Influence of Exogenous Ethylene and Mechanical Damage on Gene Expression and Physiological Parameters of Maize Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments, and Sampling
2.2. Method of BXD Isolation, Sample Preparation, and Measurement
2.3. SOD Measurement Method
2.4. CAT Measurement Method
2.5. MDA Measurement Method
2.6. Total RNA Isolation and cDNA Synthesis
2.7. qPCR Analysis
2.8. Statistical Analysis
3. Results
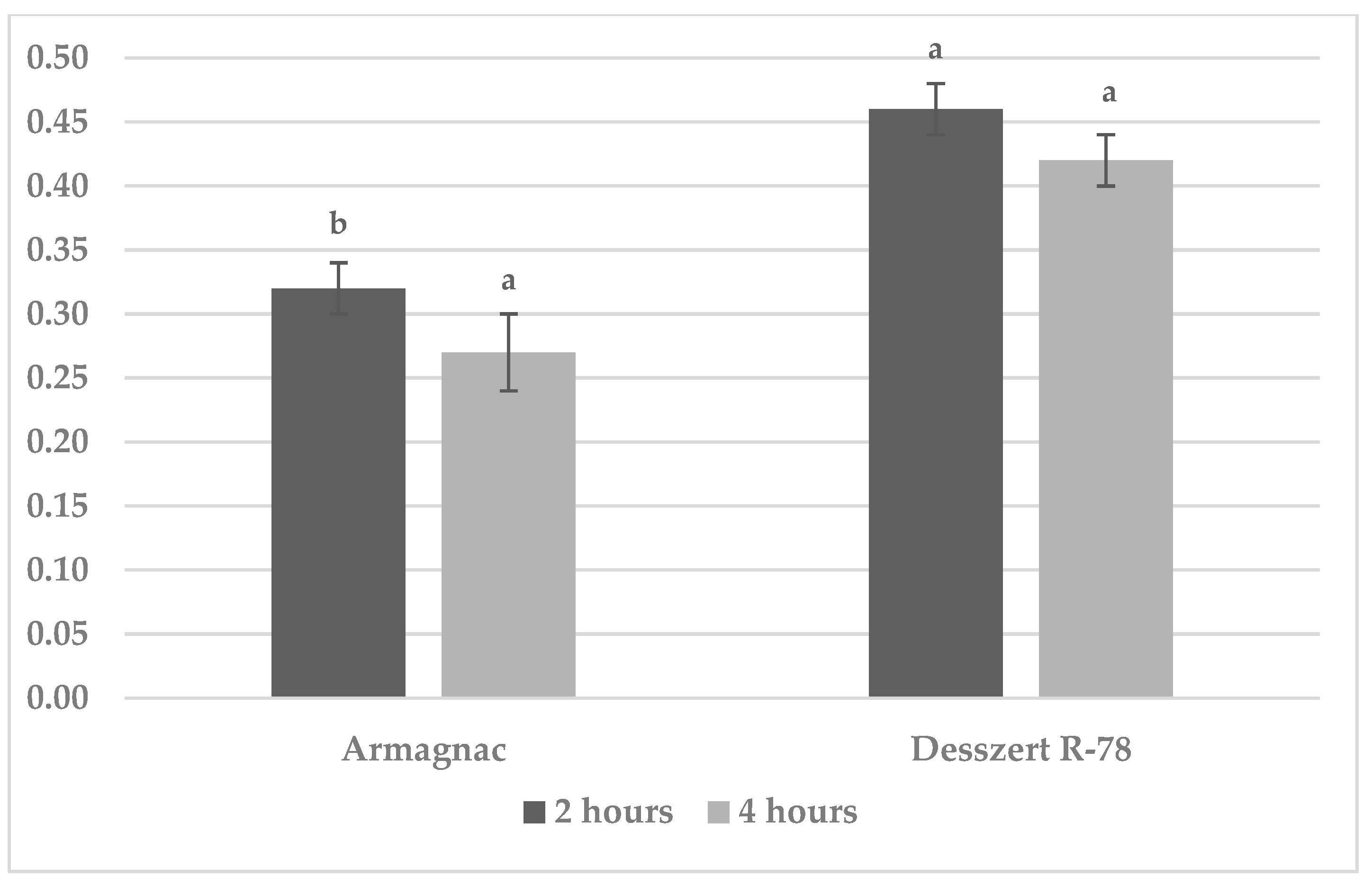
3.1. Results of Benzoxazinoid (BXD) Measurements
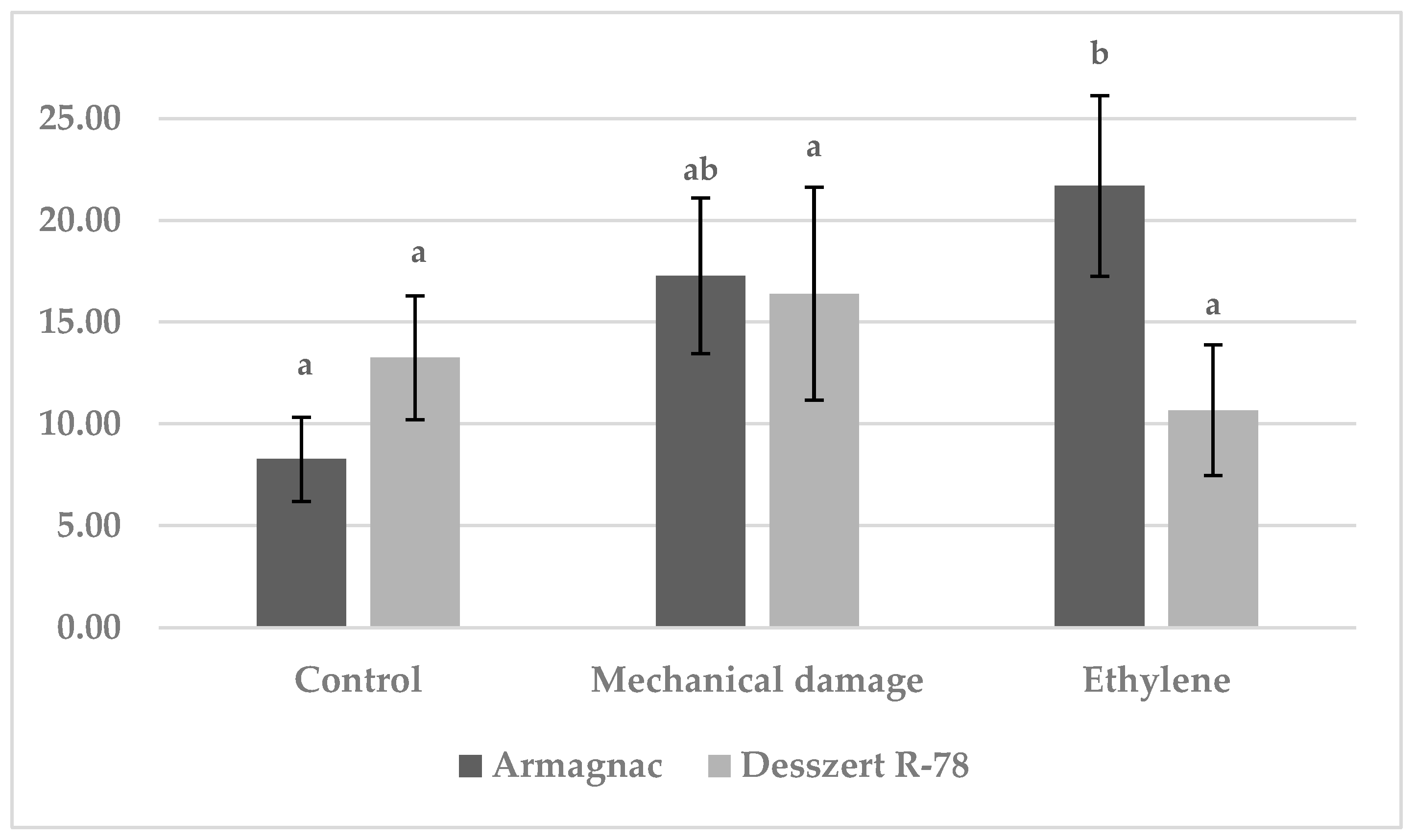
3.2. Results of SOD Measurement
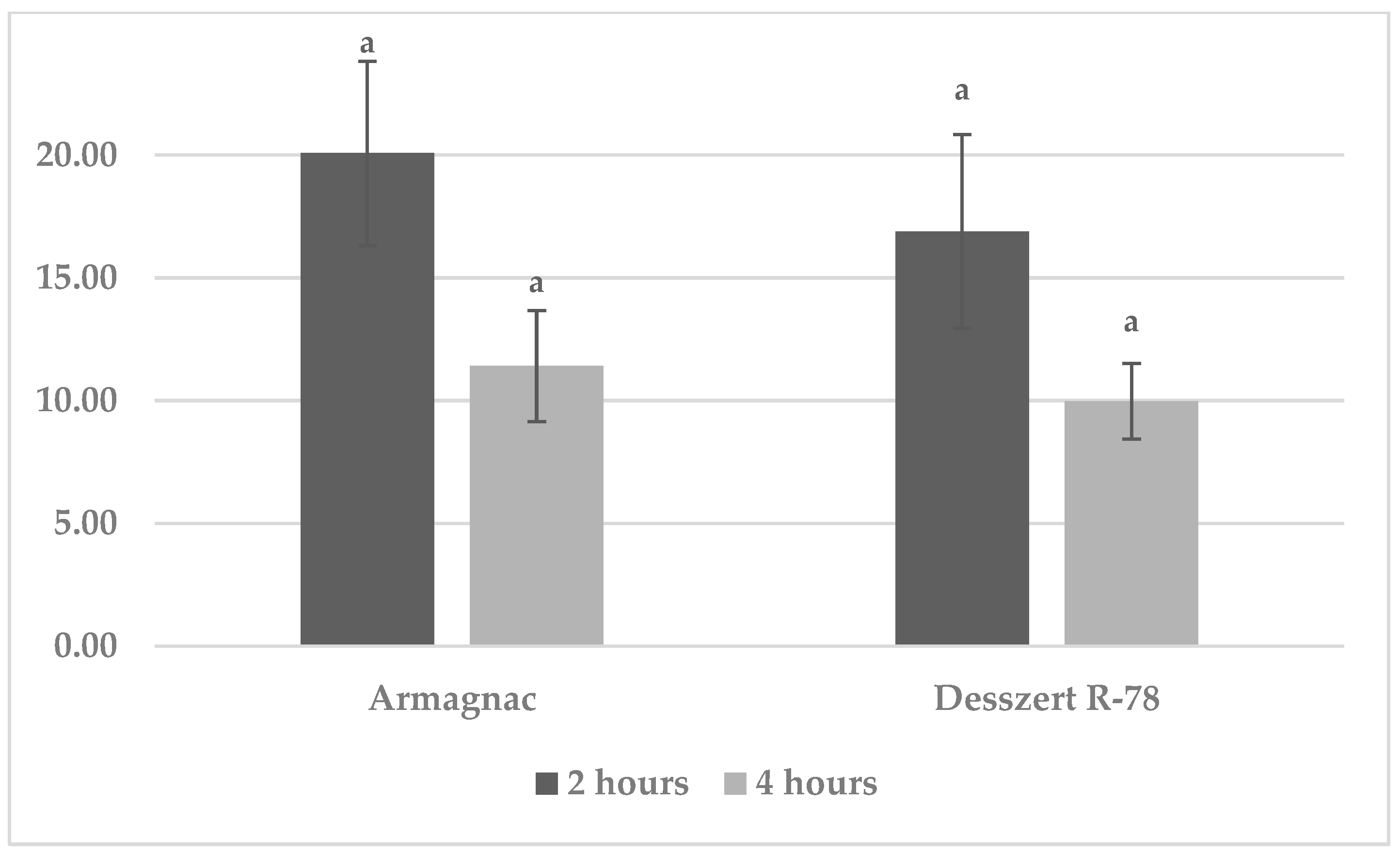
3.3. Results of CAT Measurement
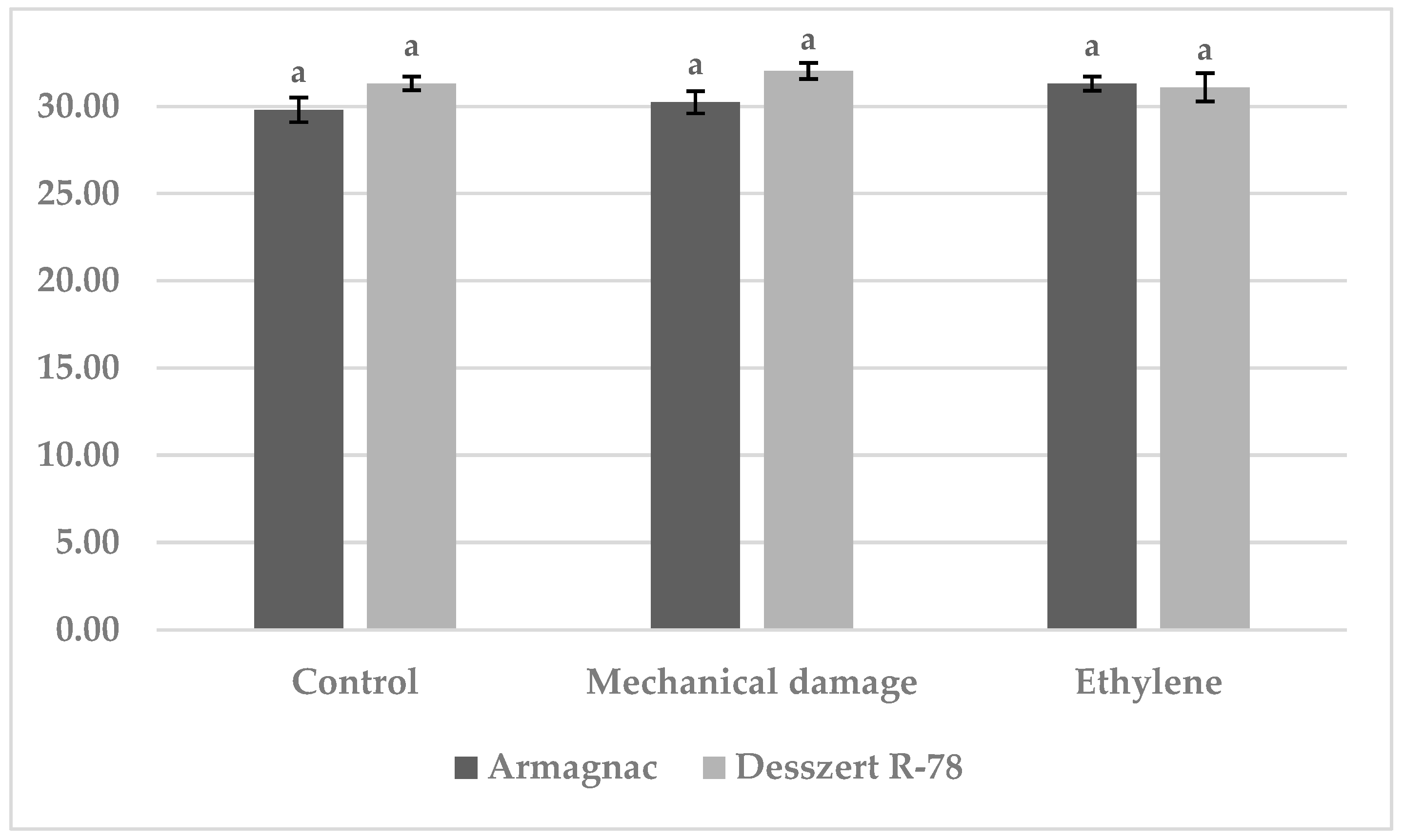
3.4. Results of MDA Measurements
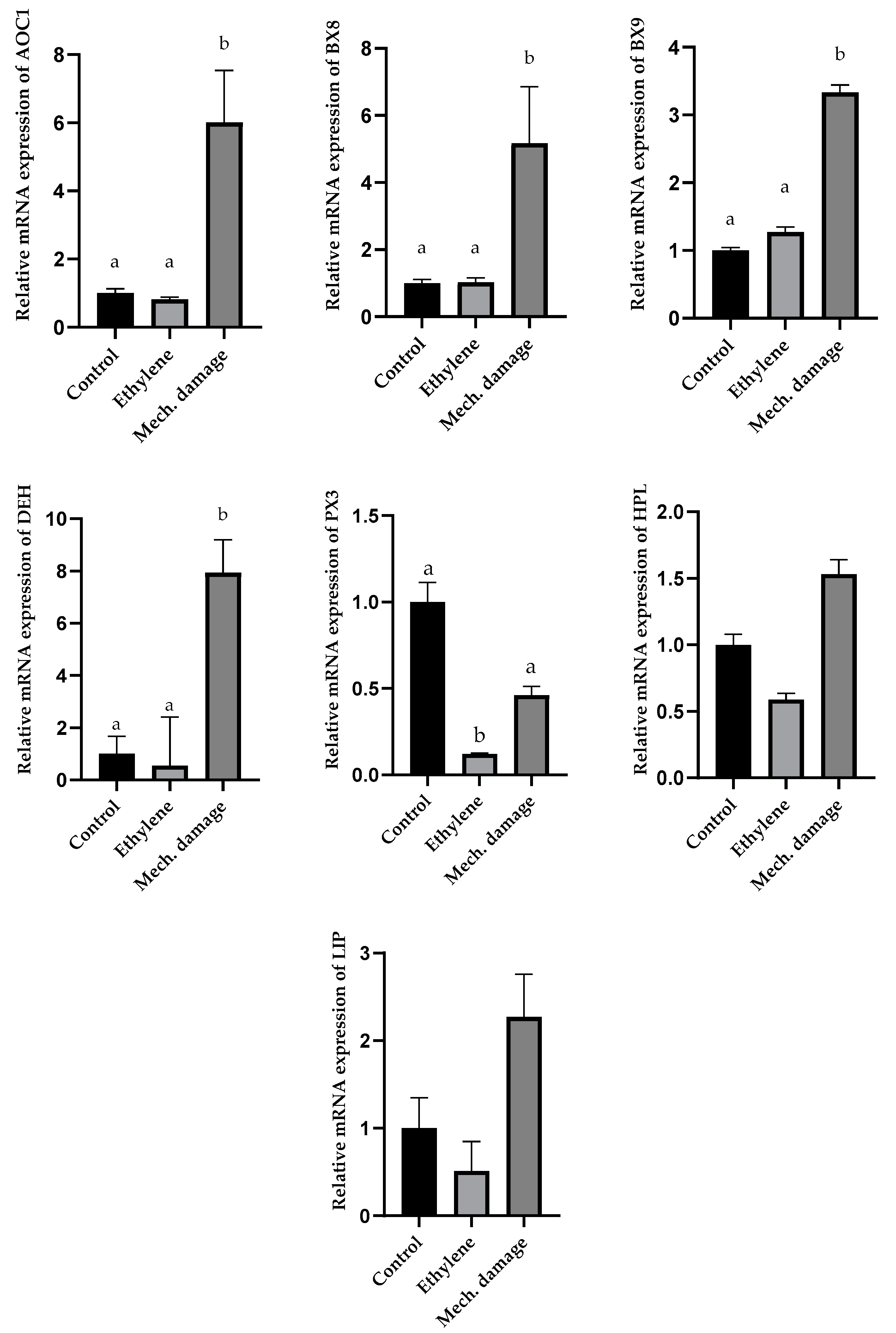
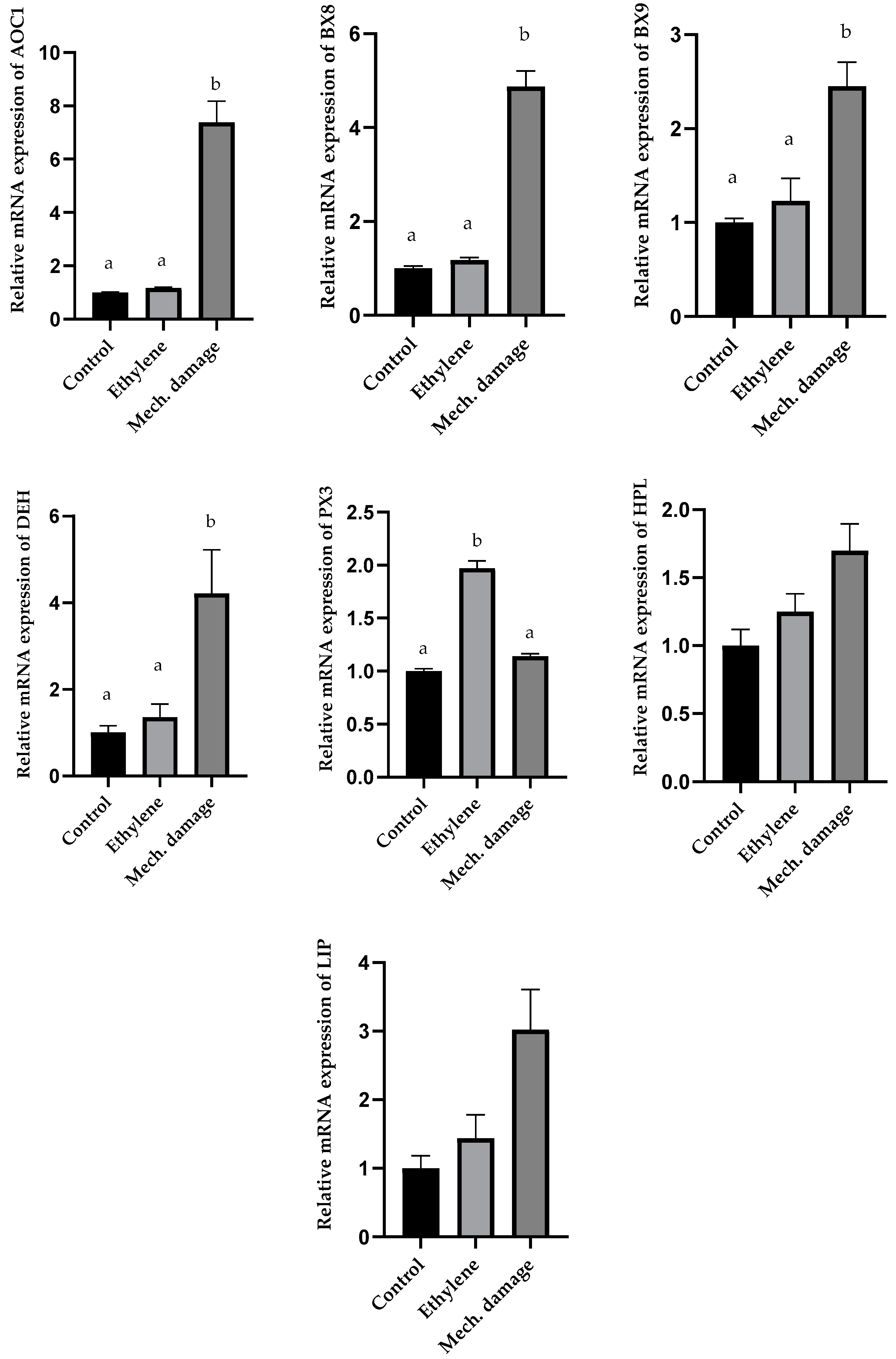
3.5. Results of Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makleit, P.; Szilvia, V.; Lóránt, S. Hazai Rozs (Secale Cereale L.) Fajták Ciklikus Hidroxámsav-Tartalma És Kiválasztása. Növénytermelés 2017, 66, 47–68. [Google Scholar]
- Makleit, P.; Nagy, A.; Veres, S.; Fónagy, A. Cyclic Hydroxamic Acid Content and Its Temporal Changes in Five Commercial Maize (Zea mays) Hybrids. Cereal Res. Commun. 2018, 46, 686–696. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic Acids Derived from 2-Hydroxy-2H-1,4-Benzoxazin-3(4H)-One: Key Defense Chemicals of Cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassão, D.G. Plant Defense and Herbivore Counter-Defense: Benzoxazinoids and Insect Herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef]
- Cambier, V.; Hance, T.; De Hoffmann, E. Variation of DIMBOA and Related Compounds Content in Relation to the Age and Plant Organ in Maize. Phytochemistry 2000, 53, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.M.; Veyrat, N.; Glauser, G.; Marti, G.; Doyen, G.R.; Villard, N.; Gaillard, M.D.P.; Köllner, T.G.; Giron, D.; Body, M.; et al. A Specialist Root Herbivore Exploits Defensive Metabolites to Locate Nutritious Tissues. Ecol. Lett. 2012, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Schullehner, K.; Dick, R.; Fiesselmann, A.; Gierl, A. Benzoxazinoid Biosynthesis, a Model for Evolution of Secondary Metabolic Pathways in Plants. Phytochemistry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Li, S.; Ma, C.; Liu, H.; Wang, L.; Qi, J.; Wu, J. ZmMPK6 and Ethylene Signalling Negatively Regulate the Accumulation of Anti-insect Metabolites DIMBOA and DIMBOA-Glc in Maize Inbred Line A188. New Phytol. 2021, 229, 2273–2287. [Google Scholar] [CrossRef]
