New Fertilisers with Innovative Chelates in Wheat Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodological Assumptions
2.2. Laboratory and Statistical Analysis Methods
3. Results
3.1. Plants
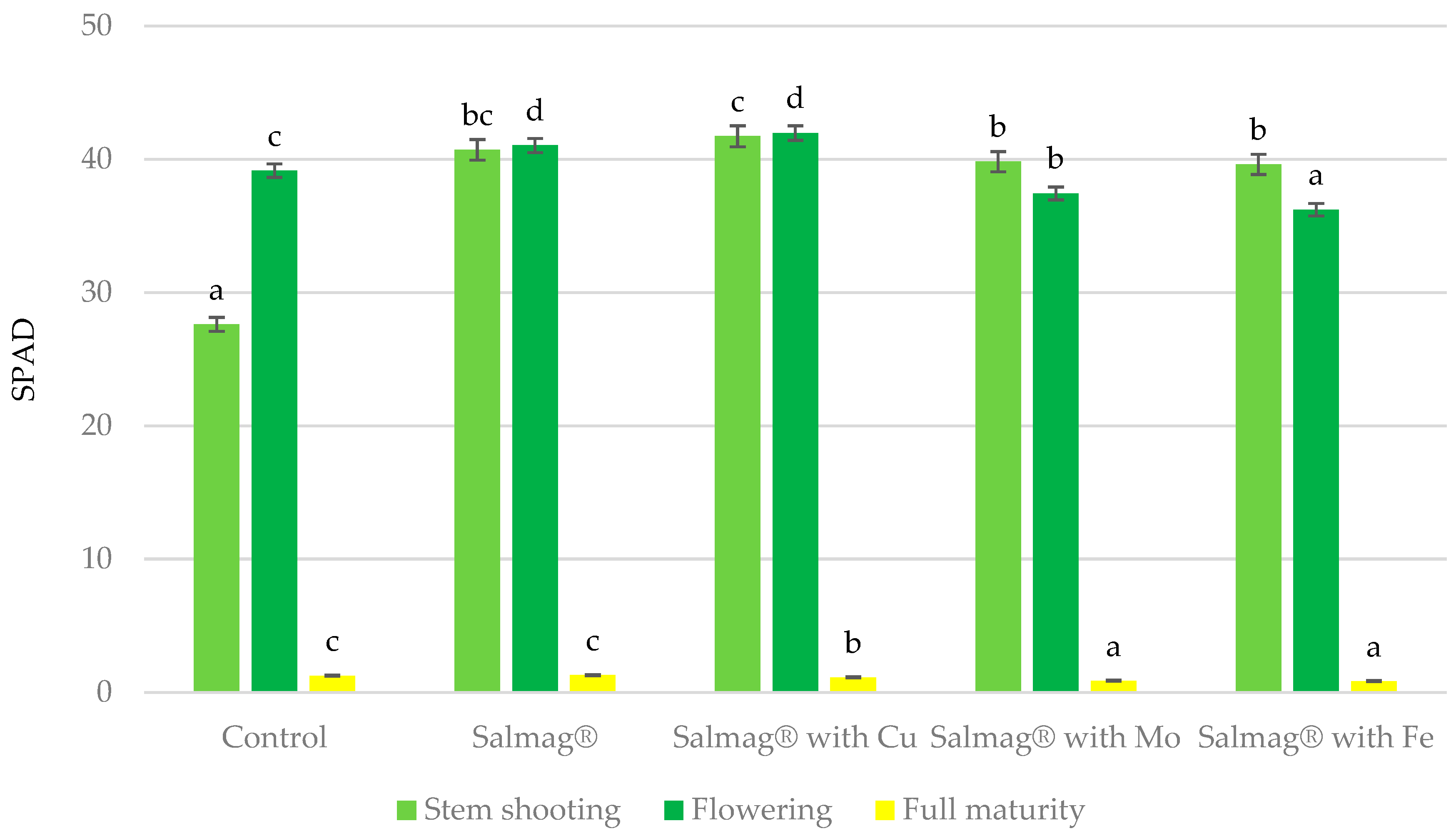
3.1.1. SPAD Index
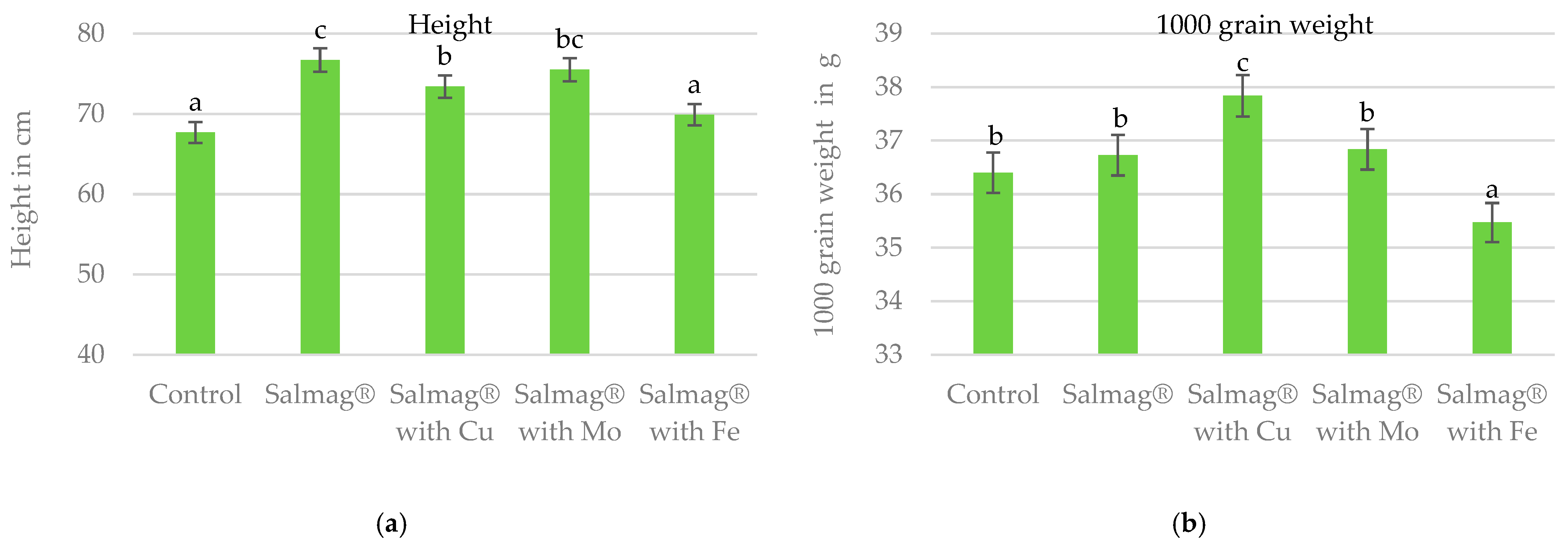
3.1.2. Plant Height
3.1.3. 1000-Grain Weight
3.1.4. Crop Yield
3.1.5. Macronutrients
3.1.6. Micronutrients
3.2. Soil Properties
3.2.1. pH
3.2.2. Macronutrients
3.2.3. Micronutrients
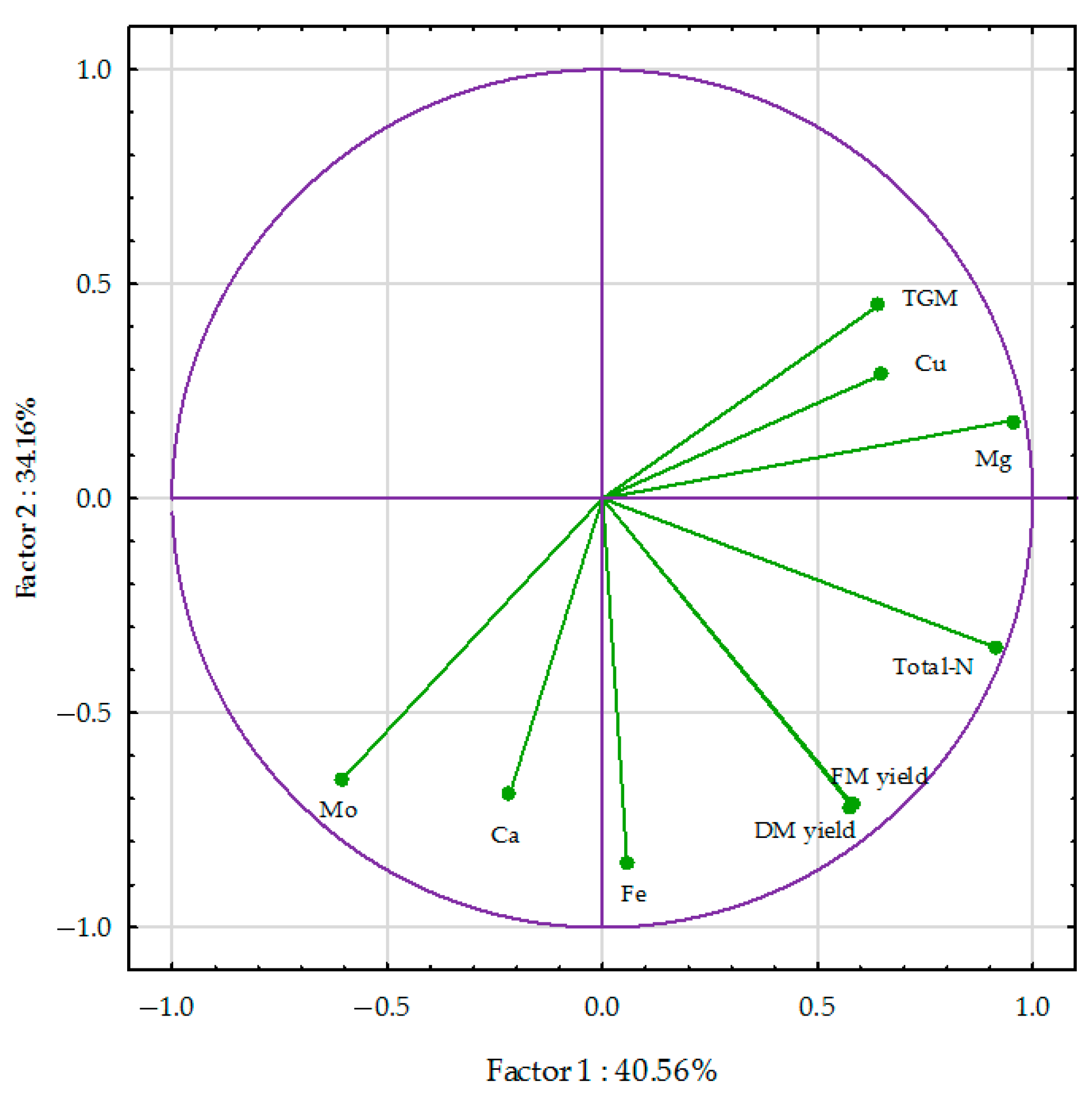
3.3. PCA Analysis and the Percentage of Variability Observed
3.3.1. Spring Wheat Grain
3.3.2. Spring Wheat Straw
3.3.3. Soil after Spring Wheat Harvest
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanisławska-Glubiak, E.; Korzeniowska, J. Analysis of the micronutrient fertiliser market against the needs for micronutrient fertilisation in Poland. Stud. Rep. Inst. Soil Sci. Plant Cultiv. (IUNG-PIB) 2016, 63, 145–161. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments, 1st ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015; p. 468. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Zheljazkov, V.D.; Gollany, H.T.; Kleber, M.; Xing, B.; Astatkie, T. Micronutrients in the soil and wheat: Impact of 84 years of organic or synthetic fertilization and crop residue management. Agronomy 2019, 9, 464. [Google Scholar] [CrossRef]
- Thapa, S.; Bhandari, A.; Ghimire, R.; Xue, Q.; Kidwaro, F.; Ghatrehsamani, S.; Maharjan, B.; Goodwin, M. Managing micronutrients for improving soil fertility, health, and soybean yield. Sustainability 2021, 13, 11766. [Google Scholar] [CrossRef]
- Denton-Thompson, S.M.; Sayer, E.J. Micronutrients in food production: What can we learn from natural ecosystems? Soil Syst. 2022, 6, 8. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Barczyk, K.; Jasiak-Małota, K.; Brodowska, M. Efficiency of using aminochelate fertilizers in plant nutrition. Przem. Chem. 2024, 103, 271–276. [Google Scholar] [CrossRef]
- Sekhon, B.S. Chelates for micronutrient nutrition among crops. Resonance 2003, 8, 46–53. [Google Scholar] [CrossRef]
- Yudaev, P.; Chistyakov, E. Chelating extractants for metals. Metals 2022, 12, 1275. [Google Scholar] [CrossRef]
- Klem-Marciniak, E.; Huculak-Mączka, M.; Marecka, K.; Hoffmann, K.; Hoffmann, J. Chemical stability of the fertilizer chelates Fe-EDDHA and Fe-EDDHSA over time. Molecules 2021, 26, 1933. [Google Scholar] [CrossRef]
- Grzesik, R.; Pankalla, E.; Kuźnik, N. Coating of calcium ammonium nitrate fertilizers with model chelates. Przem. Chem. 2022, 101, 810–814. [Google Scholar] [CrossRef]
- Saridis, G.; Chorianopoulou, S.N.; Ventouris, Y.E.; Sigalas, P.P.; Bouranis, D.L. An exploration of the roles of ferric iron chelation-strategy components in the leaves and roots of maize plants. Plants 2019, 8, 133. [Google Scholar] [CrossRef]
- Patent No. 242478. Process for Obtaining Biodegradable Micronutrient Fertiliser Components and Biodegradable Micronutrient Fertiliser Components. 2022. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.435383 (accessed on 8 July 2024).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 182. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 28 May 2024).
