Abstract
The fodder soybean (Glycine max) is an excellent leguminous forage with a high protein content and hay yield, cultivated comprehensively in alpine regions, but seasonal drought in northern regions severely impacts the growth of seedlings. Melatonin (MT) and strigolactone (SL) are critical in relieving the restraint of plant growth in water-deficient environments, but the mechanisms of MT- and SL-mediated drought resistance in fodder soybean needs to be explored. This study mainly investigated the potential morphophysiological mechanism of MT and SL treatments in protecting fodder soybean from drought stress. The fodder soybean ‘Gongnong 535’ was treated with 100 µM MT or 1 µM SL under normal, moderate, and severe water deficit conditions. The results showed that MT and SL treatments enhanced the plant growth parameters and stomatal aperture under drought stress. Moreover, the observed reductions in superoxide ion (O2.-), malondialdehyde (MDA), and relative electrical conductivity (REC), along with enhancements in the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), as well as higher levels of ascorbate (AsA), glutathione (GSH), soluble sugar (SS), soluble protein (SP), and free proline (Pro), indicated that MT and SL application effectively alleviated the oxidative damage and prevented the cell membrane disruption caused by drought stress. Additionally, MT and SL treatments improved photosynthesis and growth in fodder soybean seedlings under water stress by adjusting chlorophyll pigments, gas exchange indexes, and chlorophyll fluorescence parameters, as well as endogenous hormone levels. Simultaneously, MT and SL influenced the expression of genes associated with photosynthesis and antioxidant defenses, as well as phytohormone concentrations. Notably, the protective effect of the SL treatment was superior to that of MT in water-deficient conditions. This study contributes to further understanding the defensive mechanism of MT and SL against drought stress.
1. Introduction
Drought stress is a primary and widespread abiotic stress that constrains plant yield and quality in countries or regions with water scarcity [1,2]. Drought stress induces stomatal closure, accumulates reactive oxygen species (ROS), and decreases photosynthetic capacity in plants, thereby affecting plant normal growth [3]. Although plants develop a set of self-defense mechanisms in response to water deficiency [4], the self-regulation capacity of plants is limited. Prolonged and aggravated drought stress often exceeds their regulatory threshold, causing damage to their protective system and even plant death. Therefore, exploring feasible methods to enhance plant drought resistance is crucial. Among these methods, the application of exogenous hormones is considered an effective and economical strategy to mitigate the unfavorable impact of water deficiency stress in plants [5]. Thus, selecting an appropriate phytohormone is the main effective way to improve a plant’s capacity for drought resistance.
Melatonin (MT) is a widely present endogenous hormone in plants, and it has an important role in mitigating diverse biotic and abiotic stressors [6,7]. In recent years, some research findings demonstrate that MT can increase plant drought resistance by regulating the plant’s physiological metabolism. In response to water-deficient environments, MT treatment reduces the concentration of ROS and stabilizes cell membrane structures [8]. Furthermore, by activating antioxidant enzymes and non-enzyme antioxidant content, MT strengthens antioxidant capacity during drought stress [9]. Actually, MT has also been testified in multiple plants to improve the chloroplast structure, photosynthetic electron transfer rate, and light capture ability under water deficiency conditions [8,9,10]. Additionally, exogenous MT collaborates with other endogenous hormones, such as IAA, GA, CTK, JA, and ABA, to suppress the water deficit stress response [11]. Meanwhile, drought resistance is enhanced through MT treatment by regulating the gene expression levels related to antioxidants, photosynthesis, and phytohormones [10,11]. Evidently, these research findings provide a theoretical reference for applying MT to improve plant drought tolerance. Nevertheless, there have been relatively few studies that explore the change rule of antioxidant defenses, photosynthesis, hormone synthesis, and the key gene expression of forage subjected to drought conditions by applying exogenous MT.
Strigolactone (SL) is a class of carotenoid-derived phytohormones that plays a vital function in improving adaptability to environmental changes [12]. Notably, there have been sporadic studies on the application of SL to improve plant drought tolerance. SL treatment increases photosynthetic efficiency and inhibits chlorophyll degradation in winter wheat (Triticum aestivum L.) during drought periods [13]. In yellow pepper (Capsicum chinense), by reducing the malondialdehyde (MDA) content and activating antioxidant enzymes, SL mediates the antioxidant defense process under water stress [14], while in crabapple (Malus spectabilis), SL decreases the influence of drought stress on the phenotype [15]. In addition, SL can also regulate ABA content, participate in photosynthetic reactions, and increase the gene expression of plants under drought stress [16,17]. It is evident that SL has potentiality in improving plant adaptation to drought environments. However, our understanding of the mechanism of improved drought resistance mediated by SL is still limited. Particularly, the effect of SL on fodder soybean seedlings in the process of drought stress has not been investigated.
Fodder soybean (Glycine max) is an annual legume species known for its high-quality forage, primarily distributed in temperate and cold temperate regions [18]. Due to its tall stature, lush stems and leaves, shade tolerance, high protein content, and rich rhizobium, fodder soybean is often intercropped with silage corn in production. This practice not only increases the forage yield and quality, but also reduces the use of fertilizers, thereby decreasing environmental pollution [19]. However, fodder soybean is sensitive to water deficiency during the seedling stage; thus, its growth and yield are severely impacted by seasonal drought in northern regions [20]. Therefore, it is imperative to further understanding how MT and SL contribute to coping with water deficiency stress during the development of fodder soybean seedlings, including changes in phenotype, photosynthetic characteristics, antioxidant defense systems, and important genes expression. For the research aim, we hypothesized that the application of MT and SL can improve fodder soybean tolerance to drought stress by regulating morphophysiological responses and key genes expression. The hypothesis was validated by examining three distinct questions: (1) whether the application of kMT and SL improves the tolerance to drought of fodder soybean seedlings, (2) whether treatments with MT and SL effectively affect physiological metabolic processes under drought stress, and (3) whether MT or SL is more beneficial in improving growth and reducing drought damage during the seedling stage of fodder soybean. This study provides further insights into the mechanism by primarily exploring the morphological and physiological changes that come about during the alleviation of drought damage through MT and SL application.
2. Materials and Methods
2.1. Plant Material and Treatments
The experiment was developed in an artificial climate chamber of the Horticultural Experimental Station at Northeast Agricultural University (E 126°68′, N 45°72′). The average photoperiod was 13 h, with a relative humidity of 55% ± 5%, temperature of 30 °C/20 °C (day/night), and illumination of 1000 ± 46 µmol·m−2·s−1. Seeds of the ‘Gongnong 535’ fodder soybean that were plump, uniformly colored, and consistent in size were selected and sown in PVC seedling pots, with dimensions of diameter 16 cm × height 20 cm. Each pot contained 800 g of mixed substrates, including nutrient soil (Pindstrup, Pinstow, Denmark) and sand (Shahe, Haerbing, China) in a ratio of 2:1, and 10 seeds were sown per pot. Watering was performed every 3 days, and 8 seedlings with uniform growth potential were kept per pot in the true leaf stage. Hence, there were 40 plants for each treatment, with 5 replicates per treatment. When the seedlings reached the V3 stage, natural drought treatment was initiated. Based on preliminary experiments, the spraying of distilled water, 100 μM MT (Merck, Darmstadt, Germany), or 1 μM SL (Merck, Darmstadt, Germany) per plant took place at 20:00 each day. The spraying was performed until the leaves were fully covered with droplets but not dripping, using 40 mL each time per pot. Pot soil moisture was controlled using a gravimetric method, with three drought stress levels set: 80% (normal water supply, NW), 50% (moderate drought, MD), and 30% (severe drought, SD) of the relative water content of soil. For each drought level, four treatments were applied: no spraying (NS), water spraying (WS), MT spraying (MS), and SL spraying (SS), resulting in a total of 12 treatments. Morphological and physiological indexes were measured on the day each drought level was reached. The third mature leaf from the base was collected for each treatment. Samples requiring freezing were frozen at −80 °C for subsequent experiment.
2.2. Measurements of Growth and Morphological Indexes
Growth and morphological traits were assessed through measurement. Plant height, leaf area, biomass, and root morphological indexes were measured using methods previously described in published articles from our research group [21,22]. For stomatal traits, fresh leaves were cut into 2 × 5 mm rectangles (avoiding veins) and fixed in 10 ml of 2.5% glutaraldehyde for 1–2 h, then washed 2–3 times with 0.1 mol phosphate-buffered saline. Next, the samples were dried using a freeze dryer (Hitachi, Tokyo, Japan) for 4 min after dehydration and plated on metal film. Finally, observations were made with an electron microscope (Hitachi, Tokyo, Japan) [22]. Each measurement index was repeated 10 times.
2.3. Determination of Photosynthesis Parameters
Photosynthetic indexes were determined through a photosynthetic instrument (LI-COR, Lincoln, NE, USA). Chlorophyll fluorescence indexes were assessed through a chlorophyll fluorescence monitor (Walz, Nuremberg, Germany), following a 30 min dark adaptation period [21]. Chlorophyll (Chl) content was measured utilizing the method of 95% alcohol extraction [22], with five replicates per sample.
2.4. Measurement of Antioxidant Enzymes, Antioxidants, and Osmotic Solutes
The activity of peroxidase (POD) was quantified by the guaiacol measurement [22]. The superoxide dismutase (SOD) enzyme was determined via the NBT photoreduction technique [23], and catalase (CAT) activity was assessed through UV absorption measurement [23]. The ascorbate (AsA) content was determined following Lu et al. (2022) [23], while the glutathione (GSH) content was detected according to Guri (1983) [24]. The soluble sugar (SS) content was evaluated utilizing the anthrone colorimetric assay [22], while the soluble protein (SP) content was quantified by the Coomassie brilliant blue technique [22]. The free proline (Pro) content was assessed through the sulfosalicylic acid technique [22].
2.5. Measurement of Malondialdehyde, Relative Electrical Conductivity, and Superoxide Anion
The relative electrical conductivity (REC) was assessed with a conductometer [22]. The malondialdehyde (MDA) content was measured employing TBA measurement [22], and the superoxide anion (O2.−) content was measured via the hydroxylamine oxidation technique [23]. Each measurement index was replicated 3 times to ensure accuracy.
2.6. Measurement of Endogenous Hormone Indexes
The levels of abscisic acid (ABA), jasmonic acid (JA), indole acetic acid (IAA), and zeatin riboside (ZR) were measured using kits provided by the Chemical Control Laboratory of China Agricultural University [25].
2.7. Measurement of Gene Expression
The relative expression of pivotal genes was quantified using qRT-PCR. The experiment was conducted using a Quantagene q225 Real-Time PCR System (Kubotechnology, Beijing, China), and the protocols were in accordance with the ChamQTM Universal SYBR® qPCR Master Mix kit (Vazyme, Nanjing, China). The relative expression of genes was determined by the 2−∆∆Ct method. The primers of the qRT-PCR are listed in Table S1.
