Toxic Effects of Bt-(Cry1Ab+Vip3Aa) Maize (“DBN3601T’’ Event) on the Asian Corn Borer Ostrinia furnacalis (Guenée) in Southwestern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of the ACB
2.2. Maize Variety for Testing
2.3. Determination of ACB Susceptibility to Insecticidal Proteins Expressed by the Bt Maizes
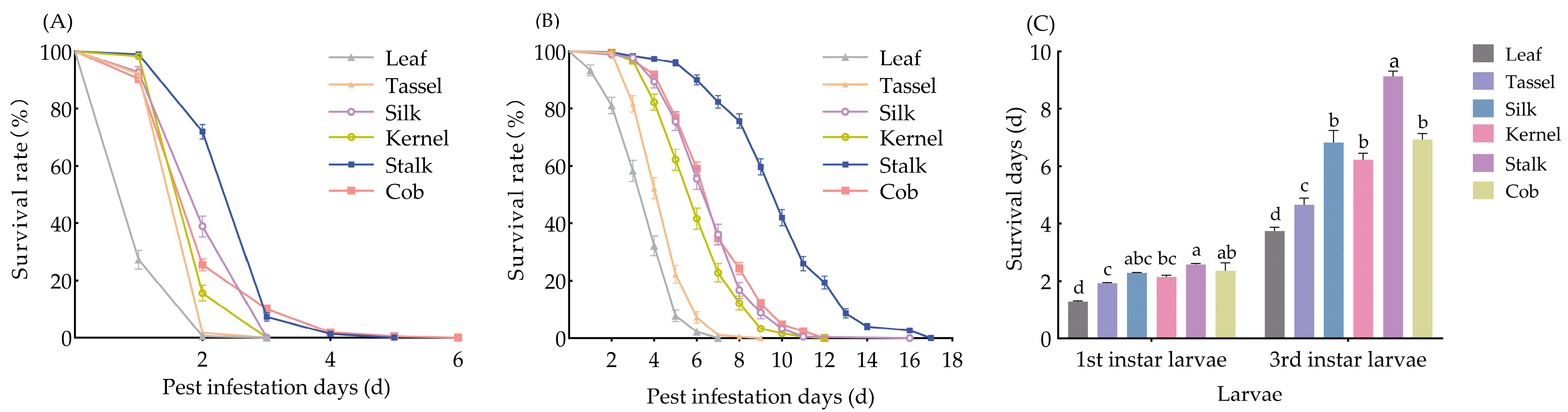
2.4. Determination of Insecticidal Protein Expression in Different Tissues of Bt Maize and Tissue Bioassays
2.5. Field Evaluation
2.6. Data Analysis
3. Results
3.1. Susceptibility of the ACB to Bt Insecticidal Proteins Expressed by the Bt Maize
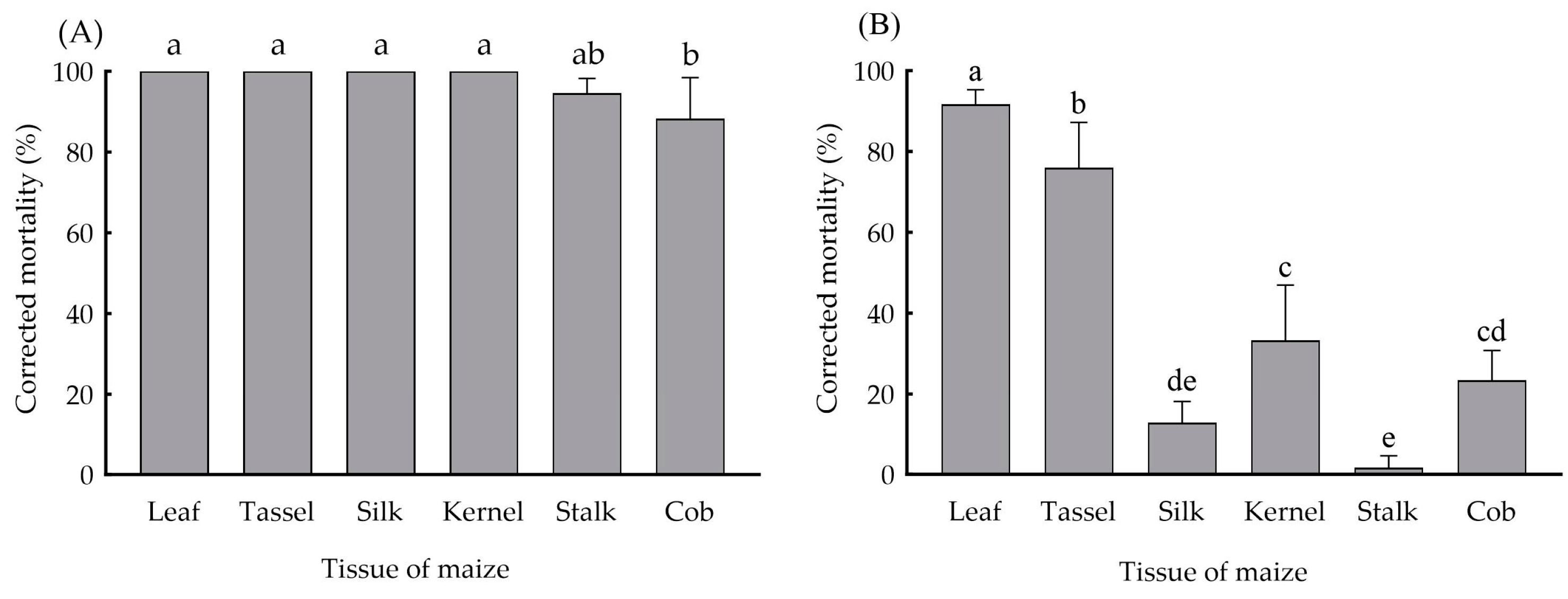
3.2. Expression of Insecticidal Proteins in Different Tissues of Bt-(Cry1Ab+Vip3Aa) Maize
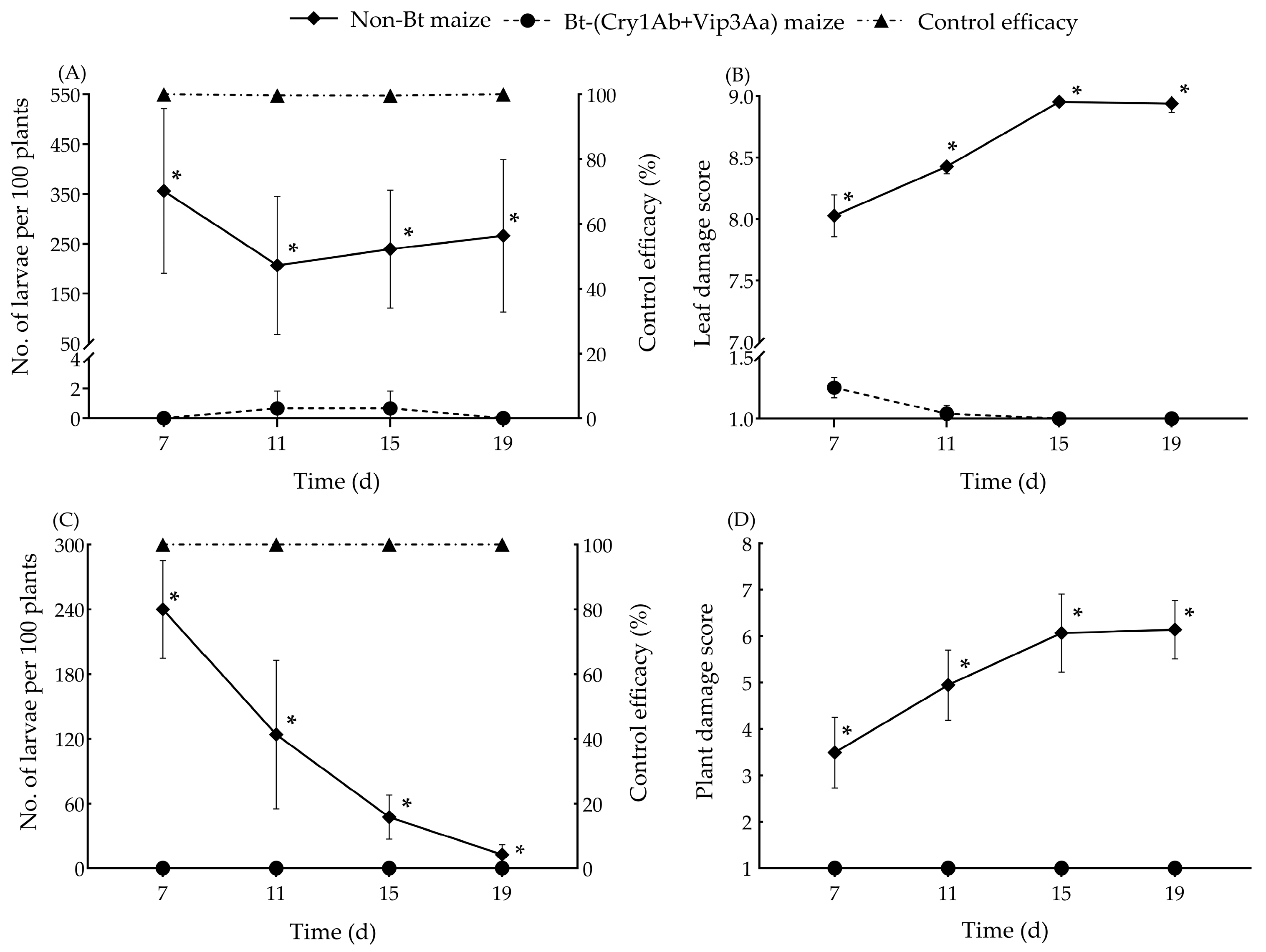
3.3. Field Evaluation for Resistance Level of the Bt Maize to ACB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.Y.; Lu, X.; He, K.L.; Zhou, D.R. Review of history, present situation and prospect of the Asian corn borer research in China. J. Shengyang Agri. Univ. 2000, 5, 402–412. [Google Scholar]
- Nafus, D.M.; Schreiner, I.H. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Int. J. Pest Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Mutuura, A.; Munroe, E. Taxonomy and distribution of the European corn borer and allied species: Genus Ostrinia (Lepidoptera: Pyralidae). Mem. Entomol. Soc. Can. 1970, 102, 1–112. [Google Scholar] [CrossRef]
- Tai, H.K.; Bai, S.H.; Gu, Z.L.; Liu, Z.; Wang, G.Q.; Li, A.; Zhang, F.; Wang, Z.Y. Population dynamics and major damage region of Asian corn borer Ostrinia furnacalis in Dehong prefecture of Yunnan province. Plant Prot. 2016, 42, 171–176. [Google Scholar]
- Li, Q.C.; Shi, J.; Huang, C.L.; Guo, J.F.; He, K.L.; Wang, Z.Y. Asian corn borer (Ostrinia furnacalis) infestation increases Fusarium verticillioides infection and Fumonisin contamination in maize and reduces the yield. Plant Dis. 2023, 107, 1557–1564. [Google Scholar] [CrossRef]