- Salmag® Specification. Available online: https://grupaazoty.com/upload/1/files/2024/nawozy/Specification%20Salmag%205.02.2024_EN.pdf (accessed on 5 June 2024).
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plants Properties, 1st ed.; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warszawa, Poland, 1998.
- ISO 10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13.3. 2017. Available online: http://statistica.io (accessed on 28 December 2023).
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrient Deficiencies in Global Crop Production. Springer Science & Business Media B.V.: Berlin, Germany, 2008. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2001; p. 403. [Google Scholar]
- Grześkowiak, A. Vademecum on Fertilisation or Basic and Practical Information on Sustainable Fertilisation; Grupa Azoty Zakłady Chemiczne „Police“ S.A., Police, 2016, 1–112. Available online: https://polifoska.pl/images/pliki/vademecum-nawozenia.pdf (accessed on 5 July 2024).
- Rana, M.S.; Hu, C.X.; Shaaban, M.; Imran, M.; Afzal, J.; Moussa, M.G.; Elyamine, A.M.; Bhantana, P.; Saleem, M.H.; Syaifudin, M.; et al. Soil phosphorus transformation characteristics in response to molybdenum supply in leguminous crops. J. Environ. Manag. 2020, 268, 110610. [Google Scholar] [CrossRef]
- Rana, M.S.; Sun, X.C.; Imran, M.; Ali, S.; Shaaban, M.; Moussa, M.G.; Khan, Z.; Afzal, J.; Binyamin, R.; Bhantana, P.; et al. Molybdenum-induced effects on leaf ultra-structure rhizosphere phosphorus transformation in Triticum aestivum, L. Plant Physiol. Biochem. 2020, 153, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Tahir, M.M.; Rahim, N. Effect of N fertilizer source and timing on yield and N use efficiency of rainfed maize (Zea mays L.) in Kashmir—Pakistan. Geoderma 2013, 195, 87–93. [Google Scholar] [CrossRef]
- Szulc, P.; Bocianowski, J.; Kruczek, A.; Szymanska, G.; Roszkiewicz, R. Response of two cultivar types of maize (Zea mays L.) expressed in protein content and its yield to varied soil resources of N and Mg and a form of nitrogen fertilizer. Polish J. Environ. Stud. 2013, 22, 1845–1853. Available online: http://www.pjoes.com/pdf-89154-23013?filename=Response%20of%20Two%20Cultivar.pdf (accessed on 8 July 2024).
