Abstract
Sour rot (SR) is a disease complex that affects grape berries during ripening and can cause severe yield losses and deterioration of wine quality. The etiology and epidemiology of the disease remain uncertain, which has severely limited the development of specific, targeted management strategies. In this study, a network meta-analysis was applied to data collected through a previous systematic literature review for statistically comparing the efficacy of different methods for the control of SR and some filamentous fungi isolated from rotten berries. Use of either synthetic fungicides (CHEM) and natural compounds or biocontrol microorganisms (BIO) provided partial and variable control of SR; however, the efficacy of BIO was similar to, or higher than, that provided by CHEM. Agronomic practices (AGRO) had a significant but lower effect on SR. The integration of different control methods (IPM) provided better and less variable disease control than any single method. Natural compounds, such as zeolites and bicarbonates, and microorganisms (e.g., yeasts Candida and Aureobasidium) are also promising alternatives to synthetic fungicides in SR control.
1. Introduction
Sour rot (SR) is a grapevine disease complex caused by a community of microorganisms including yeasts, bacteria, and filamentous fungi [1,2,3]. Recently, this disease has received increased interest because of its effect on yield losses and reduced wine quality in several grape production regions of the world [4,5,6,7].
Commonly, the first symptoms of SR are oxidation of the grape skin, turning it light-brown in color in both red and white varieties [8,9], followed by softening and disaggregation of the internal tissues. Affected berries ooze fermented grape pulp that smells of acetic acid and drips onto other berries within the cluster [10,11,12,13]. The SR-associated microorganisms penetrate berries through cracks and wounds in the berry skin that are caused by both abiotic (e.g., rain, high humidity, hail, and berry abrasion) and biotic (e.g., pests and birds) factors, including fungal pathogens such as Botrytis cinerea [14] and Erysiphe necator [15], feeding activity of berry moths such as the tortricid Lobesia botrana [16] and wasps [17], or oviposition of Drosophila suzuki flies [18]. The typical smell of affected berries [19] is caused by the activity of acetic acid bacteria (AAB) such as Acetobacter, Gluconobacter, and Gluconacetobacter, which oxidate the ethanol produced by the fermentation to acetic acids of berry sugars by non-Saccharomyces yeasts [20]. Some filamentous spoilage fungi such as Aspergillus, Penicillium, Cladosporium, Alternaria, and Rhizopus can finally grow on rotten berries [2,21].
The etiology and epidemiology of the disease are still not completely understood, which has severely limited the development of specific, targeted management strategies [9]. Recently, a systematic literature review was conducted by Brischetto et al. [22] with the aim of collecting and synthesizing the available literature on SR, focusing on its etiology, epidemiology, and control. Disease control strategies are mainly based on the integration of cultural and chemical control methods [23,24]. SR can be partially managed through practices focused on creating less conducive environment for the disease, reducing fruit fly infestations, preventing berry damages, choosing a trellis system that reduces canopy density, or managing the canopy to optimize air movement and reduce humidity. Leaf removal on the fruit zone is the most applied technique, able to decrease the incidence and severity of SR because it reduces the density of the canopy, increasing air movement, solar radiation, and temperature while reducing the relative humidity within the fruiting zone. Also, leaf removal increases the penetration of plant protection products (PPPs) into the fruit zone [23,25,26,27]. Chemical programs using PPPs can also contribute to the direct control of SR development. For instance, Tjamos et al. [24] proved that the treatments with fludioxonil were effective in controlling the incidence of SR caused by Aspergillus spp., while the treatments with cyprodinil were ineffective. However, the increase in restrictions of the use of PPPs determines the need of developing alternative SR management practices that can effectively control the disease.
In this work, we used the results of the above-mentioned systematic literature review [22] to statistically compare methods for the control of SR and of some filamentous fungi isolated from rotten berries through a network meta-analysis. Network meta-analysis allows for the integration and analysis of results obtained from different sources of information, considering all possible correlations [28], for determining the overall effectiveness of numerous disease control methods. This multi-strategy analysis permits the use of a large number of individual studies and is built on the principle that overall knowledge is based on the collection and combination of results from individual studies and observations [29,30,31,32].
2. Materials and Methods
2.1. Database of Studies
A database concerning studies of SR control was assembled based on the systematic literature review conducted by Brischetto et al. [22]. The research methodology for this systematic review was described by Brischetto et al. [22] and was conducted by using the three most relevant online bibliographic databases: (i) Scopus (https://www.scopus.com/, accessed on 17 October 2023); (ii) Web of Science Core Collection (http://webofknowledge.com/WOS, accessed on 17 October 2023); and (iii) Google Scholar (https://scholar.google.com/, accessed on 17 October 2023). Database searches were conducted in English. The search terms “sour rot”, “grape”, and “vitis vinifera” were combined into search strings using wildcards (*) and connectors (AND, OR) for identifying works for inclusion.
The search string provided papers in which SR was associated with different microorganisms, including the yeasts Pichia spp., Candida spp., and Hanseniaspora spp., and/or the AAB Gluconobacter spp. and Acetobacter spp., which have been frequently associated with SR for their capability to produce ethanol, acetic acid, and other compounds [13,20]. Other papers reported the occurrence or co-occurrence of filamentous fungi such as Aspergillus spp., Penicillium spp., Rhizopus spp., Alternaria spp., and Cladosporium spp., which may worsen the berry rot [21], spoil wine [12], and/or produce mycotoxins under vineyard conditions [2,33]. As we were interested in the control of the disease complex, we did not select papers based on the microorganisms that have been associated with SR by different authors.
To be included in the database for meta-analysis, papers had to meet the following criteria: (i) at least one treatment type was applied once to several times in a season to control berry rot; (ii) treatments were applied in the field or laboratory with either natural or artificial inoculum; (iii) berry rot was assessed as incidence of affected bunches (referred to as X for the meta-analysis); and (iv) the experiment had a suitable experimental design with at least three replicates and an untreated control (NT). Papers dealing with insecticides for controlling Drosophila spp. flies (Diptera: Dosophilidae) were not included in the analysis because we focused on methods for direct control of the disease rather than insect vectors.
Sixteen papers were considered in aggregate, for a total of 67 studies, with a study being a single independent experiment in which at least two treatments were compared, one of them being an untreated control (NT). Overall, 573 entries (i.e., combination of treatment and disease/causal agent) were considered [34]. These entries were split in three datasets: (i) 142 entries conducted on SR in field experiments between 1988 and 2022 in Canada, Greece, Italy, Spain, and USA; (ii) 66 entries conducted on Aspergillus spp. in field experiments between 2002 and 2006 in Greece; and (iii) 368 entries carried out in laboratory conditions with the following filamentous fungi: Aspergillus spp. (189 entries), Rhizopus stolonifer (47 entries), Fusarium oxysporum (44 entries), Ulocladium sp. (44 entries), and Penicillium comune (44 entries).
For field experiments, most of the studies were conducted with a randomized complete block design, with four replicate blocks. Grape variety and treatment (i.e., disease control methods) varied among studies, as summarized in Table 1 for datasets (i) and (ii) and in Table 2 for dataset (iii). The treatments were grouped into the four following categories: CHEM (chemical), based on the application of synthetic fungicides only (e.g., fludioxonil, cyprodinil, and carbendazim); BIO (biological), based on the application of biological control agents (BCAs, e.g., Aureobasidium pullulans, Candida sake, and Ulocladium oudemansii), natural products (e.g., zeolite, calcium chloride, and sodium bicarbonate), or their combination; AGRO (agronomical), based on leaf removal; and IPM (integrated pest management), based on the integration of AGRO, BIO, and/or CHEM.

Table 1.
Main characteristics of the studies conducted in the field. Treatments were grouped into four categories: AGRO, based on agronomic practices; BIO, based on the application of biological control agents, natural products, or their combination; CHEM, based on the application of chemical fungicides only; and IPM, integrated (based on the integration of AGRO, BIO, and/or CHEM).

Table 2.
Main characteristics of studies conducted using biological control agents to control filamentous fungi that were associated with berries affected by sour rot through artificial inoculation of berries under laboratory conditions.
The laboratory studies, which included three replicates, evaluated the efficacy of BCAs (Table 2). In these studies, intact berries were taken from the vineyard during the ripening period and rinsed in water, surface-disinfected, washed again to remove disinfectants, and finally wounded with a sterile needle. The treatment methods with BCAs were different, comprising the immersion of wounded berries in a suspension of each BCA (108 CFU/mL) [42,43] or pipetting the suspension of each yeast (containing 106 CFU/mL) into the wound [3]. Following treatment, berries were inoculated with fungal spore suspensions (containing 104–107 cells/mL) pipetted into each wound [3] or sprayed by using a hand sprayer [42,43]. After inoculation, berries were air-dried, placed in plastic boxes or bags, and kept at temperatures of 22–25 °C for six days depending on the study.
2.2. Meta-Analysis
A network meta-analysis was conducted to evaluate the effect of the treatment in reducing disease incidence compared to the non-treated control [44,45]. For each study and treatment (including the non-treated control), mean disease incidence data were extracted from the publication and used to conduct the analysis. For each study, disease incidence (X) from treated (T) and non-treated plots (NT) were used to estimate treatment effect (L) according to the following equation: LT = ln(XT) − ln(XNT). The log incidence of treatments was used because the distribution of ln(X) is closer to a normal distribution with respect to the distribution of X.
