The Influence of the Hybrid Compound Nd(NO3)3@Zn-MOF on the Growth of Vanilla (Vanilla planifolia Jacks. ex Andrews) Cultured In Vitro: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zn-MOF Synthesis
2.2. Nd(NO3)3@Zn-MOF Synthesis and Mechanochemical Doping
2.3. X-ray Powder Diffraction (XRD)
2.4. In Vitro Establishment and Multiplication
2.5. Effect of Nd(NO3)3@Zn-MOF on the In Vitro Growth of V. planifolia
2.6. Determination of Chlorophyll and Carotenoid Content
2.7. Determination of Phenolic Compounds and Antioxidant Capacity
2.8. Experimental Design and Statistical Analysis
3. Results
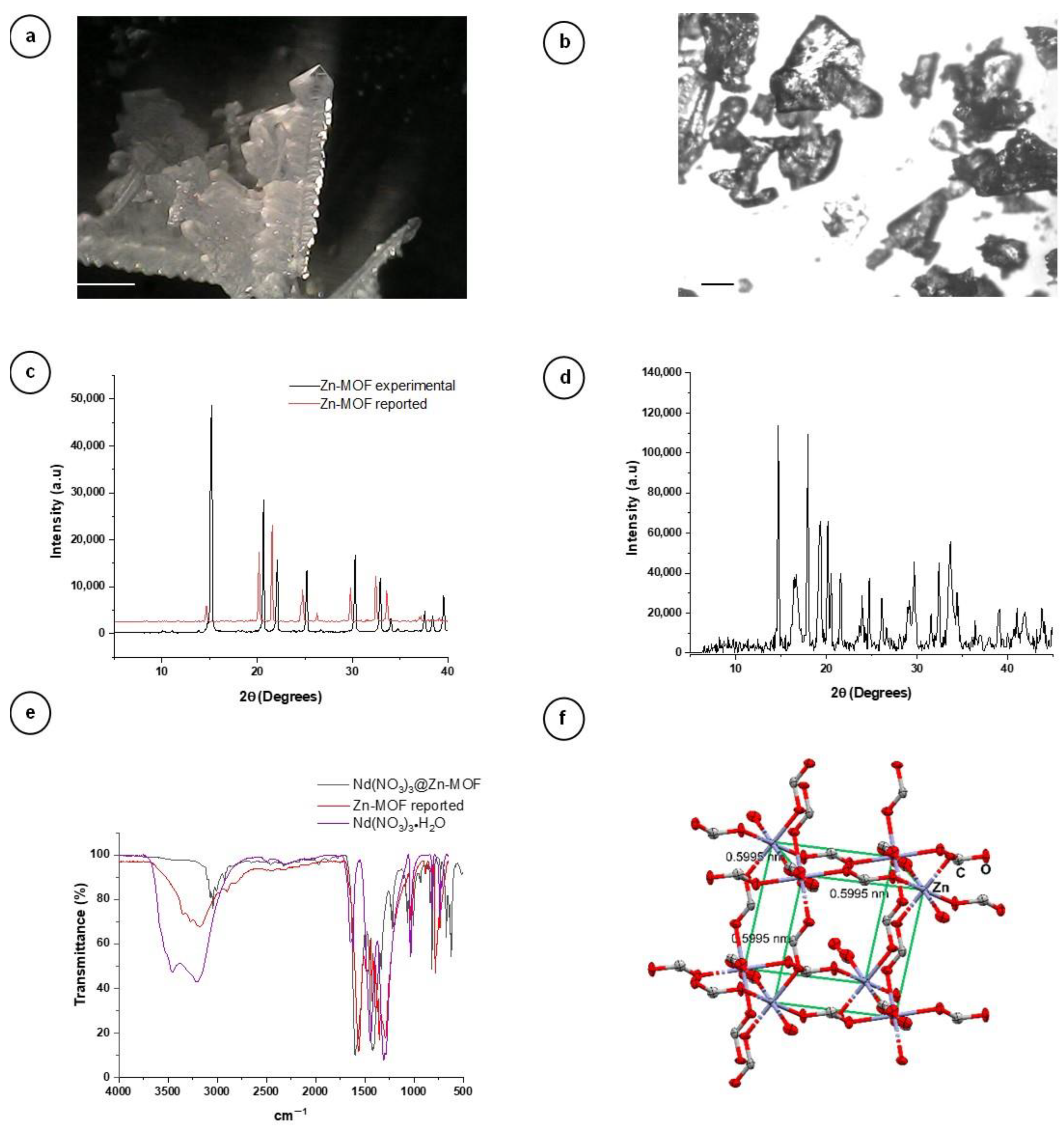
3.1. Characterization of the Nd(NO3)3@Zn-MOF by X-ray Powder Diffraction (XRD)
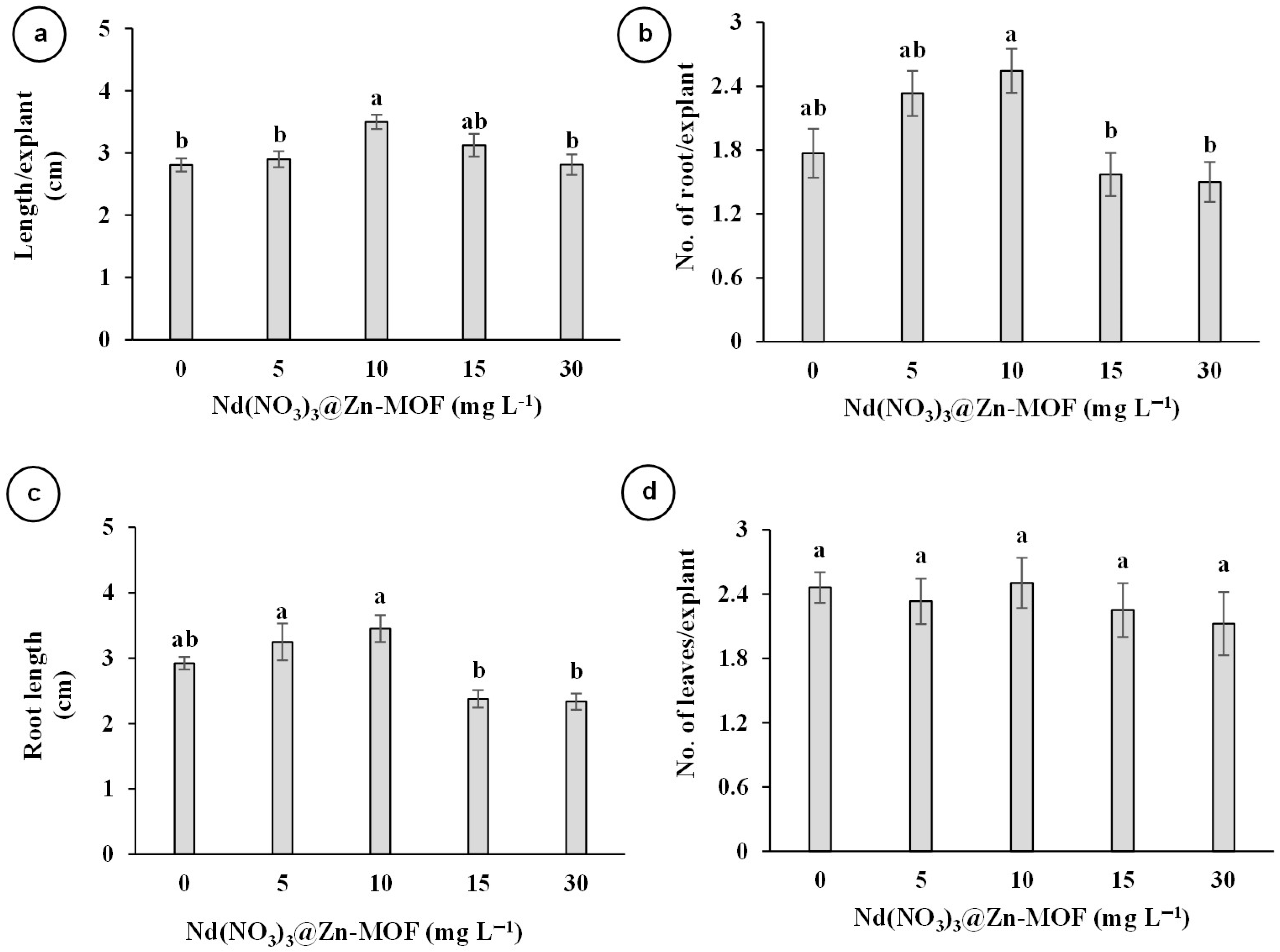
3.2. Effect of Nd(NO3)3@Zn-MOF on the In Vitro Growth of V. planifolia
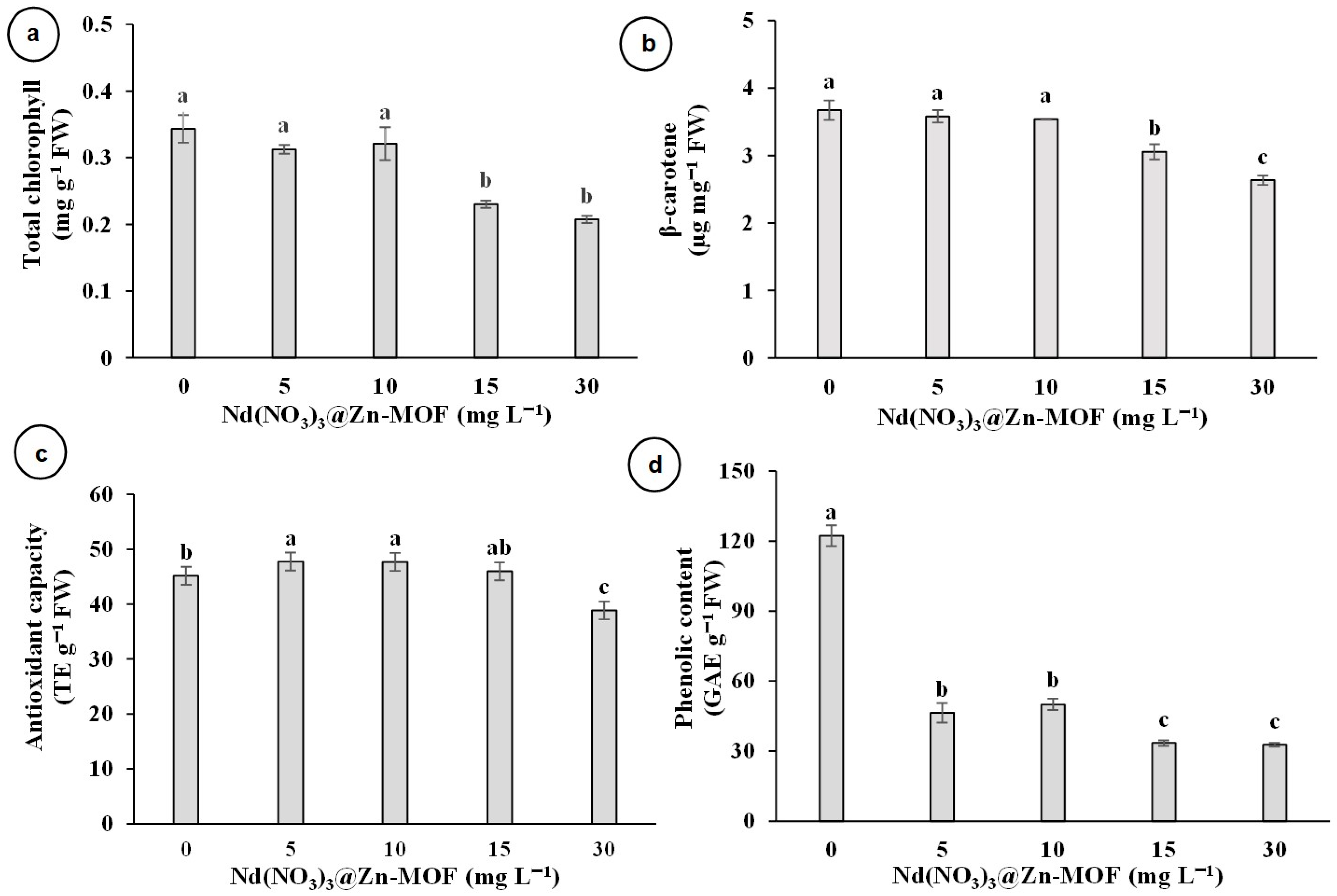
3.3. Effect of Nd(NO3)3@Zn-MOF on Biochemical Parameters of In Vitro Plantlets of V. planifolia
4. Discussion
4.1. Evaluating the Effect of Nd(NO3)3@Zn-MOF on the In Vitro Growth of V. planifolia
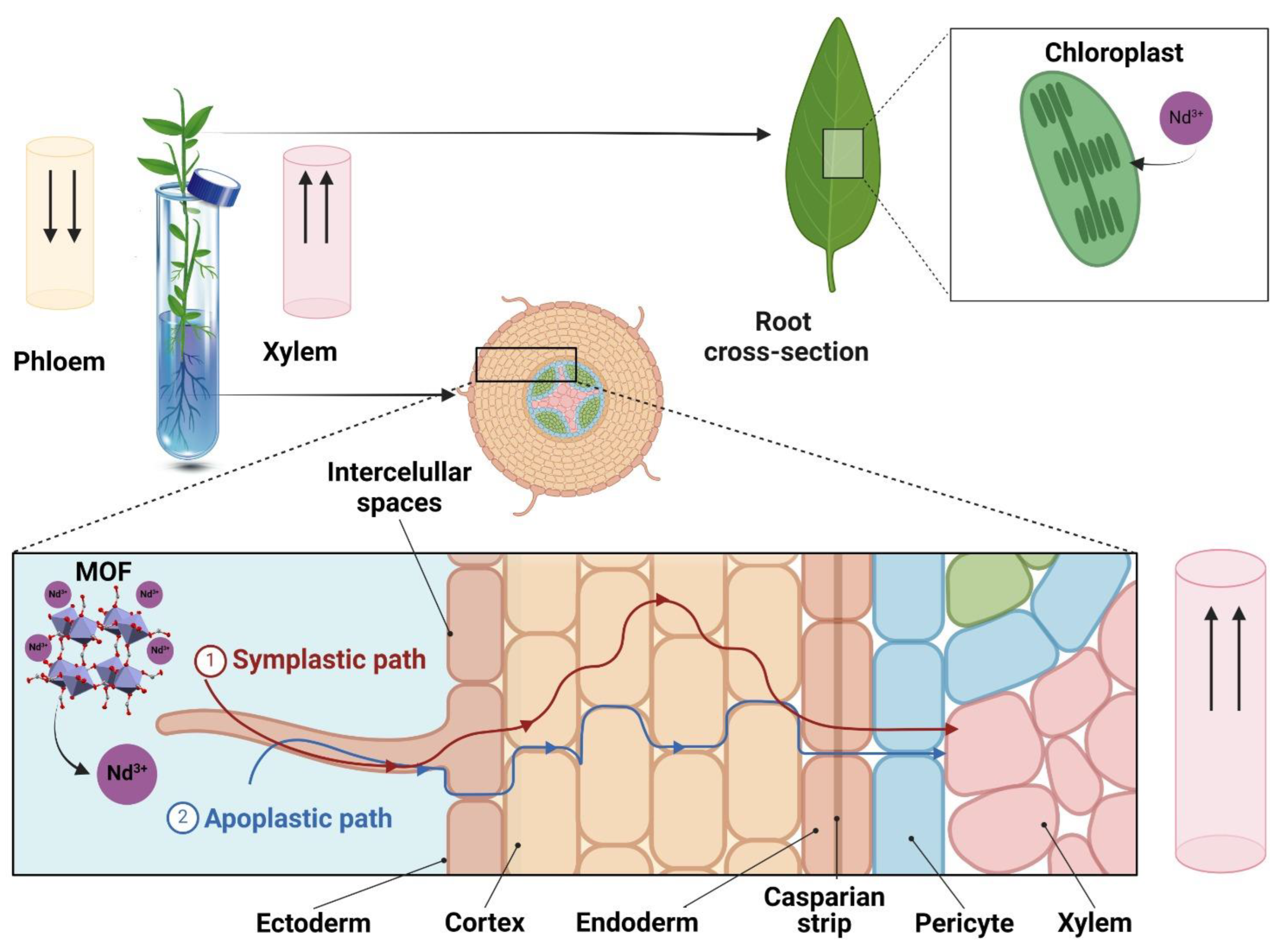
4.2. Effect of Nd(NO3)3@Zn-MOF on Biochemical Parameters of In Vitro Plantlets of V. planifolia
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Swetha, S.; Janani, B.; Khan, S.S. A critical review on the development of metal-organic frameworks for boosting photocatalysis in the fields of energy and environment. J. Clean. Prod. 2022, 333, 130164. [Google Scholar] [CrossRef]
- Sikder, A.; Pearce, A.K.; Parkinson, S.J.; Napier, R.; Reilly, R.K. Recent trends in advanced polymer materials in agriculture related applications. ACS Appl. Polym. Mater. 2021, 3, 1203–1217. [Google Scholar] [CrossRef]
- Chauchan, D.; Rishabh, A.O.; Mangalaraja, R.V.; Ashfaq, M.; Talreja, N. Metal-organic framework as an emerging material: A novel plant growth stimulant. In Nanotecnology-Based Sustainable Alternatives for the Management of Plant Diseases; Balestra, G.M., Fortunati, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 323–339. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, M.; Wang, Y.; Pan, S.; Sun, X.; Zhao, R.; Sun, Z.; Gao, R.; Guo, Z.; Xu, Y.; et al. A pH/cellulase dual stimuli-responsive cellulose-coated metal-organic framework for eco-friendly fungicide delivery. Chem. Eng. J. 2023, 462, 142190. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Bauchan, G.; Mowery, J.; Zavalij, P. Development of metal-organic framework for gaseous plant hormone encapsulation to manage ripening of climacteric produce. J. Agric. Food Chem. 2016, 64, 5164–5170. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Trickett, C.A.; Alahmadi, S.B.; Alshammari, A.S.; Yaghi, O.M. Calcium L-Lactate frameworks as naturally degradable carriers for pesticides. J. Am. Chem. Soc. 2017, 139, 8118–8121. [Google Scholar] [CrossRef]
- Addelhameed, R.M.; Abdelhameed, R.E.; Kamel, H.A. Iron-based metal-organic-frameworks as fertilizers for hydroponically grown Phaseolus vulgaris. Mater Lett. 2019, 237, 72–79. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Liu, X.; Yi, X.; Zhou, Z.; Liu, D. Multifunctional β-cyclodextrin MOF-derived porous carbon as efficient herbicides adsorbent and potassium fertilizer. ACS Sustain. Chem. Eng. 2019, 7, 14479–14489. [Google Scholar] [CrossRef]
- Wu, K.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Optimization of metal-organic (citric acid) frameworks for controlled release of nutrients. RSC Adv. 2019, 9, 32270e32277. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.A.M.; Telford, R.; Livesey, T.C.; Katsikogianni, M.; Kelly, A.L.; Terry, L.R.; Ting, V.P.; Nayak, S. Zirconium-based MOFs and their biodegradable polymer composites for controlled and sustainable delivery of herbicides. ACS Appl. Bio Mater. 2022, 5, 3972–3981. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Cao, L.; Wang, C.J.; Zheng, L.; Li, Y.Y.; Cao, C.; Huang, Q. Metabolic pathways reveal the effect of fungicide loaded metal-organic frameworks on the growth of wheat seedlings. Chemosphere 2022, 307, 135702. [Google Scholar] [CrossRef]