- Ding, X.; Yang, M.; Huang, H.; Chuan, Y.; He, X.; Li, C.; Zhu, Y.; Zhu, S. Priming Maize Resistance by Its Neighbors: Activating 1,4-Benzoxazine-3-Ones Synthesis and Defense Gene Expression to Alleviate Leaf Disease. Front. Plant Sci. 2015, 6, 830. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.C.; Hipskind, J.D.; Wood, K.V.; Nicholson, R.L. Separation and Quantification of Cyclic Hydroxamic Acids and Related Compounds by High-Pressure Liquid Chromatography. J. Agric. Food Chem. 1988, 36, 57–60. [Google Scholar] [CrossRef]
- Hartenstein, H.; Lipmann, T.; Sicker, D. An Efficient Procedure for the Isolation of Pure 2,4-Dihydroxy-7-Methoxy-2H-1,4- Benzoxazin-3(4H)-One (DIMBOA) from Maize. Indian J. Heterocycl. Chem. 1992, 2, 75–76. [Google Scholar]
- Sarker, U.; Oba, S. Catalase, Superoxide Dismutase and Ascorbate-Glutathione Cycle Enzymes Confer Drought Tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, M.W.P.C.; Alberda, M.; Van Der Plas, L.H.W. Role of Oxidative Damage in Tulip Bulb Scale Micropropagation. Plant Sci. 1997, 130, 207–216. [Google Scholar] [CrossRef]
- Alici, E.; Arabaci, G. Determination of SOD, POD, PPO and Cat Enzyme Activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Baryla, A.; Laborde, C.; Montillet, J.