The within-study variance was estimated for each treatment of a single study as s2 = V/(n2), where V is the residual error component of the study, n is the number of replicates, and is the mean disease incidence. To calculate V for each study, different approaches were used [45,46]: (i) when the original data were available, V was extracted directly from the ANOVA table of the study (i.e., the residual variance or mean square error); (ii) when the study did not include the ANOVA table but did include the least significant difference (LSD), V was calculated as (n (LSD/1.96)2/2); (iii) when only the significant mean separation was provided (i.e., significant differences between means denoted by letters in a graph or a table), the estimated LSD was computed as the average between the smallest observed significant difference and the largest observed non-significant difference.
The meta-analysis was conducted with the software R (v. 3.4.0; package “metafor”) [47,48]. A multivariate random effects model was fitted via linear (mixed-effects) models by using the rma.mv function. The model was fitted in the form Y~N(μ, Σ + S), where ~N indicates a multivariate normal distribution, µ is the expected value for the different treatments, Σ is the between-study variance–covariance matrix, and S is the within-study variance–covariance matrix. The restricted maximum-likelihood estimation (REML) method was used for model fitting. Random effects were specified in the form ~inner|outer, with the outer factor corresponding to the study identification and the inner factor corresponding to the treatment type (i.e., the disease control method).
To assess the nature of the residual heterogeneity, I2 statistics were calculated as proposed by [49] and [50] by also running a similar model but with fixed effects; the I2 statistic was based on the relation between the variance–covariance matrix of models with fixed and random effects in the following form: (vcov(random)[1] − vcov(fixed)[1])/vcov(random)[1]).
Treatment effects were presented as L, with negative values of L indicating that SR incidence was lower in the treated plot than in the NT control (i.e., the treatment reduced the disease incidence compared to the untreated control). Standard errors and confidence intervals were likewise calculated for these values of L. A Wald-type test statistic was used to determine whether the mean differences in L were significantly different from zero, namely, whether the disease incidence in the treated plots ln(XT) differed from that in the untreated plots ln(XNT). The percentage of disease reduction relative to the control was also estimated as (1 − exp(L)) × 100, and the 95% confidence intervals were calculated as by [44,51].
3. Results
In dataset (i), there were 54 entries for BIO, which considered the efficacy on SR of different BCAs (i.e., Candida spp., Aureobasidium pullulans, and Ulocladium oudemansii) applied in the vineyard alone and/or in combination with substances of natural origin (e.g., chitosan, sodium bicarbonate, fatty acids, and zeolites), or with fungicides (specifically, copper or sulfur). For AGR, there were 24 entries dealing with leaf removal for reducing moisture of the bunch during ripening. In particular, the leaf removal was applied pre-bloom in 20.8% of these studies (<BBCH 61), after bloom in 37.5% (>BBCH 61), in full bloom in 37.5%, and during veraison in 4.2% (BBCH 81).
For CHEM, the 21 entries considered the efficacy of synthetic fungicides used alone, specifically aminopyrimidines (mepanypirin, cyprodinil), phthalates (procymidone), benzodioxoles (fludoxionil), and benzimidazoles (carbendazim). Finally, for IPM, the six entries combined leaf removal, fungicides, and/or natural compounds.
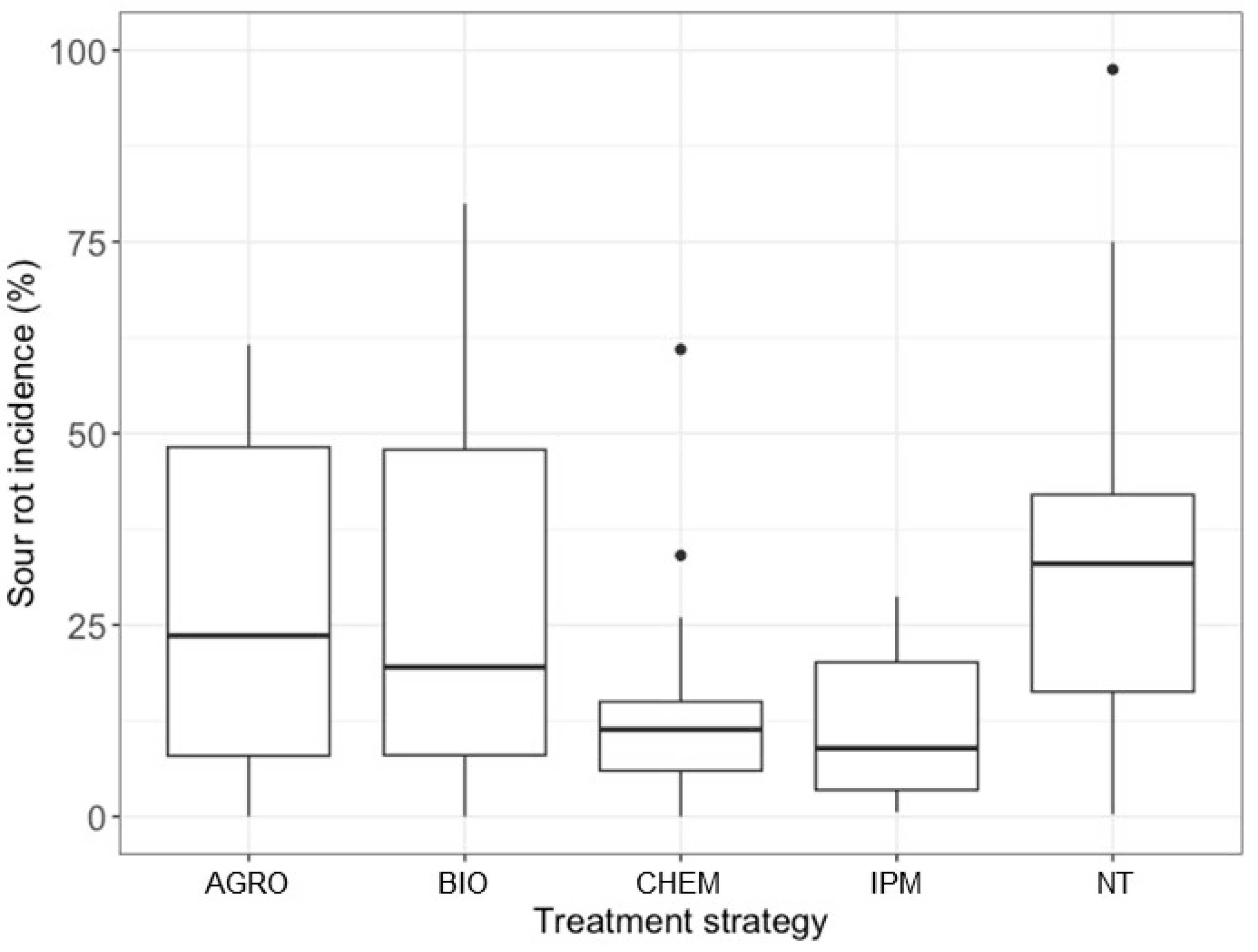
SR incidence in the NT plots of the studies conducted in the field ranged from 0.3% to 97.5%, with 90% of the values ranging from 4.4% to 73.8%, indicating a wide range of epidemiological situations (Figure 1).

Figure 1.
Box plot representing the distribution of grape sour rot incidence in different field studies in which disease control treatments were grouped as follows: AGRO, based on agronomic practices; BIO, based on the application of biological control agents, natural products, or their combination; CHEM, based on the application of chemical fungicides only; and IPM, integrated (based on the integration of AGRO, BIO, and/or CHEM). NT means non-treated control.
The average disease incidence was 33.9% (±3.7%) in the NT plots and 24.4% (±2.2%) in the treated plots. Disease incidence showed high variability among treatments (Figure 1). For example, with the AGRO and BIO treatments, 90% of disease incidence values ranged from 0.4% to 59.8% and from 1.8% to 70.7%, respectively. Lower variability was observed for CHEM, where 90% of the disease incidence values ranged from 0% to 34%.
