Abstract
This study examines the impact of line spacing (X: 24 m, Y: 9 m, Z: 6.5 m) and orientation to tree lines on the growth, yield, and quality of red clover (Trifolium pratense L.) in a temperate, irrigated agroforestry system (2 ha) in Szarvas, Hungary. Three sampling locations were distinguished between the east and west oriented tree lines: the north (N) side, middle (M) strip, and south (S) side of the tree lines. The highest red clovers were observed in the 6.5 m spacing (mean height 69.3 ± 7.2 cm), although yields were similar across 24 m, 9 m, and 6.5 m spacings (2.9 t ha−1, 2.3 t ha−1, and 2.7 t ha−1 dry matter, respectively). Orientation significantly influenced all forage quality parameters, with the north side showing earlier developmental stages and higher proportions of immature flowers (41–59%). Managing the spatial arrangement of red clover in agroforestry systems can help optimize forage quality by mitigating variations in plant maturity.
1. Introduction
There is an immediate need for increasing adaptive capacity in agriculture to long-term climatic trends and increasing variability in weather patterns [1]. Production systems such as monocrop cultivation monocultures are no longer sustainable [2,3], as monocultures have evident adverse effects on soil and water quality, as well as on the conservation of biodiversity [3]. It is also largely responsible for soil nutrient depletion and soil erosion [3]. Alternatively, agroforestry systems (AFS) combine herbaceous crops or legumes with perennial woody crops to create diversified, sustainable, and climate-resilient systems [4]. Agroforestry, the integration of trees into agricultural landscapes, serves as an adaptation strategy that can help mitigate climate change by significantly increasing carbon sequestration in both soil and vegetation [5].
As we face the challenges of climate change and seek sustainable solutions, it is crucial to consider how environmental factors influence competitive dynamics within agroforestry systems. Vaccaro [6] summarized how environmental factors such as solar radiation and water availability influence the competition dynamics between trees and arable crops in agroforestry systems: in regions above 45 degrees latitude, there is more competition for light between trees and crops due to lower solar radiation. In Mediterranean areas, water availability is usually the most limiting factor. Arid environments face competition for light or nutrients, while temperate climates deal with water scarcity [6]. According to Ehret et al. [7] in temperate agroforestry systems, light availability and quality are expected to be the most important constraints throughout the growing season. In addition, other studies have shown that competition for soil organic matter, and nutrient availability is also an issue [7,8].
The challenges highlighted above occur for a variety of reasons, such as the age of the trees, the space between the trees, the design of the agroforestry systems at different elevations, and the spatial variation of the soil. A study on apple–soybean intercropping systems found that older trees occupied a larger soil space and had greater fine-root biomass density, affecting interspecific competition and soil spatial distribution [9]. Different tree spacing in agroforestry systems alters microclimate and soil properties, affecting decomposition rates, e.g., willow leaves in agroforestry treatments showed greater traits and influenced litter decomposition compared to monoculture, highlighting the impact of tree spacing on agroecosystem processes [10]. Crop density plays a crucial role in optimizing yield in agroforestry systems, e.g., in a jujube/cotton agroforestry system, cotton yield was significantly affected by plant density, with the highest yield attained at an intermediate density due to intraspecific and interspecific competitions [11]. A study in Belgium analyzed the spatial variability of soil organic carbon, acidity, and nutrient status in arable agroforestry fields; the results highlighted significantly higher soil organic carbon and nutrient concentrations in the vicinity of trees in field boundaries, impacting soil conditions up to at least 30 m into the field [12].
Woody line rows also have an impact on non-cropped vegetation in agroforestry. The weed community is also affected by competition for water and nutrient supply, as well as for light [13]. The effect of different tillage can be detected in the arable field by comparing the weed cover and composition of the border line and the inner part of the field [14], but in agroforestry, the shading effect of woody lines is even greater than in arable land [15].
Legumes play a crucial role in agroforestry, offering a range of benefits such as nutrient fixation, soil improvement, and biodiversity enhancement. Forage legumes not only provide food and feed to animals, but also improve soil productivity and act as soil-conserving components of agricultural and agroforestry systems. The inclusion of legumes in agroforestry systems is crucial for sustainable food and nutritional security without compromising long-term soil fertility [16]. Integrating legumes in agroecosystems can reduce the negative impacts of production by limiting major nitrogen fertilizers in the greenhouse gas (GHG) emission processes and simplifying cropping practices [16,17].
Red clover’s use in arable rotations can make significant contributions to sustainable intensification, preserving environmental integrity, and resolving food access and distribution equalities. Red clover is also valued for its nitrogen-fixing ability and nutritional properties for ruminants [18]. Red clover has been compared to lucerne for ruminant feeding, showing differences in crude protein (CP) concentrations and NDF digestibility, with red clover having lower CP and NDF concentrations and greater organic matter digestibility [19]. Red clover contributes to environmental protection and soil fertility, providing ecosystem services such as atmospheric nitrogen fixation, soil conservation, and increased soil microbial activity [20]. It can fix in excess of 350 kg/ha of nitrogen, making it beneficial for cropping systems [21]. Red clover faces challenges related to drought, with breeding for increased adaptation becoming essential [22,23]. Inclusion of early- versus late-maturing red clover varieties is being explored to reduce disproportionality in the yield between spring and summer growth [24].
This study examines the impact of line spacing (X: 24 m, Y: 9 m, Z: 6.5 m) and east–west orientation of tree lines on the growth (plant height, number of stem, number of flowers, SPAD) yield, and quality (crude protein, fiber, ash, fat content) of red clover (Trifolium pratense L.) in a temperate, irrigated agroforestry system.
2. Materials and Methods
2.1. Site Description
The experiment was set up at the agroforestry research site (2.7 ha) of the Hungar-ian University of Agriculture and Life Sciences (MATE), Institute of Environmental Sciences (IES), Research Center for Irrigation and Water Management (ÖVKI), in Szarvas, Hungary.