- Wu, Q.L.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Trajectory modeling revealed a southwest-northeast migration corridor for Fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 2021, 28, 649–661. [Google Scholar] [CrossRef]
- Huang, Y.R.; Lv, H.; Dong, Y.Y.; Huang, W.J.; Hu, G.; Liu, Y.; Chen, H.; Geng, Y.; Bai, J.; Guo, P.; et al. Mapping the spatio-temporal distribution of Fall armyworm in China by coupling multi-factors. Remote Sens. 2022, 14, 4415. [Google Scholar] [CrossRef]
- Jiang, C.X.; Zhang, X.Y.; Wu, J.Q.; Feng, C.H.; Ma, L.; Hu, G.; Li, Q. The source areas and migratory pathways of the Fall armyworm Spodoptera frugiperda (Smith) in Sichuan province, China. Insect 2022, 13, 935. [Google Scholar] [CrossRef]
- He, R.R.; Xu, P.Z. Population dynamics and control strategy of five species of corn borer in east and west Sichuan [China]. SW China J. Agric. Sci. 1989, 2, 48–53. [Google Scholar]
- Siegwart, M.; Thibord, J.-B.; Olivares, J.; Hirn, C.; Elias, J.; Maugin, S.; Lavigne, C. Biochemical and molecular mechanisms associated with the resistance of the European corn borer (Lepidoptera: Crambidae) to lambda-cyhalothrin and first monitoring tool. J. Econ. Entomol. 2017, 110, 598–606. [Google Scholar] [CrossRef]
- Li, G.P.; Wu, K.M. Commercial strategy of transgenic insect-resistant maize in China. J. Plant Prot. 2022, 49, 17–32. [Google Scholar]
- Li, H.H.; Liu, C.Y.; Zhang, H.W.; Wang, X.J.; Tang, Q.L.; Wang, Y.H. Global genetically modified crop industrialization trends in 2022. J. Agric. Sci. Technol. 2023, 25, 6–16. [Google Scholar]
- Thompson, G.D.; Dalmacio, S.C.; Criador, A.R., IV; Alvarez, E.R.; Hechanova, R.F. Field performance of TC1507 transgenic corn hybrids against Asian corn borer in the Philippines. Philipp. Agric. Sci. 2010, 93, 375–383. [Google Scholar]
- Reay-Jones, F.P.F.; Wiatrak, P. Evaluation of new transgenic corn hybrids producing multiple Bacillus thuringiensis toxins in South Carolina. J. Entomol. Sci. 2011, 46, 152–164. [Google Scholar] [CrossRef]
- Archer, T.L.; Schuster, G.; Patrick, C.; Cronholm, G.; Bynum, E.D., Jr.; Morrison, W. Whorl and stalk damage by European and Southwestern corn borers to four events of Bacillus thuringiensis transgenic maize. Crop Prot. 2000, 19, 181–190. [Google Scholar] [CrossRef]
