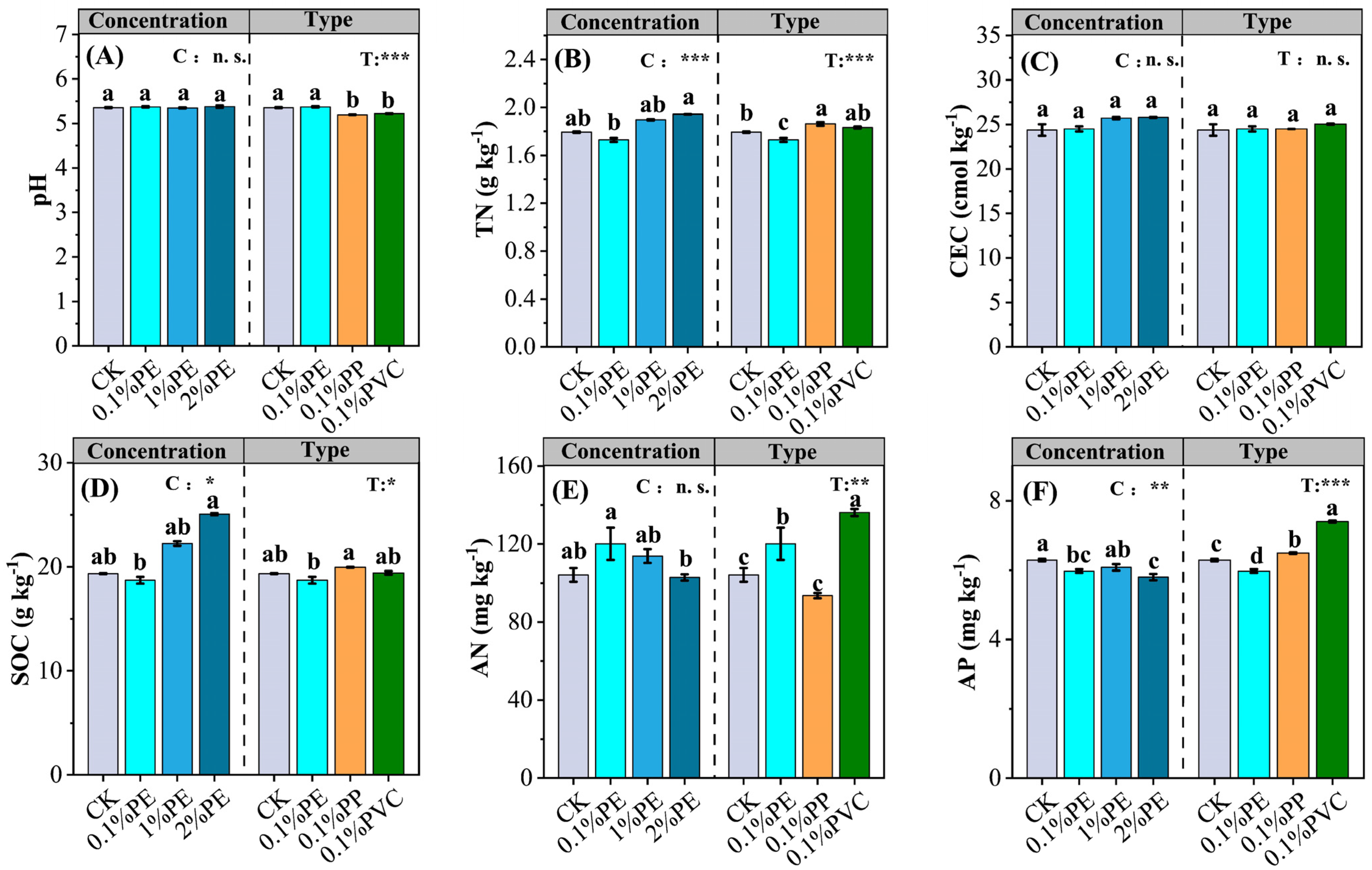

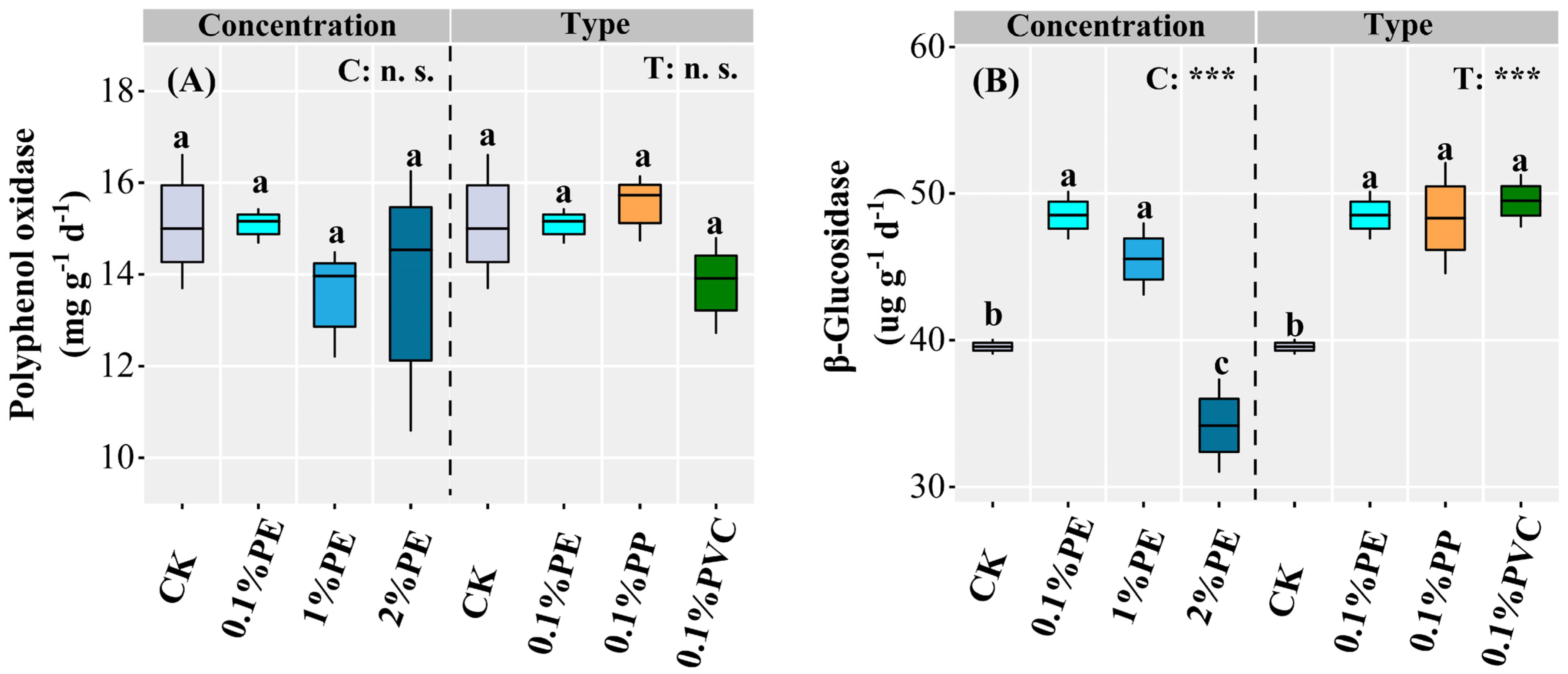
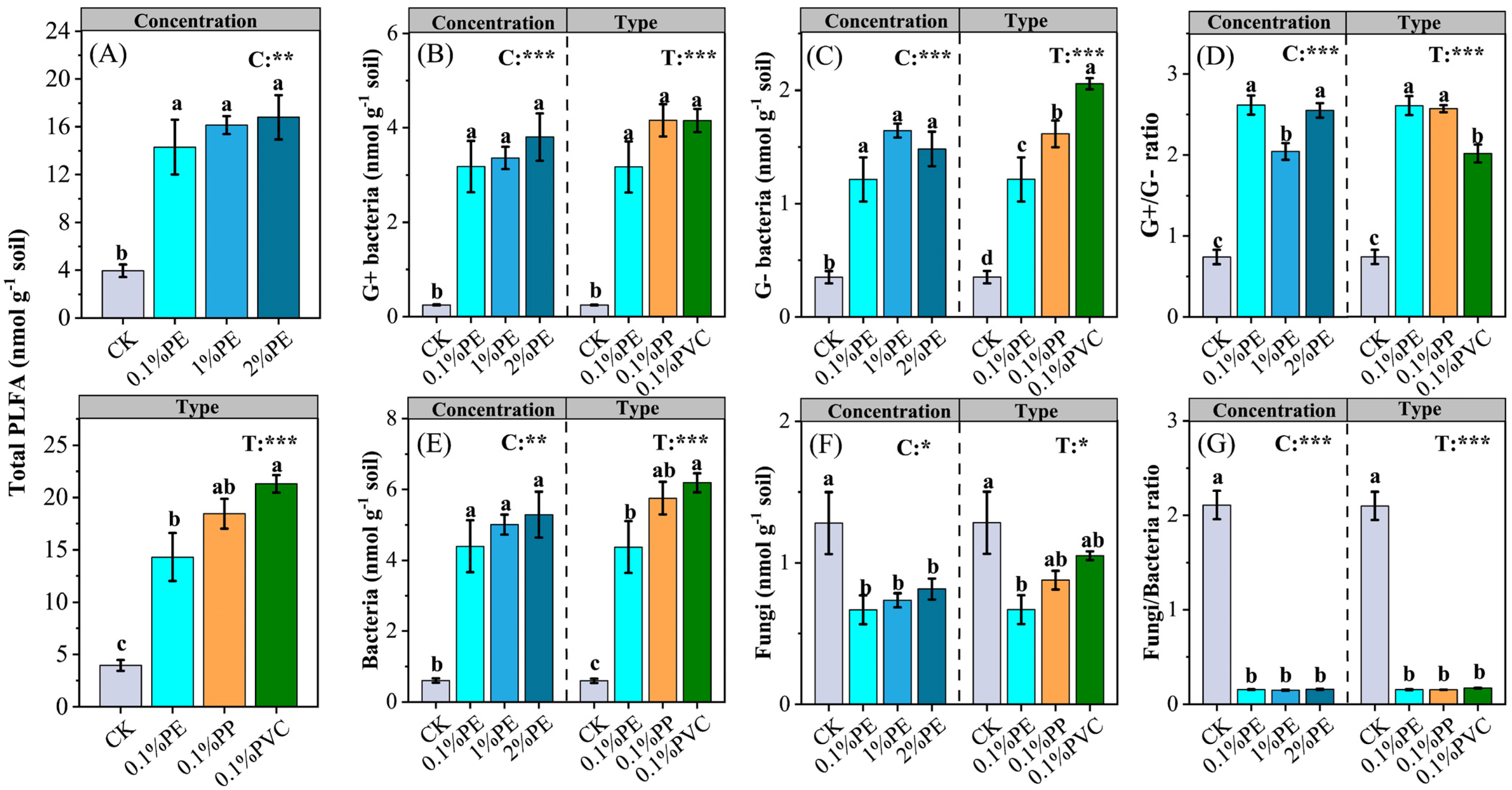
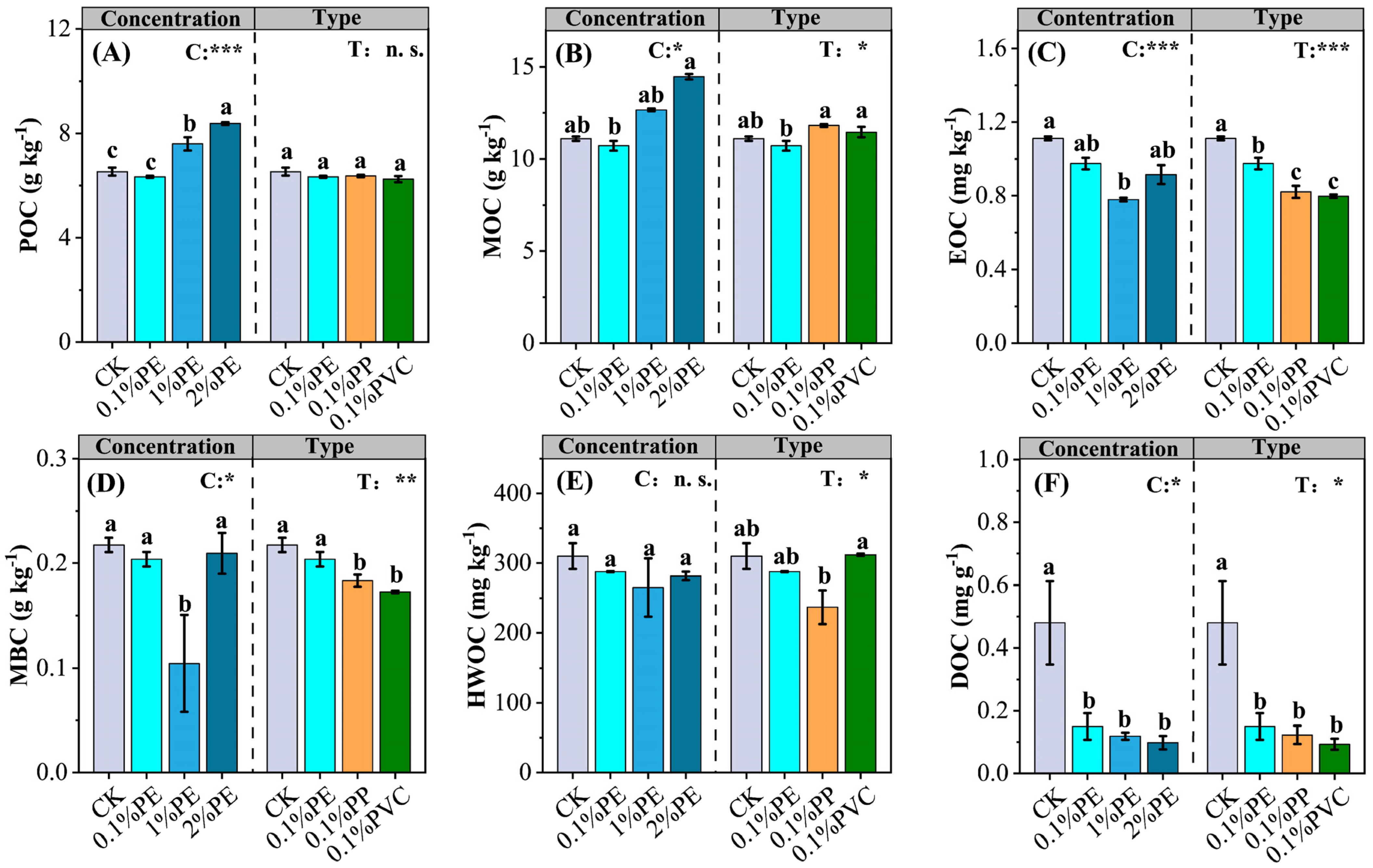
| Vegetable Crops | Species | Crop Season | Fertilization Period | Nitrogen Fertilization a (kg N ha−1) |
|---|---|---|---|---|
| 1st | Flowering Chinese Cabbage (Brassica parachinensis L. H. Bailey) | 26 December 2021–16 February 2022 | 21 December 2021 | 168 |
| 2nd | Flowering Chinese Cabbage (Brassica parachinensis L. H. Bailey) | 9 March 2022–24 April 2022 | 12 March 2022 | 168 |
| 3rd | Water spinach (Ipomoea aquatica Forsk) | 21 August 2022–29 September 2022 | 14 August 2022 | 168 |
| Variant | Treat | Percentage of Different Particles (%) | |||
|---|---|---|---|---|---|
| 2–5 mm | 0.25–2 mm | 0.053–0.25 mm | <0.053 mm | ||
| MP concentration | CK | 12.86 ± 1.91 a | 60.07 ± 0.93 a | 17.71 ± 0.76 ab | 9.37 ± 1.52 ab |