- Mondal, T.; Mondal, S.; Bose, S.; Sengupta, D.; Ghori, K.U.; Saha, S.K. Pure White light emission from a rare earth-free intrinsic metal-organic framework and its applications in a WLED. J. Mater. Chem. C 2018, 9, 15891–15899. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siquiera, J.O.; Guilherme, L.R.G. Rare earth elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira-Junior, J.C.; Ribeiro, J.S.; Fernández, G.C.; Guerra-Mariano, G.; Rezende-Trindade, V.D.; Rodríguez-Dos Reis, A. Hormesis in plant: Physiological and biochemical responses. Ecotoxiol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, C.; Tai, P.; Sun, L.; Chen, Z. The exposure of gadolinium at environmental relevant levels induced genotoxic effects in Arabidopsis thaliana L. Ecotoxicol. Environ. Saf. 2021, 215, 112138. [Google Scholar] [CrossRef]
- Agathokleous, E.; Zhou, B.; Geng, C.; Xu, J.; Santains, C.J.; Feng, Z.; Tack, F.M.G.; Ringklebe, J. Mechanisms of cerium induced stress in plants: A meta-analysis. Sci. Total Environ. 2022, 852, 158352. [Google Scholar] [CrossRef]
- Jessat, J.; John, W.A.; Moll, H.; Vogel, M.; Steudter, R.; Drobot, B.; Hubner, R.; Stumpf, T.; Sachs, S. Localization and chemical speciation of Eu(III) in Brassica napus plants. Ecotoxicol. Environ. Saf. 2023, 254, 114741. [Google Scholar] [CrossRef]
- Guo, T.; He, D.; Liu, Y.; Li, J.; Wang, F. Lanthanum promotes Solanum nigrum L. growth and phytoremediation of cadmium and lead through endocytosis: Physiological and biochemical response heavy metal uptake and visualization. Sci. Total Environ. 2024, 912, 168915. [Google Scholar] [CrossRef]
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of rare earth elements (REEs) and their roles in plant growth: A review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Merino, F.; Gómez-Trejo, L.F.; Ruvalcaba-Ramírez, R.; Trejo-Téllez, L. Lanthanides as beneficial elements for plants. In Beneficial Chemical Elements of Plants; Pandey, S., Tripathi, D.K., Sinch, V.P., Sharma, S., Chauhan, D.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 349–369. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Wang, Y. Changues in endogenous hormone levels and redox status during enhanced adventitious rooting by rare earth elements neodymium of Dendrobium desinflorum. J. Rare Earth 2008, 26, 869. [Google Scholar] [CrossRef]
- Rezaee, A.; Hale, B.; Santos, R.M.; Chiang, Y.W. Accumulation and toxicity of lanthanum and neodymium in horticultural (Brassica chinesis L. and Helianthus annus L). Can. J. Chem. Eng. 2018, 96, 2263–2272. [Google Scholar] [CrossRef]