- Graeber, J.V.; Nafziger, E.D.; Mies, D.W. Evaluation of transgenic, Bt -containing corn hybrids. J. Prod. Agric. 1999, 12, 659–663. [Google Scholar] [CrossRef]
- Zhang, S.M.; Yang, F.; Zhao, N.; Xia, W.; Shang, Y.; Yan, W.; Li, C.C.; Li, F.W. Advance of research and development of genetically modified maize in China. Manag. Agric. Sci. Technol. 2022, 41, 72–76. [Google Scholar]
- Sun, D.D.; Quan, Y.D.; Wang, Y.Q.; Wang, Z.Y.; He, K.L. Resistance of transgenic Bt maize (Ruifeng 125, DBN9936 & DBN9978) to Asian corn borer. Plant Prot. 2021, 47, 206–211. [Google Scholar]
- Zhao, S.Y.; Yang, X.M.; Liu, D.Z.; Sun, X.X.; Li, G.P.; Wu, K.M. Performance of the domestic Bt corn event expressing pyramided Cry1Ab and Vip3Aa19 against the invasive Spodoptera frugiperda (JE Smith) in China. Pest Manag. Sci. 2023, 79, 1018–1029. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yang, X.M.; Wang, W.H.; Wu, K.M. Insecticidal effects of transgenic maize Bt-Cry1Ab, Bt-Vip3Aa, and Bt-Cry1Ab+ Vip3Aa against the Oriental armyworm, Mythimna separata (Walker) in Southwest China. Toxins 2024, 16, 134. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.H.; Kang, G.D.; Yang, X.M.; Wu, K.M. Toxic effects of Bt-(Cry1Ab+ Vip3Aa) maize on storage pest Paralipsa gularis (Zeller). Toxins 2024, 16, 92. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of migration in Mythimna separata (Walker) in China: A review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ. Entomol. 2011, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Cong, X.P.; Wang, X.P.; Lei, C.L. A review of geographic distribution, overwintering and migration in Spodoptera exigua Hübner (Lepidoptera: Noctuidae). J. Entomol. Res. Soc. 2011, 13, 39–48. [Google Scholar]
- Qiao, L. The Studies on Biology and Rearing of Ostrinia furnacalis (Guenée). Master’s Thesis, Northwest Agriculture and Forestry University, Shanxi, China, 2008. [Google Scholar]