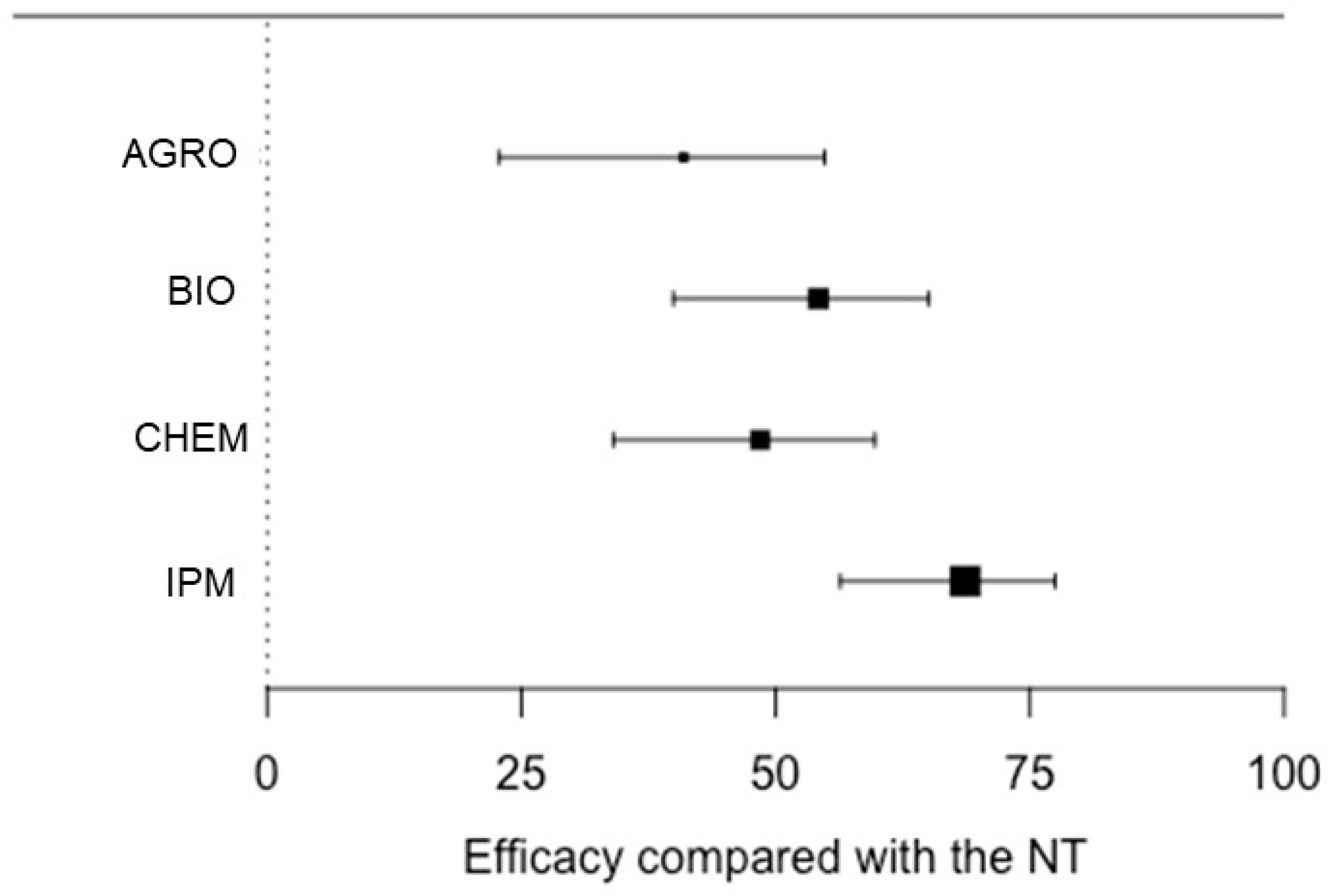
Heterogeneity testing indicated that the results of the meta-analysis were consistent and robust. The test for residual heterogeneity rejected the null hypothesis of homogeneity across studies (QE = 5253, df = 136, p < 0.0001), and the values of I2 were >70% for all treatments except for the integrated one (Table 3). Therefore, the heterogeneity in the estimated L values was mainly due to the among-studies variability and not the sampling errors in each study. The average values of L were significantly less than zero for all treatments (i.e., estimated SR incidence was lower in the treated than in the untreated plots; Table 3). Pairwise comparison by linear contrast showed that the L value estimated for IPM (−1.15) was significantly lower than those for all other treatment categories (p ≤ 0.025; Table 4), with an average disease reduction of 68.6% with respect to NT (Figure 2).

Table 3.
Effect on reduction in sour rot incidence compared to the untreated control of four treatment categories: CHEM (application of chemical fungicides only); BIO (application of biological control agents, natural products, or their combinations); AGRO (application of agronomical practices only); or IPM (integration of chemical fungicides with strategies from the other categories).

Table 4.
Pairwise comparison of the effect on reduction in sour rot incidence compared to the non-treated control for different treatment categories: CHEM (application of chemical fungicides only); BIO (application of biological control agents, natural products, or their combinations); AGRO (application of agronomical practices only); or IPM (integration of chemical fungicides with strategies from the other categories).

Figure 2.
Efficacy of different treatments on the control of grape sour rot in the field expressed as the percentage of disease reduction relative to the non-treated control (NT), as estimated using the meta-analysis. Treatments were grouped as follows: AGRO, based on agronomic practices; BIO, based on the application of biological control agents, natural products, or their combination; CHEM, based on the application of chemical fungicides only; and IPM, integrated (based on the integration of AGRO, BIO, and/or CHEM). Whiskers show 95% confidence intervals. Dots represent the average efficacy of treatments, and dot size increases with the precision of estimates.
The L value estimated for BIO was not significantly different from, but was lower than, those estimated for both AGRO and CHEM (Table 4); therefore, BIO provided better disease control than both AGRO and CHEM, with an average disease reduction of 54.1% (Figure 2).
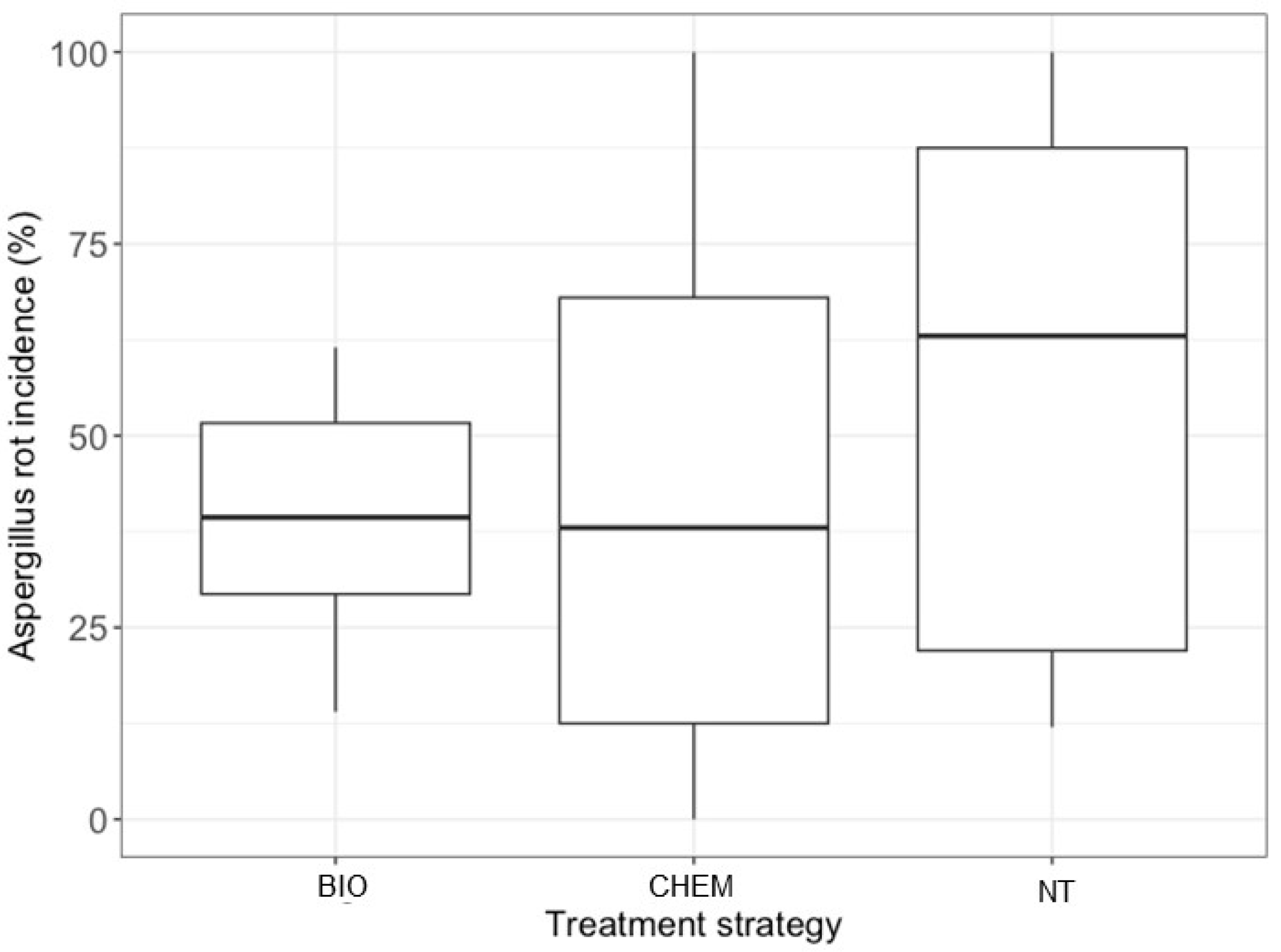
In dataset (ii), 10 entries and 38 were conducted to evaluate the effect of A. pullullans (BIO) and fludioxonil, cyprodinil, or carbendazim (CHEM) on the reduction in Aspergilli isolated from affected bunches. Aspergillus rot incidence ranged from 12% to 100% (Figure 3), with an average of 58.9% (±8.1%) in NT bunches.

Figure 3.
Box plots representing the distribution of the incidence of Aspergilla isolated from rotten berries that had been treated in the field to control sour rot. Treatments were grouped as follows: BIO, based on the application of biological control agents, natural products, or their combination; and CHEM, based on the application of chemical fungicides only. NT means non-treated control.