Hungary has a temperate continental climate; the specific area of the experimental site is described as a warm and dry climate region. The meteorological data of the experimental year (2022) were collected at an automatic weather station (Agromet Solar, Boreas Ltd., Hungary) located 1300 m from the experimental site (Figure 1). Total rainfall in the year of the experiment was 343.5 mm, 33% less than the average annual rainfall in the region (515 mm). The experimental year was not only drier but also warmer, with average annual temperatures (12.5 °C) above the long-term average for the region (10.8 °C).
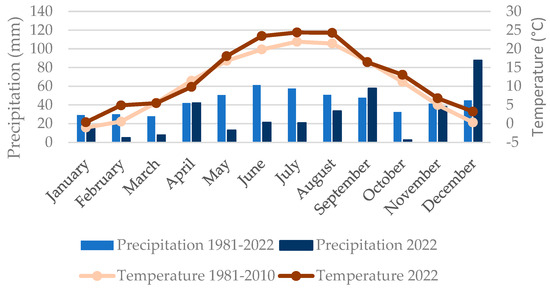
Figure 1.
Monthly precipitation and mean monthly temperature in the experimental year 2022 and 1981–2010 in Szarvas.
The experimental site (46°51′06.8″ N 20°31′16.3″ E) is located on the Great Hungarian Plain in a 1 km wide flat valley surrounded by oxbow lake of the River Körös, which is an island-like area. Due to river regulation measures, the area has been free from flooding since the second half of the 19th century. The soils of the area are formed on the alluvial deposits of the Körös river, mainly on clay and silty clay bedrock. The soil type of the experimental site is Vertisols, with a clay texture, 6.7 pH(KCl), <0.5 m/m% total carbonate, and 1.1% total organic carbon.
The weed infestation in the experimental area is low, but is influenced by the orientation of sampling locations. The total weed cover was 0.6 ± 0.4%, 0.7 ± 0.3%, 3.0 ± 2.6% in the N, M, S locations, with Lolium perenne, Adonis aestivalis, Capsella bursa-pastoris, and Cirsium arvense being the most abundant on 29 April, and <0.1 ± <0.1%, 0.0 + 0.0%, 0.2 ± 0.1%, with Convolvulus arvense being the only weed present on 17 August. Contributing to the low weed cover was the fact that 2022 was an extremely dry year with only Convolvulus arvense as a weed. Also, according to Den Hollander et al. [25] other clover species like Persian clover, red clover, alsike clover, berseem clover, and crimson clover provide good weed suppression. The results of the weed survey are supported by the examination of areas of 1 m2 [14,26].
2.2. Red Clover Cultivation Technology
Before the red clover was sown, the experimental area served as an energy plantation and was left fallow in 2020 following its conversion. The cultivation of red clover began with ploughing, disking, and seedbed preparation, which took place between December 2020 and March 2021. The DIANA variety was sown at a rate of 12 kg ha−1 on 25 March 2021. During the first year, irrigation was performed on 8–9 July using surface water (50 mm) from the oxbow lake of the Körös River, followed by the first mowing on 16 July. A second irrigation of 50 mm was conducted on 28–29 July. The seed was harvested on 5 October in the first year. In the second year, 2022, the first mowing occurred on 19 May. Due to the extremely dry conditions, three irrigations were necessary: on 24 May (45 mm), 7 June (90 mm), and 20 June (50 mm). The second mowing was carried out on 6 August 2022. The water consumption for red clover production is 500 to 700 mm per year, with the highest usage occurring from July to August, when up to 4.5 mm per day is consumed [27].
The water quality of the oxbow lake is excellent fresh water for irrigation (specific electrical conductivity 371 µS/cm, sodium adsorption ratio 1.2) [28]. The irrigation method used was sprinkler irrigation with a drum sprinkler.
2.3. Experimental Design and Sampling Locations
Prior to the present experiment, there was an energy plantation (Salix alba L., clone 77 and 82, named “Naperti”) in the research area between 2014 and 2019 for experimental purposes, with irrigation using reused water from an intensive catfish farm. In 2020, the plantation was transformed into an agroforestry system consisting of 7 rows of trees simulating alley cropping arrangement [29]. This transformation effectively created a semi-industrial pilot agroforestry system. Between the rows of willow trees, there are 3 fields of different widths (X: 24 m, Y: 9 m, Z: 6.5 m) in two replicates for intercropping (Figure 2). The orientation of the tree rows is east–west, which is the recommended orientation to reduce the shading of arable land by trees [30]. Since the trees were planted in April 2014, they reached their 9th growing season in 2022. In January 2021, the above-ground biomass of the willows was harvested in the year of red clover sowing, and in the second year of red clover sowing, the trees produced their second annual shoot. The spacing between the trees in the rows was 0.5 m.
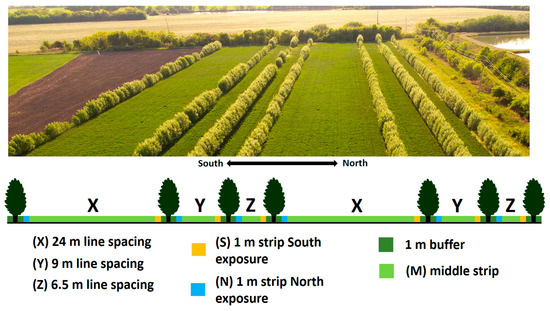
Figure 2.
Photo and layout of the experimental site. Line spacing means the distance between the tree lines (X, Y, Z).The 1 m strip with north exposure, 1 m strip with south exposure, and middle strip arerefer to the orientation. The 1 m buffer is the zone between the tree lines and the red clover area.
The buffer zone was “beside the crop line, which did not receive any soil disturbance, or crop production” [13]. The zone was approximetly 1 m wide in 2022, so the real widths of the plots were as follows: 22 m, 7 m, 4.5 m. Each plot was 275 metres long, so the areas of plots X, Y, and Z were, in order, 6050 m2, 1925 m2, 1237.5 m2.