- Shi, K.; Liu, C.; Liu, D.; Lyu, K.M.; Chen, J.; Wang, X. The accumulation and effect of rare earth element neodymium on the root of rice seedlings. Environ. Sci. Pollut. Res. 2021, 28, 48656–48665. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Calinoiu, L.F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- Manokari, M.; Priyadharshini, S.; Cokulraj, M.; Dey, A.; Faisal, M.; Alatar, A.A.; Alok, A.; Shekhawat, M.S. Exogenous implications of silver nitrate on direct and indirect somatic embryogenesis and germination of cold stored synseeds of Vanilla planifolia Jacks. ex Andrews. S. Afr. J. Bot. 2022, 150, 129–138. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Metabolite transformation and β-D- glucosidase activity during the high hydrostatic pressure assisted curing of vainilla beans (Vanilla planifolia) to improve phenolic compounds formation. Food Chem. 2022, 384, 132497. [Google Scholar] [CrossRef] [PubMed]
- Peña-Barrientos, A.; Perea-Flores, M.J.; Martínez-Gutiérrez, H.; Patrón-Soberano, O.A.; González-Jiménez, F.E.; Vega-Cuellar, M.A.; Dávila-Ortiz, G. Physicochemical, microbiological, and structural relationship of vanilla beans (Vanilla planifolia, Andrews) during traditional curing process and use of its waste. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100445. [Google Scholar] [CrossRef]
- Serrano-Fuentes, M.K.; Gómez-Merino, F.C.; Cruz-Izquierdo, S.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Gamma radiation (Co60) induces mutation during in vitro multiplication of vainilla (Vanilla planifolia Jacks. ex Andrews). Horticulturae 2022, 8, 503. [Google Scholar] [CrossRef]
- Watteyn, C.; Rubens, B.; Azofeifa-Bolaños, J.B.; Solano, F.; Pérez-Silva, A.; Karremans, A.P.; Muys, B. Cultivation potential of vanilla crop wild relatives in two contrasting land use systems. Eur. J. Agron. 2023, 149, 126890. [Google Scholar] [CrossRef]
- Rodríguez-Deméneghi, M.; Aguilar-Rivera, N.; Gheno-Heredia, Y.A.; Armas-Silva, A. Cultivo de vainilla en México: Tipología, características, producción, prospectiva, agroindustrial e innovaciones biotecnológicas como estrategia de sustentabilidad. Sci. Agropecu. 2023, 14, 93–109. [Google Scholar] [CrossRef]