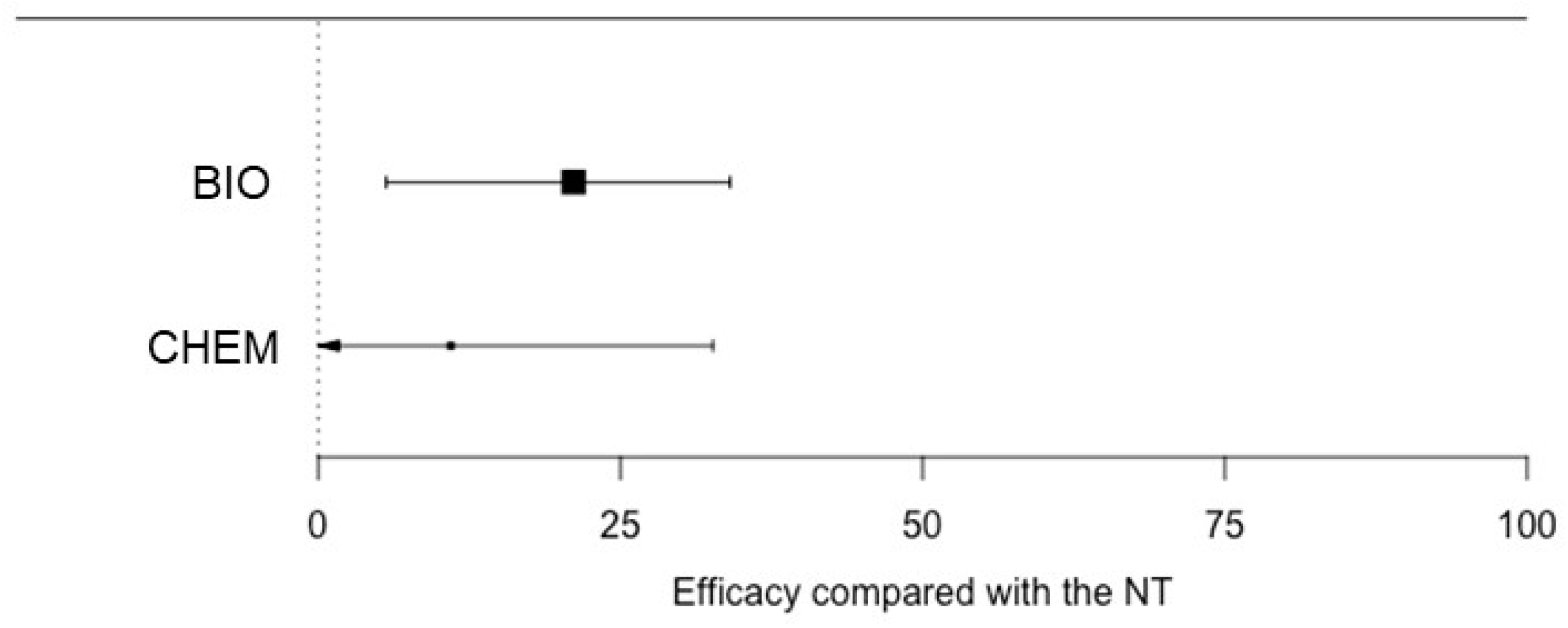
Disease incidence showed higher variability among plots treated with CHEM (Figure 3), with values ranging from 0% to 100%. The test for residual heterogeneity rejected the null hypothesis of homogeneity across studies (QE = 693, df = 63, p < 0.0001), and the value of I2 was >95% for CHEM only (Table 3). The average values of L were significantly less than zero for BIO, but not for CHEM (Table 5). The L value estimated for BIO (−0.24) was not significantly different from, but was lower than, that estimated for CHEM (−0.12; p = 0.213). Therefore, BIO provided slightly better efficacy than CHEM, with an average reduction of 21.1% compared to NT (Figure 4).

Table 5.
Effect in the reduction in Aspergillus spp. incidence compared to the untreated control of two treatment categories: CHEM (application of chemical fungicides only); and BIO (application of biological control agents, natural products, or their combinations).

Figure 4.
Efficacy (%) of different treatments on the incidence of Aspergilli isolated from rotten berries that had been treated to control sour rot, relative to the non-treated control (NT), as estimated with the meta-analysis. Treatments were grouped as follows: BIO, based on the application of biological control agents, natural products, or their combination; and CHEM, based on the application of chemical fungicides only. Whiskers show 95% confidence intervals. Dots represent the average efficacy of treatments, and dot size increases with the precision of estimates.
Incidence of berry rot caused by artificial inoculation of berries with fungi isolated from sour rot-damaged grapes (dataset (iii)) ranged from 10% to 100% in NT, with an average of 86.9% (±7.6%). The average rot incidence was 86.7% (±1.6%) in berries that had been treated with BCAs, with a wide range of 17.9% to 100%. The test for residual heterogeneity rejected the null hypothesis of homogeneity across the studies (QE = 1607, df = 366, p < 0.0001), and the heterogeneity in the estimated L value was mainly due to the among-studies (I2 = 94.6%). The average values of L for the treated groups was significantly less than zero (−0.265; confidence interval 95%: −0.502, −0.029; p = 0.028). Overall, the treatment provided a disease reduction of 23.3% compared to NT.
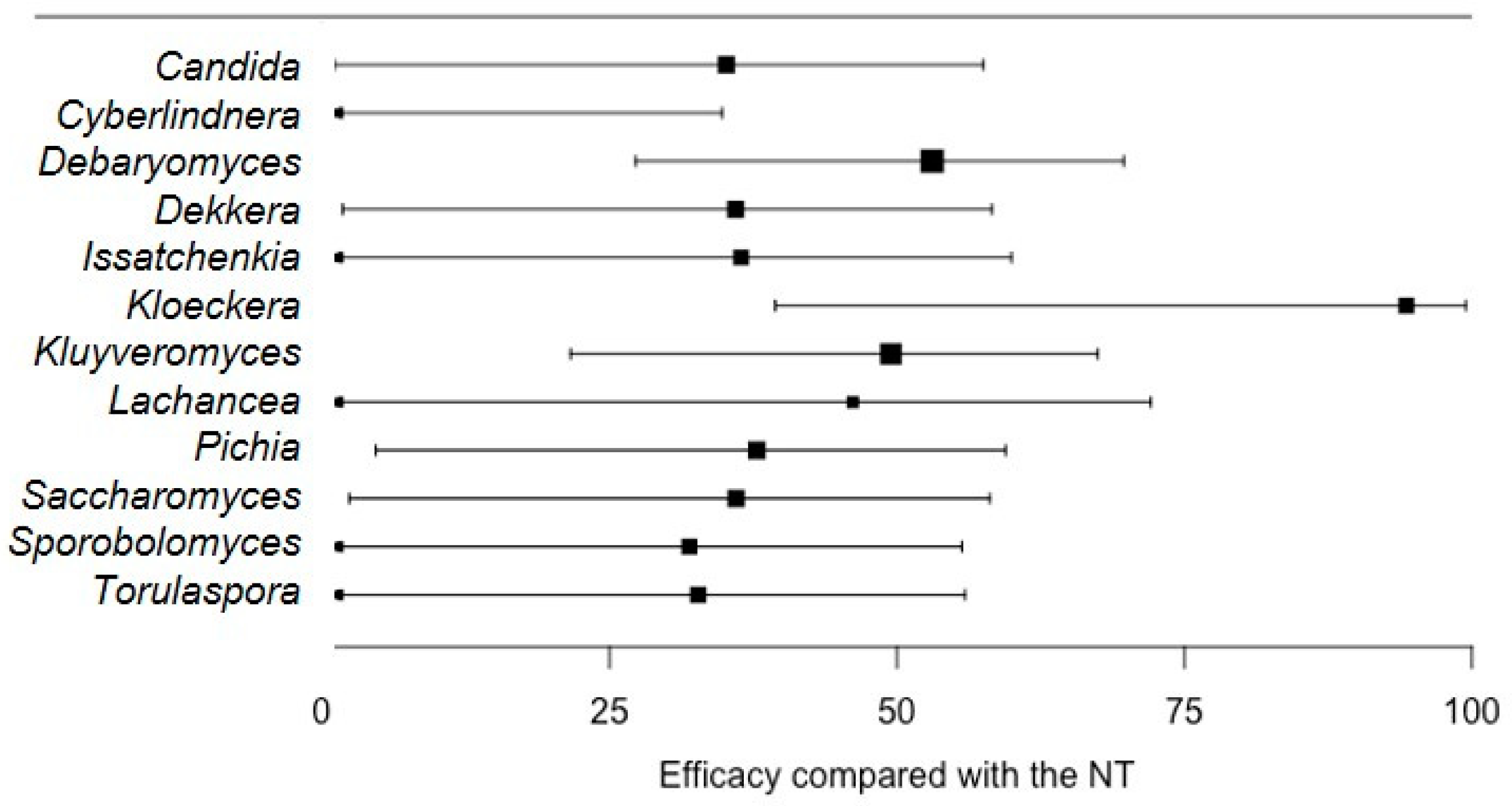
When dataset (iii) was explored for Aspergillus spp. only, the average disease incidence was 81.8% (±12.5%) in NT, with values ranging from 10% to 100%. The test for residual heterogeneity rejected the null hypothesis of homogeneity across studies (QE = 1231, df = 176, p < 0.0001), and the values of I2 were >80% for all the tested microorganisms, except for Kloeckera sp. (I2 = 39.4%) and Lachancea sp. (I2 = 48.9%). The average values of L for the treated groups were significantly less than zero for Candida spp., Debaryomyces sp., Dekkera sp., Kloeckera sp., Kluyveromyces sp., Pichia sp., and Saccharomyces spp. (Table 6). The highest disease reduction was observed with Kloeckera sp. (94.3%) (Figure 5).

Table 6.
Effect of biocontrol microorganisms on the reduction in grape berry rot incidence caused by Aspergilli isolated from berries showing sour rot symptoms, as compared to the untreated control under laboratory conditions.

Figure 5.
Efficacy of different microorganisms on the reduction in the incidence of grape berry rot caused by Aspergilli (% of berries) relative to the non-treated control (NT), as estimated with the meta-analysis. Aspergilli had been isolated from rotten berries showing sour rot symptoms, and were artificially inoculated on healthy, wounded berries. Whiskers show 95% confidence intervals. Dots represent the average efficacy of treatments, and dot size increases with the precision of estimates.
The L value estimated for Kloeckera sp. (−2.86) was significantly lower than that estimated for Candida spp. (−0.43; p = 0.049), Cyberlindnera sp. (0.07; p = 0.018), Sporobolomyces sp. (−0.38; p = 0.045), and Torulaspora sp. (−0.40; p = 0.046) (Table 6).