By testing the three different line spacings (plots of the intercrop) (X: 24 m, Y: 9 m, Z: 6.5 m) between the trees, we looked for the optimum line spacing at which the intercrop plant could grow best. The orientation of the tree lines was east–west. Three sampling locations were distinguished according to the position relative to the tree lines: north (N) is the northern sides of the tree lines, highly affected by the shadows of the trees during the season; middle (M) is the strip equidistant from the tree (1 m strips in the center of the red clover plots); and south (S) is the southern sides of the tree lines. By differentiating between sites, we aimed to investigate the effect of the shadow of the tree lines on the development of red clover (Figure 2). All three aforementioned positions (N, M, S) were selected for each line spacing (X, Y, Z); for the number of replicates, see Section 2.4, Section 2.5 and Section 2.6. Remote sensing and ground observations indicated that the vegetation was uniformly developed and the area was homogeneous, justifying the use of fewer replicates. Additionally, the experimental site’s layout, constrained by the pilot agroforestry system, limited the number of feasible replicates.
The N and S positions represent strips 1 m wide and 275 m long; the M position represents the area of the red clover parcel minus the areas of N and S. Red clover (Trifolium pratense L.) is a perennial crop, and measurements were made in its second year. This was a one-year study. Because the experiment was conducted between tree lines, the shading effects of tree growth varied, making comparisons between years difficult, as these were very fast-growing willow clones (77, 82) [31].
2.4. Assay of Phenologycal Parameters
The phenological measurements of the plants were recorded on 12 July 2022, during the phenological phase of full flowering. The following parameters of red clover were studied: root length, shoot length, number of shoots, plant weight, and the number and colour of flowers. For phenological measurements, 5 red clover individual was collected along 5 straight lines perpendicular to the tree lines at the sampling positions indicated above in each line spacing (X, Y, Z) and in each orientation (N, M, S), resulting in 25 replications per each position. The individual plants were dug up and transported to the laboratory, where the roots were washed. The length of the longest shoot, which can be taken as the height of the plant, was measured with a tape measure, and the numbers of stem branches and flowers were counted. The plant weight without the root was measured with a bench scale (Metripond Plus CAS MWP 1500, Metripond Plus Mérlegtechnika Kft. 6800 Hódmezővásárhely, Bajcsy Zsilinszky utca 70).
For plant biomass measurements, a 1 m2 red clover area was mown (27 June 2022) along 4 straight lines perpendicular to the tree lines at the sampling positions indicated above in each line spacing (X, Y, Z) and in each orientation (N, M, S), resulting in 4 replications per position. The biomass was measured with a bench scale (Metripond Plus CAS CS, Metripond Plus Mérlegtechnika Kft. 6800 Hódmezővásárhely, Bajcsy Zsilinszky utca 70).
2.5. Forage Quality of Red Clover
Although the aim of the crop was to harvest seeds from the second growth, four different parameters (crude protein, crude fat, crude fiber, crude ash) of the red clover biomass were investigated for a fiber feed. Plant samples were taken from the mown red clover during biomass measurements, resulting in 4 replicates per sampling position (see Section 2.4). The fodder quality was determined in the laboratory in the Agricultural Science Specialization of the MATE University Laboratory Center in Kaposvár. Crude protein content (%) was determined using the Kjeldahl method (EN ISO 5983-2:2009). For the determination of crude fiber content (%), the sample was treated with boiling sulphuric acid and potassium hydroxide, then filtered, dried, weighed, and ashed at 475–500 °C. The weight loss on ashing indicated the crude fiber content (Commission Regulation (EC) No 152/2009). Crude ash content (%) was determined by incineration of dried samples (ISO 5984:1992). The content of crude fat (%) was determined by the Soxhlet extraction method (according to Hungarian standard MSZ 6830-19:1979) [32].
2.6. Soil Moisture Measurements
Soil moisture measurements were conducted on three occasions (31 May, 13 June, and 27 June 2022) 6–7 days after the irrigation events. Measurements were taken along 8 straight lines perpendicular to the tree rows at the specified sampling positions (X, Y, Z) in each orientation (N, M, S) and directly within the tree lines, resulting in 8 replications per position. A custom-built soil penetrometer (model PEN 100M150) was used, capable of penetrating up to 1 m. However, due to the extremely high resistance of the soil, measurements were limited to the top 50 cm.
The penetrometer measures soil moisture content by detecting impedance between the probe tip and the shaft, with wet soil showing lower impedance and dry soil showing higher impedance. Calibration tables developed using soil samples from sandy and clay soils were used to convert the measured impedance into gravimetric moisture content (percent by weight), which was then displayed and stored in the device’s internal memory. Although the instrument is no longer commercially available, its unique calibration ensured accurate readings under the study’s specific conditions.
2.7. Statistical Analyses
The collected data underwent two-way analysis of variance (ANOVA) using IBM SPSS Statistics software (version 25.0) to assess the variability and validate the results. Pearson correlation was used to examine the relationships between the plant phenology parameters. The evaluation of the line spacing and orientation was carried out with a one-way ANOVA and Tukey’s post hoc test.
3. Results
3.1. Phenology
Red clover stem length varied significantly with orientation (north, middle, south), line spacing (X, Y, Z), and their interaction (Table 1).

Table 1.
Two-way ANOVA output of line spacing and orientation for phenology data.
Stem lengths ranged from 37.5 cm to 86.3 cm. In line X, the mean stem length was 63.4 ± 10.8 cm, with the south side showing shorter plants compared to the north side and the middle strip. In line Y, the mean stem length was 66.6 ± 8.9 cm, with the tallest plants in the middle strip and no significant differences between the north and south sides. In line Z, the mean stem length was 69.3 ± 7.2 cm, with no significant differences according to orientation (Table 2).

Table 2.
Phenology data of red clover.