- Wierzbowska, J.; Żuk-Gołaszewska, K. The impact of nitrogen fertilization and Rhizobium inoculation on the yield and quality of Trigonella foenum-graecum L. J. Elem. 2014, 19, 1109–1118. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N.; Brodowska, M. Role of humic acids-based fertilisers and nitrogen fertilisers in the regulation of the macroelement content in maize biomass. J. Elem. 2023, 28, 1289–1309. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, M.; Marshall, F.M. The role of organic vs. inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecol. Eng. 2010, 36, 1733–1740. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Brodowska, M.S. Content of trace elements in soil fertilized with potassium and nitrogen. Agriculture 2020, 10, 398. [Google Scholar] [CrossRef]
- Czarnecki, S.; Düring, R.A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. Soil 2015, 1, 23–33. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S. Potassium and nitrogen fertilization vs. trace element content of maize (Zea mays L.). Agriculture 2021, 11, 96. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Brodowska, M.S.; Kordala, N. Trace element contents in maize following the application of organic materials to reduce the potential adverse effects of nitrogen. Materials 2023, 16, 215. [Google Scholar] [CrossRef]
- Miner, G.L.; Delgado, J.A.; Ippolito, J.A.; Barbarick, K.A.; Stewart, C.E.; Manter, D.K.; Del Grosso, S.J.; Halvorson, A.D.; Floyd, B.A.; D’Adamo, R. Influence of long-term nitrogen fertilization on crop and soil micronutrients in a no-till maize cropping system. Field Crops Res. 2018, 228, 170–182. [Google Scholar] [CrossRef]
- Ali, N.S.; Hassan, W.F.; Janno, F.O. Soil iron and nitrogen availability and their uptake by maize plants as related to mineral and bio nitrogen fertilizers application. Agric. Biol. J. North Am. 2015, 6, 118–122. [Google Scholar] [CrossRef]
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation factor of selected heavy metals in Zea mays. J. Health Pollut. 2019, 9, 191207. Available online: https://www.journalhealthpollution.org/doi/pdf/10.5696/2156-9614-9.24.191207 (accessed on 8 July 2024). [CrossRef]
- Schoffer, J.T.; Sauve, S.; Neaman, A.; Ginocchio, R. Role of leaf litter on the incorporation of copper-containing pesticides into soils under fruit production: A review. J. Soil Sci. Plant Nutr. 2020, 10, 990–1000. [Google Scholar] [CrossRef]
- Jenkins, S.N.; Murphy, D.V.; Waite, I.S.; Rushton, S.P.; O’Donnell, A.G. Ancient landscapes and the relationship with microbial nitrification. Sci. Rep. 2016, 6, 30733. [Google Scholar] [CrossRef]