2.8. Data Analysis
All data analyses were determined by SPSS (v. 20.00). The difference between treatments under the same degree of drought was evaluated using one-way ANOVA by Duncan’s test, and statistical difference was considered significant (p < 0.05) The analysis results were presented through the mean as well as standard deviation (SD). The figure of the impact of MT and SL on gene expression level in fodder soybean under drought stress was performed in ‘heatmap’ package ‘R’ (version 4.0.3), and column charts were displayed by GraphPad Prism (v. 9.00).
3. Results
3.1. Impact of MT and SL on the Growth of Fodder Soybean under Drought Stress
The plant height, leaf area, and above- and belowground biomass of fodder soybean showed a decreased trend with the severity of drought (Table 1). Applying MT and SL can considerably reduce the influence on the growth of fodder soybean of water-deficient environments. Under the MD condition, spraying MT and SL increased plant height by 2.45% and 8.29%, leaf area by 6.99% and 8.74%, aboveground biomass by 10.14% and 11.59%, and belowground biomass by 11.11% and 16.67% compared with spraying water, respectively, but those changes were not significant (Table 1). Meanwhile, the effects of MT and SL spraying on the leaf area and aboveground and belowground biomass of fodder soybean reached an outstanding level under the SD condition compared to spraying water (Table 1). Notably, the SL application significantly enhanced both aboveground and belowground biomass compared to MT under the SD treatment.

Table 1.
Effects of MT and SL on growth parameters of fodder soybean under drought stress.
3.2. Impact of MT and SL on Morphological Characteristics of Fodder Soybean under Drought Stress
3.2.1. Changes in Root System
As the drought intensified, the root morphology of fodder soybean was enormously impacted (Figure S1). However, when MT and SL were sprayed, the detrimental impact of water deficiency stress on fodder soybean root growth was mitigated. Compared to spraying water, spraying MT and SL markedly altered the root length and surface area under the NW condition (p < 0.05). In MD stress, spraying SL increased the root surface area by 15.03%, reaching a prominent level, while spraying SL played a certain role in the root surface area, but did not reach a significant level (Table 2). Under SD stress, compared with spraying water, spraying MT and SL distinctly increased the root length by 32.81% and 34.33%, root surface area by 8.81% and 10.19%, and average root diameter by 4.65% and 6.98% (p < 0.05). Moreover, the effect of spraying SL on the root indexes in fodder soybean is more remarkable than that of spraying MT under SD stress.

Table 2.
Effects of MT and SL on root indexes of fodder soybean under drought stress.
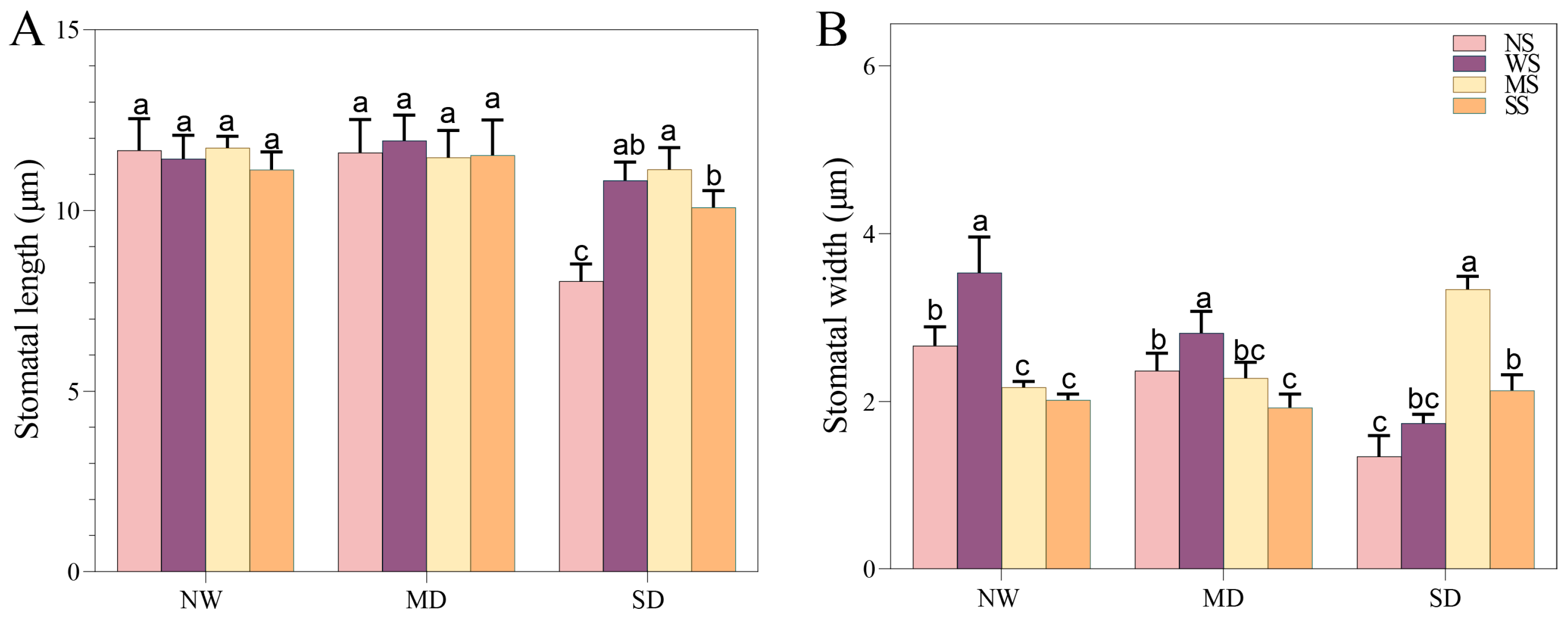
3.2.2. Changes in Stomatal Traits
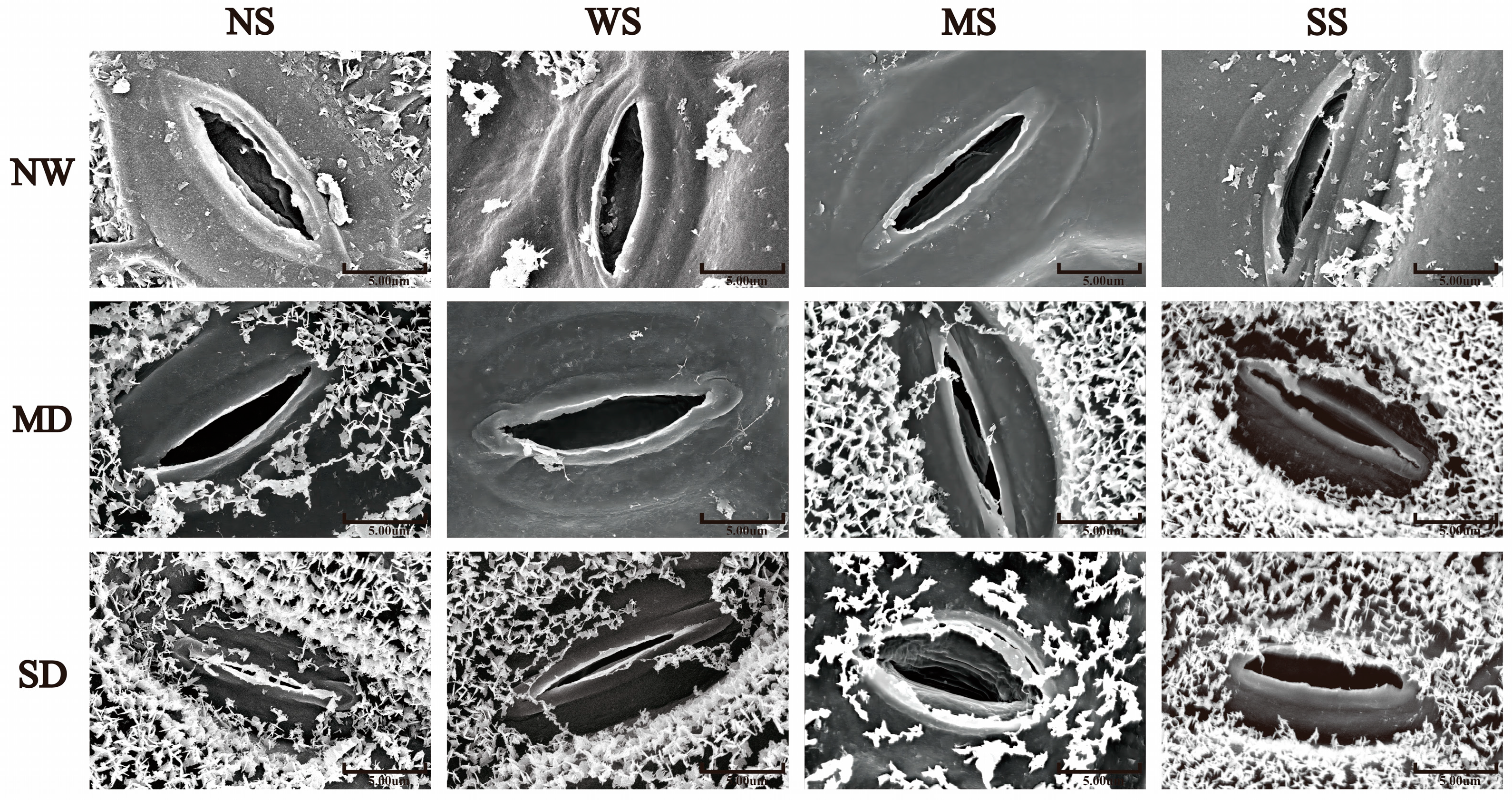
With the development of soil drought, the length and width of the stomata in fodder soybean notably decreased, and the stomata gradually closed (Figure 1). The MT and SL treatments showed a positive effect on the stomatal traits of fodder soybean, including stomatal aperture, length and width. Compared with no spraying treatment (NS), spraying water significantly increased the width of the stomata, while MT and SL treatments obviously decreased the width of the stomata under NW conditions (Figure 1 and Figure 2). Under MD stress, compared with spraying water, spraying MT and SL decreased stomatal length by 3.86% and 3.35% (p < 0.05), and stomatal width by 19.21% and 31.67%, respectively (p < 0.05) (Figure 2). Under SD conditions, the stomatal aperture of fodder soybean apparently became smaller or even closed under both no spraying (NS) and spraying water treatments (WS) (Figure 1), while MT and SL treatments maintained the stomatal status. Additionally, the treatment with MT most significantly lessened the adverse impact of water deficit stress on the stomatal traits of fodder soybean across all treatments under SD conditions.
