Genome-Wide Identification of the Shaker Potassium Channel Family in Chinese Cabbage and Functional Studies of BrKAT1 in Yeast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification of Shaker K+ Channel Family Members
2.2. Phylogenetic Tree Analysis, Gene Structure, and Chromosome Position
2.3. Intraspecific Collinearity
2.4. Interspecific Collinearity
2.5. Promoter Analysis
2.6. Plant Culture, Gene Cloning, Vector Construction, and Yeast Transformation
2.7. Yeast Assays
3. Results
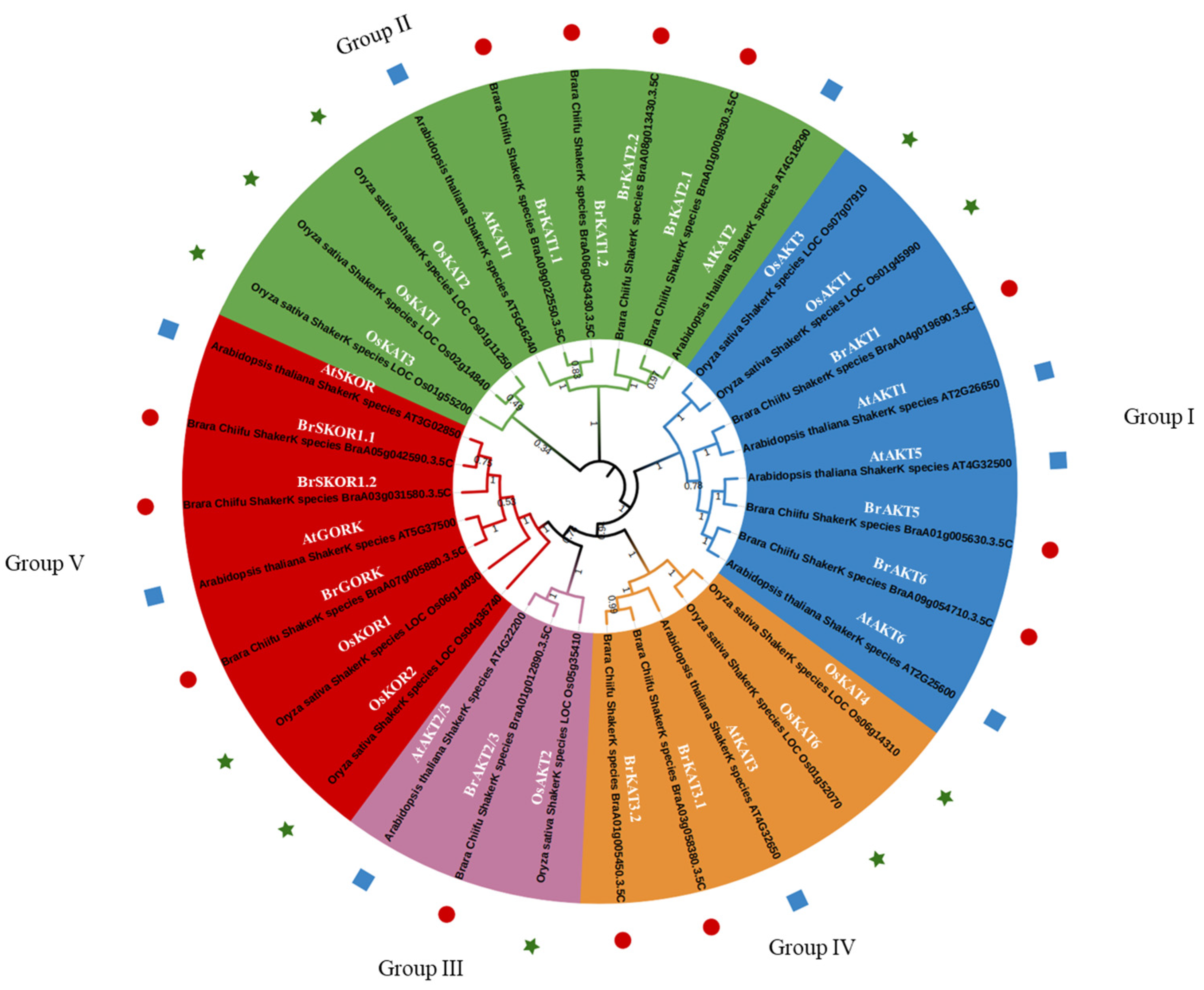
3.1. Phylogenetic Tree Analysis
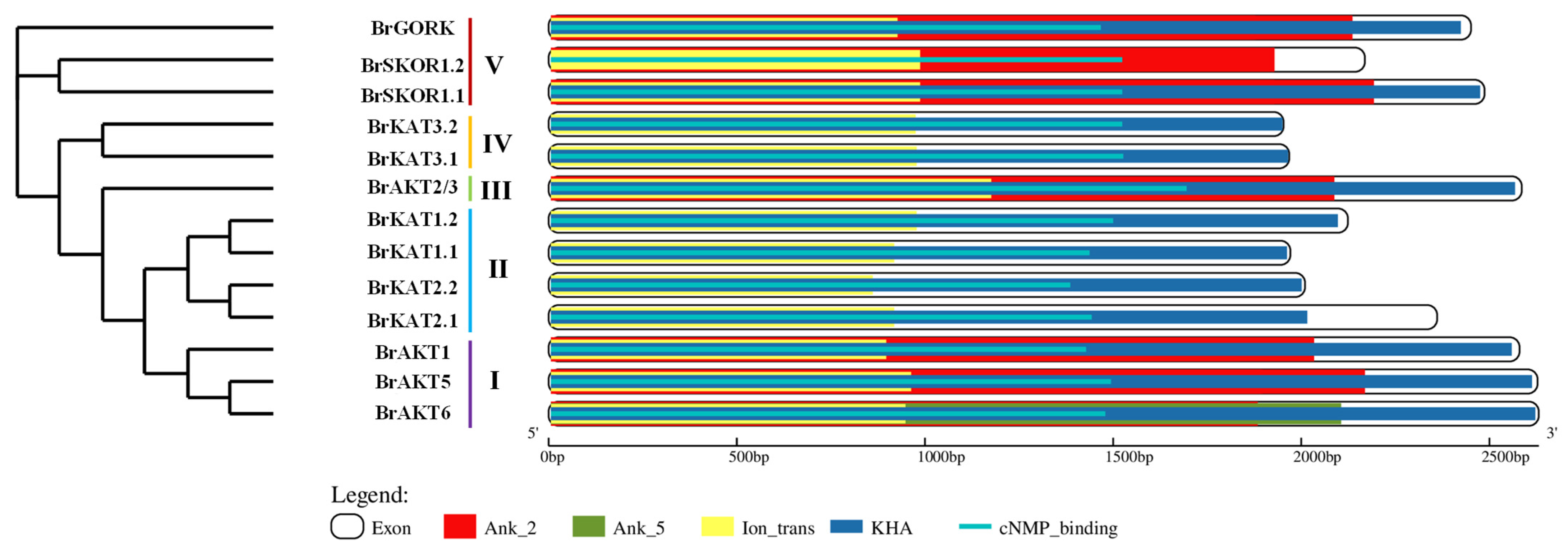
3.2. Protein Domains of the Chinese Cabbage Shaker K+ Channel
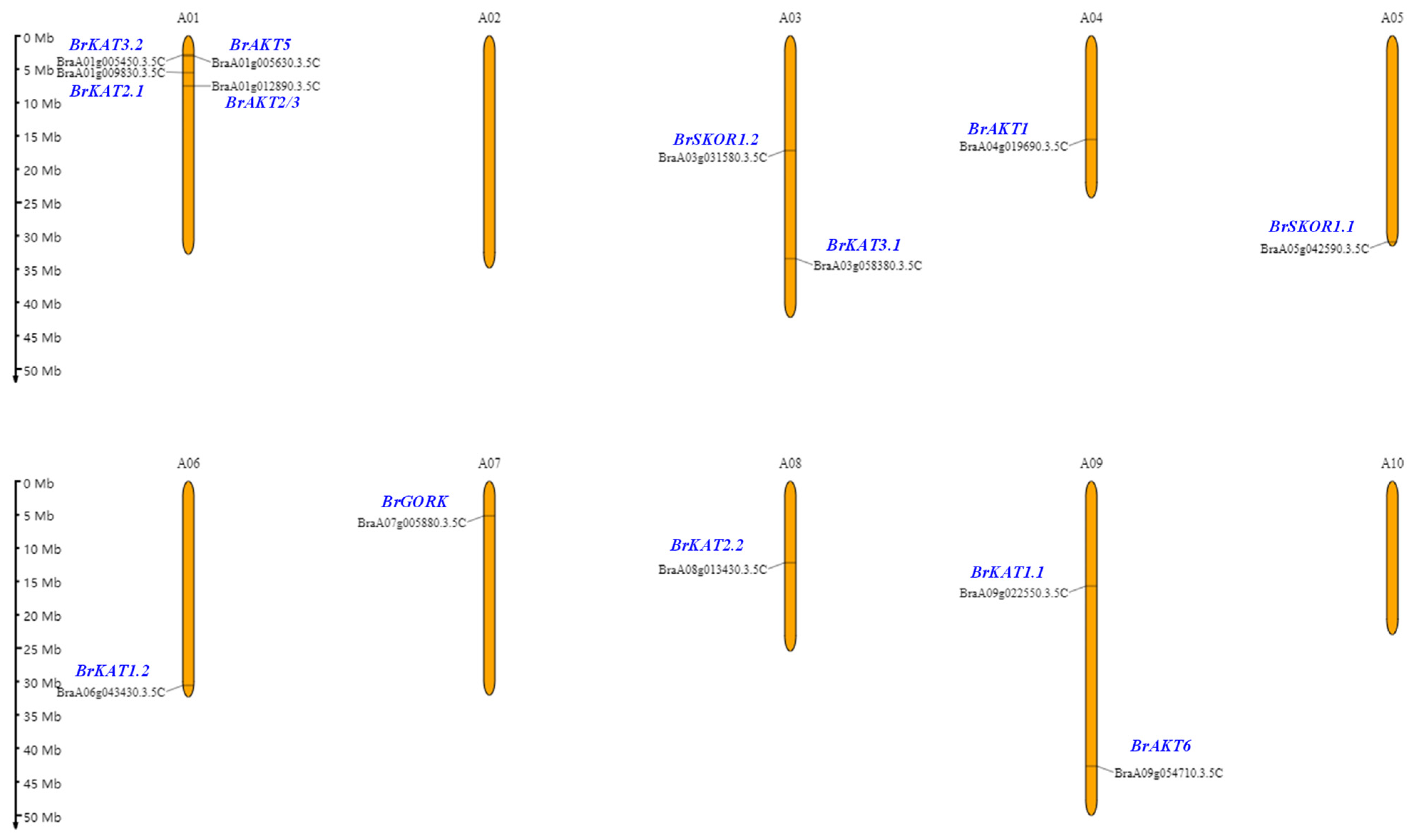
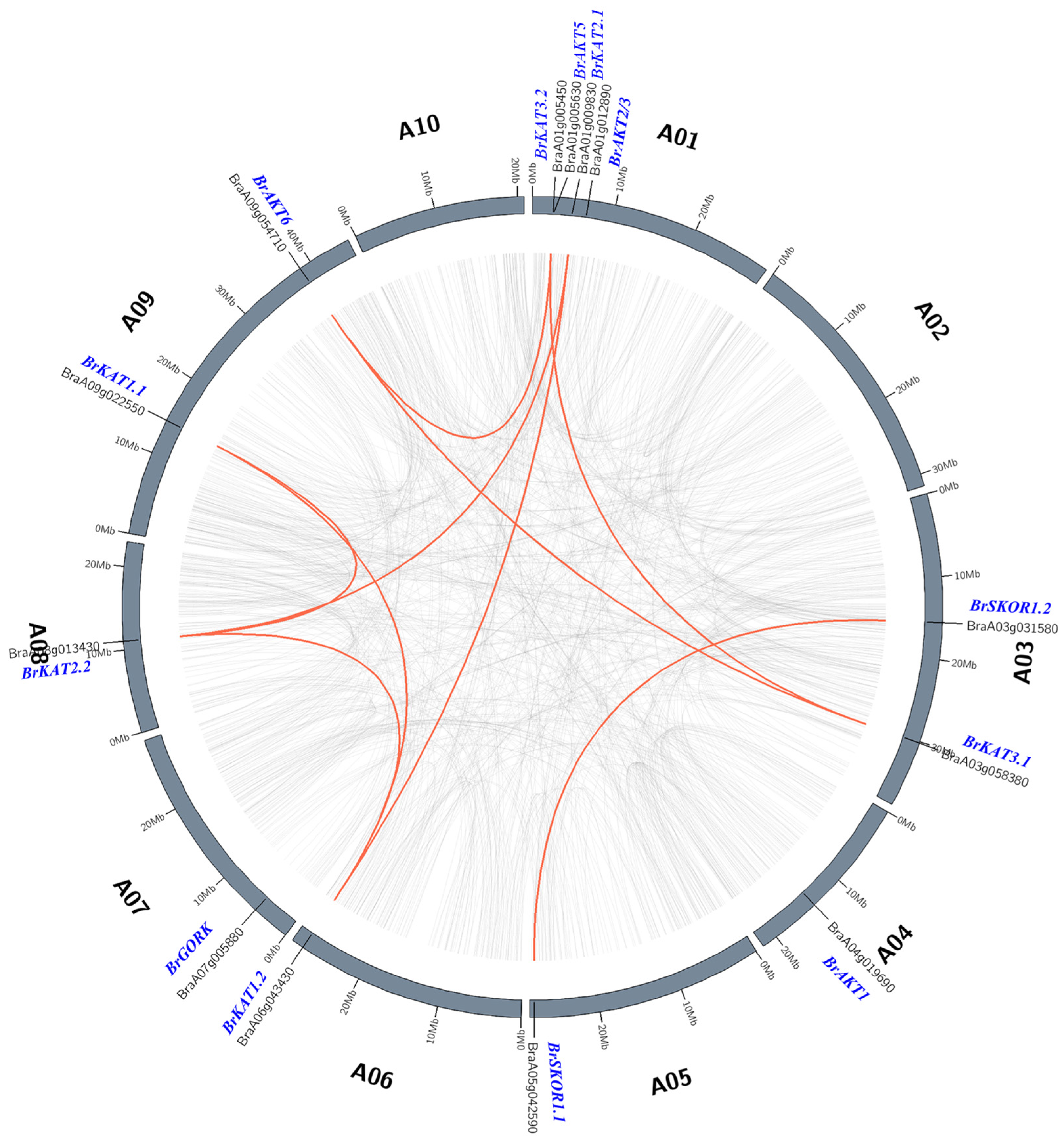
3.3. Chromosome Position and Intraspecific Collinearity of Shaker K+ Channels in Chinese Cabbage
3.4. Interspecific Collinearity
3.5. Promoter Analysis
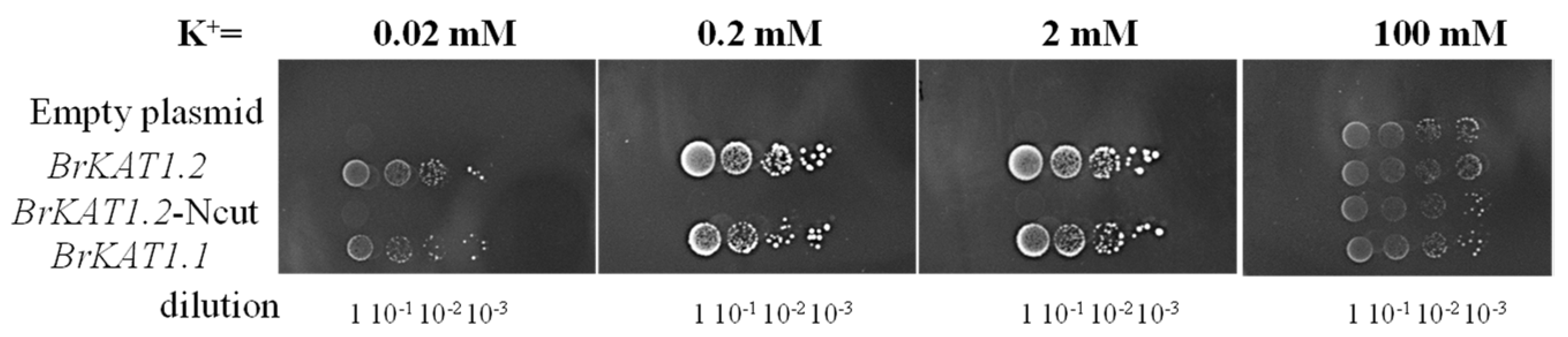
3.6. Both BrKAT1 Proteins Have Potassium Absorption Functions, and the N-Terminus Is Necessary for Maintaining Potassium Channel Function
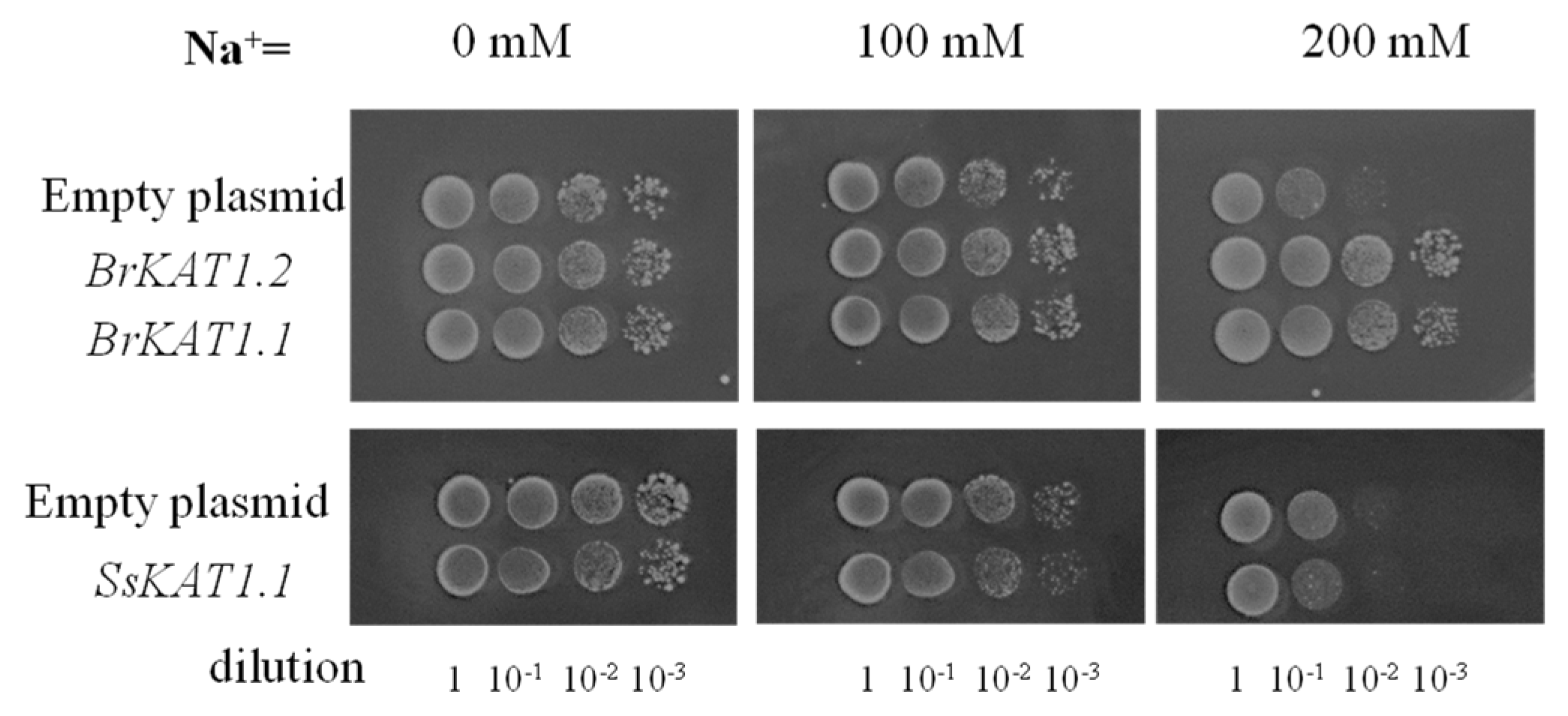
3.7. BrKAT1 Enhances Cell Salt Tolerance
4. Discussion
4.1. Gene Expansion Exists in the Shaker K+ Channel of Chinese Cabbage, and the Segmental Duplication of the Genes in Group II and Group V as the Main Reason for the Above Phenomenon
4.2. The Two Homologs of Group II, BrKAT1.1 and BrKAT1.2, Have Similar Potassium Uptake and Salt Tolerance Ability but Possess Distinct Promoter Elements
4.3. The N-Terminus Is Necessary for Maintaining the Function of BrKAT1
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, G.; Nong, T.; Hunpatin, O.S.; Shi, C.; Su, X.; Xu, F.; Wang, Y.; Zhang, Z.; Ning, Y.; Liu, H.; et al. Genome-wide identification of Shaker K(+) channel family in Nicotiana tabacum and functional analysis of NtSKOR1B in response to salt stress. Front. Plant Sci. 2024, 15, 1378738. [Google Scholar] [CrossRef] [PubMed]