| 0.1%PE | 6.99 ± 0.91 a | 60.71 ± 1.93 a | 20.64 ± 2.82 a | 11.66 ± 3.84 a | |
| 1%PE | 9.15 ± 3.07 a | 66.59 ± 3.08 a | 13.58 ± 1.7 b | 10.68 ± 1.68 ab | |
| 2%PE | 12.17 ± 3.18 a | 69.02 ± 5.47 a | 13.11 ± 2.04 b | 5.7 ± 0.53 ab | |
| One-way ANOVA | n. s. | n. s. | * | n. s. | |
| MP type | CK | 12.86 ± 1.91 b | 60.07 ± 0.93 a | 17.71 ± 0.76 ab | 9.37 ± 1.52 a |
| 0.1%PE | 6.99 ± 0.91 c | 60.71 ± 1.93 a | 20.64 ± 2.82 a | 11.66 ± 3.84 a | |
| 0.1%PP | 18.44 ± 1.75 a | 59.23 ± 3.22 a | 14.1 ± 1.13 b | 8.23 ± 2.03 a | |
| 0.1%PVC | 12.87 ± 0.71 b | 62.44 ± 1.5 a | 16.37 ± 1.94 ab | 8.33 ± 2.48 a | |
| One-way ANOVA | ** | n. s. | n. s. | n. s. | |
| Parameter | MWD (mm) | GMD (mm) | FD | Different Particle Sizes of Soil Water-Stable Aggregates (mm) | |||
|---|---|---|---|---|---|---|---|
| 2–5 mm | 0.25–2 mm | 0.053–0.25 mm | <0.053 mm | ||||