- Kha-Lok, T.; Wan-Abdullah, W.; Ong-Abdullah, J.; Lamasudin, D.U.; Wee, C.Y.; Mohd-Yusoff, M.H.Y.; Loh, J.Y.; Chen, W.H.; Lai, K.S. Calcium lignosulfonate modulates physiological and biochemical responses to enhance shoot multiplication in Vanilla planifolia Andrews. Physiol. Mol. Biol. Plants 2023, 29, 377–392. [Google Scholar] [CrossRef]
- Sorcia-Morales, M.; Gómez-Merino, F.; Sánchez-Segura, L.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Multi-walled carbon nanotubes improved development during in vitro multiplication of sugarcane (Saccharum spp.) in a semi-automated bioreactor. Plants 2021, 10, 2015. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Spinoso-Castillo, J.L. Utilización de nanopartículas de plata en la micropropagación de plantas. Mundo Nano. Rev. Interdiscip. Nanocienc. Nanotecnol. 2023, 16, e00063. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Harbone, J.B. Phenolic compounds. In Phytochemical Methods: A Guide, to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1973; pp. 33–88. [Google Scholar]
- Biehler, E.; Mayer, F.; Hoffman, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C56. [Google Scholar] [CrossRef] [PubMed]
- Payet, B.; Cheong-Sing, A.S.; Smadja, J. Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J. Agric. Food Chem. 2006, 54, 7270–7276. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Peña-Rodríguez, R.; Rivera, J.M.; Armenta-Jaime, E.; Haggeo-Desirena, E.; Molina-González, J.A. Doping of a Zn-MOF with Eu3+ and Tb3+ for application in the manufacture of a WLED. J. Mater. Chem. C 2021, 9, 15091–15899. [Google Scholar] [CrossRef]
- Zhou, X.; Miao, Y.; Suslick, K.S.; Dlott, D.D. Mechanochemistry of metal-organic framework under pressure and shock. Acc. Chem. Res. 2020, 53, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Glowniak, S.; Szczesniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal-organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Gratz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Sun, D. Stimuli responsive structural changes in metal-organic frameworks. Chem. Commun. 