- Very, A.A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef] [PubMed]
- Jegla, T.; Busey, G.; Assmann, S.M. Evolution and Structural Characteristics of Plant Voltage-Gated K(+) Channels. Plant Cell 2018, 30, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, D.; Basset, M.; Lepetit, M.; Conejero, G.; Gaymard, F.; Astruc, S.; Grignon, C. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J. 1996, 9, 195–203. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A role for the AKT1 potassium channel in plant nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef]
- Mouline, K.; Very, A.A.; Gaymard, F.; Boucherez, J.; Pilot, G.; Devic, M.; Bouchez, D.; Thibaud, J.B.; Sentenac, H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K(+) channel in Arabidopsis. Gene Dev. 2002, 16, 339–350. [Google Scholar] [CrossRef]
- Lacombe, B.; Pilot, G.; Michard, E.; Gaymard, F.; Sentenac, H.; Thibaud, J.B. A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 2000, 12, 837–851. [Google Scholar]
- Lebaudy, A.; Vavasseur, A.; Hosy, E.; Dreyer, I.; Leonhardt, N.; Thibaud, J.B.; Véry, A.A.; Simonneau, T.; Sentenac, H. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. USA 2008, 105, 5271–5276. [Google Scholar] [CrossRef]
- Huang, Y.N.; Yang, S.Y.; Li, J.L.; Wang, S.F.; Wang, J.J.; Hao, D.L.; Su, Y.H. The rectification control and physiological relevance of potassium channel OsAKT2. Plant Physiol. 2021, 187, 2296–2310. [Google Scholar] [CrossRef]
- Reintanz, B.; Szyroki, A.; Ivashikina, N.; Ache, P.; Godde, M.; Becker, D.; Palme, K.; Hedrich, R. AtKC1, a silent Arabidopsis potassium channel α-subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA 2002, 99, 4079–4084. [Google Scholar] [CrossRef]