| MWD | 1 | 0.824 ** | −0.364 | 0.934 ** | −0.156 | −0.498 * | −0.538 * |
| GMD | 1 | −0.817 ** | 0.661 ** | 0.15 | −0.275 | −0.910 ** | |
| FD | 1 | −0.132 | −0.483 * | 0.052 | 0.964 ** | ||
| Indicators | SOC Mineralization | ||
|---|---|---|---|
| MR | CM | ||
| Soil carbon fractions | POC | −0.526 * | −0.251 |
| MOC | −0.231 | −0.503 * | |
| EOC | −0.511 * | 0.798 ** | |
| MBC | −0.05 | 0.344 | |
| HWOC | −0.143 | 0.434 | |
| DOC | −0.299 | 0.543 * | |
| Response | Factors | Explains (%) | Pseudo-F | p | Response | Factors | Explains (%) | Pseudo-F | p |
|---|---|---|---|---|---|---|---|---|---|
| Soil aggregate stability | CM | 27.7 | 6.1 | 0.008 ** | Soil organic carbon mineralization | pH | 71.6 | 40.4 | 0.002 ** |
| MBC | 17.6 | 4.8 | 0.038 * | Total PLFA | 17.5 | 24.2 | 0.004 ** | ||
| Total PLFA | 12.4 | 4.1 | 0.04 * | TN | 5.2 | 12.8 | 0.004 ** | ||