4. Discussion
In this work, a network meta-analysis was conducted using 16 papers (for a total of 67 studies and 573 entries) on the control of SR and some filamentous fungi isolated from rotten berries, which were retrieved through a previous systematic literature review [22]. The size of this dataset ensured the representativeness of the results. We used the term “sour rot” in the literature search because we were more interested in the control of the disease complex (which is mainly caused by a consortium of non-Saccharomyces yeasts and AAB that usually evolve during the disease) rather than on single microorganisms of the complex. Since some papers mentioned some filamentous fungi (e.g., Aspergillus spp., Cladosporium spp., Rhizopus spp., etc.) as components of SR, usually as final invaders of spoiled bunches, we dedicated a specific analysis to these fungi. Studies on the control of these fungi as single rotting agents have not been considered; for instance, studies on Aspergillus spp. for their ability to accumulate the mycotoxin ochratoxin A in grape berries with no reference to SR were not considered [52,53].
Overall, our meta-analysis showed partial and variable control of SR with either synthetic fungicides (CHEM) and natural compounds or biocontrol microorganisms (BIO). This result may be related to the etiology of SR, which involves microbial communities that include non-Saccharomyces, AAB, and eventually saprophytic or weak pathogenic filamentous fungi [2,20,21]. The composition of these microbial communities changes in different grape-growing areas, agricultural contexts, and seasons [54]. Clearly, plant protection products may be more or less effective depending on their activity against the specific microorganisms involved in the SR etiology in a specific agricultural situation.
The complexity of SR’s etiology is likely the reason why the integration of different control methods (IPM) provided better and less variable disease control than other methods, with different plant protection products likely able to differently control different microorganisms. The integration of different methods in an IPM approach also meets the request of switching from synthetic fungicides to low-impact options for disease control [55,56]. Indeed, it is already known that the combination of agronomic practices and BCAs guarantees the greater efficacy of the latter [57].
Synthetic fungicides of different chemical groups were used in studies included in our meta-analysis. The most-used active ingredients belong to the anilino-pyrimidines and phenylpyrroles groups (e.g., cyprodinil, fludioxonil), commonly applied to control gray mold worldwide [45] and secondary bunch rots caused by filamentous fungi (EU pesticide database). Some active ingredients evaluated for SR control are no longer approved for their use in agriculture (procymidone, carbendazim, fenarimol) and, based on our dataset, several active ingredients currently authorized for the control of gray mold have not been tested for their efficacy in controlling SR. These active ingredients include the multisite folpet, succinate dehydrogenase inhibitors (e.g., boscalid), uncouplers of oxidative phosphorylation, and others. Their evaluation may enlarge the fist of synthetic fungicides useful for SR management in vineyards, leading to possible improvements in chemical control, even though the use of synthetic fungicides late in ripening leads to negative impacts and possible residues in the final products [2].
Leaf removal, which was the most investigated agronomic practice used in the selected studies (AGRO), had a significant but low effect on SR in our meta-analysis. This practice, consisting in the removal of basal leaves of shoots in the cluster zone, creates a less conducive environment for SR by facilitating air movement and reducing humidity in the bunch zone [9,10,23,58,59].
Leaf removal was also used as a component of IPM. When combined with CHEM or BIO, leaf removal may improve the distribution of BCA on bunches [60] in addition to modifying the grape’s microclimate. However, microclimatic conditions can influence the survival, growth, and efficacy of BCAs [61,62]. For example, Calvo-Garrido et al. [63] found that leaf removal reduced the population of the BCA C. sake on grapes under the concentration threshold for effective SR suppression by exposing the yeast to abiotic factors constraining survival in field conditions, such as temperature, relative humidity, and sunlight. Therefore, integration of leaf removal and BCAs should be carefully evaluated.
In our meta-analysis, the efficacy of BIO (which includes natural compounds and biocontrol microorganisms) was similar to, or higher than, that provided by synthetic fungicides. This is a very interesting result because disease control with natural alternatives usually provides less and more variable control than with chemical pesticides in several pathosystems [64].
Zeolites and carbonates were among the most considered natural compounds for SR control; they were used in eight studies, while chitosan or fatty acids were used in only four. Zeolites are inorganic crystalline minerals characterized by a structure based on an infinitely extended three-dimensional framework, with cations bonded within this framework by electrostatic forces; these cations are exchangeable through diffusion in a medium containing another cation and add antimicrobial properties to the substance [65].
Zeolites, which also absorb water and reduce moisture on plant surfaces, show broad-spectrum antimicrobial activity and long-lasting active periods when modified with metal ions such as Ag+, Cu2+, Fe2+, and Zn2+ [66,67,68,69,70,71], and are capable of bonding with mycotoxins [72]. Modified natural zeolites show multiple modes of microbicidal action, including disruption of the cell wall, cell membrane damage, and the alteration of multiple cellular functions through the interaction with proteins, lipides, and DNA, as well as inducing of oxidative stress [73]. Thus, zeolites have potential for large use in crop protection [74]. In viticulture, zeolites have also been used to control downy and powdery mildews and Botrytis bunch rot [75,76,77,78].
Carbonates and bicarbonates are inorganic salts with well-known broad-spectrum activity in controlling a wide range of fungi, including food spoilage organisms and plant pathogens [79,80]. For example, ammonium, potassium, and sodium bicarbonates are able to directly inhibit fungal colony growth and spore development, leading to collapse and shrinkage most likely because of changes in the pH and osmotic pressure of cells [5,81,82,83]. Bicarbonates can also induce resistance, as demonstrated in citrus fruit [84]. Interestingly, sodium bicarbonate increases the efficacy of the biocontrol yeast Hanseniaspora uvarum against B. cinerea in table grapes [85]. Sodium bicarbonate also inhibits the growth of bacteria and yeasts [86].
The yeasts Candida spp. and A. pullulans were the most-used microorganisms for biocontrol in the field, and species of Candida, Pichia, Kloeckera, and Saccharomyces were tested under laboratory conditions. It is important to note that these yeasts are among the most common inhabitants of the grape berry surface during ripening [20,87,88,89], meaning that they can stably occupy this particular ecological niche, which is a prerequisite for BCA effectiveness. Stable colonization of the grape berry surface by yeasts (e.g., A. pullulans), microcracks and wounds (the pathway for SR microorganisms entering the berry pulp), high tolerance to different ecological stresses (e.g., desiccation and irradiation) [90,91], competition for space and nutrients [92,93,94], and antagonistic activity through the production of extracellular chitinases and β-1-3-glucanases [95] have been proven to be effective against B. cinerea, Penicillium spp., and Aspergillus spp. [96,97,98,99], as well as other microorganisms [100,101,102].
In contrast with yeasts, no studies were retrieved in the international literature on the effect of bacteria on SR control [22]. However, two Italian papers published in proceedings of annual technical meetings on crop protection (called Giornate Fitopatologiche) showed that Bacillus subtilis [103] and B. amyloliquefaciens [104] exhibited similar efficacy to fungicides (specifically, a mixture of fludioxonil and cyprodinil) in controlling SR. As Bacillus spp. have been largely investigated as biocontrol agents for B. cinerea control in grapes [90,105] through the production of secondary metabolites [106] and induction of resistance [107], research on the use of Bacillus-based biofungicides against SR should be further investigated. Spore-forming Bacillus species also have the advantages of a long shelf-life, a wide spectrum of activity, and a generally high compatibility with most synthetic fungicides [108].
The efficacy of BCAs in our meta-analysis is encouraging, considering that a previous meta-analysis on the biocontrol of main grapevine diseases highlighted the difficulty of drawing practical guidelines for BCA use against main grape diseases because of the great variability of microorganisms, application timing and frequency, methods for efficacy assessment, and varying environmental conditions [109]. However, further research is needed to better define the following key aspects: (i) the efficacy of BCAs against the main causal agents of SR; (ii) the biocontrol mechanisms (competition, parasitism, antibiosis, or induced resistance) that are more likely to be effective against SR-related microorganisms; and (iii) application timing under vineyard conditions. Research on the biocontrol of B. cinerea showed that the efficacy of different BCAs is strictly related to the timing of application, the plant substrate to be colonized in relation to the plant growth stage, the environmental conditions in relation to BCA requirements, and their modes of action [110].
Our meta-analysis did not include studies on the control Drosophila spp. flies in vineyards. However, Drosophila melanogaster, D. suzukii, and other species play a key role in SR development through a multitrophic interaction in which insect vectors of SR microorganisms, both epiphytically and in the gut, cause skin opening through the ovipositor, modify pulp composition, and hinder wound healing trough larval activities [13,18,20,22]. Therefore, control of Drosophila spp. can be a component of an integrated SR control scheme. However, the use of insecticides has promoted rising insecticide resistance, has negative effects on natural enemies and outbreaks of secondary pests, and has pre-harvest interval restriction [111,112,113]. Therefore, the alternative control of trough predators, parasitoids, and/or entomopathogens, mass trapping, attract and kill techniques, and repellents that have been applied to reduce direct damage to grapes caused by D. suzukii [112,114,115,116] should be explored for the control of fly populations in relation to SR.
5. Conclusions
The results of this study show that the use of alternatives to synthetic fungicides are promising for the control of SR in vineyards; these results may be related to the broad spectrum and multiple modes of action of these compounds, which may act against the multiple yeasts, bacteria, and filamentous fungi that together and/or in succession are involved in the SR complex. Among these alternatives, zeolites and bicarbonates were used more frequently than other natural compounds. These alternatives also include BCAs based on selected strains of those microorganisms (e.g., yeasts Candida and Aureobasidium) that usually colonize the grape berry surfaces, including microcracks and wounds, during ripening and are characterized by competition and antagonistic capacities against multiple microorganisms. Potential synergism between natural compounds and BCAs, which has been proven in other systems, should be investigated. Finally, an integration of direct SR control with the reduction in Drosophila spp. populations through non-chemical alternatives should be explored further.