- Song, Y.Y.; Zhou, D.R.; He, K.L. Studies on mass rearing of Asian corn borer: Development of a satisfactory non-agar semi-artificial diet and its use. J. Plant Prot. 1999, 26, 324–328. [Google Scholar]
- Wang, W.H.; Zhang, D.D.; Zhao, S.Y.; Wu, K.M. Susceptibilities of the invasive Fall armyworm (Spodoptera frugiperda) to the insecticidal proteins of Bt maize in China. Toxins 2022, 14, 507. [Google Scholar] [CrossRef]
- Liang, G.M.; Tan, W.J.; Guo, Y.Y. Improvement of artificial feeding technology of cotton bollworm. Plant Prot. 1999, 25, 16–18. [Google Scholar]
- He, K.L.; Wang, Z.Y.; Zhou, D.R.; Wen, L.P.; Song, Y.Y. Methodologies and criteria for evaluating maize resistance to the Asian corn borer. J. Shengyang Agri. Univ. 2000, 31, 439–443. [Google Scholar]
- Lit, M.C.; Adalla, C.B.; Lantin, M.M. Host plant resistance to the Asiatic corn borer, Ostrinia furnacalis, in the Philippines. In Proceedings of the International Symposium on Methodologies for Developing Host Plant Resistance to Maize Insects, Veracruz, Mexico, 9–14 March 1987. [Google Scholar]
- Wang, Y.Q.; Zhao, W.L.; Han, S.; Wang, L.X.; Chang, X.; Liu, K.Q.; Quan, Y.D.; Wang, Z.Y.; He, K.L. Seven years of monitoring susceptibility to Cry1Ab and Cry1F in Asian corn borer. Toxins 2023, 15, 137. [Google Scholar] [CrossRef]
- He, K.L.; Wang, Z.Y.; Wen, L.; Bai, S.X.; Ma, X.; Yao, Z. Determination of baseline susceptibility to Cry1Ab protein for Asian corn borer (Lep., Crambidae). J. Appl. Entomol. 2005, 129, 407–412. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, S.; Long, Y.; Wang, Y.Q.; Zhao, W.L.; Shwe, S.M.; Wang, Z.Y.; He, K.L.; Bai, S.X. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis in relation to Cry1Ab toxins in China. Toxins 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Li, G.P.; Huang, J.R.; Ji, T.J.; Tian, C.H.; Zhao, X.C.; Feng, H.Q. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis related to Cry1 toxins in the Huanghuaihai summer corn region of China. Pest Manag. Sci. 2020, 76, 4311–4317. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.H.; Wang, Z.Y.; He, K.L.; Xie, H.C.; Wang, Y.Q. Insecticidal activity and synergism of combinations of Bacillus thuringiensis Cry and Vip3Aa against Ostrinia furnacalis. Plant Prot. 2023, 49, 310–315. [Google Scholar]