| HWOC | 10.3 | 4.2 | 0.016 * | AP | 1.9 | 6.7 | 0.028 * | ||
| CEC | 4.4 | 1.9 | 0.142 | MBC | 0.3 | 1.1 | 0.292 | ||
| DOC | 3.6 | 1.6 | 0.21 | CEC | 0.2 | 0.6 | 0.496 | ||
| β-glucosidase | 2.5 | 1.1 | 0.332 | PPO | 0.3 | 0.8 | 0.4 | ||
| PPO | 2.1 | 1 | 0.424 | HWOC | 0.2 | 0.5 | 0.51 | ||
| AN | 3.9 | 2 | 0.18 | DOC | 0.2 | 0.5 | 0.43 | ||
| POC | 0.4 | 0.2 | 0.882 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wan, Q.; Zhou, P.; Li, H.; Liu, Y.; Lu, Y.; Li, B. Microplastics Can Inhibit Organic Carbon Mineralization by Influencing Soil Aggregate Distribution and Microbial Community Structure in Cultivated Soil: Evidence from a One-Year Pot Experiment. Agronomy 2024, 14, 2114. https://doi.org/10.3390/agronomy14092114
Chen Z, Wan Q, Zhou P, Li H, Liu Y, Lu Y, Li B. Microplastics Can Inhibit Organic Carbon Mineralization by Influencing Soil Aggregate Distribution and Microbial Community Structure in Cultivated Soil: Evidence from a One-Year Pot Experiment. Agronomy. 2024; 14(9):2114. https://doi.org/10.3390/agronomy14092114
Chicago/Turabian StyleChen, Zonghai, Quan Wan, Pengyu Zhou, Haochen Li, Yige Liu, Ying Lu, and Bo Li. 2024. "Microplastics Can Inhibit Organic Carbon Mineralization by Influencing Soil Aggregate Distribution and Microbial Community Structure in Cultivated Soil: Evidence from a One-Year Pot Experiment" Agronomy 14, no. 9: 2114. https://doi.org/10.3390/agronomy14092114
APA StyleChen, Z., Wan, Q., Zhou, P., Li, H., Liu, Y., Lu, Y., & Li, B. (2024). Microplastics Can Inhibit Organic Carbon Mineralization by Influencing Soil Aggregate Distribution and Microbial Community Structure in Cultivated Soil: Evidence from a One-Year Pot Experiment. Agronomy, 14(9), 2114. https://doi.org/10.3390/agronomy14092114