2020, 56, 9416–9432. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Javanbakht, S.; Baradaran, B.; Shahbazi, M.A.; Hashemzaei, M.; Mokhtarzadeh, A.; Santos, H.A. Synthesis and therapeutic potential of stimuli responsive metal-organic frameworks. Chem. Eng. J. 2021, 408, 127233. [Google Scholar] [CrossRef]
- Wang, W.; Yang, F.; Yang, Y.; Wang, Y.Y.; Liu, B. Rational synthesis of a stable rod MOF for ultrasensitive detection of nitenpyram and nitrofurazone in natural water systems. J. Agric. Food Chem. 2022, 70, 15682–15692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, X.; Zhao, C.; Yuan, Z.; Zhang, D.; Zhao, H.; Yang, N.; Guo, K.; He, Y.; Hu, J.; et al. A pH responsive MOF for site-specific delivery of fungicide to control citrus disease of Botrytis cinerea. Chem. Eng. J. 2022, 431, 133351. [Google Scholar] [CrossRef]
- Oroojalian, F.; Karimzadeh, S.; Javanbakht, S.; Hejazi, M.; Baradaran, B.; Webster, T.J.; Mokhtarzadeh, A.; Varma, R.S.; Kesharwani, P.; Sahebkar, A. Current trends in stimuli-responsive nanotheranostics based on metal-organic frameworks for cancer therapy. Mater. Today 2022, 57, 192–224. [Google Scholar] [CrossRef]
- Guan, Q.; Fang, Y.; Wu, X.; Ou, R.; Zhang, X.; Xie, H.; Tang, M.; Zeng, G. Stimuli responsive metal-organic frameworks materials towards advanced smart application. Mater. Today 2023, 64, 138–164. [Google Scholar] [CrossRef]
- Bolaji-Umar, O.; Amudalat-Ranti, L.; Shehu-Abdulbaki, A.; Lukman-Bola, A.; Khadijat-Abdulhamid, A.; Ramat-Biola, M.; Oluwagbenga, V.K. Stresses in Plants: Biotic and Abiotic; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Crop physiology and biotechnology. In Plant Physiology Development and Metabolism; Springer: Singapore, 2023; pp. 809–830. [Google Scholar] [CrossRef]
- Grosjean, N.; Purwadi, I.; Sirguey, C.; Chalot, M.; Jean, M.L.; Van der Ent, A.; Blaudez, D. Rare earth elements in plants: Transfer, transport, accumulation, impacts and perspectives. Adv. Bot. Res. 2024, 109, 19–61. [Google Scholar] [CrossRef]
- Shahin, L.; Zhang, L.; Mohnen, D.; Urbanowicz, B.R. Insights into pectin O-acetylation in the plant cell wall: Structure, synthesis, and modification. Cell Surf. 2023, 9, 100099. [Google Scholar] [CrossRef] [PubMed]