The number of shoots per plant was also influenced by line spacing, orientation, and their interaction (Table 1). The mean shoot numbers were 6.2 ± 2.0 for line X, 7.2 ± 2.6 for line Y, and 7.3 ± 2.7 for line Z. In line Y, the middle strip had significantly fewer shoots than the north and south sides. For the north strips, line X had fewer shoots compared to lines Y and Z (Table 2).
Fresh weight per plant varied from 2.2 g to 37.3 g and was significantly affected by orientation, line spacing, and their interaction (Table 1). In line X, the mean plant mass was 11.2 ± 5.8 g, with the highest mass in the middle strip and no significant differences between the north and south sides. In line Y, the mean plant mass was 14.4 ± 7.5 g, with the middle strip significantly higher than the north side. In line Z, the mean plant mass was 14.4 ± 6.9 g, with the highest mass in the middle strip and no significant orientation differences (Table 2). There were strong correlations between red clover weight and stem length and between the number of stems and the number of flowers (Table 3).

Table 3.
Pearson correlation of phenology parameters.
SPAD values, indicating chlorophyll content, were significantly affected by line spacing and orientation, but not their interaction (Table 1). Mean SPAD values were 54.3 ± 5.3 for line X, 53.1 ± 7.0 for line Y, and 51.2 ± 5.8 for line Z. SPAD values were significantly higher in the north strip than in the middle and south strips across all line spacings (Table 2).
The total number of flowers per plant was significantly influenced by orientation, line spacing, and their interaction (Table 1). The mean flower counts were 6.6 ± 4.6 for line X, 7.8 ± 5.2 for line Y, and 8.5 ± 4.9 for line Z. The lowest flower count was observed on the north side, and the highest in the middle strip across all line spacings. In the southern strip, line X had significantly fewer flowers than lines Y and Z (Table 2). Flower maturity varied significantly with orientation, with a higher proportion of purple (immature) flowers on the north side, indicating earlier developmental stages (Figure 3). This is supported by the correlation between SPAD values and number of purple/brown/total flowers, as chlorophyll content depends on the flowering stage (Table 3). According to Saadat et al. [33], the differences in SPAD values in red clover could be attributed to the plants flowering at different times.
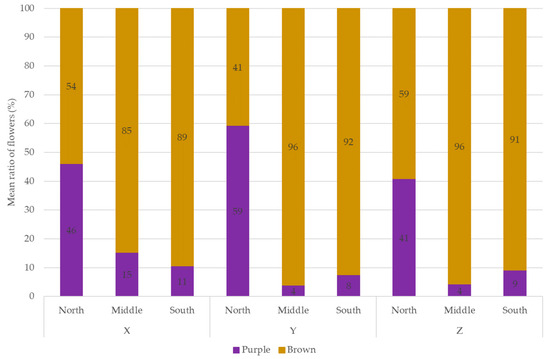
Figure 3.
Distribution of flowers by maturity stage (colour). Line spacings X: 24 m, Y: 9 m, and Z: 6.5 m.
3.2. Forage Quality
Forage quality in terms of crude protein, crude fat, crude fiber, and crude ash was analysed at different line spacings and in different orientations. The orientation (north, middle, south) had a significant influence on all examined forage quality parameters at the p < 0.001 level, except for crude fiber, which was significant at the p < 0.01 level (Table 4). Line spacing significantly influenced only crude fiber (p < 0.001). The interaction of line spacing and orientation was significant in one case, specifically for crude ash (p = 0.01).

Table 4.
Two-way ANOVA results for the effects of line spacing and orientation on forage quality parameters.
The crude protein content of red clover varied between 15.6% and 19.3% in the experiment. The highest mean protein value (18.1 ± 0.8%) was recorded at the widest line spacing (24 m, X), with Y and Z line spacings showing lower values (17.5 ± 0.7% and 17.5 ± 1.2%, respectively); however, the differences were not significant. Orientation significantly affected protein content, with the north side of the tree line having the highest mean value (X: 18.4 ± 0.4%; Y: 18.2 ± 0.7%; Z: 18.9 ± 0.4%) and the middle section having the lowest (Figure 4).
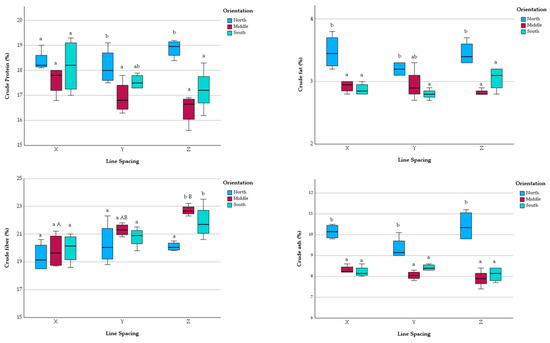
Figure 4.
Forage quality results of red clover. The letters ‘a’ and ‘b’ indicate significant differences among different orientations within the same line spacing, while ‘A’ and ‘B’ denote significant differences among different line spacings (X, Y, or Z) within the same orientation, respectively, at the p = 0.05 level. X: 24 m Y: 24 m Z: 6.5 m.
The crude fat content ranged from 2.7% to 3.8%. No significant differences were found between the line spacings (X: 3.1 ± 0.3%; Y: 3.0 ± 0.2%; Z: 3.1 ± 0.3%). Orientation had a significant effect, with the north side showing the highest mean value (X: 3.5 ± 0.3%; Y: 3.2 ± 0.1%; Z: 3.5 ± 0.2%), while no significant differences were observed between the middle and south sides (Figure 4).
The crude fiber content ranged from 18.5% to 23.5%. Line spacing significantly affected crude fiber content, with mean values increasing as line spacing decreased (X: 19.7 ± 1.0%; Y: 20.8 ± 1.0%; Z: 21.6 ± 1.3%). This was the only forage quality parameter showing a strong negative relationship with row spacing (Table 5). Orientation also significantly influenced crude fiber content, with the north side having the lowest mean value (X: 19.4 ± 1.0%; Y: 20.3 ± 1.5%; Z: 20.1 ± 0.3%), particularly significant in the narrowest (Z) line spacing (Figure 4).