- Chakroun, M.; Bel, Y.; Caccia, S.; Abdelkefi-Mesrati, L.; Escriche, B.; Ferré, J. Susceptibility of Spodoptera frugiperda and S. exigua to Bacillus thuringiensis Vip3Aa insecticidal protein. J. Invertebr. Pathol. 2012, 110, 334–339. [Google Scholar] [CrossRef]
- Liang, J.G.; Zhang, D.D.; Li, D.Y.; Zhao, S.Y.; Wang, C.Y.; Xiao, Y.T.; Xu, D.; Yang, Y.Z.; Li, G.P.; Wang, L.L.; et al. Expression profiles of Cry1Ab protein and its insecticidal efficacy against the invasive fall armyworm for Chinese domestic GM maize DBN9936. J. Integr. Agric. 2021, 20, 792–803. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, Z.Y.; He, K.L.; Cong, B.; Bai, S.X.; Wen, L.P. Temporal and Spatial Expression of Cry1Ab Toxin in Transgenic Bt Corn and its Effects on Asian corn borer, Ostrinia furnacalis (Guenée). Sci. Agric. Sin. 2004, 37, 1155–1159. [Google Scholar]
- Afidchao, M.M.; Musters, C.J.M.; de Snoo, G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays L.) in the Philippines. Pest Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef]
- Le, D.K.; Le, Q.K.; Tran, T.T.H.; Nguyen, D.V.; Dao, T.H.; Nguyen, T.T.; Truong, X.L.; Nguyen, Q.C.; Pham, H.P.; Phan, T.T.T.; et al. Baseline susceptibility of Asian corn borer (Ostrinia furnacalis (Guenée)) populations in Vietnam to Cry1Ab insecticidal protein. J. Asia-Pac. Entomol. 2019, 22, 493–498. [Google Scholar] [CrossRef]
- Brookes, G.; Dinh, T.X. The impact of using genetically modified (GM) corn/maize in Vietnam: Results of the first farm-level survey. GM Crop. Food. 2021, 12, 71–83. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Burkness, E.C.; Mitchell, P.D.; Moon, R.D.; Leslie, T.W.; Fleischer, S.J.; Abrahamson, M.; Hamilton, K.L.; Steffey, K.L.; Gray, M.E.; et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010, 330, 222–225. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.B.; Huckaba, R.M. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Farhan, Y.; Schaafsma, A.W. Practical resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) to Cry1F Bacillus thuringiensis maize discovered in Nova Scotia, Canada. Sci Rep. 2019, 9, 18247. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, E.; Estrada, A.; Alpuerto, V.; Head, G. Monitoring Cry1Ab susceptibility in Asian corn borer (Lepidoptera: Crambidae) on Bt corn in the Philippines. Crop Prot. 2011, 30, 554–559. [Google Scholar] [CrossRef]
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef]
- Kaur, G.; Guo, J.G.; Brown, S.; Head, G.P.; Price, P.A.; Paula-Moraes, S.; Ni, X.Z.; Dimase, M.; Huang, F.N. Field-evolved resistance of Helicoverpa zea (Boddie) to transgenic maize expressing pyramided Cry1A. 105/Cry2Ab2 proteins in northeast Louisiana, the United States. J. Invertebr. Pathol. 2019, 163, 11–20. [Google Scholar] [CrossRef]
- Farinós, G.P.; De La Poza, M.; Hernández-Crespo, P.; Ortego, F.; Castañera, P. Resistance monitoring of field populations of the corn borers Sesamia nonagrioides and Ostrinia nubilalis after 5 years of Bt maize cultivation in Spain. Entomol. Exp. Appl. 2004, 110, 23–30. [Google Scholar] [CrossRef]
- García, M.; García-Benítez, C.; Ortego, F.; Farinós, G.P. Monitoring insect resistance to Bt maize in the European Union: Update, challenges, and future prospects. J. Econ. Entomol. 2023, 116, 275–288. [Google Scholar] [CrossRef]
- Siegfried, B.D.; Hellmich, R.L. Understanding successful resistance management: The European corn borer and Bt corn in the United States. GM Crop. Food. 2012, 3, 184–193. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, H.N.; Lu, Y.H.; Yang, Y.H.; Wu, K.M.; Tabashnik, B.E.; Wu, Y.D. Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat. Biotechnol. 2015, 33, 169–174. [Google Scholar] [CrossRef]
| Inhibition. Effect | Bt Event (Protein) | N | LC50/GIC50 (95%FL) ng/g | LC95/GIC95 (95%FL) ng/g | Slope ± SE | χ2 | df |
|---|---|---|---|---|---|---|---|
| LC | DBN9936 (Cry1Ab) | 360 | 51.42 (45.11–58.90) a | 190.15 (150.32–261.01) a | 2.90 ± 0.25 | 15.83 | 13 |