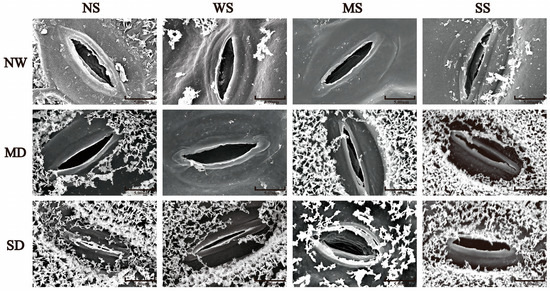
Figure 1.
Effects of MT and SL on stomatal traits in fodder soybean under drought stress. Scale bars = 5 μm. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL.
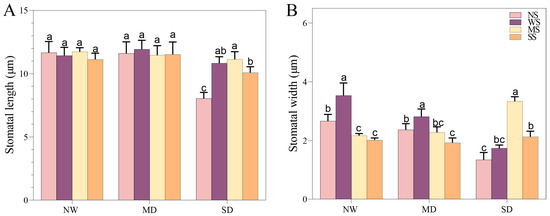
Figure 2.
Effects of MT and SL on stomatal length and width in fodder soybean under drought stress. (A) stomatal length, (B) stomatal width. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 10).
3.3. Impact of MT and SL on Photosynthetic System in Fodder Soybean under Drought Stress
3.3.1. Changes in Gas Exchange Parameters
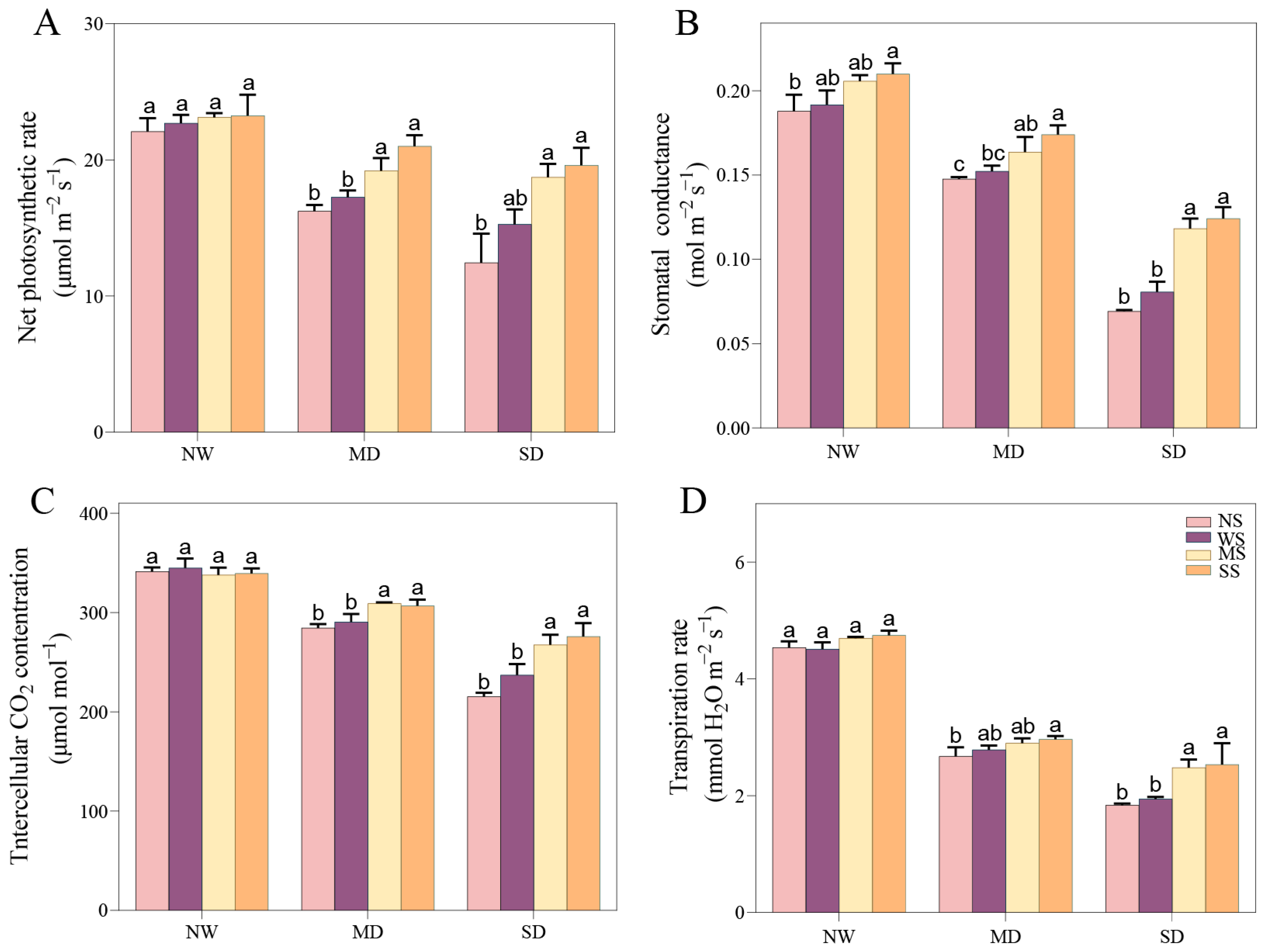
As the drought stress intensified, the photosynthetic indexes consistently decreased, including Pn, Gs, Ci, and Tr. However, the application of MT and SL significantly ameliorated these gas exchange parameters of fodder soybean under drought stress. Under MD stress, spraying MT and SL improved the Pn, Gs, Ci and Tr compared with spraying water, with changes in Pn and Ci reaching an outstanding level (p < 0.05), (Figure 3). Meanhile, under SD stress, Pn, Gs, Ci and Tr remarkably increased after spraying MT and SL compared to no spraying and spraying water.
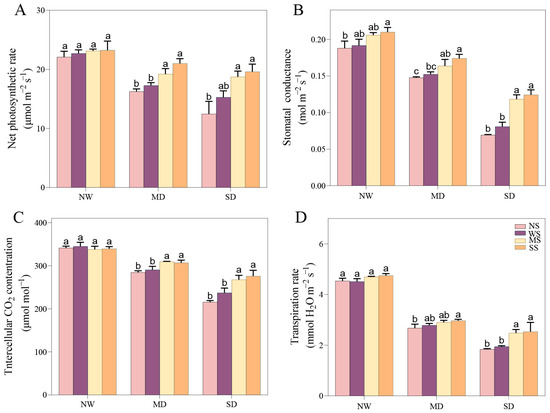
Figure 3.
Effects of MT and SL on gas exchange in fodder soybean under drought stress. (A) Net photosynthetic rate (Pn); (B) stomatal conductance (Gs); (C) intercellular CO2 concentration (Ci); (D) transpiration rate (Tr). NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 5).
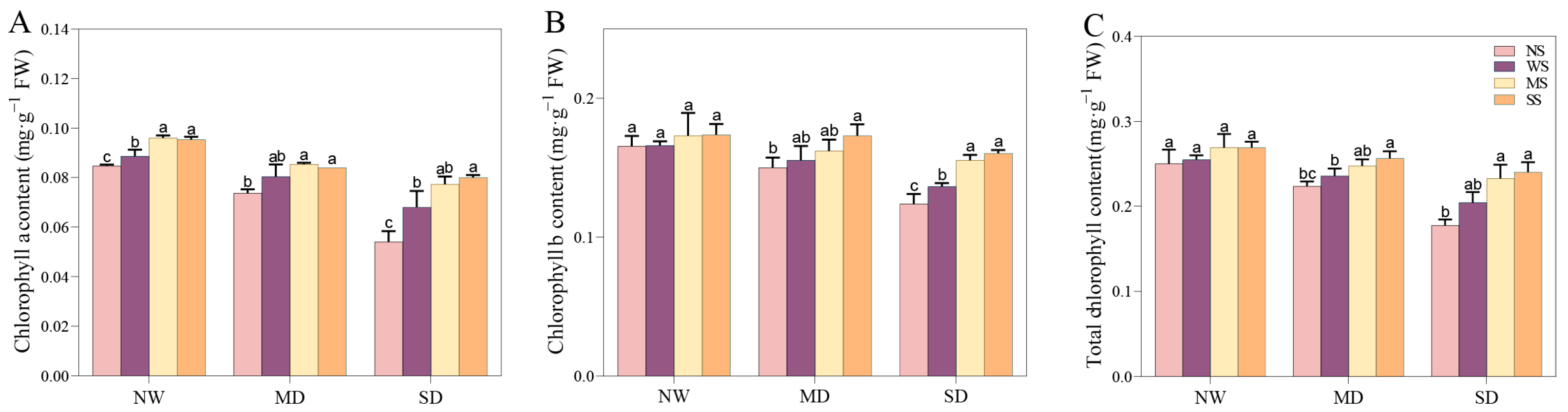
3.3.2. Changes in Chlorophyll Pigments
Water shortages obviously prevented the synthesis of photosynthetic pigments. As the water deficit intensified, the levels of Chl a, Chl b, and total Chl generally decreased (Figure 4). Notably, treatments with MT and SL enhanced the chlorophyll content in water deficit conditions. During MD stress, spraying MT increased Chl a, Chl b, and total Chl content by 6.33%, 4.29%, and 4.99% respectively, relative to spraying water. Similarly, spraying SL resulted in increases of 4.04%, 11.37%, and 8.88% for the same parameters (Figure 4). Furthermore, under SD stress, the MT and SL treatments were more prominent in improving Chl a, Chl b, and total Chl content than those under MD stress. Specifically, spraying SL increased the Chl a and Chl b content by 17.65% and 17.64%, respectively, demonstrating significant differences.
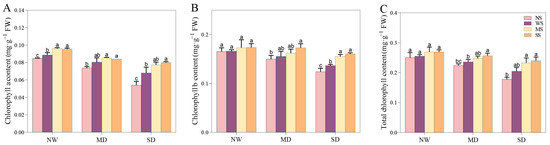
Figure 4.
Effects of MT and SL on chlorophyll pigments in fodder soybean under drought stress. (A) Chlorophyll a content; (B) chlorophyll b content; (C) total chlorophyll content. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 5).