Table 5.
Pearson correlation of parameters of forage quality and line spacing.
The crude ash content varied from 7.4% to 11.2%. No significant differences were found between line spacings (X: 8.9 ± 1.0%; Y: 8.6 ± 0.6%; Z: 8.8 ± 1.3%). Orientation significantly impacted the crude ash content, with the north side showing the highest mean values (X: 10.2 ± 0.4%; Y: 9.4 ± 0.5%; Z: 10.4 ± 0.7%), which were significant across all line spacings (Figure 4).
3.3. Yield
The two-way ANOVA result indicates that red clover biomass (d.m.) was only significantly affected by position relative to the tree line, but not by line spacing (Table 6).

Table 6.
Two-way ANOVA results for the effects of line spacing and orientation on red clover yield.
The lowest red clover biomass mass was measured on the north side of the tree line for each line spacing (Table 7).

Table 7.
Yield of red clover.
The highest biomass mass was measured in the middle strip of each line for all line spacings. The dry matter content of red clover was lower on the northern side of the tree line than in the middle and southern strips (the difference was significant in the X and Z strips). The specific yield of red clover per line spacing was calculated by taking the biomass measured in each strip (N, M, S) and weighting it according to the area of the strips, resulting in an average yield per unit area. There was no significant difference between the specific yields calculated in the different line spacings (p = 0.276). If we consider the values measured in the middle band of the widest line space as a conventional field reference value (3.01 t/ha), the specific yields in the line spaces X, Y, and Z were 3.8%, 22.8%, and 10.7% lower than the conventional values.
3.4. Soil Moisture
Soil moisture was measured three times, each time 6–7 days after irrigation. At the first measurement date, the soil moisture in the fasciculus at a depth of 5–18 cm was lower than that measured in the intercrop soil. At the same time, the higher water use of the trees did not extend horizontally to the northern and southern strips (~1.5 m from the tree line) of red clover soil. In all strips, similar soil moisture levels were measured throughout the soil profile. The second measurement date was preceded by 90 mm irrigation. Neither the orientation nor the tree line had any effect on the soil moisture, as the curves of the mean values seem to confirm. At the third time of measurement, it can again be seen that the different strips had similar moisture contents, but in the 7–15 cm soil layer, the higher water use of the willows is reflected in the lower soil moisture values (Figure 5).
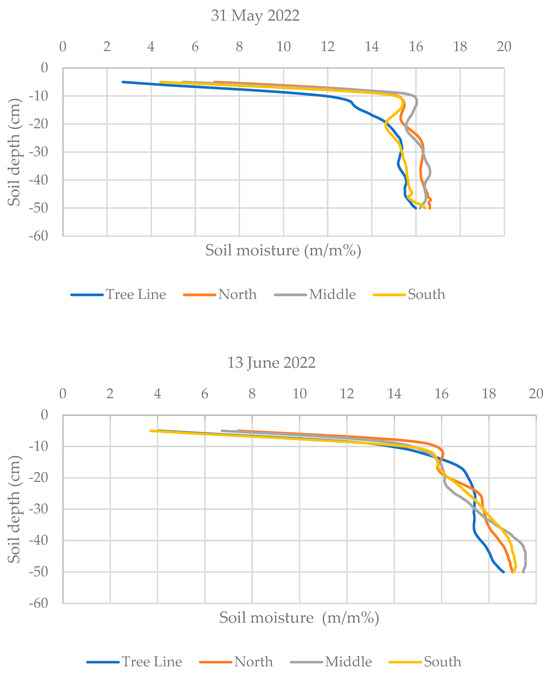
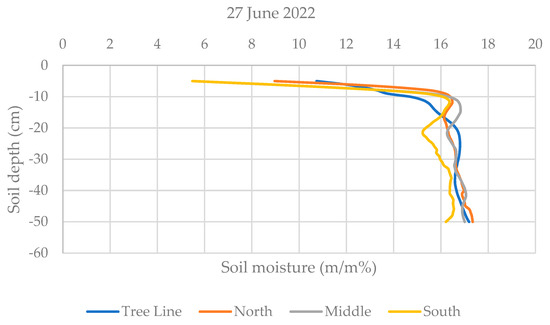
Figure 5.
Soil moisture distribution by depth (5–50 cm) and orientation at three different dates. The lines in the graph represent a moving average for the 5 cm soil layer and the repetition was 8.
4. Discussion
Through evaluating line spacing by plant height values, we found that the narrowest line spacing (Z: 6.5 m) had the highest red clovers (mean 69.3 ± 7.2 cm), and in this line spacing, there was no difference in values by orientation. According to Radinovič et al. [34], 46 red clover varieties and cultivars (including DIANA) were tested over two years, with a maximum height of 65.27 cm in the first year and 50.23 cm in the second year. In our experiment, the maximum plant height was 86.3 cm. Although the plant heights were higher than in other studies, the mass of the individual plants was lower (ranged from 2.2 g to 37.3 g). According to Radinovič et al. [34], the lowest green mass yield was 75.33 g plant−1. However, according to Nadeem et al. [24], the plant weight ranged from 12.1 to 17.0 g plant−1 at the first harvest of the second experimental yield in the case of six cultivars. According to the weight of the red clover plant, the highest weight was measured in the middle strips in all line spacings; there were no significant differences in line X, Y, Z.
The SPAD values, which indicate chlorophyll content and plant health [35], were found to have a strong positive correlation with the number of flowers in red clover. This suggests that higher SPAD values may be associated with a greater number of flowers in red clover plants. SPAD values also showed a positive correlation with the number of purple flowers and a negative correlation with brown flowers. This is because the chlorophyll content of the plant decreases as flowering progresses [36,37], whereas it is high at the beginning of flowering. Thus, both the flower colour and SPAD values indicate the different maturity states of the red clover in our experiment. Flower maturity varied significantly with orientation, with a higher proportion of purple (immature) flowers (41–59% of total flowers) on the north side, indicating earlier developmental stages, than in the middle or south strips (4–15% of total flowers). SPAD values were significantly higher in the north strip (53.4–57.1), also showing the earlier maturity of the plants, than in the middle and south strips (49.0–55.2) across all line spacings.