- Adhikari, L.; Missaoui, A.M. Nodulation response to molybdenum supplementation in alfalfa and its correlation with root and shoot growth in low pH soil. J. Plant Nutr. 2017, 40, 2290–2302. [Google Scholar] [CrossRef]
- Ge, X.X.; Vaccaro, B.J.; Thorgersen, M.P.; Poole, F.L.; Majumder, E.L.; Zane, G.M.; De Leon, K.B.; Lancaster, W.A.; Moon, J.W.; Paradis, C.J.; et al. Iron- and aluminium-induced depletion of molybdenum in acidic environments impedes the nitrogen cycle. Environ. Microbiol. 2019, 21, 152–163. [Google Scholar] [CrossRef]
- Shcherbakova, E.N.; Shcherbakov, A.V.; Andronov, E.E.; Gonchar, L.N.; Kalenskaya, S.M.; Chebotar, V.K. Combined pre-seed treatment with microbial inoculants and Mo nanoparticles changes composition of root exudates and rhizosphere microbiome structure of chickpea (Cicer arietinum L.) plants. Symbiosis 2017, 73, 57–59. [Google Scholar] [CrossRef]
- Wen, X.; Hu, C.X.; Sun, X.C.; Zhao, X.H.; Tan, Q.L.; Liu, P.J.; Xin, J.; Qin, S.Y.; Wang, P.C. Characterisation of vegetable nitrogen uptake and soil nitrogen transformation in response to continuous molybdenum application. J. Plant Nutr. Soil Sci. 2018, 181, 516–527. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E. Chemical properties of soil in four-field crop rotations under organic and conventional farming systems. Agronomy 2020, 10, 1045. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Zafar, M.; Sultan, T. Changes in soil properties and microbial indices across various management sites in the mountain environments of Azad Jammu and Kashmir. Commun. Soil Sci. Plant Anal. 2010, 41, 768–782. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Sharma, S.; Dhaliwal, S.S. Effects of sewage sludge and rice straw compost on yield, micronutrient availability and soil quality under rice-wheat system. Commun. Soil Sci. Plant Anal. 2019, 50, 1943–1954. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Borowik, A.; Kordala, N. Sewage sludge as a tool in limiting the content of trace elements in Avena sativa L. on the soil polluted with diesel oil. Materials 2021, 14, 4003. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. The effectiveness of foliar applications of synthesized zinc-amino acid chelates in comparison with zinc sulfate to increase yield and grain nutritional quality of wheat. Eur. J. Agron. 2013, 45, 68–74. [Google Scholar] [CrossRef]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. Iron(II)-amino acid chelates alleviate salt-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci. Horticult. 