- Geiger, D.; Becker, D.; Vosloh, D.; Gambale, F.; Palme, K.; Rehers, M.; Anschuetz, U.; Dreyer, I.; Kudla, J.; Hedrich, R. Heteromeric AtKC1middle dotAKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 2009, 284, 21288–21295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, L.; Li, H.D.; Xu, J.; Wu, W.H. Potassium channel alpha-subunit AtKC1 negatively regulates AKT1-mediated K(+) uptake in Arabidopsis roots under low-K(+) stress. Cell Res. 2010, 20, 826–837. [Google Scholar] [CrossRef]
- Jeanguenin, L.; Alcon, C.; Duby, G.; Boeglin, M.; Cherel, I.; Gaillard, I.; Zimmermann, S.; Sentenac, H.; Very, A.A. AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 2011, 67, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferrière, N.; Thibaud, J.B.; Sentenac, H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ache, P.; Becker, D.; Ivashikina, N.; Dietrich, P.; Roelfsema, M.R.G.; Hedrich, R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000, 486, 93–98. [Google Scholar] [CrossRef]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F.; Porée, F.; Boucherez, J.; Lebaudy, A.; Bouchez, D.; Véry*, A.A.; et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Ivashikina, N.; Becker, D.; Ache, P.; Meyerhoff, O.; Felle, H.H.; Hedrich, R. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 2001, 508, 463–469. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, J.; Zhou, X.R.; Yuan, H.J.; Kumar, T.; Luan, S.; Wang, S.M. ZxAKT1 is essential for K(+) uptake and K(+) /Na(+) homeostasis in the succulent xerophyte Zygophyllum xanthoxylum. Plant J. 2017, 90, 48–60. [Google Scholar] [CrossRef]