Orientation (north, middle, south) consistently influenced the forage quality parameters of red clover, with the north side generally showing higher protein and ash content and lower fiber content. The higher fiber content is an indication of lower-quality forage, as it is characterized by more difficult digestibility and lower protein content [38]. According to our results, the lowest crude fiber and the highest crude protein contents of red clover were measured on the north side of the tree line (Figure 4), which means that the highest-quality red clover was grown in the shade of the trees. Since shade resulted in a lower yield, the improved yield quality is likely to have been an indirect effect of shade. According to Mikhailov et al. [39] where red clover grew at the edge of the forest, with lighting conditions limited to about 50% of full illumination, the plants did not “overheat” and had a higher content of ascorbic acid. In addition, other environmental conditions, especially temperature and soil moisture, affect the level of vitamins in red clover, which may indirectly impact the fiber content [39].
The concentration of rumen-degradable protein decreased with advancing plant development in red clover cultivars, indicating a decrease in crude protein content as the plant matured [40]. According to our results, the lowest protein content was measured in the middle and south strips (Figure 4). This may be due to the fact that red clover in these locations was more mature (Figure 3), whereas the plants on the north side of the tree line were at an earlier stage of development at the time of measurement. The growth stages of red clover influenced the crude protein content of red clover: early flowering, 19.5%, late flowering, 14.0%, sowing, 13.2% [41]. On the other hand, there was a strong negative correlation between the protein and fiber content of red clover (Figure 4, Table 2), which was due to the fact that as the protein content decreased during ripening, the fiber content increased [41], also confirming the different developmental stages of the red clover. Our results for crude ash content are similar to the results for protein content, as the ash content was higher in the northern plants at an earlier stage of development (Figure 4). According to Markovič et al. [42] as the red clover matured, the content of crude ash decreased from the first to the third stage of growth.
Red clover is a valuable forage crop, with dry matter yields ranging from 9 to 18 t ha−1 year−1 (two mowing in one year) in Ukraine [43]. The mean annual production of established red clover is 17.0 ± 0.48 t DM ha−1 in New Zealand [44]. The mean dry matter yield of the first main harvesting season ranges from 7.4 t ha−1 in Serbia and Norway to 13.4 t ha−1 in Switzerland. In our experiment, the yield of the second mowing of the two-year-old red clover was significantly lower (Table 7). The difference was probably mainly due to the climate requirements of the red clover, as the wetter and cooler weather in northern Europe is more favorable for its environmental needs. The recommended growing conditions for red clover can be summarised as follows: soil pH, 6.0–7.0; temperature, 20–25 °C; annual rainfall from 550 mm; and well-drained soil with a salinity of 0–0.75 dS m−1 [20]. Red clover yields in the 24, 9, and 6.5 m rows were 2.9 t ha−1, 2.3 t ha−1, and 2.7 t ha−1 dry matter, respectively, with no significant difference between them.
The soil moisture measurements indicate that irrigation temporarily homogenized soil moisture levels across different strips and orientations. However, willow water use significantly affected soil moisture at certain depths, as is particularly evident for lower soil moisture values at 5–18 cm depth near the tree line. Over time, the effect of tree water uptake became apparent, especially in areas closer to the trees, suggesting that the proximity to the tree line does influence soil moisture dynamics in red clover fields. From the soil moisture values, it can be concluded that the developmental stages of the plants in the different strips were mainly influenced by other factors, e.g., light stress or temperature stress [45], rather than the available water supply, which deserves further investigation.
5. Conclusions
The study shows that orientation significantly influences both forage quality parameters and growth of red clover in agroforestry systems. The study focused on red clover in its second year of growth, limited by the one-year observation. North-facing strips had higher protein and ash contents and lower fiber content, indicating superior forage quality influenced by environmental factors such as light availability. In addition, the central strips had higher plant mass, stem length, and flower number due to optimal light and resource conditions, while the north-facing strips had higher SPAD values and a greater proportion of immature flowers, suggesting delayed development due to limited light. These results highlight the critical role of spatial arrangement and orientation in optimising red clover growth and quality in agroforestry systems and emphasise the importance of effective management practices to improve forage quality in different parts of the field. Future research should aim to conduct longer-term studies to verify these findings and further investigate the influence of spatial arrangement and orientation over multiple growing seasons.