-L.; Triantaphylidès, C.; Chagvardieff, P. Evaluation of Lipid Peroxidation as a Toxicity Bioassay for Plants Exposed to Copper. Environ. Pollut. 2000, 109, 131–135. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Q.; Geng, B.; Shi, J.; Zhu, H.; Sun, Y.; Yang, Q.; Yang, B.; Guo, Z. Jasmonate Biosynthesis Enzyme Allene Oxide Cyclase 2 Mediates Cold Tolerance and Pathogen Resistance. Plant Physiol. 2023, 193, 1621–1634. [Google Scholar] [CrossRef]
- Han, G.-Z. Evolution of Jasmonate Biosynthesis and Signaling Mechanisms. EXBOTJ 2016, 68, 1323–1331. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Pandian, S.; Rakkammal, K.; Largia, M.J.V.; Thamilarasan, S.K.; Balaji, S.; Zoclanclounon, Y.A.B.; Shilpha, J.; Ramesh, M. Jasmonates in Plant Growth and Development and Elicitation of Secondary Metabolites: An Updated Overview. Front. Plant Sci. 2022, 13, 942789. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, M.; Erb, M. Integration of Two Herbivore-induced Plant Volatiles Results in Synergistic Effects on Plant Defence and Resistance. Plant Cell Environ. 2019, 42, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.; Michaelson, L.V.; Haslam, R.; Bellec, Y.; Gissot, L.; Marion, J.; Da Costa, M.; Boutin, J.-P.; Miquel, M.; Tellier, F.; et al. The Very-Long-Chain Hydroxy Fatty Acyl-CoA Dehydratase PASTICCINO2 Is Essential and Limiting for Plant Development. Proc. Natl. Acad. Sci. USA 2008, 105, 14727–14731. [Google Scholar] [CrossRef]