Author Contributions
Conceptualization, V.R. and G.F.; methodology, V.R. and G.F.; formal analysis, G.F.; data curation, C.B. and G.F.; writing—original draft preparation, C.B. and G.F.; writing—review and editing, V.R. and G.F.; supervision, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
C.B. conducted this study within the Doctoral School on the Agro-Food System (AgriSystem) at the Università Cattolica del Sacro Cuore, Piacenza, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hewstone, N.; Valenzuela, J.; Muñoz, C. Nueva variedad de uva de mesa. Agric.-Cult. Tec. 2007, 67, 201–204. [Google Scholar]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Toro, M.E.; Combina, M.; Castellanos de Figueroa, L.I.; Vazquez, F. Biocontrol of fungi isolated from sour rot infected table grapes by Saccharomyces and other yeast species. Postharvest Biol. Technol. 2013, 86, 456–462. [Google Scholar]
- Barata, A.; Campo, E.; Malfeito-Ferreira, M.; Loureiro, V.; Cacho, J.; Ferreira, V. Analytical and sensorial characterization of the aroma of wines produced with sour rotten grapes using GC-O and GC-MS: Identification of key aroma compounds. J. Agric. Food Chem. 2011, 59, 2543–2553. [Google Scholar]
- Nigro, F.; Schena, L.; Ligorio, A.; Pentimone, I.; Ippolito, A.; Salerno, M.G. Control of table grape storage rots by pre-harvest applications of salts. Postharvest Biol. Technol. 2006, 42, 142–149. [Google Scholar]
- Oriolani, E.J.A.; Rodríguez, M.C.; Combina, M. Complejo parasitario de la podredumbre ácida de los racimos de la vid, en Mendoza y San Juan Argentina. Congr. Latinoam. Vitic. Enol. 2007, 11, 26–30. [Google Scholar]
- Wei, Y.; Wang, C.; Zhao, X.; Shang, Q.; Liu, Z. Identification and biological characteristics of the pathogenic sf-19 strain of grape sour rot from Beijing. Phytopathology 2015, 105, 147. [Google Scholar]
- Gravot, E.; Blancard, D.; Fermaud, M.; Lonvaud, A.; Joyeux, A. Sour rot. I: Etiology. Research into the causes of this form of rot of grapes in Bordeaux vineyards. Phytoma 2001, 543, 36–39. [Google Scholar]
- Hall, M.E.; Loeb, G.M.; Wilcox, W.F. Control of sour rot using chemical and canopy management techniques. Am. J. Enol. Vitic. 2018, 69, 342–350. [Google Scholar]
- Bisiach, M.; Minervini, G.; Zerbetto, F. Possible integrated control of grapevine sour rot. Vitis 1986, 25, 118–128. [Google Scholar]
- Guerzoni, E.; Marchetti, R. Analysis of yeast flora associated with grape sour rot and of the chemical disease markers. Appl. Environ. Microbiol. 1987, 53, 571–576. [Google Scholar] [PubMed]
- Barata, A.; González, S.; Malfeito-Ferreira, M.; Querol, A.; Loureiro, V. Sour rot damaged grapes are sources of wine spoilage yeasts. FEMS Yeast Res. 2008, 8, 1008–1017. [Google Scholar]
- Hall, M.E.; Loeb, G.M.; Cadle-Davidson, L.; Evans, K.J.; Wilcox, W.F. Grape sour rot: A four-way interaction involving the host, yeast, acetic acid bacteria, and insects. Phytopathology 2018, 108, 1429–1442. [Google Scholar]
- Marchetti, R.; Guerzoni, M.E.; Gentile, M. Research on the etiology of a new disease of grapes: Sour rot. Vitis 1984, 23, 55–65. [Google Scholar]
- Gadoury, D.M.; Seem, R.C.; Wilcox, W.F.; Henick-Kling, T.; Conterno, L.; Day, A.; Ficke, A. Effects of diffuse colonization of grape berries by Uncinula necator on bunch rots, berry microflora, and juice and wine quality. Phytopathology 2007, 97, 1356–1365. [Google Scholar]
- Moschos, T. Yield loss quantification and assessment of economic injury level for the anthophagous generation of the European grapevine moth Lobesia botrana Den. Et Schiff. (Lepidoptera: Tortricidae). Int. J. Pest Manag. 2005, 51, 81–89. [Google Scholar]
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Berlin, Germany, 1990. [Google Scholar]
- Ioriatti, C.; Guzzon, R.; Anfora, G.; Ghidoni, F.; Mazzoni, V.; Villegas, T.R.; Dalton, D.T.; Walton, V.M. Drosophila suzukii (Diptera: Drosophilidae) contributes to the development of sour rot in grape. J. Econ. Entomol. 2018, 111, 283–292. [Google Scholar]
- Pinto, L.; Malfeito-Ferreira, M.; Quintieri, L.; Silva, A.C.; Baruzzi, F. Growth and metabolite production of a grape sour rot yeast-bacterium consortium on different carbon sources. Int. J. Food Microbiol. 2019, 296, 65–74. [Google Scholar]
- Barata, A.; Santos, S.C.; Malfeito-Ferreira, M.; Loureiro, V. New insights into the ecological interaction between grape berry microorganisms and Drosophila flies during the development of sour rot. Microb. Ecol. 2012, 64, 416–430. [Google Scholar] [PubMed]
- Crandall, S.G.; Spychalla, J.; Crouch, U.T.; Acevedo, F.E.; Naegele, R.P.; Miles, T.D. Rotting grapes don’t improve with age: Cluster rot disease complexes, management, and future prospects. Plant Dis. 2022, 106, 2013–2025. [Google Scholar]
- Brischetto, C.; Rossi, V.; Fedele, G. Knowledge gaps on grape sour rot inferred from a systematic literature review. Front. Plant Sci. 2024, 15, 1415379. [Google Scholar] [CrossRef]
- Stapleton, J.J.; Grant, R.S. Leaf removal for nonchemical control of the summer bunch rot complex of wine grapes in the San Joaquin valley. Plant Dis. 1992, 76, 205–208. [Google Scholar]
- Tjamos, S.E.; Antoniou, P.P.; Kazantzidou, A.; Antonopoulos, D.F.; Papageorgiou, I.; Tjamos, E.C. Aspergillus niger and Aspergillus carbonarius in Corinth raisin and wine-producing vineyards in Greece: Population composition, ochratoxin A production and chemical control. J. Phytopathol. 2004, 152, 250–255. [Google Scholar]
- Duncan, R.A.; Stapleton, J.J.; Leavitt, G.M. Population dynamics of epiphytic mycoflora and occurrence of bunch rots of wine grapes as influenced by leaf removal. Plant Pathol. 1995, 44, 956–965. [Google Scholar]
- Sholberg, P.L.; O’gorman, D.T.; Haag, P.D. Identification and control of grape sour rot causal agents in British Columbia. Can. J. Plant Pathol. 2009, 31, 498–499. [Google Scholar]
- Vogel, A.; Breeden, S.; Brannen, P.; Blaauw, B.; Hickey, C. Grape Sour Rot. In UGA Cooperative Extension Circular 1212; University of Georgia Cooperative Extension Service: Athens, GA, USA, 2020. [Google Scholar]
- Madden, L.V.; Piepho, H.P.; Paul, P.A. Statistical models and methods for network meta-analysis. Phytopathology 2016, 106, 792–806. [Google Scholar]
- Madden, L.V.; Paul, P.A. Meta-Analysis for evidence synthesis in plant pathology: An overview. Phytopathology 2011, 101, 16–30. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Cooper, H.; Hedges, L.V. Research synthesis as a scientific process. In The Handbook of Research Rynthesis and Meta-Analysis, 2nd ed.; Cooper, H., Hedges, L.V., Valentine, J.C., Eds.; SAGE: Thousand Oaks, CA, USA, 2009; pp. 3–16. [Google Scholar]
- Hunter, J.E.; Schmidt, F.L. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings, 2nd ed.; SAGE Inc.: Thousand Oaks, CA, USA, 2004. [Google Scholar]
- Battilani, P.; Pietri, A. Ochratoxin A in Grapes and Wine. Mycotoxins in Plant Disease: Under the Aegis of COST Action 835 ‘Agriculturally Important Toxigenic Fungi 1998–2003’, EU Project (QLK 1-CT-1998-01380), and ISPP ‘Fusarium Committee’; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; pp. 639–643. [Google Scholar]
- Ojiambo, P.S.; Scherm, H. Biological and application-oriented factors influencing plant disease suppression by biological control: A meta-analytical review. Phytopathology 2006, 96, 1168–1174. [Google Scholar]
- Schena, L.; Nigro, F.; Soleti Ligorio, V.; Yaseen, T.; Ippolito, A.; El Ghaouth, A. Biocontrol activity of bio-coat and biocure against postharvest rots of table grapes and sweet cherries. Acta Hortic. 2005, 682, 2115–2120. [Google Scholar]
- Dimakopoulou, M.; Tjamos, S.E.; Antoniou, P.P.; Pietri, A.; Battilani, P.; Avramidis, N.; Markakis, E.A.; Tjamos, E.C. Phyllosphere grapevine yeast Aureobasidium pullulans reduces Aspergillus carbonarius (sour rot) incidence in wine-producing vineyards in Greece. Biol. Control 2008, 46, 158–165. [Google Scholar]
- Calvo-Garrido, C.; Viñas, I.; Elmer, P.A.G.; Usall, J.; Teixidó, N. Candida sake CPA-1 and other biologically based products as potential control strategies to reduce sour rot of grapes. Lett. Appl. Microbiol. 2013, 57, 356–361. [Google Scholar] [PubMed]