Author Contributions
Conceptualization, Á.K. and B.B.; methodology, M.J. and Á.S.; formal analysis, M.Z.; investigation, Á.K., Z.D. and I.K.; data curation, Á.K.; writing—original draft preparation, Z.D.; writing—review and editing, Á.K.; visualization, N.T.; supervision, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the project ‘The feasibility of the circular economy during national defense activities’ of 2021 Thematic Excellence Programme of the National Research, Development and Innovation Office under grant no.: TKP2021-NVA-22, led by the Centre for Circular Economy Analysis.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our sincere gratitude to the Hungarian company SZARVASI MEDICAGO Ltd. for providing us with the red clover seeds for our experiment and for managing the plant protection tasks. Their generous contribution and support were essential to the success of this study. Thank you for your commitment to the advancement of agricultural research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tripathi, J.; Variyar, P.S. Food Safety and Methods to Ensure Food Security in the Face of Climate Change. CABI Rev. 2021, 16, 1–11, PAVSNNR202116015. [Google Scholar] [CrossRef]
- Faye, J.B.; Braun, Y.A. Soil and Human Health: Understanding Agricultural and Socio-Environmental Risk and Resilience in the Age of Climate Change. Health Place 2022, 77, 102799. [Google Scholar] [CrossRef] [PubMed]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; Tourdonnet, S.; Valantin-Morison, M. Mixing Plant Species in Cropping Systems: Concepts, Tools and Models. A Review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef]
- Smith, J.; Pearce, B.D.; Wolfe, M.S. Reconciling Productivity with Protection of the Environment: Is Temperate Agroforestry the Answer? Renew. Agric. Food Syst. 2013, 28, 80–92. [Google Scholar] [CrossRef]
- Dmuchowski, W.; Baczewska-Dąbrowska, A.H.; Gworek, B. The Role of Temperate Agroforestry in Mitigating Climate Change: A Review. For. Policy Econ. 2024, 159, 103136. [Google Scholar] [CrossRef]
- Vaccaro, C. Environmental and Biological Implications for Understorey Crop Growth in Temperate Silvoarable Agroforestry Systems. Ph.D. Thesis, Eidgenössische Technische Hochschule (ETZ), Zürich, Switzerlan, 2023; p. 122. [Google Scholar]
- Ehret, M.; Graß, R.; Wachendorf, M. The Effect of Shade and Shade Material on White Clover/Perennial Ryegrass Mixtures for Temperate Agroforestry Systems. Agrofor. Syst. 2015, 89, 557–570. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Barthès, B.G.; Saby, N.P.A.; Parent, T.; Dupraz, C.; Bernoux, M.; Chenu, C. Impact of Alley Cropping Agroforestry on Stocks, Forms and Spatial Distribution of Soil Organic Carbon — A Case Study in a Mediterranean Context. Geoderma 2015, 259–260, 288–299. [Google Scholar] [CrossRef]
- Sun, Y.; Bi, H.; Xu, H.; Duan, H.; Peng, R.; Wang, J. Below-Ground Interspecific Competition of Apple (Malus pumila M.)–Soybean (Glycine max L. Merr.) Intercropping Systems Based on Niche Overlap on the Loess Plateau of China. Sustainability 2018, 10, 3022. [Google Scholar] [CrossRef]
- Coleman, B.R.; Martin, A.R.; Thevathasan, N.V.; Gordon, A.M.; Isaac, M.E. Leaf Trait Variation and Decomposition in Short-Rotation Woody Biomass Crops under Agroforestry Management. Agric. Ecosyst. Environ. 2020, 298, 106971. [Google Scholar] [CrossRef]
- Wang, Q.; Han, S.; Zhang, L.; Zhang, D.; Van Der Werf, W.; Evers, J.B.; Sun, H.; Su, Z.; Zhang, S. Density Responses and Spatial Distribution of Cotton Yield and Yield Components in Jujube (Zizyphus jujube)/Cotton (Gossypium hirsutum) Agroforestry. Eur. J. Agron. 2016, 79, 58–65. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees Increase Soil Organic Carbon and Nutrient Availability in Temperate Agroforestry Systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Kun, Á.; Simon, B.; Zalai, M.; Kolozsvári, I.; Bozán, C.; Jancsó, M.; Körösparti, J.T.; Kovács, G.P.; Gyuricza, C.; Bakti, B. Effect of Mulching on Soil Quality in an Agroforestry System Irrigated with Reused Water. Agronomy 2023, 13, 1622. [Google Scholar] [CrossRef]
- Zalai, M.; Keresztes, Z.; Dorner, Z. Effects of Field Margins on the Weed Composition of Organic Cereals. Landsc. Manag. Funct. Biodivers. 2014, 100, 147–152. [Google Scholar]
- Reid, R.; Ferguson, I.S. Development and Validation of a Simple Approach to Modelling Tree Shading in Agroforestry Systems. Agrofor. Syst. 1992, 20, 243–252. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, M.R.; Arif, M.; Mahala, D.M.; Kumar, D.; Ghasal, P.C.; Yadav, K.C.; Verma, R.K. Multiple Agroecosystem Services of Forage Legumes towards Agriculture Sustainability: An Overview. Indian J. Agri. Sci. 2020, 90, 1367–1377. [Google Scholar] [CrossRef]
- Rosenstock, T.; Tully, K.; Arias-Navarro, C.; Neufeldt, H.; Butterbach-Bahl, K.; Verchot, L. Agroforestry with N2-Fixing Trees: Sustainable Development’s Friend or Foe? Curr. Opin. Environ. Sustain. 2014, 6, 15–21. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J.; Davies, W.P. Red Clover (Trifolium pratense) in Conservation Agriculture: A Compelling Case for Increased Adoption. Int. J. Agric. Sustain. 2018, 16, 342–366. [Google Scholar] [CrossRef]
- Sousa, D.O.; Hansen, H.H.; Hallin, O.; Nussio, L.G.; Nadeau, E. A Two-year Comparison on Nutritive Value and Yield of Eight Lucerne Cultivars and One Red Clover Cultivar. Grass Forage Sci. 2020, 75, 76–85. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J. The Use of Red Clover (Trifolium pratense) in Soil Fertility-Building: A Review. Field Crops Res. 2018, 221, 38–49. [Google Scholar] [CrossRef]