- Li, J.; Gao, Z.; Zhou, L.; Li, L.; Zhang, J.; Liu, Y.; Chen, H. Comparative transcriptome analysis reveals K+ transporter gene contributing to salt tolerance in eggplant. BMC Plant Biol. 2019, 19, 67. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Fang, Q.; Chang, X.; Sun, M.; Li, W.; Li, Y. GmAKT1 is involved in K(+) uptake and Na(+)/K(+) homeostasis in Arabidopsis and soybean plants. Plant Sci. 2021, 304, 110736. [Google Scholar] [CrossRef]
- Ahmad, I.; Mian, A.; Maathuis, F.J. Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. J. Exp. Bot. 2016, 67, 2689–2698. [Google Scholar] [CrossRef]
- Feng, X.; Liu, W.; Cao, F.; Wang, Y.; Zhang, G.; Chen, Z.H.; Wu, F. Overexpression of HvAKT1 improves drought tolerance in barley by regulating root ion homeostasis and ROS and NO signaling. J. Exp. Bot. 2020, 71, 6587–6600. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Q.; Qi, K.; Xie, Z.; Yin, H.; Wang, P.; Wang, R.; Huang, Z.; Zhang, S.; Wang, L.; et al. Identification of Shaker K(+) channel family members in Rosaceae and a functional exploration of PbrKAT1. Planta 2019, 250, 1911–1925. [Google Scholar] [CrossRef] [PubMed]
- Azeem, F.; Ijaz, U.; Ali, M.A.; Hussain, S.; Zubair, M.; Manzoor, H.; Abid, M.; Zameer, R.; Kim, D.S.; Golokhvast, K.S.; et al. Genome-Wide Identification and Expression Profiling of Potassium Transport-Related Genes in Vigna radiata under Abiotic Stresses. Plants 2021, 11, 2. [Google Scholar] [CrossRef]
- Feng, C.; He, C.; Wang, Y.; Xu, H.; Xu, K.; Zhao, Y.; Yao, B.; Zhang, Y.; Zhao, Y.; Idrice Carther, K.F.; et al. Genome-wide identification of soybean Shaker K(+) channel gene family and functional characterization of GmAKT1 in transgenic Arabidopsis thaliana under salt and drought stress. J. Plant Physiol. 2021, 266, 153529. [Google Scholar] [CrossRef]