- Calzarano, F.; Valentini, G.; Arfelli, G.; Seghetti, L.; Manetta, A.C.; Metruccio, E.G.; Di Marco, S. Activity of Italian natural chabasite-rich zeolitites against grey mould, sour rot and grapevine moth, and effects on grape and wine composition. Phytopathol. Mediterr. 2019, 58, 307–322. [Google Scholar]
- Carbó, A.; Torres, R.; Usall, J.; Marín, A.; Chiralt, A.; Teixidó, N. Novel film-forming formulations of the biocontrol agent Candida sake CPA-1: Biocontrol efficacy and performance at field conditions in organic wine grapes. Pest Manag. Sci. 2019, 75, 959–968. [Google Scholar]
- Calderone, F.; Vitale, A.; Panebianco, S.; Lombardo, M.F.; Cirvilleri, G. COS-OGA applications in Organic Vineyard manage major airborne diseases and maintain postharvest quality of wine grapes. Plants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Calzarano, F.; Seghetti, L.; Pagnani, G.; Di Marco, S. Italian zeolitites in the control of grey mould and sour rot and their effect on leaf reflectance, grape and wine. Agriculture 2020, 10, 580. [Google Scholar] [CrossRef]
- McLaughlin, R.J.; Wilson, C.L.; Droby, S.; Ben-Arie, R.; Chalutz, E. Biological Control of Postharvest Diseases of Grape, Peach, and Apple with the Yeasts Kloeckera apiculata and Candida guilliermondii. Plant Dis. 1992, 76, 470–473. [Google Scholar]
- Fiori, S.; Urgeghe, P.P.; Hammami, W.; Razzu, S.; Jaoua, S.; Migheli, Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [Google Scholar]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar]
- González-Domínguez, E.; Fedele, G.; Caffi, T.; Delière, L.; Sauris, P.; Gramaje, D.; Ramos-Saez de Ojer, J.L.; Díaz-Losada, E.; Díez-Navajas, A.M.; Bengoa, P.; et al. A network meta-analysis provides new insight into fungicide scheduling for the control of Botrytis cinerea in vineyards. Pest Manag. Sci. 2019, 75, 324–332. [Google Scholar]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. A quantitative review of tebuconazole effect on Fusarium head blight and deoxynivalenol content in wheat. Phytopathology 2007, 97, 211–220. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar]
- CoreTeam, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar]
- Jackson, D.; White, I.R.; Riley, R.D. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat. Med. 2012, 31, 3805–3820. [Google Scholar]
- Ngugi, H.K.; Esker, P.D.; Scherm, H. Meta-analysis to determine the effects of plant disease management measures: Review and case studies on soybean and apple. Phytopathology 2011, 101, 31–41. [Google Scholar]
- Zouhair, S.; Qjidaa, S.Q.; Selouane, A.; Bouya, D.; Decock, C.; Bouseta, A. Effect of five fungicides on growth and ochratoxin A production by two Aspergillus carbonarius and Aspergillus niger isolated from Moroccan grapes. SAJEB 2014, 4, 118–126. [Google Scholar]
- Laaziz, A.; Qjidaa, S.; El Hammoudi, Y.E.; Hajjaji, A.; Bouseta, A. Chemical control of fungal growth and ochratoxin A production by Aspergillus isolated from Moroccan grapes. S. Asian J. Exp. Biol. 2017, 7, 84–91. [Google Scholar]
- Gao, F.; Chen, J.; Xiao, J.; Cheng, W.; Zheng, X.; Wang, B.; Shi, X. Microbial community composition on grape surface controlled by geographical factors of different wine regions in Xinjiang, China. Food Res. Int. 2019, 122, 348–360. [Google Scholar]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Anfora, G. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar]
- Rossi, V.; Sperandio, G.; Caffi, T.; Simonetto, A.; Gilioli, G. Critical success factors for the adoption of decision tools in IPM. Agronomy 2019, 9, 710. [Google Scholar] [CrossRef]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanisms of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar]
- Zoecklein, B.W.; Wolf, T.K.; Duncan, N.W.; Judge, J.M.; Cook, M.K. Effects of fruit zone leaf removal on yield, fruit composition, and fruit rot incidence of Chardonnay and White Riesling (Vitis vinifera L.) grapes. Am. J. Enol. Vitic. 1992, 43, 139–148. [Google Scholar]
- McFadden-Smith, W.; Gubler, W.D. Sour Rot. In Compendium of Grape Diseases, Disorders, and Pests, 2nd ed; Wilcox, W.F., Gubler, W.D., Uyemoto, J.K., Eds.; APS Press: St. Paul, MN, USA, 2015; pp. 87–90. [Google Scholar]
- Wurms, K.; Chee, A.; Elmer, P.; Agnew, R.; Wood, P.; Chee, A.A. Developing new biologically based products for control of botrytis bunch rot. Part 1: Developing a new natural product for mid-season botrytis control–NP2 moves closer to the market. Wine Vitic. J. 2011, 26, 64–72. [Google Scholar]
- Jackson, A.M.; Whipps, J.M.; Lynch, J.M. Effects of temperature, pH and water potential on growth of four fungi with disease biocontrol potential. World J. Microbiol. Biotechnol. 1991, 7, 494–501. [Google Scholar]
- Fedele, G.; González-Domínguez, E.; Rossi, V. Influence of environment on the biocontrol of Botrytis cinerea: A systematic literature review. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; De Cal, A., Melgarejo, P., Magan, N., Eds.; Springer Nature: Switzerland, 2020; pp. 61–82. [Google Scholar]
- Calvo-Garrido, C.; Viñas, I.; Usall, J.; Rodríguez-Romera, M.; Ramos, M.C.; Teixidó, N. Survival of the biological control agent Candida sake CPA-1 on grapes under the influence of abiotic factors. J. Appl. Microbiol. 2014, 117, 800–811. [Google Scholar]
- Altieri, V.; Battilani, P.; Camardo Leggieri, M.; Fedele, G.; Ji, T.; Rossi, V.; Salotti, I. Current situation and prospective for effective biocontrol of main grape diseases. In Advances in Bioprotection of Plants Against Diseases; Sharma, S., Minshad, A., Eds.; BDS Publishing: Cambridge, UK, 2023; in press. [Google Scholar]
- Bish, D.; Ming, D. Natural Zeolites: Occurrence, Prop- Erties, Applications, 45; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2018; p. 69. [Google Scholar]
- Ferreira, L.; Fonseca, A.M.; Botelho, G.; Aguiar, C.A.; Neves, I.C. Antimicrobial activity of faujasite zeolites doped with silver. Micropor. Mesopor. Mater. 2012, 160, 126–132. [Google Scholar]
- Septommy, C.; Sa’adah, N.; Mu’arofah, B. The effect of natural zeolite (Ag-zeolite) modified with silver against the inhibition of Candida albicans. JDS Dent. Soc. 2020, 5, 56–60. [Google Scholar]
- Alswat, A.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Saleh, T.A. Copper oxide nanoparticles-loaded zeolite and its characteristics and antibacterial activities. J. Mater. Sci. Technol. 2017, 33, 889–896. [Google Scholar]
- Savi, G.D.; Cardoso, W.A.; Furtado, B.G.; Bortolotto, T.; Da Agostin, L.O.V.; Nones, J.; Zanoni, E.T.; Montedo, O.R.K.; Angioletto, E. New ion-exchanged zeolite derivatives: Antifungal and antimycotoxin properties against Aspergillus flavus and aflatoxin b1. Mater. Res. Express 2017, 4, 085401. [Google Scholar]
- Nikolov, A.; Dobreva, L.; Danova, S.; Miteva-Staleva, J.; Krumova, E.; Rashev, V.; Vilhelmova-Ilieva, N. Natural and modified zeolite clinoptilolite with antimicrobial properties: A review. Acta Microbiol. Bulgarica 2023, 39, 147–161. [Google Scholar]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimi- crobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar]
- Panayotova, M.; Mintcheva, N.; Gemishev, O.; Tyuliev, G.; Gicheva, G.; Djerahov, L. Preparation and antimicrobial properties of silver nanoparticles supported by natural zeolite clinoptilolite. Bulg. Chem. Commun. 2018, 50, 211–218. [Google Scholar]
- Nikolov, A.; Doneva, L.; Danova, S.; Miteva-Staleva, J.; Kru-Mova, E.; Rashev, V.; Vilhelmova-Ilieva, N. Modified natural zeolite clinoptilolite with antibacterial, antifungal and antiviral properties. In Proceedings of the EUROCLAY 2023. International Conference of European Clay Groups Association, Bari, Italy, 24–27 July 2023. [Google Scholar]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar]
- Polat, İ.; Ünlü, A.; Keçeci, M.; Özdemir, M.; Öztop, A.; Çalışkan, S. Efficiency of zeolite as alternative product for controlling downy mildew (Plasmopara viticola) in table grape. J. Turk. Phytopathol. 2018, 47, 93–103. [Google Scholar]
- Calzarano, F.; Seghetti, L.; Pagnani, G.; Metruccio, E.G.; Di Marco, S. Control of grapevine downy mildew by an Italian copper chabasite-rich Zeolitite. Agronomy 2022, 12, 1528. [Google Scholar] [CrossRef]
- La Torre, A.; Righi, L.; Iovino, V.; Battaglia, V. Evaluation of copper alternative products to control grape downy mildew in organic farming. J. Plant Pathol. 2019, 101, 1005–1012. [Google Scholar]