2014, 165, 91–98. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.H.; Afify, A.S.; Shanab, S.M.; Shalaby, E.A.; Hafez, R.M. Biotechnological studies on Arthrospira platensis biomass cultivated in enriched culture with chelated leather waste and chelated glycinate. Biomass Conv. Bioref. 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Najizadeh, A.; Khoshgoftarmanesh, A.H. Effects of foliar applied zinc in the form of ZnSO4 and Zn-amino acid complexes on pistachio nut yield and quality. J. Plant Nutr. 2019, 42, 2299–2309. [Google Scholar] [CrossRef]
- Yeboah, S.; Asibuo, J.; Oteng-Darko, P.; Adjei, E.A.; Lamptey, M.; Danquah, E.O.; Waswa, B.; Butare, L. Impact of foliar application of zinc and magnesium aminochelate on bean physiology and productivity in Ghana. Int. J. Agron. 2021, 2021, 9766709. [Google Scholar] [CrossRef]
- Soltani, S.M.; Chaleshtori, M.H.; Talab, K.T.; Vahed, H.S.; Katigari, M.S. Rice growth improvement, bio-fortification, and mitigation of macronutrient requirements through foliar application of zinc and iron- glycine chelate and zinc sulfate. J. Plant Nutr. 2023, 46, 1777–1786. [Google Scholar] [CrossRef]
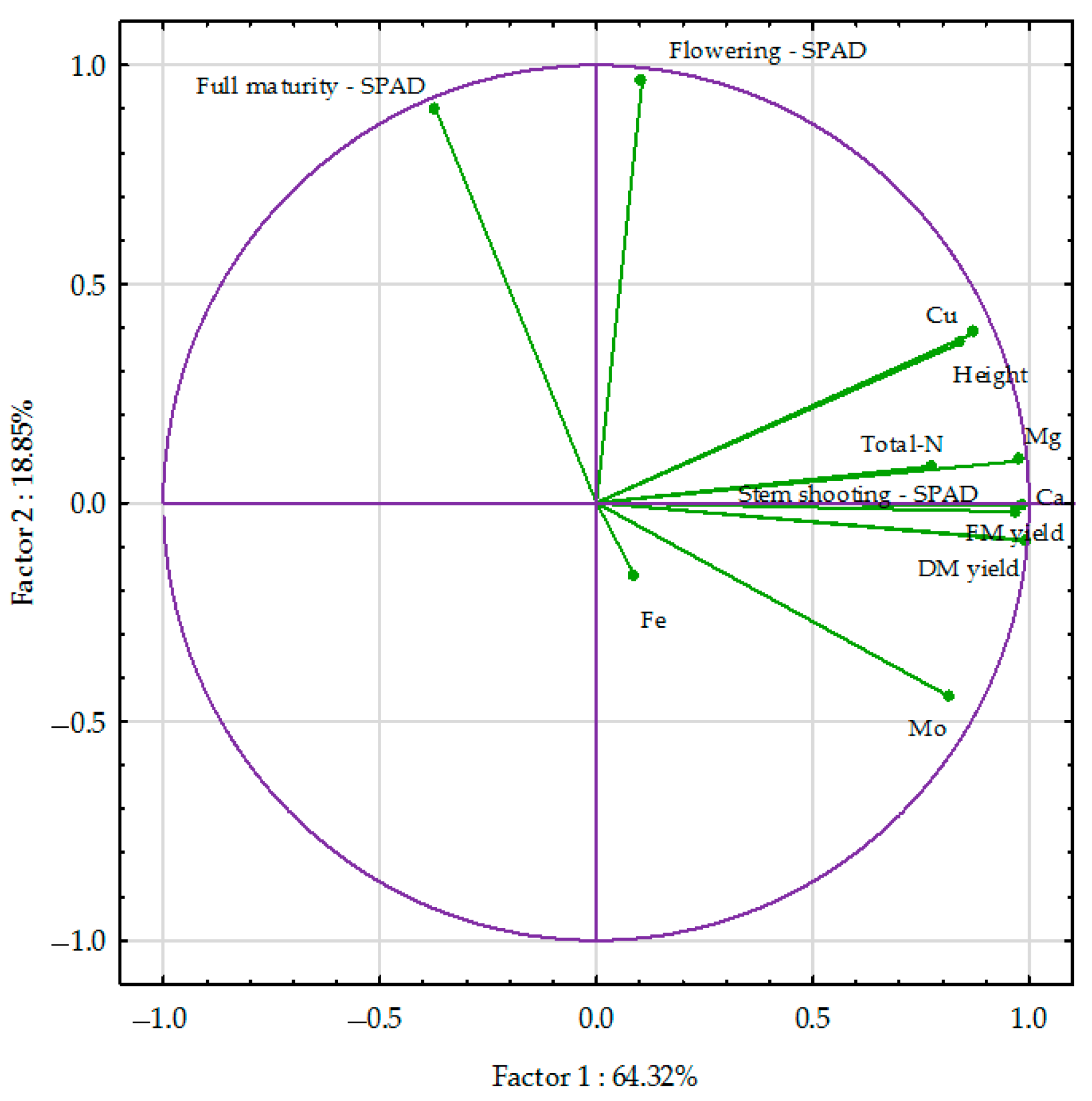
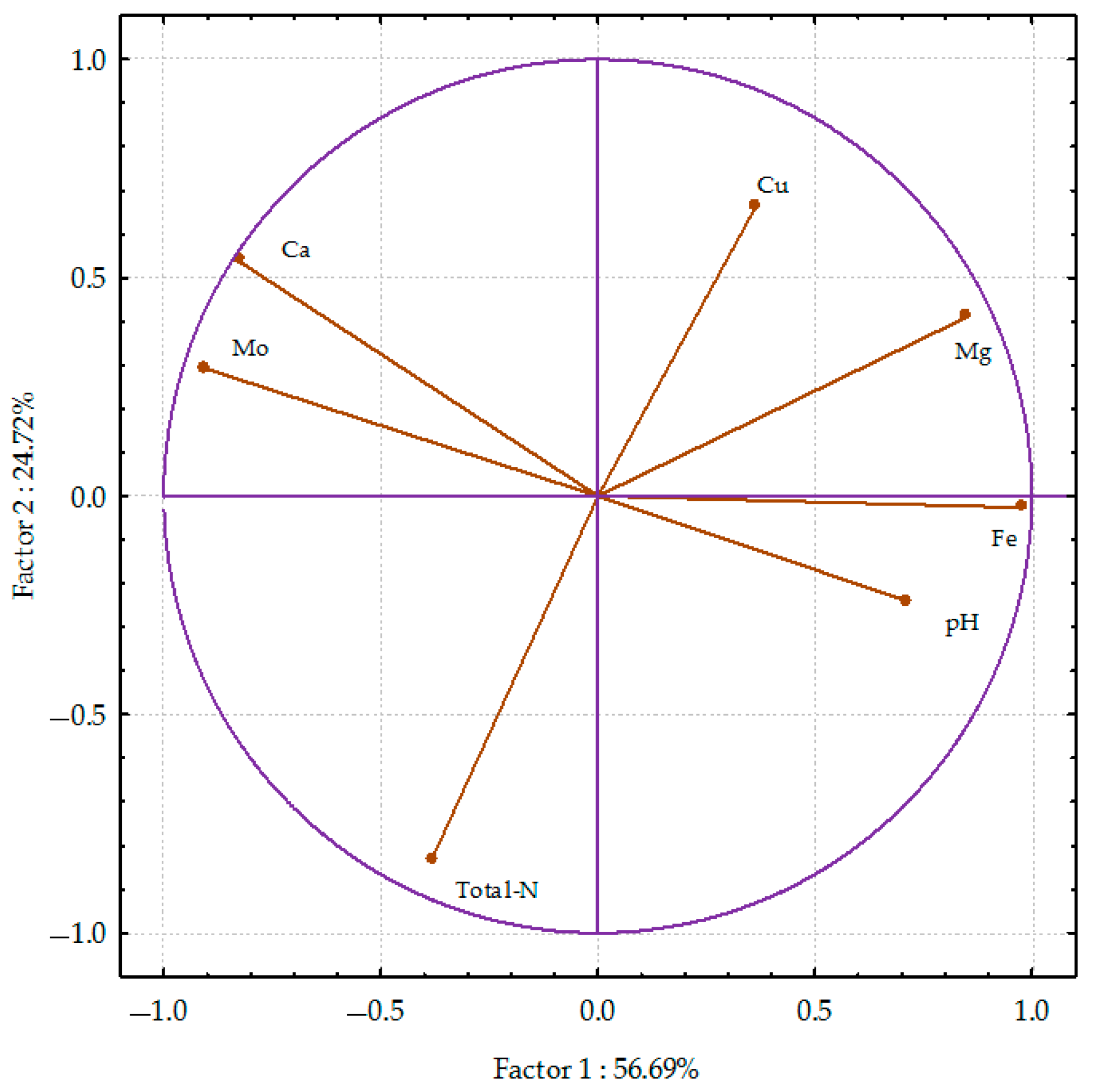
| Fertiliser | Yield FM | Yield DM | Total-N | Ca | Mg | Cu | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| g pot−1 | Content in g kg−1 DM | Content in mg kg−1 DM | ||||||
| Grain | ||||||||
| Control | 12.98 ± 0.13 a | 11.79 ± 0.12 a | 25.46 ± 0.25 a | 0.315 ± 0.004 a | 1.024 ± 0.002 a | 1.883 ± 0.002 b | 1.346 ± 0.039 a | 35.47 ± 0.46 a |
| Salmag® | 21.23 ± 0.38 b | 19.48 ± 0.36 b | 38.36 ± 0.46 d | 0.315 ± 0.002 a | 1.058 ± 0.006 b | 1.910 ± 0.007 b | 1.354 ± 0.024 ab | 39.65 ± 0.68 b |
| Salmag® with Cu | 22.57 ± 0.28 c | 20.66 ± 0.23 c | 35.84 ± 0.47 c | 0.317 ± 0.005 a | 1.059 ± 0.010 b | 1.911 ± 0.046 b | 1.301 ± 0.032 a | 36.11 ± 0.59 a |
| Salmag® with Mo | 22.33 ± 0.33 c | 20.51 ± 0.28 c | 30.80 ± 0.42 b | 0.316 ± 0.007 a | 1.014 ± 0.004 a | 1.813 ± 0.018 a | 1.486 ± 0.016 c | 37.37 ± 1.09 a |
| Salmag® with Fe | 21.13 ± 0.60 b | 19.37 ± 0.54 b | 31.64 ± 0.36 b | 0.338 ± 0.002 b | 1.027 ± 0.005 ab | 1.887 ± 0.009 b | 1.417 ± 0.010 bc | 41.56 ± 0.82 b |
| LSD0.01 | 0.67 | 0.60 | 1.04 | 0.011 | 0.031 | 0.059 | 0.068 | 1.97 |
| Straw | ||||||||
| Control | 20.97 ± 0.45 a | 15.61 ± 0.40 a | 10.92 ± 0.22 a | 0.834 ± 0.020 a | 0.675 ± 0.017 a | 1.897 ± 0.046 a | 0.833 ± 0.008 a | 66.98 ± 3.45 b |