- Yang, Y.; Han, J.; Zhang, Y.; Lin, S.; Liang, M.; Zhao, L.; Song, Z.; Filiz, E. Genome-Wide Identification and Characterization of the Shaker-Type K+ Channel Genes in Prunus persica (L.) Batsch. Int. J. Genom. 2022, 2022, 5053838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Y.; Wang, H.; Wang, X.; Lv, M.; Yang, P.; Zhang, L. Identification and Characterization of Shaker K(+) Channel Gene Family in Foxtail Millet (Setaria italica) and Their Role in Stress Response. Front. Plant Sci. 2022, 13, 907635. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Qi, G.-N.; Li, J.; Xu, Z.-J.; Wu, W.-H.; Wang, Y. The Os-AKT1 Channel Is Critical for K+ Uptake in Rice Roots and Is Modulated by the Rice CBL1-CIPK23 Complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef]
- Sun, D.; Xu, J.; Wang, H.; Guo, H.; Chen, Y.; Zhang, L.; Li, J.; Hao, D.; Yao, X.; Li, X. Genome-Wide Identification and Expression Analysis of the PUB Gene Family in Zoysia japonica under Salt Stress. Plants 2024, 13, 788. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Kong, W.; Shi, J.; Yang, B.; Yu, S.; Zhao, P.; Guo, Z.; Zhu, H. Genome-Wide Analysis of the Wall-Associated Kinase (WAK) Genes in Medicago truncatula and Functional Characterization of MtWAK24 in Response to Pathogen Infection. Plants 2023, 12, 1849. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.L.; Zhou, J.Y.; Yang, S.Y.; Huang, Y.N.; Su, Y.H. Functional and Regulatory Characterization of Three AMTs in Maize Roots. Front. Plant Sci. 2020, 11, 884. [Google Scholar] [CrossRef]
- Duan, H.-R.; Ma, Q.; Zhang, J.-L.; Hu, J.; Bao, A.-K.; Wei, L.; Wang, Q.; Luan, S.; Wang, S.-M. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 2015, 395, 173–187. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, A.; Sun, J.; Chen, X.; Liu, M.; Zhao, P.; Jiang, W.; Tang, Z. Identification of Shaker K(+) channel family members in sweetpotato and functional exploration of IbAKT1. Gene 2021, 768, 145311. [Google Scholar] [CrossRef]