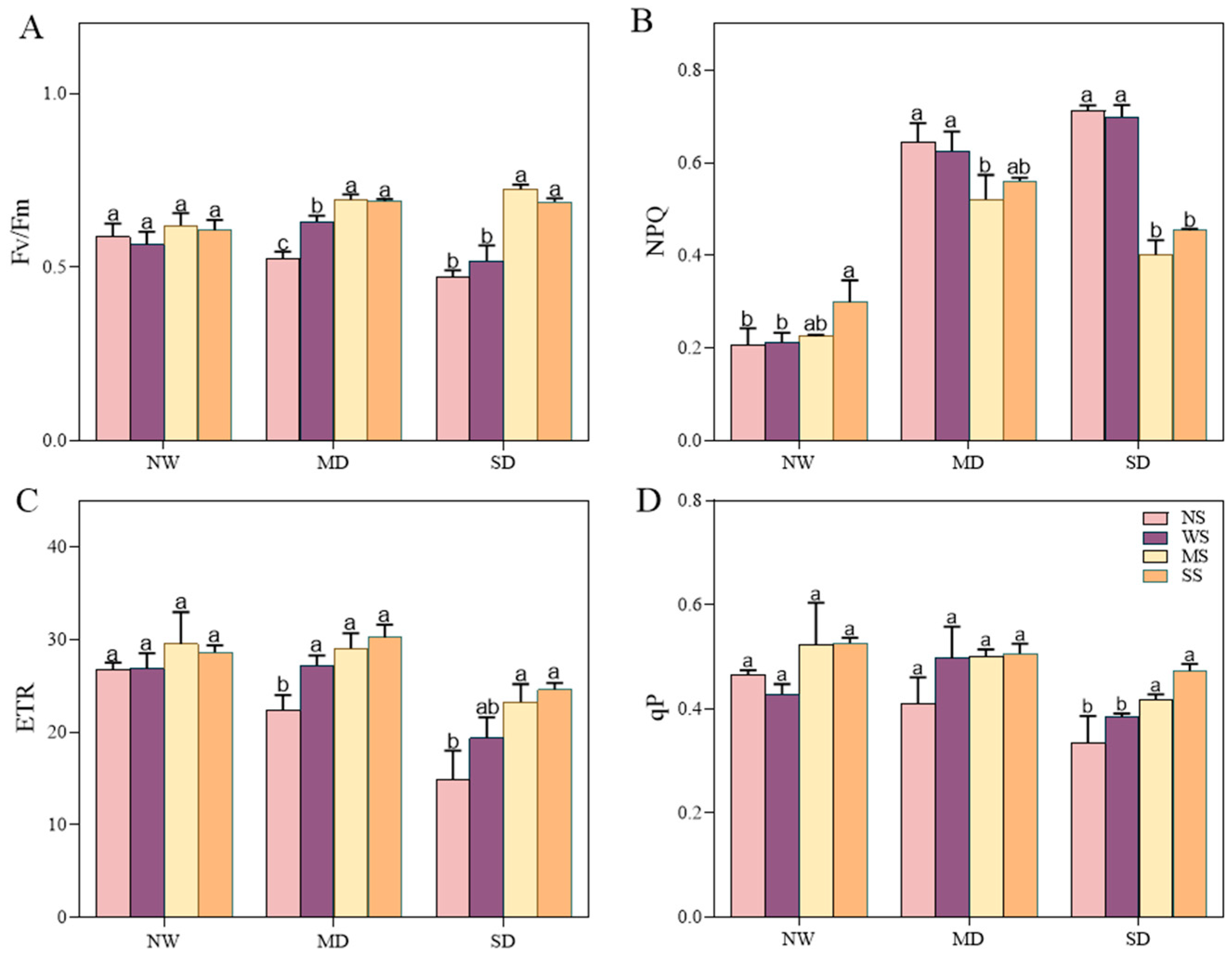
3.3.3. Analysis of Chlorophyll Fluorescence Changes
Water deficiency stress showed an adverse effect on the chlorophyll fluorescence of fodder soybean, including Fv/Fm, ETR, qP and NPQ. Conversely, the use of MT and SL treatments resulted in remarkable changes in the chlorophyll fluorescence of fodder soybean seedlings under different drought stresses (Figure 5). Under NW conditions, the Fv/Fm, ETR and qP showed slight changes between different treatments, while the NPQ exhibited a prominent increase after spraying SL. Under MD stress, in comparison to spraying water, spraying MT increased the Fv/Fm, ETR and qP by 9.88%, 6.74%, 0.93%, and the NPQ decreased by 20.95%, with a significant change in Fv/Fm and NPQ (p < 0.05, Figure 5). Meanwhile, spraying SL increased the Fv/Fm, ETR and qP by 9.29%, 11.03%, 1.81%, and the NPQ decreased by 14.83%. Under SD stress, compared to NS and WS treatments, spraying MT and SL significantly changed chlorophyll fluorescence parameters; among them, the Fv/Fm increased the most, by 39.64% and 32.75%, respectively, while the NPQ decreased the most obviously, by 42.61% and 35.13%, respectively.
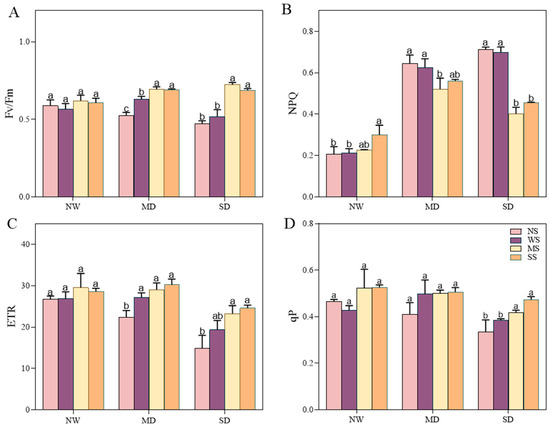
Figure 5.
Effects of MT and SL on chlorophyll fluorescence indexes in fodder soybean under drought stress. (A) Maximum quantum yield of photosystem II (Fv/Fm); (B) non-photochemical quenching (NPQ); (C) electron transport rate (ETR); (D) photochemical quenching (qP). NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 5).
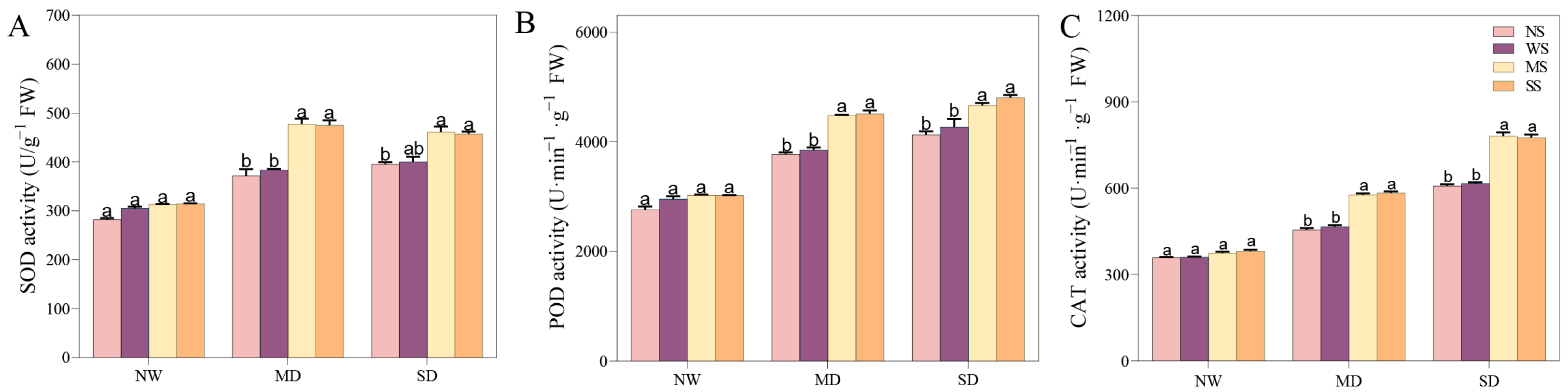
3.4. Impact of MT and SL on Antioxidant Enzyme Activity in Fodder Soybean under Drought Stress
Water deficiency stress extensively activated antioxidant enzymes. When fodder soybean seedlings were treated with MT and SL, the change in the SOD, POD, and CAT enzymes was visible, with a dramatic increase prior to both NS and WS treatments under the same drought gradient (Figure 6). Under MD stress, treatment with MT enhanced the SOD, POD, and CAT enzymes by 24.56%, 16.40%, and 23.64%, respectively, while SL treatment increased them by 23.92%, 17.20% and 25.04% compared to spraying water (Figure 6). Under SD treatment, MT and SL treatments elicited a positive response and significantly increased enzyme activities compared with spraying water. In detail, the alterations in POD and CAT activities following MT application reached a significant level. Similarly, the SOD, POD, and CAT enzymes increased by 14.3%, 12.7%, and 25.9% following SL application, respectively.
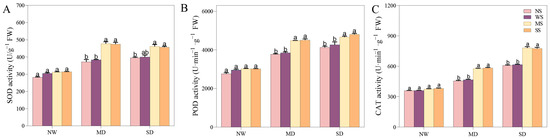
Figure 6.
Effects of MT and SL on SOD, POD, CAT enzymes in fodder soybean under drought stress. (A) SOD activity; (B) POD activity; (C) CAT activity. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 5).