- Kobayashi, F.; Maeta, E.; Terashima, A.; Kawaura, K.; Ogihara, Y.; Takumi, S. Development of Abiotic Stress Tolerance via bZIP-Type Transcription Factor LIP19 in Common Wheat. J. Exp. Bot. 2008, 59, 891–905. [Google Scholar] [CrossRef]
- Bate, N.J.; Sivasankar, S.; Moxon, C.; Riley, J.M.C.; Thompson, J.E.; Rothstein, S.J. Molecular Characterization of an Arabidopsis Gene Encoding Hydroperoxide Lyase, a Cytochrome P-450 That Is Wound Inducible1. Plant Physiol. 1998, 117, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Van Camp, W.; Van Montagu, M.; Inzé, D.; Asada, K. Superoxide Dismutase in Plants. Crit. Rev. Plant Sci. 1994, 13, 199–218. [Google Scholar] [CrossRef]
- Sen Raychaudhuri, S.; Deng, X.W. The Role of Superoxide Dismutase in Combating Oxidative Stress in Higher Plants. Bot. Rev. 2000, 66, 89–98. [Google Scholar] [CrossRef]
- Berwal, M.K.; Ram, C. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. In Abiotic and Biotic Stress in Plants; Bosco De Oliveira, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-811-2. [Google Scholar]
- Sharma, I.; Ahmad, P. Catalase. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–148. ISBN 978-0-12-799963-0. [Google Scholar]