- Mun, J.H.; Kwon, S.J.; Yang, T.J.; Seol, Y.J.; Jin, M.; Kim, J.A.; Lim, M.H.; Kim, J.S.; Baek, S.; Choi, B.S.; et al. Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol. 2009, 10, R111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Sun, R.; Hou, X.; Zheng, H.; Zhang, F.; Zhang, Y.; Liu, B.; Liang, J.; Zhuang, M.; Liu, Y.; et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Zhang, Y.; Hu, L.; Zhai, M.; Xuan, J. Genome-wide identification and expression analysis of auxin response factor (ARF) gene family in pecan indicates its possible roles during graft union formation. Sci. Hortic. 2023, 322, 112401. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. Brassica rapa Genome Sequencing Project, C., The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Chen, H.; Zhang, Z.; Wu, J.; Wang, X. A near-complete genome assembly of Brassica rapa provides new insights into the evolution of centromeres. Plant Biotechnol. J. 2023, 21, 1022–1032. [Google Scholar] [CrossRef]
- Li, W.H.; Gojobori, T.; Nei, M. Pseudogenes as a paradigm of neutral evolution. Nature 1981, 292, 237–239. [Google Scholar] [CrossRef]
- Tian, R.; Yang, Y.; Chen, M. Genome-wide survey of the amino acid transporter gene family in wheat (Triticum aestivum L.): Identification, expression analysis and response to abiotic stress. Int. J. Biol. Macromol. 2020, 162, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Cherel, I. Regulation of K+ channel activities in plants: From physiological to molecular aspects. J. Exp. Bot. 2004, 55, 337–351. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Li, X.; Kong, W.; Chen, R.; Liu, J.; Guo, H.; Zhou, J. Phosphorylation regulation of nitrogen, phosphorus, and potassium uptake systems in plants. Crop J. 2023, 11, 1034–1047. [Google Scholar] [CrossRef]