- Bortolotti, P.P.; Nannini, R. Trials Against Botrytis cinerea Through the Use of Different Active Substances, with Particular Attention to Natural Products. In Atti, Giornate Fitopatologiche, Chianciano Terme (Siena), 8–11 Marzo 2016, Volume Secondo; Alma Mater Studiorum, Universitá di Bologna: Bologna, Italy, 2016; pp. 489–495. [Google Scholar]
- Porter, L.L. Bicarbonate Inhibition of Select Phytopathogenic Fungi: Mechanistic Studies and Disease Control Implications; Cornell University: Ithaca, NY, USA, 1993. [Google Scholar]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar]
- Smilanick, J.L.; Mansour, M.F.; Margosan, D.A.; Gabler, F.M.; Goodwine, W.R. Influence of pH and NaHCO3 on effectiveness of imazalil to inhibit germination of Penicillium digitatum and to control postharvest green mold on citrus fruit. Plant Dis. 2005, 89, 640–648. [Google Scholar]
- Venditti, T.; Molinu, M.G.; Dore, A.; Agabbio, M.; D’hallewin, G. Sodium carbonate treatment induces scoparone accumulation, structural changes, and alkalinization in the albedo of wounded citrus fruits. J. Agric. Food Chem. 2005, 53, 3510–3518. [Google Scholar]
- Türkkan, M.; Özcan, M.; Erper, İ. Antifungal effect of carbonate and bicarbonate salts against Botrytis cinerea, the casual agent of grey mould of kiwifruit. Akad. Ziraat Derg. 2017, 6, 107–114. [Google Scholar]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium carbonate and bicarbonate treatments induce resistance to postharvest green mould on citrus fruit. Postharvest Biol. Technol. 2014, 87, 61–69. [Google Scholar]
- Qin, X.; Xiao, H.; Xue, C.; Yu, Z.; Yang, R.; Cai, Z.; Si, L. Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol. Technol. 2015, 100, 160–167. [Google Scholar]
- Corral, L.G.; Post, L.S.; Montville, T.J. Antimicrobial activity of sodium bicarbonate: A research note. J. Food Sci. 1988, 53, 981–982. [Google Scholar]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [PubMed]
- Varela, C.; Borneman, A.R. Yeast found in vineyards and wineries. Yeast 2017, 34, 111–128. [Google Scholar]
- Chavan, P.; Mane, S.; Kulkarni, G.; Shaikh, S.; Ghormade, V.; Nerkar, D.P.; Shouche, Y.; Deshpande, M.V. Natural yeast flora of different varieties of grapes used for wine making in India. Food Microbiol. 2009, 26, 801–808. [Google Scholar]
- Elad, Y.; Köhl, J.; Fokkema, N.J. Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic yeasts. Phytopathology 1994, 84, 1193–1200. [Google Scholar]
- Köhl, J.; Molhoek, W.M.L.; Van der Plas, C.H.; Fokkema, N.J. Effect of Ulocladium atrum and other antagonists on sporulation of Botrytis cinerea on dead lily leaves exposed to field conditions. Phytopathology 1995, 85, 393–400. [Google Scholar]
- Fokkema, N.J. The role of saprophytic fungi in antagonism against Drechslera sorokiniana (Helminthosporium sativum) on agar plates and on rye leaves with pollen. Physiol. Plant Pathol. 1973, 3, 195–205. [Google Scholar]
- Lima, G.; Ippolito, A.; Nigro, F.; Salerno, M. Effectiveness of Aureobasidium pullulans and Candida oleophila against postharvest strawberry rots. Postharvest Biol. Technol. 1997, 10, 169–178. [Google Scholar]
- Raspor, P.; Miklic-Milek, D.; Avbelj, M.; Cadez, N. Biocontrol of grey mould disease on grape caused by Botrytis cinerea with autochthonous yeasts. Food Technol. Biotechnol. 2010, 48, 336–343. [Google Scholar]
- Castoria, R.; De Curtis, F.; Lima, G.; Caputo, L.; Pacifico, S.; De Cicco, V. Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: Study on its modes of action. Postharvest Biol. Technol. 2001, 22, 7–17. [Google Scholar]
- Filonow, A.B.; Vishniac, H.S.; Anderson, J.A.; Janisiewicz, W.J. Biological control of Botrytis cinerea in apple by yeasts from various habitats and their putative mechanisms of antagonism. Biol. Control 1996, 7, 212–220. [Google Scholar]
- Ippolito, A.; El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 2000, 19, 265–272. [Google Scholar]
- Kalogiannis, S.; Tjamos, S.E.; Stergiou, A.; Antoniou, P.P.; Ziogas, B.N.; Tjamos, E.C. Selection and evaluation of phyllosphere yeasts as biocontrol agents against grey mould of tomato. Eur. J. Plant Pathol. 2006, 116, 69–76. [Google Scholar]
- Di Canito, A.; Mateo-Vargas, M.A.; Mazzieri, M.; Cantoral, J.; Foschino, R.; Cordero-Bueso, G.; Vigentini, I. The role of yeasts as biocontrol agents for pathogenic fungi on postharvest grapes: A review. Foods 2021, 10, 1650. [Google Scholar] [CrossRef]
- Bozoudi, D.; Tsaltas, D. The multiple and versatile roles of Aureobasidium pullulans in the vitivinicultural sector. Fermentation 2018, 4, 85. [Google Scholar] [CrossRef]
- Rathnayake, R.M.S.P.; Savocchia, S.; Schmidtke, L.M.; Steel, C.C. Characterisation of Aureobasidium pullulans isolates from Vitis vinifera and potential biocontrol activity for the management of bitter rot of grapes. Eur. J. Plant Pathol. 2018, 151, 593–611. [Google Scholar]
- Lima, G.; Ippolito, A.; Nigro, F.; Romanazzi, G.; Schena, L.; Gatto, M.A.; Salerno, M. Lotta biologica contro marciumi postraccolta di uva da tavola, fragola e actinidia con Aureobasidium pullulans e Candida oleophila. Inf. Agrar. 1996, 45, 79–84. [Google Scholar]
- Benuzzi, M.; Ladurner, E.; Fiorentini, F. Efficacy of Serenade, New Bacillus subtilis-Based Biofungicide, in Controlling the Pathogenic Microorganisms of Crops. In Giornate Fitopatologiche 2006, Riccione (RN), Atti, Volume Secondo; Università di Bologna: Bologna, Italy, 2006; pp. 429–436. [Google Scholar]
- Bugiani, R.; Cavazza, F.; Franceschelli, F.; Landi, M.; Preti, M. Contenimento Del Marciume Acido Del Grappolo in Pre-Vendemmia con Diversi Prodotti Naturali. In Giornate Fitopatologiche 2020, Bologna (BO), Atti, Volume Secondo; Università di Bologna: Bologna, Italy, 2020; pp. 419–428. [Google Scholar]
- Mari, M.; Guizzardi, M.; Brunelli, M.; Folchi, A. Postharvest biological control of grey mould (Botrytis cinerea Pers.: Fr.) on fresh-market tomatoes with Bacillus amyloliquefaciens. Crop Prot. 1996, 15, 699–705. [Google Scholar]
- Leifert, C.; Li, H.; Chidburee, S.; Hampson, S.; Workman, S.; Sigee, D.; Epton, H.A.; Harbour, A. Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J. Appl. Bacteriol. 1995, 78, 97–108. [Google Scholar]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Emmert, E.A.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar]
- Altieri, V.; Rossi, V.; Fedele, G. Integration of mathematical modeling and target-based application of biocontrol agents for the control of Botrytis cinerea in vineyards. Pest Manag. Sci. 2024, 80, 4352–4360. [Google Scholar] [PubMed]
- Altieri, V.; Rossi, V.; Fedele, G. Efficacy of preharvest application of biocontrol agents against gray mold in grapevine. Front. Plant Sci. 2023, 14, 1154370. [Google Scholar]
- Klick, J.; Yang, W.Q.; Lee, J.C.; Bruck, D.J. Reduced spray programs for Drosophila suzukii management in berry crops. Int. J. Pest Manag. 2016, 62, 368–377. [Google Scholar]
- Haye, T.; Girod, P.; Cuthbertson, A.G.S.; Wang, X.G.; Daane, K.M.; Hoelmer, K.A.; Baroffio, C.; Zhang, J.P.; Desneux, N. Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J. Pest Sci. 2016, 89, 643–651. [Google Scholar]
- Hutchison, W.D.; Wold-Burkness, S. Spotted wing Drosophila. FruitEdge, 2018; University of Minnesota: Extension, St. Paul, MN, USA. Available online: https://www.fruitedge.umn.edu/swdpestprofile (accessed on 6 August 2024).
- Walton, V.M.; Burrack, H.J.; Dalton, D.T.; Isaacs, R.; Wiman, N.; Ioriatti, C. Past, present and future of Drosophila suzukii: Distribution, impact and management in United States berry fruits. Acta Hortic. 2016, 1117, 87–94. [Google Scholar]
- Wiman, N.G.; Dalton, D.T.; Anfora, G.; Biondi, A.; Chiu, J.C.; Daane, K.M.; Gerdeman, B.; Gottardello, A.; Hamby, K.A.; Isaacs, R.; et al. Drosophila suzukii population response to environment and management strategies. J. Pest Sci. 2016, 89, 653–665. [Google Scholar]
- Leach, H.; Moses, J.; Hanson, E.; Fanning, P.; Isaacs, R. Rapid harvest schedules and fruit removal as non-chemical approaches for managing spotted wing Drosophila. J. Pest Sci. 2018, 91, 219–226. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).