- Wiche, O.; Pourret, O. The role of root carboxylate release on rare earth element (hyper) accumulation in plants a biogeochemical perspective on rhizosphere chemistry. Plant Soil 2023, 492, 79–90. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Kovarikova, M.; Tomaskova, I.; Soudek, P. Rare earth elements plants. Biol. Plant. 2019, 63, 20–32. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Accumulator plants and hormesis. Environ. Pollut. 2021, 274, 116526. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquia-Jiménez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.T.; Guevara-González, R.G. Plant hormesis: Revising of the concepts of biostimulation, elicitation and their application in a sustainable agricultural production. Sci. Total Environ. 2023, 864, 164883. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Téllez, L.I.; Gómez-Trejo, L.F.; Gómez-Merino, F.C. Beneficial elements: Novel players in plant biology for innovate crop production. Front. Plant Sci. 2023, 14, 1303462. [Google Scholar] [CrossRef]
- Ramírez-Antonio, V.J.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Hidalgo-Contreras, J.V. Neodymium stimulates growth, nutrient concentration, and metabolism in sugarcane in hydroponics. Sugar Tech 2023, 25, 1385–1395. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M.; Ibrahim, A.A.; Alsadon, A. Potential roles of melatonin and sulfur in alleviation of lanthanum toxicity in tomato seedlings. Ecotoxicol. Environ. Saf. 2019, 180, 656–667. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Balakrishna, A.; Liew, K.X.; Kuijer, H.N.J.; Xiao, T.T.; Blilou, I.; Al-Babili, S. Installing the neourospora carotenoid pathway in the plants enables cytosolic formation of provitamin A and its sequestration in lipid droplets. Mol. Plant 2023, 16, 1066–1081. [Google Scholar] [CrossRef]