- Marten, I.; Hoshi, T. The N-terminus of the K channel KAT1 controls its voltage-dependent gating by altering the membrane electric field. Biophys. J. 1998, 74, 2953–2962. [Google Scholar] [CrossRef]
- Marten, I.; Hoshi, T. Voltage-dependent gating characteristics of the K+ channel KAT1 depend on the N and C termini. Proc. Natl. Acad. Sci. USA 1997, 94, 3448–3453. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, M.; Jia, Y.; Yang, F.; Zhang, Y.; Xu, X.; Li, X.; Yang, F.; Lei, J.; Wang, Y.; et al. Structural basis for the activity regulation of a potassium channel AKT1 from Arabidopsis. Nat. Commun. 2022, 13, 5682. [Google Scholar] [CrossRef]


| Name | Primers (5′ to 3′) |
|---|---|
| BrKAT1.1-F | actatagggaatattaagcttATGTCCATCTCTTGCACCAGAAACT |
| BrKAT1.1-R | tgatggatatctgcagaattcTCAGCTTGATGAGAAAAACAAATGA |
| BrKAT1.2Ncut-F | actatagggaatattaagcttATGTGGCTTGTCCTTCTAGTTATTTACT |
| BrKAT1.2Ncut-R | tgatggatatctgcagaattcTCAATGTCTACCTTCAAACTCAATTTG |
| BrKAT1.2-F | actatagggaatattaagcttATGCCAATCTCTTGTACCAGAAACT |
| BrKAT1.2-R | tgatggatatctgcagaattcTCAATGTCTACCTTCAAACTCAATTTG |
| Protein Name | Gene_ID | Length | MW (kDa) | pI |
|---|---|---|---|---|
| BrKAT3.2 | BraA01g005450.3.5C | 651 | 74.51 | 7.24 |
| BrAKT5 | BraA01g005630.3.5C | 876 | 98.16 | 6.94 |
| BrKAT2.1 | BraA01g009830.3.5C | 787 | 90.79 | 9.18 |
| BrAKT2/3 | BraA01g012890.3.5C | 862 | 97.92 | 6.73 |
| BrSKOR1.2 | BraA03g031580.3.5C | 723 | 82.54 | 6.84 |
| BrKAT3.1 | BraA03g058380.3.5C | 656 | 75.19 | 8.19 |
| BrAKT1 | BraA04g019690.3.5C | 860 | 97.44 | 7.9 |