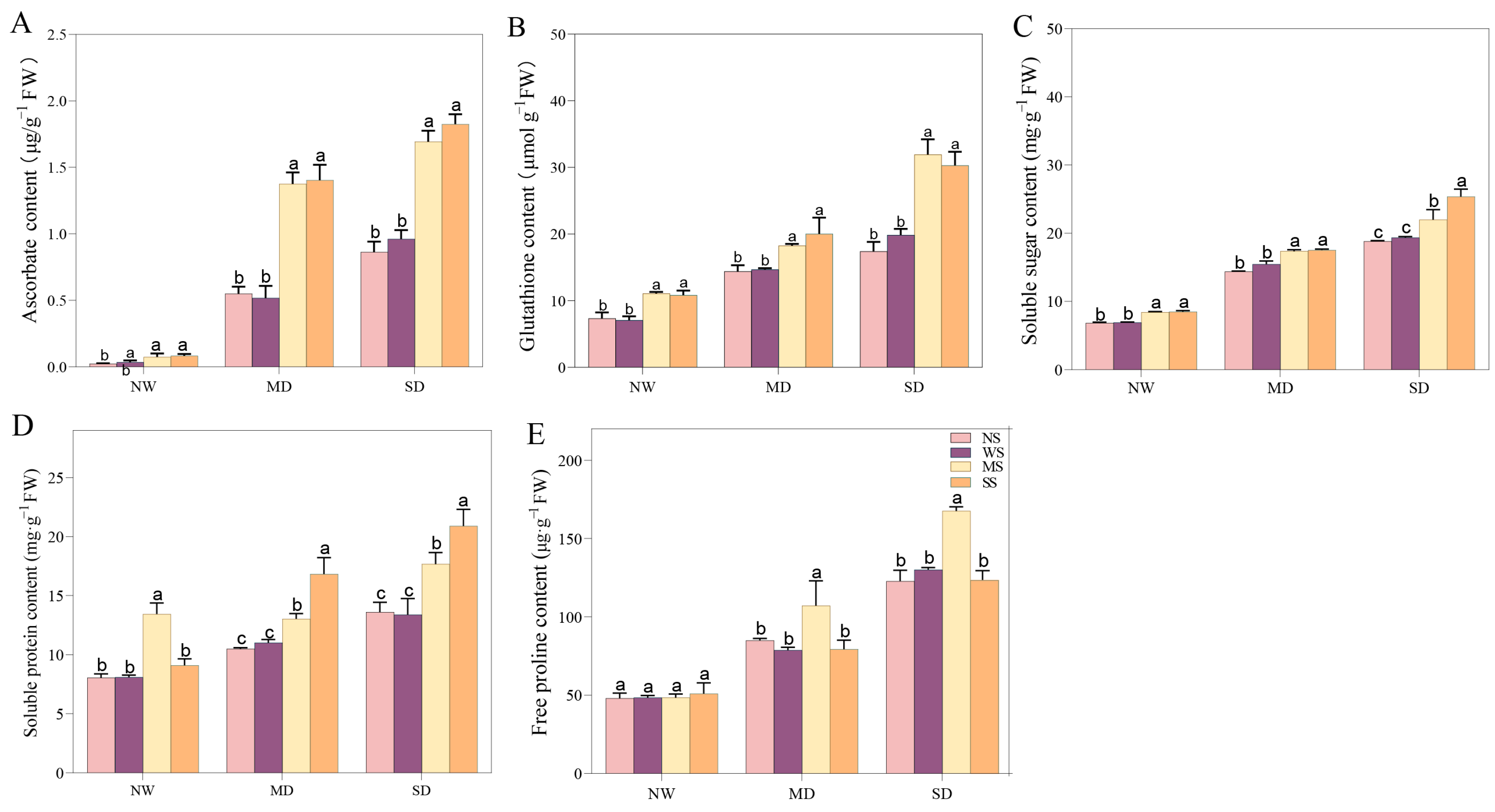
3.5. Impact of MT and SL on Antioxidants and Osmotic Solutes under Drought Stress
Spraying MT and SL prominently enhanced AsA and GSH content, when subjected to different drought stresses. Evidently, under MD stress, the AsA content of the MT and SL treatments was 1.65 and 1.71 times higher than that of the spraying water treatment, while GSH increased by 24.40% and 36.62%, respectively (Figure 7). Moreover, fodder soybean seedlings with MT and SL treatments exhibited more ASA and GSH content under SD stress than under MD stress. Specifically, AsA content increased 1.28 and 1.33 times compared with spraying water under SD stress, while GSH content increased by 65.92% and 69.50%, respectively (Figure 7). Spraying MT and SL significantly increased the SS, SP, and Pro content in different drought conditions (Figure 7). Under MD stress, the SS, SP, and Pro content with MT treatment were enhanced by 12.31%, 18.29%, and 36.08% compared to spraying water, respectively, reaching a distinguishable level (p < 0.05), whereas exogenous SL significantly increased the SS and SP content compared to water spraying, by 13.50% and 52.85%, respectively. (Figure 7). Under SD stress, compared with spraying water, the change trend of SS, SP, and Pro content when spraying MT and SL was similar to that under MD stress. Notably, spraying SL had a substantial enhancing effect on SP content under SD stress, elevating it by 60.2%. Pro also changed remarkably after spraying MT compared to spraying water, increasing by 28.81%.
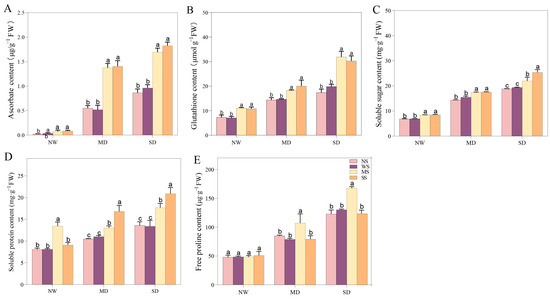
Figure 7.
Effects of MT and SL on antioxidants and osmotic solutes in fodder soybean under drought stress. (A) Ascorbate content; (B) glutathione content; (C) soluble sugar content; (D) soluble protein content; (E) free proline content. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 5).
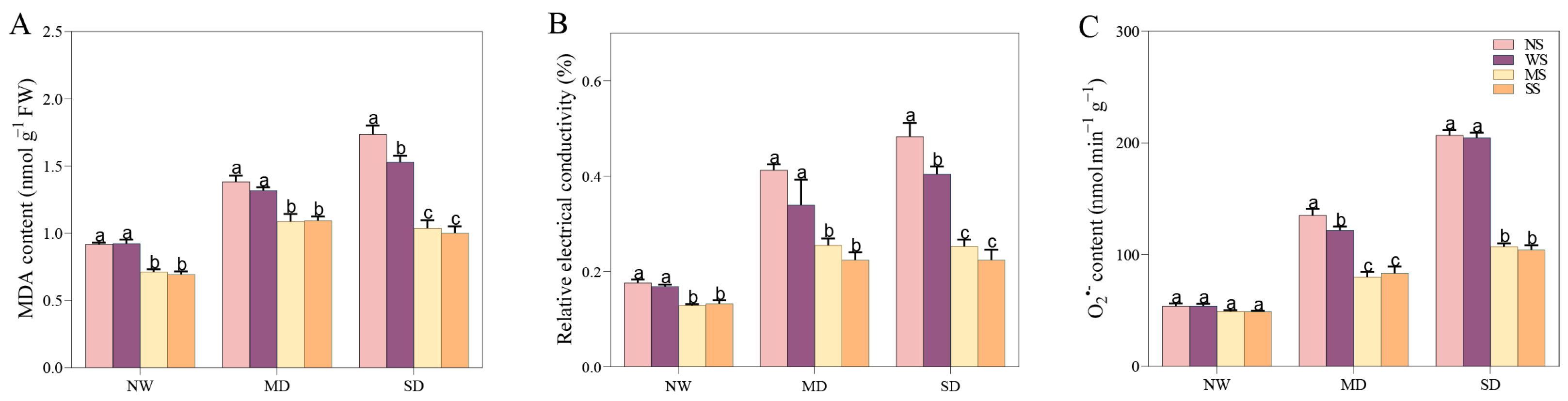
3.6. Impact of MT and SL on Cell Membrane and Oxidative Damage in Fodder Soybean under Drought Stress
A water deficit environment apparently leads to the cell membrane disruption and oxidative damage of fodder soybean seedlings, as demonstrated by a substantial increase in the levels of MDA, REC, and O2.− (Figure 8). Conversely, treatments with MT and SL reduced damage of the cell membrane under both MD and SD stress. Under MD stress, MDA, REC, and O2.− decreased by 17.59%, 24.78% and 34.37% after spraying MT, respectively. Meanwhile, MDA, REC and O2.− decreased by 16.98%, 33.92%, and 31.63% after spraying SL, respectively (Figure 8). Obviously, the change trend of spraying MT and SL on MDA, REC, and O2.− under SD stress was similar to that under the MD treatment, but the change range was more remarkable under SD stress than under MD treatment. Especially, O2.− content after spraying MT and SL decreased by 47.62% and 49.05%, respectively.
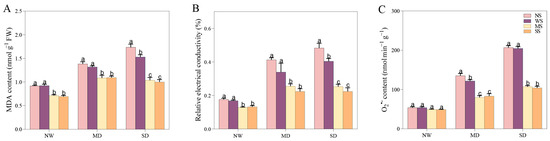
Figure 8.
Effects of MT and SL on cell membrane and oxidative damage in fodder soybean under drought stress. (A) MDA content; (B) REC content; (C) O2.− content. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought condition by one-way ANOVA (p < 0.05, n = 5).
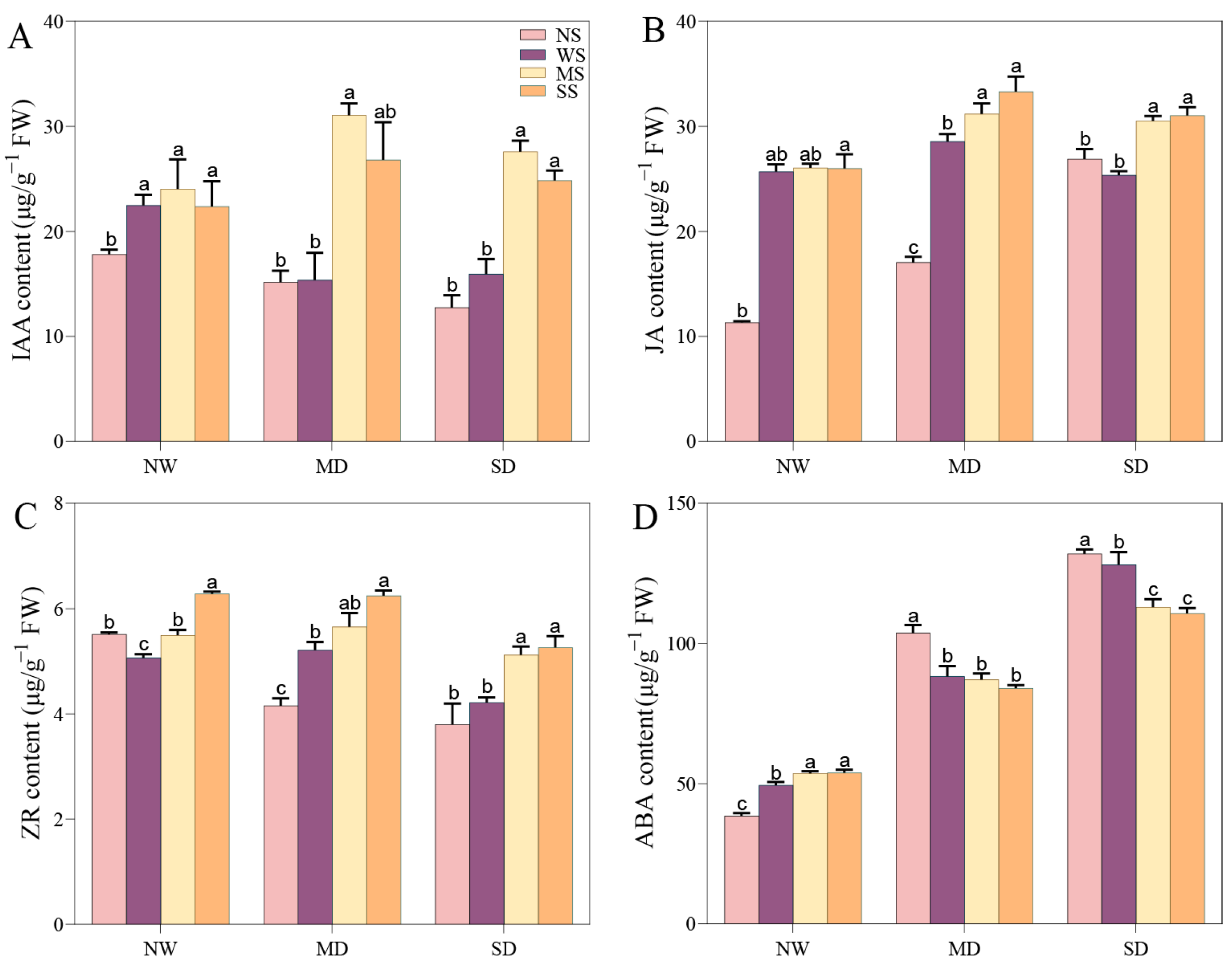
3.7. Impact of MT and SL on Phytohormone Content in Fodder Soybean under Drought Stress
The content of IAA and ZR prominently declined as the drought severity increased, whereas the change trend of JA and ABA content was reversed. However, spraying MT and SL outstandingly increased the IAA, JA, and ABA content to a significant level compared to no spraying under NW conditions, while the content of ZR obviously changed in comparison with spraying water (p < 0.05). Under MD stress, IAA, JA, and ZR increased by 103.42%, 14.99% and 8.40% with spraying MT compared with spraying water, and applying SL led to a 74.48%, 13.08%, and 19.72% increase, respectively. By contrast, ABA content with spraying water, MT, and SL obviously declined compared to no spraying under MD stress (p < 0.05). Under SD stress, the change patterns of IAA, JA, ZR, and ABA while spraying MT and SL were similar to those in the MD treatment. Prominently, the content of ABA significantly decreased with the application of MT and SL compared to spraying water under the SD treatment, by 11.84% and 13.55%, respectively (Figure 9).