- Dridi, N.; Brito, P.; Bouslimi, H.; Ferreira, R.; Martins-Dias, S.; Cacador, I.; Sleimi, N. Physiological and biochemical behaviours, and antioxidant response of Helianthus annus under lanthanum and cerium stress. Sustainability 2022, 14, 4153. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, X.D.; Zhao, B.; Wang, Y.C. Reduction of hyperhydricity in the culture of Lepidium meyenii shoots by the addition of rare earth elements. Plant Growth Regul. 2007, 52, 151–159. [Google Scholar] [CrossRef]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Madhu; Sharma, A.; Kaur, A.; Tyagi, S.; Upadhyay, S.K. Glutathione peroxidases in plants: Innumerable role in abiotic stress tolerance and plant development. J. Plant Growth Regul. 2023, 42, 598–613. [Google Scholar] [CrossRef]
- Staszek, P.; Krasuska, U.; Ciacka, K.; Gniazdowska, A. ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxidants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Eroofeva, E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, Y.; Wang, W.; Min, K.; Ling, W.; Ma, W.; Zhang, W.; Hou, X.; Wei, L.; Liu, Q.; et al. Dose-Dependent Effect on Plant Growth of Exposure to Metal–Organic Framework MIL-101(Cr). Environ. Sci. Technol. 2024, 58, 8009–8019. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Cruz, C.A.; De Jesús García-Zárate, X.; Spinoso-Castillo, J.L.; Peña-Rodríguez, R.; Colorado-Peralta, R.; Sánchez-Páez, R.; Bello-Bello, J.J. The Influence of the Hybrid Compound Nd(NO3)3@Zn-MOF on the Growth of Vanilla (Vanilla planifolia Jacks. ex Andrews) Cultured In Vitro: A Preliminary Study. Agronomy 2024, 14, 1880. https://doi.org/10.3390/agronomy14091880
Cruz-Cruz CA, De Jesús García-Zárate X, Spinoso-Castillo JL, Peña-Rodríguez R, Colorado-Peralta R, Sánchez-Páez R, Bello-Bello JJ. The Influence of the Hybrid Compound Nd(NO3)3@Zn-MOF on the Growth of Vanilla (Vanilla planifolia Jacks. ex Andrews) Cultured In Vitro: A Preliminary Study. Agronomy. 2024; 14(9):1880. https://doi.org/10.3390/agronomy14091880
Chicago/Turabian StyleCruz-Cruz, Carlos Alberto, Xóchitl De Jesús García-Zárate, José Luis Spinoso-Castillo, Rodolfo Peña-Rodríguez, Raúl Colorado-Peralta, Ricardo Sánchez-Páez, and Jericó Jabín Bello-Bello. 2024. "The Influence of the Hybrid Compound Nd(NO3)3@Zn-MOF on the Growth of Vanilla (Vanilla planifolia Jacks. ex Andrews) Cultured In Vitro: A Preliminary Study" Agronomy 14, no. 9: 1880. https://doi.org/10.3390/agronomy14091880