- Zeng, C.-Q.; Liu, W.-X.; Hao, J.-Y.; Fan, D.-N.; Chen, L.-M.; Xu, H.-N.; Li, K.-Z. Measuring the Expression and Activity of the CAT Enzyme to Determine Al Resistance in Soybean. Plant Physiol. Biochem. 2019, 144, 254–263. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase Function in Plants: A Focus on Arabidopsis Mutants as Stress-Mimic Models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A Major Locus Controlling Malondialdehyde Content under Water Stress Is Associated with Fusarium Crown Rot Resistance in Wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Diani, Z.; Yassine, O.; Aissam, S.; Hsissou, D.; El Modafar, C. Induction of Early Oxidative Events in Soft Wheat Leaves Inoculated with Septoria Tritici and Their Relationship to Resistance of Moroccan Cultivars. Int. J. Agric. Biol. 2009, 11, 351–359. [Google Scholar]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Ahmed, S.S.; He, Y.M. Exogenous Abscisic Acid Increases Antioxidant Enzymes and Related Gene Expression in Pepper (Capsicum Annuum) Leaves Subjected to Chilling Stress. Genet. Mol. Res. 2012, 11, 4063–4080. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Ashraf, M.A.; Rasheed, R.; Iqbal, M.; Ibrahim, M.; Ashraf, S. Heat Shock Increases Oxidative Stress to Modulate Growth and Physico-Chemical Attributes in Diverse Maize Cultivars. Int. Agrophysics 2016, 30, 519–531. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Sun, S.; Lu, X.; Li, Q.; Li, L.; Wang, K.; Liu, J. Effects of Salt Stress on the Antioxidant Activity and Malondialdehyde, Solution Protein, Proline, and Chlorophyll Contents of Three Malus Species. Life 2022, 12, 1929. [Google Scholar] [CrossRef] [PubMed]

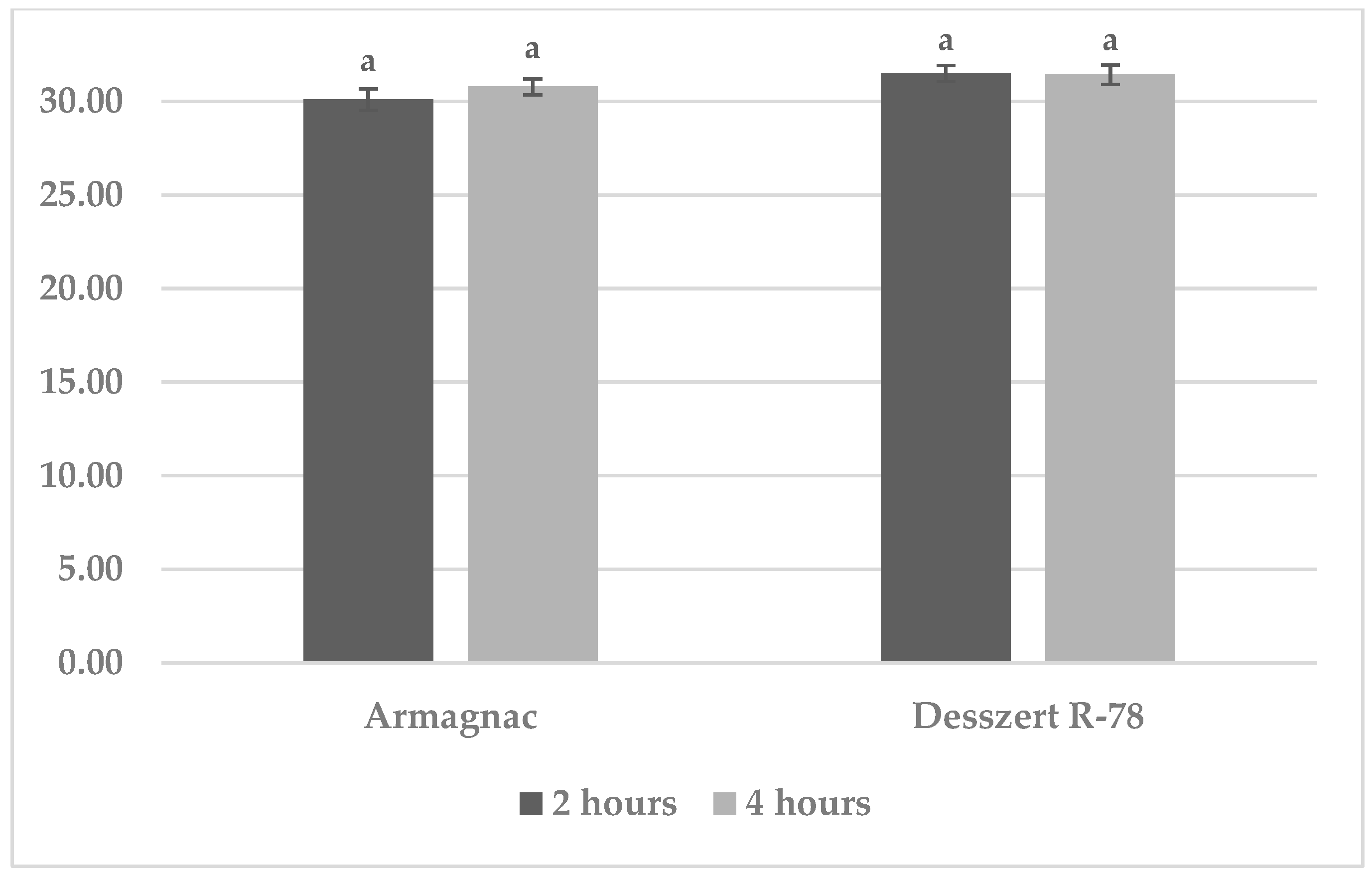
| Chemical Compound | Treatment | Sampling 2 h after Treatments | Sampling 4 h after Treatments |
|---|---|---|---|
| DIMBOA | Control | 27.93 ± 8.56 a1 | 12.17 ± 2.79 a1 |
| Mechanical damage | 34.62 ± 8.43 a1 | 32.62 ± 6.54 b1 | |
| Ethylene | 38.87 ± 9.08 a1 | 28.56 ± 5.27 b1 | |
| HMBOA | Control | 12.36 ± 2.10 a1 | 11.90 ± 2.57 a1 |
| Mechanical damage | 19.41 ± 2.01 a1 | 13.88 ± 2.55 a1 | |
| Ethylene | 15.00 ± 3.75 a1 | 15.43 ± 1.77 a1 | |
| DIMBOA+ HMBOA | Control | 40.29 ± 7.75 a1 | 24.07 ± 5.27 a1 |
| Mechanical damage | 54.04 ± 8.43 a1 | 46.50 ± 6.52 b1 | |
| Ethylene | 53.87 ± 8.79 a1 | 43.99 ± 4.99 b1 |
| Chemical Compound | Treatment | Sampling 2 h after Treatments | Sampling 4 h after Treatments |
|---|---|---|---|
| DIMBOA | Control | 26.45 ± 6.76 a1 | 72.11 ± 7.87 b2 |