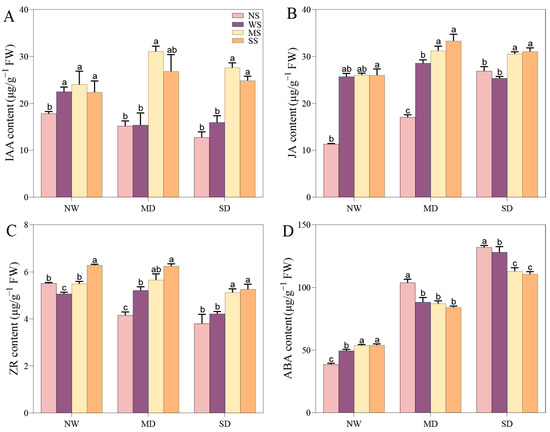
Figure 9.
Effects of MT and SL on phytohormone content in fodder soybean under drought stress. (A) IAA content; (B) JA content; (C) ZR content; (D) ABA content. NW: normal water supply; MD: moderate drought; SD: severe drought; NS: no spraying; WS: spraying water; MS: spraying MT; SS: spraying SL. The bars indicate the mean ± SD. The different letters are significant in different treatments under the same drought conditions by one-way ANOVA (p < 0.05, n = 5).
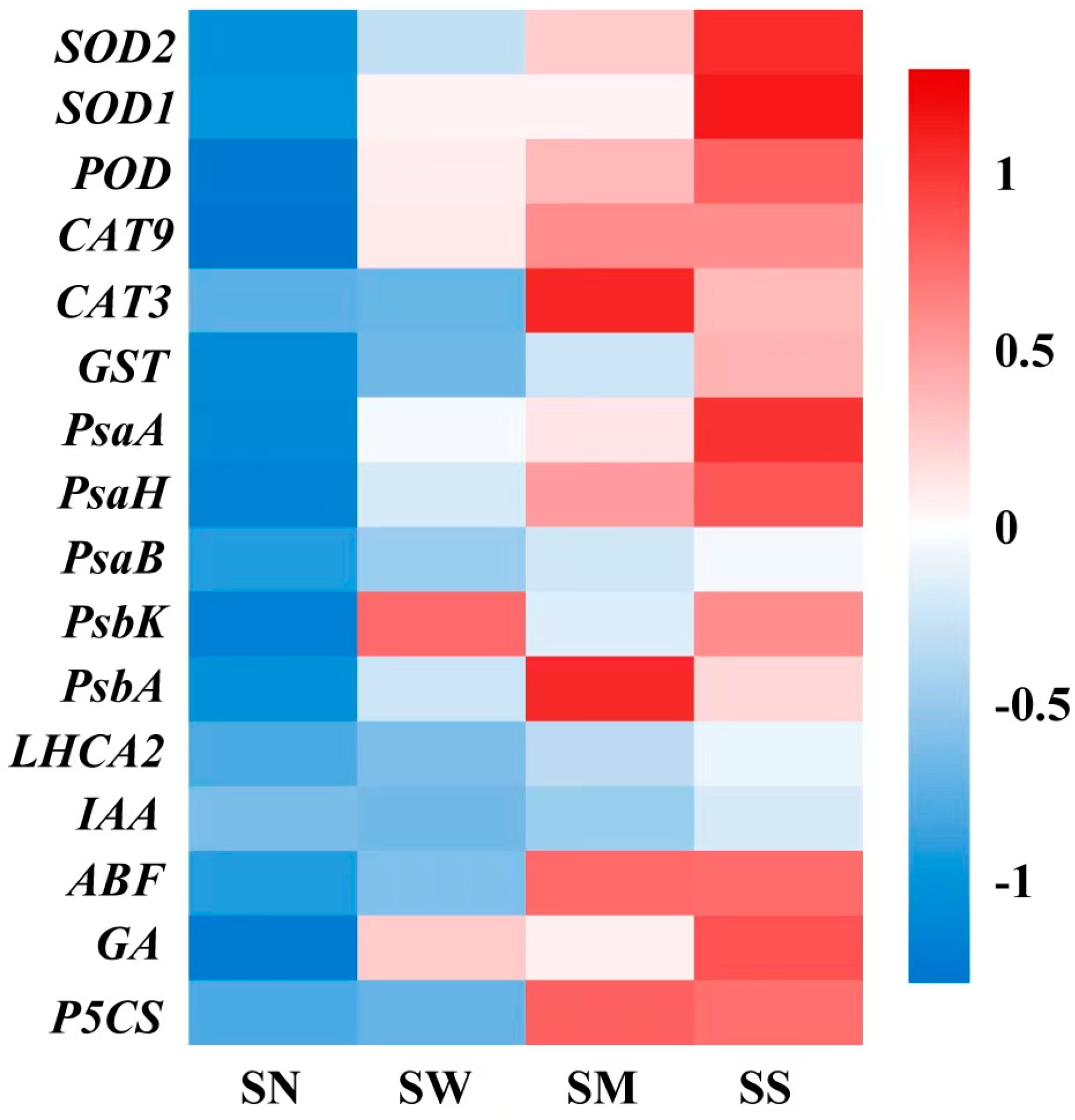
3.8. Impact of MT and SL on Gene Expression Level in Fodder Soybean under Drought Stress
The expression of antioxidant enzyme genes (SOD1, SOD2, POD, CAT9, CAT3, GST), photosynthetic protein and electron transfer genes (PsaA, PsaB, PsaH, PsaK, PsbA, LHCA2), hormone anabolic genes (IAA, ABF, GA), and the proline synthesis key gene (P5CS) were significantly affected by drought stress (Figure S1 and Figure 10). Both the MT and SL treatments significantly modulated these genes’ expression, with notable increases observed under SD stress compared to spraying water. Specifically, the gene expression levels were substantially increased with the MT and SL treatments relative to both no spraying and spraying water. (p < 0.05, Figure 10). Remarkably, the positive effect of the SL treatment was more prominent than that of the MT treatment.
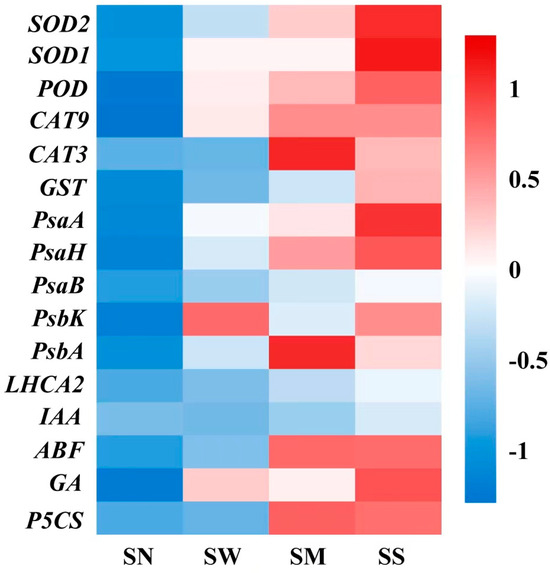
Figure 10.
Effects of MT and SL on gene relative expression levels in fodder soybean under severe drought stress. SN: no spraying; SW: spraying water; SM: spraying MT; SS: spraying SL. The abscissa indicates different treatments, the ordinate indicates different genes. The color intensity in the grid represents the genes’ relative expression levels after normalization and standardization under different treatments.
4. Discussion
Abiotic stress significantly impacts plant growth and morphological characteristics, which directly reflect the plant’s stress response. Previous studies have found that MT application decreased the degree of leaf wilting and increased leaf area, plant height, and biomass in ryegrass (Lolium perenne L.) and wheat [26,27]. Furthermore, the application of SL improved the growth and root morphology of tobacco (Nicotiana tabacum L.) under water deficiency [17]. This study found that water deficit stress significantly restrained the growth and morphological characteristics of fodder soybean seedlings. However, treatment with MT and SL effectively mitigated the adverse impacts of water deficit stress, as evidenced by an improved leaf area, plant height, biomass, root features, and stomatal status. (Table 1, Figure 1 and Figure 2). This finding was similar to changes in the growth and morphology of naked oats (Avena nuda L.) [7], maize (Zea mays L.) [12], and crabapple [15] with MT or SL treatments under drought stress.
Drought stress induced the producing and accumulating of ROS within plant cells, triggering oxidative stress and lipid peroxidation [24]. However, MT and SL treatments effectively decreased ROS concentrations, thereby mitigating damage from drought stress [14,28]. In this study, drought stress was induced to increase levels of O2.−, MDA, and membrane leakage probed by REC, resulting in ROS excessive accumulation, aggravating lipid peroxidation and impairing the membrane integrity in fodder soybean. However, the application of MT and SL markedly decreased these parameters and alleviated oxidative damage under MD and SD stress (Figure 8). This was consistent with the earlier research that exogenous hormones significantly reduced the build-up of ROS in Phoebe sheareri seedlings in a water-deficient environment [28]. Plants mainly activate antioxidant enzymes and non-enzymatic antioxidants in water deficit conditions. SOD is the primary enzyme contributing to converting O2.− into H2O2 and O2, thus maintaining low oxygen free radical levels to prevent membrane damage. CAT specifically breaks down H2O2 to prevent its toxic buildup and oxidative damage to cells, while POD also contributes to ROS neutralization [29]. In fodder soybean, the activities of the SOD, POD, and CAT enzymes were consistently enhanced as the water deficit intensified, while MT and SL treatments not only increased these enzyme activities, but also remarkably regulated the expression of encoding their genes, including SOD1, SOD2, POD, CAT3, CAT9 (Figure 10 and Figure S2). We speculated that spraying MT and SL had an obviously positive effect on the antioxidative defense system by regulating gene expression related to their biosynthesis or catabolism. Moreover, previous research also has demonstrated that the application of phytohormones evidently activated antioxidant enzymes in buckwheat (Fagopyrum Tataricum (L.) Gaertn) [28], soybean [30], and maize [31] under drought stress, resulting in reducing MDA content and ROS concentrations, which further confirms the finding that MT and SL treatments are beneficial to suppressing oxidative stress and avoiding cell injury during the seedling stage of fodder soybean under water deficit conditions.