| BrSKOR1.1 | BraA05g042590.3.5C | 829 | 94.05 | 6.65 |
| BrKAT1.2 | BraA06g043430.3.5C | 688 | 79.05 | 6.4 |
| BrGORK | BraA07g005880.3.5C | 817 | 94.11 | 6.05 |
| BrKAT2.2 | BraA08g013430.3.5C | 670 | 76.94 | 8.67 |
| BrKAT1.1 | BraA09g022550.3.5C | 657 | 75.69 | 7.11 |
| BrAKT6 | BraA09g054710.3.5C | 877 | 98 | 6.64 |
| Gene Pairs | Ks | Ka | Ka/Ks |
|---|---|---|---|
| BrKAT1.1-BrKAT2.2 | 0.17 | 1.11 | 0.15 |
| BrKAT2.2-BrKAT1.2 | 0.21 | 1.16 | 0.18 |
| BrKAT1.1-BrKAT1.2 | 0.05 | 0.30 | 0.18 |
| BrKAT2.1-BrKAT2.2 | 0.09 | 0.47 | 0.20 |
| BrKAT1.2-BrKAT2.1 | 0.16 | 1.48 | 0.11 |
| BrSKOR1.1-BrSKOR1.2 | 0.04 | 0.35 | 0.10 |
| BrAKT6-BrKAT3.1 | 0.74 | 4.65 | 0.16 |
| BrKAT3.1-BrKAT3.2 | 0.06 | 0.22 | 0.28 |
| BrAKT6-BrKAT3.2 | 0.56 | 4.55 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.-Y.; Gu, Z.-C.; Hao, D.-L. Genome-Wide Identification of the Shaker Potassium Channel Family in Chinese Cabbage and Functional Studies of BrKAT1 in Yeast. Agronomy 2024, 14, 1954. https://doi.org/10.3390/agronomy14091954
Zhou J-Y, Gu Z-C, Hao D-L. Genome-Wide Identification of the Shaker Potassium Channel Family in Chinese Cabbage and Functional Studies of BrKAT1 in Yeast. Agronomy. 2024; 14(9):1954. https://doi.org/10.3390/agronomy14091954
Chicago/Turabian StyleZhou, Jin-Yan, Ze-Chen Gu, and Dong-Li Hao. 2024. "Genome-Wide Identification of the Shaker Potassium Channel Family in Chinese Cabbage and Functional Studies of BrKAT1 in Yeast" Agronomy 14, no. 9: 1954. https://doi.org/10.3390/agronomy14091954
APA StyleZhou, J.-Y., Gu, Z.-C., & Hao, D.-L. (2024). Genome-Wide Identification of the Shaker Potassium Channel Family in Chinese Cabbage and Functional Studies of BrKAT1 in Yeast. Agronomy, 14(9), 1954. https://doi.org/10.3390/agronomy14091954