| Salmag® | 52.23 ± 0.41 b | 33.94 ± 0.38 c | 14.00 ± 0.12 c | 1.491 ± 0.029 c | 1.044 ± 0.043 c | 2.007 ± 0.001 b | 0.858 ± 0.002 ab | 63.79 ± 1.94 ab |
| Salmag® with Cu | 53.14 ± 0.43 bc | 34.19 ± 0.17 c | 14.56 ± 0.06 d | 1.309 ± 0.045 b | 0.981 ± 0.019 bc | 2.008 ± 0.020 b | 0.848 ± 0.018 ab | 87.14 ± 0.24 c |
| Salmag® with Mo | 53.33 ± 0.50 c | 34.21 ± 0.14 c | 12.32 ± 0.29 b | 1.504 ± 0.031 c | 0.953 ± 0.034 b | 2.004 ± 0.067 b | 0.872 ± 0.014 b | 57.78 ± 1.99 a |
| Salmag® with Fe | 50.61 ± 0.71 d | 32.95 ± 0.44 b | 14.25 ± 0.08 cd | 1.291 ± 0.027 b | 0.953 ± 0.045 b | 1.932 ± 0.025 a | 0.861 ± 0.012 ab | 87.22 ± 2.96 c |
| LSD0.01 | 0.91 | 0.59 | 0.46 | 0.092 | 0.087 | 0.101 | 0.031 | 6.19 |
| Fertiliser | pH | Total-N | Ca | Mg | Cu | Mo | Fe |
|---|---|---|---|---|---|---|---|
| Content in g kg−1 DM | Content in mg kg−1 DM | ||||||
| Control | 5.94 ± 0.04 d | 1.067 ± 0.067 a | 4.882 ± 0.029 a | 2.070 ± 0.066 ab | 5.472 ± 0.095 a | 0.922 ± 0.024 a | 9754 ± 206 bc |
| Salmag® | 6.03 ± 0.03 c | 1.022 ± 0.051 a | 5.694 ± 0.092 b | 2.232 ± 0.066 c | 5.854 ± 0.181 a | 0.940 ± 0.007 a | 9957 ± 213 bc |
| Salmag® with Cu | 5.88 ± 0.05 b | 1.033 ± 0.088 a | 5.987 ± 0.088 c | 2.208 ± 0.033 c | 7.383 ± 0.117 b | 0.929 ± 0.013 a | 10256 ± 196 c |
| Salmag® with Mo | 5.83 ± 0.01 ab | 1.044 ± 0.019 a | 6.695 ± 0.078 d | 2.176 ± 0.015 bc | 5.472 ± 0.221 a | 0.937 ± 0.016 a | 9602 ± 210 b |
| Salmag® with Fe | 5.81 ± 0.02 z | 1.044 ± 0.051 a | 8.428 ± 0.094 e | 2.024 ± 0.050 a | 5.854 ± 0.268 a | 1.018 ± 0.008 a | 8513 ± 191 a |
| LSD0.01 | 0.06 | n.s. | 0.207 | 0.130 | 0.847 | n.s. | 579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodowska, M.S.; Wyszkowski, M.; Grzesik, R. New Fertilisers with Innovative Chelates in Wheat Cultivation. Agronomy 2024, 14, 1832. https://doi.org/10.3390/agronomy14081832
Brodowska MS, Wyszkowski M, Grzesik R. New Fertilisers with Innovative Chelates in Wheat Cultivation. Agronomy. 2024; 14(8):1832. https://doi.org/10.3390/agronomy14081832
Chicago/Turabian StyleBrodowska, Marzena S., Mirosław Wyszkowski, and Ryszard Grzesik. 2024. "New Fertilisers with Innovative Chelates in Wheat Cultivation" Agronomy 14, no. 8: 1832. https://doi.org/10.3390/agronomy14081832
APA StyleBrodowska, M. S., Wyszkowski, M., & Grzesik, R. (2024). New Fertilisers with Innovative Chelates in Wheat Cultivation. Agronomy, 14(8), 1832. https://doi.org/10.3390/agronomy14081832