AsA and GSH are significant substrates in the AsA-GSH cycle and serve as non-enzymatic antioxidants. They are crucial for regulating intracellular ROS levels and preserving cell membrane integrity [29]. This research found that water deficit stress decreased the levels of AsA and GSH, whereas treatment with MT and SL noticeably elevated their content under identical drought conditions. Simultaneously, MT and SL treatments dramatically decreased levels of O2.− and MDA under the same conditions. The results indicated that AsA and GSH increase improved cell membrane stability; thus, oxidative damage was detoxified by MT and SL under drought stress. The reason was possiblly that the increased AsA and GSH content from the MT and SL treatments promoted AsA-GSH cycling activity, clearing excessive ROS, thereby enhancing the antioxidant capacity of fodder soybean under drought stress. This is supported by the previous research on maize [30]. In addition, Pro, SP, and SS are efficient antioxidants and osmotic solutes. Under water deficit stress, they prevent some large molecules in cells from reacting with ROS, and have a vital function in maintaining cell turgor pressure and stomatal status. Thus, Pro, SP, and SS increase plants’ adaptability to water-deficient environments [32,33]. This study found that the SS and SP content of fodder soybean with MT and SL treatments was higher than that of spraying water during MD and SD stress periods, while the levels of O2.−, MDA, and REC decreased (Figure 7 and Figure 8). This suggested that MT and SL are beneficial in reducing the oxidative damage and cell membrane disruption of fodder soybean by scavenging ROS and regulating the osmotic balance under water deficiency stress. This finding aligns with the previous research [34]. Prominently, MT treatment most significantly increased Pro content in all treatments under MD and SD stress (Figure 7). Moreover, the expression level of gene P5CS with MT treatment, which is primarily responsible for regulating proline synthesis, was the highest in all treatments under the same water conditions (Figure S2 and Figure 10). It was inferred that the application of MT was apparently effective in promoting proline synthesis, by inducing the transcription level of key genes under drought stress [35,36].
Drought stress impairs plant photosynthesis primarily through stomatal and non-stomatal mechanisms. Stomatal factors include stomatal closure, which restricts CO2 uptake into mesophyll cells, thereby reducing CO2 fixation and conversion. Non-stomatal factors disrupt the photosynthetic electron transfer system and damage to the chloroplast structure, leading to a disordering in photosynthetic metabolism [37]. Studies on wheat [38], maize [39], melon (Cucumis melo L.) [40], and roses (Rosa rugosa Thunb.) [41] have confirmed that drought negatively affects photosynthetic pigments, photosynthetic reaction activity, and gas exchange capacity, decreasing photosynthesis and then restraining plant growth [42]. In this study, drought stress significantly promoted stomatal closure (Figure 1), reduced chlorophyll content, and regulated photosynthetic parameters. The changes were more pronounced as the drought stress intensified (Figure 3 and Figure 4). However, MT and SL treatments maintained stomatal characteristics, prevented chlorophyll degradation, and improved the photosynthetic system of fodder soybean in water-deficient environments. This result is in line with the research on Pennisetum purpureum with SL treatment under drought stress [17]. Additionally, drought stress also adversely affects chlorophyll fluorescence [43]. Our study observed that water deficit stress reduced the Fv/Fm, qP, and ETR, while the NPQ increased. Importantly, the MT and SL treatments regulated these indexes, increasing the Fv/Fm, qP, and ETR, and decreasing the NPQ (Figure 5). Collectively, these results suggest that by improving stomatal function, chlorophyll pigments, and photochemical reaction, MT and SL regulate the photosynthetic process, thereby promoting the growth of fodder soybean under drought stress, aligning with previous research [8]. Additionally, the value of Fv/Fm was low under NW conditions, which was inconsistent with previous findings that 0.75 rel. units is considered as a boundary value for fully functional photosystem II [44]. The results may be due to different plant species that have different requirements for the relative water content of soil [45]. Moreover, as fodder soybean seedlings are sensitive to water during the seedling stage, we speculated that fodder soybean seedlings may be subjected to slight water stress under NW conditions.
Plants counteract abiotic stresses by regulating the levels of endogenous hormones, ensuring sustained growth and development [46]. Among these hormones, IAA is the primary growth hormone involved in cell division, root development, senescence, and stress responses [47]. ZR regulates plant growth, development, nutrient uptake, chlorophyll content, and the transition from growth to senescence [48]. JA plays a critical function in plant response to water deficiency [49]. The results indicated that as the drought intensified, the IAA and ZR content was reduced, while the JA and ABA content was enhanced. By contrast, exogenous MT and SL positively affected IAA, JA, and ZR levels (Figure 9). We speculated that there was an interaction between the two exogenous hormones and the endogenous hormones of fodder soybean. The results were similar to previous research findings [50,51]. Notably, as the severity of the drought intensified, ABA content with MT and SL treatments outstandingly decreased, while the ABF expression level increased (Figure 9 and Figure 10). It appears that MT and SL reduced ABA content by regulating some genes’ expression in the ABA signaling pathway. In addition, an earlier study indicates that ABA can promote stomatal closure [52]. In this research, as drought intensity increased, the Pn and Gs decreased, while the stomatal aperture gradually closed, in fodder soybean. Conversely, the spraying of MT and SL significantly increased Pn and Gs and regulated the stomatal aperture under drought stress. Thus, the data suggest that both MT and SL treatments decrease the ABA content, thereby helping to maintain the stomatal opening and enhance photosynthetic efficiency. This result was consistent with the earlier finding that MT could enhance stomatal function by allowing plants in adversity to reopen their stomata [53].
5. Conclusions
Based on the research findings, MT and SL application mitigated the adverse impact of water deficiency on seedling growth and development by improving the phenotype, antioxidant defense ability, photosynthetic system, and phytohormone content. Treatments with MT and SL alleviated oxidative damage by decreasing ROS concentrations, activating antioxidant enzymes, and increasing antioxidant content under drought stress. Spraying MT and SL increased the photosynthetic efficiency of fodder soybean under drought stress, owing to an improvement in maintaining stomatal traits, increasing chlorophyll content, and protecting the photosynthetic system. Moreover, MT and SL can maintain the dynamic balance of endogenous hormones by increasing IAA, JA, and ZR levels and reducing ABA content, as well as regulating key gene expression under different drought conditions. Thus, MT and SL can be deemed as important protectors, strengthening fodder soybean’s adaptability to water-deficient environments. It is worth noting that SL treatment exhibits better effect compared to MT treatment in fodder soybean seedlings subjected to drought stress. However, further research using high-throughput sequencing technologies is needed to explore the internal mechanisms by morphophysiology, integrating the molecular. Field experiments also are necessary for verifying field-level effectiveness in future research. This research results can offer new evidence related to the mechanisms by which MT and SL enhance drought resistance in plants, and a theoretical reference for the development of application growth regulators.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14081803/s1, Figure S1: Impacts of MT and SL on root morphology of fodder soybean under drought stress. Figure S2: Changes in genes expression level in fodder soybean under NW and MD conditions; Table S1: Primers of key genes for qRT-PCR analysis.