- Stoddard, F.L.; Watson, C.A. (Eds.) Legumes in Cropping Systems; CABI: Oxfordshire, UK, 2017; ISBN 978-1-78064-675-6. [Google Scholar]
- Vleugels, T.; Saleem, A.; Dubey, R.; Muylle, H.; Borra-Serrano, I.; Lootens, P.; De Swaef, T.; Roldán-Ruiz, I. Phenotypic Characterization of Drought Responses in Red Clover (Trifolium pratense L.). Front. Plant Sci. 2024, 14, 1304411. [Google Scholar] [CrossRef]
- Yates, S.A.; Swain, M.T.; Hegarty, M.J.; Chernukin, I.; Lowe, M.; Allison, G.G.; Ruttink, T.; Abberton, M.T.; Jenkins, G.; Skøt, L. De Novo Assembly of Red Clover Transcriptome Based on RNA-Seq Data Provides Insight into Drought Response, Gene Discovery and Marker Identification. BMC Genom. 2014, 15, 453. [Google Scholar] [CrossRef]
- Nadeem, S.; Steinshamn, H.; Sikkeland, E.H.; Gustavsson, A.; Bakken, A.K. Variation in Rate of Phenological Development and Morphology between Red Clover Varieties: Implications for Clover Proportion and Feed Quality in Mixed Swards. Grass Forage Sci. 2019, 74, 403–414. [Google Scholar] [CrossRef]
- Den Hollander, N.G.; Bastiaans, L.; Kropff, M.J. Clover as a Cover Crop for Weed Suppression in an Intercropping Design. Eur. J. Agron. 2007, 26, 104–112. [Google Scholar] [CrossRef]
- Nkoa, R.; Owen, M.D.K.; Swanton, C.J. Weed Abundance, Distribution, Diversity, and Community Analyses. Weed Sci. 2015, 63, 64–90. [Google Scholar] [CrossRef]
- Stanciu, A.S.; Berchez, O. Research Regarding the Water Consumption on the Production of Red Clover. Analele Univ. Din Oradea Fasc. Protecţia Mediu. 2016, 27, 79–84. [Google Scholar]
- Kolozsvári, I.; Kun, Á.; Jancsó, M.; Palágyi, A.; Bozán, C.; Gyuricza, C. Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation. Agronomy 2022, 12, 1185. [Google Scholar] [CrossRef]
- Quinkenstein, A.; Wöllecke, J.; Böhm, C.; Grünewald, H.; Freese, D.; Schneider, B.U.; Hüttl, R.F. Ecological Benefits of the Alley Cropping Agroforestry System in Sensitive Regions of Europe. Environ. Sci. Policy 2009, 12, 1112–1121. [Google Scholar] [CrossRef]
- Burner, D.M.; Ashworth, A.J.; Laughlin, K.F.; Boyer, M.E. Using SketchUp to Simulate Tree Row Azimuth Effects on Alley Shading. Agron. J. 2018, 110, 425–430. [Google Scholar] [CrossRef]
- Kolozsvári, I.; Kun, Á.; Jancsó, M.; Bakti, B.; Bozán, C.; Gyuricza, C. Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions. Forests 2021, 12, 457. [Google Scholar] [CrossRef]
- Luque De Castro, M.D.; Garcı́a-Ayuso, L.E. Soxhlet Extraction of Solid Materials: An Outdated Technique with a Promising Innovative Future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Saadat, D.; Siller, A.; Hashemi, M. Phenology, Nitrogen Status, and Yield of Red Clover (Trifolium pretense L.) Affected by Application of Vitamin B12, Humic Acid, and Enriched Biochar. Agronomy 2023, 13, 2885. [Google Scholar] [CrossRef]
- Radinovic, I.; Vasiljevic, S.; Brankovic, G. Correlations of Morpho-Agronomic Traits and Forage Quality Properties in Diverse Red Clover (Trifolium pratense L.) Collections. J. Agric. Sci. BGD 2022, 67, 139–151. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD Value and Grain Yield Can Be Affected by Cultivar, Environment and Soil Nitrogen Content in Wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Tyutereva, E.V.; Dmitrieva, V.A.; Voitsekhovskaja, O.V. Chlorophyll B as A Source of Signals Steering Plant Development (Review). Sel’skokhozyaistvennaya Biol. [Agric. Biol.] 2017, 52, 843–855. [Google Scholar] [CrossRef]
- Sinka, L.; Zsembeli, J.; Ragán, P.; Duzs, L.; Hájos, M.T. Effect of Different Seedling Growing Methods on the SPAD, NDVI Values and Some Morphological Parameters of Four Sweet Corn (Zea mays L.) Hybrids. Agriculture 2021, 67, 177–190. [Google Scholar] [CrossRef]
- Tucak, M.; Ravlić, M.; Horvat, D.; Čupić, T. Improvement of Forage Nutritive Quality of Alfalfa and Red Clover through Plant Breeding. Agronomy 2021, 11, 2176. [Google Scholar] [CrossRef]
- Mikhailov, A.L.; Timofeeva, O.A.; Ogorodnova, U.A.; Stepanov, N.S. Effect of Soil and Growth Climatic Conditions on Vitamin Content of Red Clover (Trifolium pratense L.). J. Exp. Biol. Agric. Sci. 2020, 8, S292–S297. [Google Scholar] [CrossRef]
- Marković, J.; Lazarević, Đ.; Bekčić, F.; Prijović, M.; Vasić, T.; Živković, S.; Štrbanović, R. Protein and Carbohydrate Profiles of a Diploid and a Tetraploid Red Clover Cultivar. Agric. Food Sci. 2022, 31, 113478. [Google Scholar] [CrossRef]
- Blaser, R.E. Forage-Animal Management Systems; Virginia Agricultural Experiment Station: Blacksburg, Virginia, 1986; Volume Bulletin 86-7. [Google Scholar]
- Marković, J.; Štrbanović, R.; Petrović, M.; Dinić, B.; Blagojević, M.; Milić, D.; Spasić, N. Estimation of Red Clover (Trifolium pratense L.) Forage Quality Parameters Depending on the Cut, Stage of Growth and Cultivar. Agro-Knowl. J. 2012, 13, 31–38. [Google Scholar] [CrossRef][Green Version]
- Baystruk-Hlodan, L. Productivity of Red Clover (Trifolium pratense L.) in Various Ways of Use in Soil and Climatic Conditions of the Western Region of Ukraine. Agrivita. J. Agr.Sci 2023, 45, 147–153. [Google Scholar] [CrossRef]
- Keenan, L.; Mills, A.; Smith, M.; Brown, H.; McKenzie, S.; Moot, D. Predicitng Yield of Irrigated Red Clover (Trifolium pratense L.) Pastures in Response to Temperature. J. N. Z. Grassl. 2023, 85, 217–226. [Google Scholar] [CrossRef]
- Mustafa, M.; Szalai, Z.; Divéky-Ertsey, A.; Gál, I.; Csambalik, L. Conceptualizing Multiple Stressors and Their Consequences in Agroforestry Systems. Stresses 2022, 2, 242–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).