| Mechanical damage | 15.07 ± 2.91 a1 | 44.51 ± 8.36 ab2 | |
| Ethylene | 26.87 ± 4.31 a1 | 36.43 ± 8.01 a1 | |
| HMBOA | Control | 27.80 ± 9.19 a1 | 18.28 ± 3.03 a1 |
| Mechanical damage | 55.47 ± 24.85 a1 | 36.84 ± 7.57 ab1 | |
| Ethylene | 23.72 ± 2.36 a1 | 23.32 ± 3.80 a1 | |
| DIMBOA+ HMBOA | Control | 54.25 ± 7.69 a1 | 90.39 ± 6.47 b2 |
| Mechanical damage | 70.53 ± 23.51 a1 | 81.35 ± 3.47 ab2 | |
| Ethylene | 50.59 ± 3.72 a1 | 59.75 ± 4.41 a1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makleit, P.; Gulyás, G.; Czeglédi, L.; Veres, S. Influence of Exogenous Ethylene and Mechanical Damage on Gene Expression and Physiological Parameters of Maize Hybrids. Agronomy 2024, 14, 1950. https://doi.org/10.3390/agronomy14091950
Makleit P, Gulyás G, Czeglédi L, Veres S. Influence of Exogenous Ethylene and Mechanical Damage on Gene Expression and Physiological Parameters of Maize Hybrids. Agronomy. 2024; 14(9):1950. https://doi.org/10.3390/agronomy14091950
Chicago/Turabian StyleMakleit, Péter, Gabriella Gulyás, Levente Czeglédi, and Szilvia Veres. 2024. "Influence of Exogenous Ethylene and Mechanical Damage on Gene Expression and Physiological Parameters of Maize Hybrids" Agronomy 14, no. 9: 1950. https://doi.org/10.3390/agronomy14091950