Author Contributions
Conceptualization, F.X. and J.H.; methodology, R.L.; validation, X.L., C.W. and Q.W. (Qinyi Wang); formal analysis, J.J.; investigation, Q.W. (Qiyun Wei); resources, X.Z.; data curation, Q.Z. and Y.L.; writing—original draft preparation, F.X.; writing—review and editing, J.H.; supervision, G.C.; project administration, Y.C.; funding acquisition, F.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the China Postdoctoral Science Fund (2022MD723773), Heilongjiang Postdoctoral Fund (LBH-Z21009), Academic Backbone Project of Northeast Agricultural University (21XG25), and China’s National Key R&D Program (2022YFD1600505-7).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors sincerely appreciate the editor and reviewers for detailed comments that significantly improved the level of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and Crop Yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Guo, Z.; Sun, X.; Jiang, Y.; Xie, F.; Chen, Y. Role of Proline in Regulating Turfgrass Tolerance to Abiotic Stress. Grass Res. 2023, 3, 2. [Google Scholar] [CrossRef]
- Xu, Y.; Song, D.; Qi, X.; Asad, M.; Wang, S.; Tong, X.; Jiang, Y.; Wang, S. Physiological Responses and Transcriptome Analysis of Soybean under Gradual Water Deficit. Front. Plant Sci. 2023, 14, 1269884. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, A.; Wang, Y.; Jin, G.; Zhang, Y.; Gu, L.; Li, C.; Shao, X.; Wang, K. Physiological and Transcriptomic Insights into Adaptive Responses of Seriphidium transiliense Seedlings to Drought Stress. Environ. Exp. Bot. 2022, 194, 104736. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic Insights on Melatonin-Mediated Drought Stress Mitigation in Plants. Physiol. Plant 2021, 172, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plants. In Amino Acids in Higher Plants; CAB International: Wallingford, UK, 2015; pp. 390–435. [Google Scholar]
- Zhang, X.; Liu, W.; Lv, Y.; Bai, J.; Li, T.; Yang, X.; Liu, L.; Zhou, H. Comparative Transcriptomics Reveals New Insights into Melatonin-Enhanced Drought Tolerance in Naked Oat Seedlings. PeerJ 2022, 10, e13669. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Drought Stress Alleviator Melatonin Reconfigures Water-Stressed Barley (Hordeum vulgare L.) Plants’ Photosynthetic Efficiency, Antioxidant Capacity, and Endogenous Phytohormone Profile. Int. J. Mol. Sci. 2023, 24, 16228. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xu, T.; Wang, Z.; Fang, Y.; Xi, Z.; Zhang, Z. The Ameliorative Effects of Exogenous Melatonin on Grape Cuttings Under Water-Deficient Stress: Antioxidant Metabolites, Leaf Anatomy, and Chloroplast Morphology. J. Pineal. Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Yang, L.; Bu, S.; Zhao, S.; Wang, N.; Xiao, J.; He, F.; Gao, X. Transcriptome and Physiological Analysis of Increase in Drought Stress Tolerance by Melatonin in Tomato. PLoS ONE 2022, 17, e0267594. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and Transcriptomic Analyses of the Effects of Exogenous Melatonin on Drought Tolerance in Maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Wu, F.; Gao, Y.; Yang, W.; Sui, N.; Zhu, J. Biological Functions of Strigolactones and Their Crosstalk with Other Phytohormones. Front. Plant Sci. 2022, 13, 821563. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Emam, Y.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Lovisolo, C.; Visentin, I.; Cardinale, F.; Tahmasebi-Sarvestani, Z. The Potential of the Synthetic Strigolactone Analogue GR24 for the Maintenance of Photosynthesis and Yield in Winter Wheat Under Drought: Investigations on the Mechanisms of Action and Delivery Modes. Plants 2021, 10, 1223. [Google Scholar] [CrossRef]
- Shu, H.; Xu, K.; Li, X.; Liu, J.; Altaf, M.A.; Fu, H.; Lu, X.; Cheng, S.; Wang, Z. Exogenous Strigolactone Enhanced the Drought Tolerance of Pepper (Capsicum chinense) by Mitigating Oxidative Damage and Altering the Antioxidant Mechanism. Plant Cell Rep. 2024, 43, 106. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, L.; Liu, Y.; Yu, Y.; Li, H.; Wang, X.; Pang, Y.; Cao, H.; Sun, Q. Molecular and Physiological Mechanisms of Strigolactones-Mediated Drought Stress in Crab Apple (Malus hupehensis Rehd.) Seedlings. Sci. Hortic. 2023, 311, 111800. [Google Scholar] [CrossRef]
- Luqman, M.; Shahbaz, M.; Maqsood, M.F.; Farhat, F.; Zulfiqar, U.; Siddiqui, M.H.; Masood, A.; Aqeel, M.; Haider, F.U. Effect of Strigolactone on Growth, Photosynthetic Efficiency, Antioxidant Activity, and Osmolytes Accumulation in Different Maize (Zea mays L.) Hybrids Grown Under Drought Stress. Plant Signal Behav. 2023, 18, 2262795. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Feng, Q.; Zhang, J.; Han, X.; Zhang, L.; Yang, F.; Zhou, J. Effects of Exogenous Strigolactone on the Physiological and Ecological Characteristics of Pennisetum purpureum Schum Seedlings Under Drought Stress. BMC. Plant Bio. 2022, 22, 578. [Google Scholar] [CrossRef] [PubMed]
- Heba, S.A.S.; Mahmoud, H.A.M. Maximizing Land Use Efficiency and Productivity of Soybean and Fodder Maize Intercrops through Manipulating Sowing Schedule and Maize Harvest Regime. Agron. J. 2021, 11, 863. [Google Scholar]
- Yang, M.; Wang, F.; Xu, W.; Li, X.; Yin, H.; Tuluhong, M.; Qiu, R.; Li, B.; Cui, G. Effects of the Fermentation Quality and Microbial Community of Waxy Maize Mixed with Fodder Soybean Silage. Front. Microbiol. 2024, 15, 1405018. [Google Scholar] [CrossRef]
- Kaneko, M.; Uozumi, S.; Touno, E.; Degechi, S. No-till, No-herbicide Forage Soybean (Glycine max (L.) Merrill) Cropping System with an Italian Ryegrass (Lolium multiflorum Lam.) Living Mulch. Grassl. Sci. 2011, 57, 28–34. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, J.; Dong, L.; Sun, X.; Chen, J.; Xie, F.; Chen, Y. Shade Responses of Prostrate and Upright Turf-type Bermudagrasses. Grass Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, Z.; Cheng, L.; Jiang, J.; Liu, Y.; Liu, L.; You, C.; Liu, X.; Xie, F.; Qin, L.; et al. Comparative Study on the Morpho-Physiological Responses of White Clover Cultivars with Different Leaf Types to Water Deficiency. Agron. J. 2023, 13, 1859. [Google Scholar] [CrossRef]
- Lu, X.; Min, W.; Shi, Y.; Tian, L.; Li, P.; Ma, T.; Zhang, Y.; Luo, C. Exogenous Melatonin Alleviates Alkaline Stress by Removing Reactive Oxygen Species and Promoting Antioxidant Defence in Rice Seedlings. Front. Plant Sci. 2022, 13, 849553. [Google Scholar] [CrossRef]
- Guri, A. Variation in Glutathione and Ascorbic Acid Content Among Selected Cultivars of Phaseolus Vulgaris Prior to and After Exposure to Ozone. Can. J. Plant Sci. 1983, 63, 733–737. [Google Scholar] [CrossRef]
- Miedema, K.; Boelhouwer, J.; Otten, J.W. Determinations of Proteins and Hormones in Serum by An Immunoassay Using Antigen-Enzyme Conjugates. Clin. Chim. Acta 1972, 40, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin Suppression of Heat-Induced Leaf Senescence Involves Changes in Abscisic Acid and Cytokinkin Biosynthesis and Signaling Pathways in Perennial Ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial Effects of Melatonin in Overcoming Drought Stress in Wheat Seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Zhu, Y.; Zheng, W.; Li, M.; Hu, J.; Fei, Y.; Zhu, S. Exogenous Application of Melatonin to Mitigate Drought Stress-Induced Oxidative Damage in Phoebe Sheareri Seedlings. PeerJ 2023, 11, e15159. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jing, D.; Liu, F.; Ma, H.; Liu, X.; Peng, L. Serendipita Indica Alleviates Drought Stress Responses in Walnut (Juglans regia L.) Seedlings by Stimulating Osmotic Adjustment and Antioxidant Defense System. Appl. Microbiol. Biot. 2021, 105, 8951–8968. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.L.; Shahzad, R.; Shahzad, R.; Khan, M.A.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous Melatonin Induces Drought Stress Tolerance by Promoting Plant Growth and Antioxidant Defence System of Soybean Plants. AoB. Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Sattar, A.; Ul-Allah, S.; Ijaz, M.; Sher, A.; Butt, M.; Abbas, T.; Irfan, M.; Fatima, T.; Alfarraj, S.; Alharbi, S.A. Exogenous Application of Strigolactone Alleviates Drought Stress in Maize Seedlings by Regulating the Physiological and Antioxidants Defense Mechanisms. Cereal. Res. Commun. 2022, 50, 263–272. [Google Scholar] [CrossRef]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, H.; Wang, L.; Liu, H.; Huo, H.; Zhang, C.; Liu, A.; Zhu, A.; Hu, J.; Lin, Y.; et al. Physiological and Transcriptome Analyses Reveal Short-Term Responses and Formation of Memory Under Drought Stress in Rice. Front. Genet. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Altaf, M.A.; Mushtaq, N.; Fu, H.; Lu, X.; Zhu, G.; Cheng, S.; Wang, Z. Physiological and Transcriptome Analysis of the Effects of Exogenous Strigolactones on Drought Responses of Pepper Seedlings. Antioxidants 2023, 12, 2019. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is Proline Accumulation Per Se Correlated with Stress Tolerance or Is Proline Homeostasis A More Critical Issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Hu, H.; He, B.; Ma, L.; Chen, X.; Han, P.; Luo, Y.; Liu, Y.; Fei, X.; Wei, A. Physiological and Transcriptome Analyses Reveal the Photosynthetic Response to Drought Stress in Drought-Sensitive (Fengjiao) and Drought-Tolerant (Hanjiao) Zanthoxylum Bungeanum Cultivars. Front. Plant Sci. 2022, 13, 968714. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, D.; Li, X.; Shi, Y.; Shao, Y.; Fang, B.; Yue, J.; Wang, H.; Qin, F.; Cheng, H. Drought Effects on Photosynthetic Performance of Two Wheat Cultivars Contrasting in Drought. N. Z. J. Crop. Hortic. Sci. 2021, 49, 17–29. [Google Scholar] [CrossRef]
- Xu, J.; Guo, L.; Liu, L. Exogenous Silicon Alleviates Drought Stress in Maize by Improving Growth, Photosynthetic and Antioxidant Metabolism. Environ. Exp. Bot. 2022, 201, 104974. [Google Scholar] [CrossRef]
- Rehman, A.; Khalid, M.; Weng, J.; Li, P.; Rahman, S.U.; Shah, I.H.; Gulzar, S.; Tu, S.; Feng, N.; Niu, Q.; et al. Exploring Drought Tolerance in Melon Germplasm through Physiochemical and Photosynthetic Traits. J. Plant Growth Regul. 2024, 102, 603–618. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.; Cheng, C.; Jiang, X. Exogenous Calcium Enhances the Physiological Status and Photosynthetic Capacity of Rose Under Drought Stress. Hortic. Plant J 2024, 10, 853–865. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin Mediates the Regulation of ABA Metabolism, Free-Radical Scavenging, and Stomatal Behaviourin Two Malus Species Under Drought Stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhossieni, M. Modelling the Effects of Water Stress and Temperature on Seed Germination of Radish and Cantaloupe. J. Plant Growth Regul. 2019, 38, 1402–1411. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, H.R.; Long, S.P.; Baker, N.R.; Oquist, G.; Schreiber, U.; Lechner, E.G. Chlorophyll Fluorescence as A Probe of the Photosynthetic Competence of Leaves in the Field: A Review of Current Instrumentation. Funct. Ecol. 1989, 3, 497–514. [Google Scholar] [CrossRef]
- Geleta, R.J.; Roro, A.G.; Terfa, M.T. Phenotypic and Yield Responses of Common Bean (Phaseolus vulgaris L.) Varieties to Different Soil Moisture Levels. BMC Plant Biol. 2024, 24, 242. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of Shape during Stress: A Key Role for Auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef]
- Kakimoto, T. Biosynthesis of Cytokinins. J. Plant Res. 2003, 116, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Imran, M.; Mpovo, C.L.; Aaqil Khan, M.; Shaffique, S.; Ninson, D.; Bilal, S.; Khan, M.; Kwon, E.H.; Kang, S.M.; Yun, B.W.; et al. Synergistic Effect of Melatonin and Lysinibacillus fusiformis L. (PLT16) to Mitigate Drought Stress via Regulation of Hormonal, Antioxidants System, and Physio-Molecular Responses in Soybean Plants. Int. J. Mol. Sci. 2023, 24, 8489. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Abadia, J.; Marjani, M. Melatonin Foliar Sprays Elicit Salinity Stress Tolerance and Enhance Fruit Yield and Quality in Strawberry (Fragaria × ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin Increased Maize (Zea mays L.) Seedling Drought Tolerance by Alleviating Drought-Induced Photosynthetic Inhibition and Oxidative Damage. Acta. Physiol. Plant 2016, 38, 48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).