| DBN9501 (Vip3Aa) | 360 | >1591.20 | — | — | — | — | |
| DBN3601T (Cry1Ab+Vip3Aa) | 360 | 46.85 (40.36–54.20) a | 217.97 (168.01–311.21) a | 2.46 ± 0.22 | 15.38 | 13 | |
| GIC | DBN9936 (Cry1Ab) | 360 | 0.63 (0.01–1.66) A | 14.55 (9.90–19.26) A | 1.20 ± 0.23 | 3.10 | 13 |
| DBN9501 (Vip3Aa) | 360 | >1591.20 | — | — | — | — | |
| DBN3601T (Cry1Ab+Vip3Aa) | 360 | 0.30 (0.00–1.30) A | 14.99 (718–22.01) A | 0.97 ± 0.22 | 3.31 | 13 |
| Tissue | Cry1Ab μg/g | Vip3Aa μg/g | Total Bt Protein μg/g |
|---|---|---|---|
| V6–V8 leaf | 82.35 ± 8.39 a | 31.86 ± 6.91 a | 114.20 ± 3.22 a |
| VT tassel | 27.11 ± 3.32 b | 3.59 ± 0.11 b | 30.69 ± 3.41 b |
| R1 silk | 3.17 ± 1.21 e | 0.60 ± 0.29 d | 3.77 ± 0.96 d |
| R2 kernel | 5.45 ± 0.54 d | 3.48 ± 0.01 b | 8.92 ± 0.53 c |
| R3 Stalk | 9.55 ± 1.57 c | 1.55 ± 0.70 c | 11.09 ± 2.09 c |
| R3 cob | 9.15 ± 1.83 c | 1.84 ± 0.69 c | 10.99 ± 1.79 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, W.; Yang, X.; Kang, G.; Zhang, Z.; Wu, K. Toxic Effects of Bt-(Cry1Ab+Vip3Aa) Maize (“DBN3601T’’ Event) on the Asian Corn Borer Ostrinia furnacalis (Guenée) in Southwestern China. Agronomy 2024, 14, 1906. https://doi.org/10.3390/agronomy14091906
Li H, Wang W, Yang X, Kang G, Zhang Z, Wu K. Toxic Effects of Bt-(Cry1Ab+Vip3Aa) Maize (“DBN3601T’’ Event) on the Asian Corn Borer Ostrinia furnacalis (Guenée) in Southwestern China. Agronomy. 2024; 14(9):1906. https://doi.org/10.3390/agronomy14091906
Chicago/Turabian StyleLi, Haitao, Wenhui Wang, Xianming Yang, Guodong Kang, Zhenghao Zhang, and Kongming Wu. 2024. "Toxic Effects of Bt-(Cry1Ab+Vip3Aa) Maize (“DBN3601T’’ Event) on the Asian Corn Borer Ostrinia furnacalis (Guenée) in Southwestern China" Agronomy 14, no. 9: 1906. https://doi.org/10.3390/agronomy14091906
APA StyleLi, H., Wang, W., Yang, X., Kang, G., Zhang, Z., & Wu, K. (2024). Toxic Effects of Bt-(Cry1Ab+Vip3Aa) Maize (“DBN3601T’’ Event) on the Asian Corn Borer Ostrinia furnacalis (Guenée) in Southwestern China. Agronomy, 14(9), 1906. https://doi.org/10.3390/agronomy14091906






