Influence of Climatic Parameters and Plant Morphological Characters on the Total Anthocyanin Content of Purple Maize (Zea mays L., PMV-581) Cob Core
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Characteristics of the Localities
2.2. Biological Materials
2.3. Characteristics of the Cultivation Plots
2.4. Methodology
2.5. Statistical Methods
3. Results
3.1. Morphological Characteristics of the Plant and Cob of PMV-581 Purple Maize
3.2. Climatic Parameters of the PMV-581 Purple Maize Growing Locations
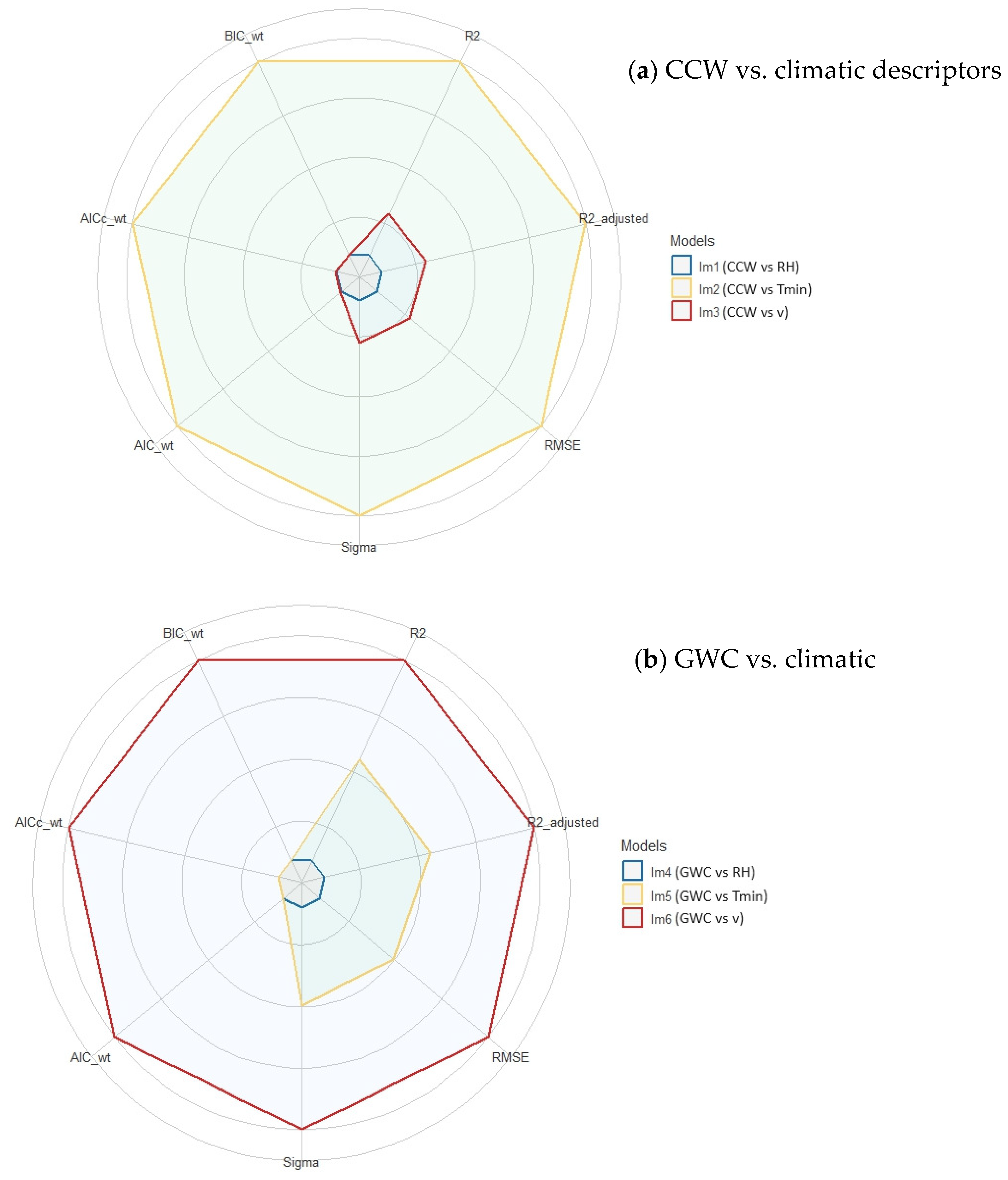
3.3. Multiple Linear Correlation between Morphological (CCW, GWC and PL) and Climatic Descriptors (Tmin, RH and v)
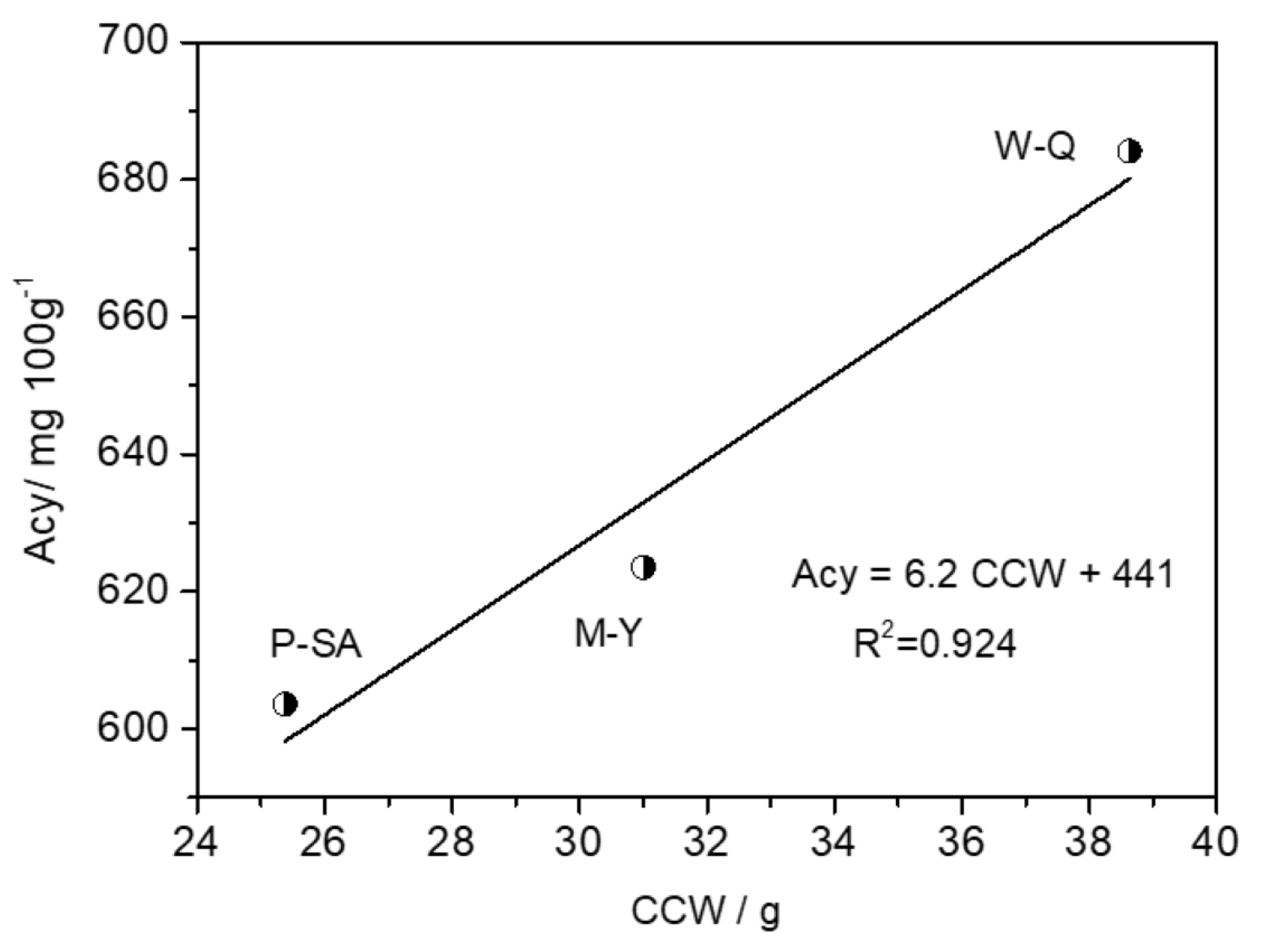
3.4. Anthocyanin Content Acy in Cob Core of PVM-581 Purple Maize: Relationship between Morphological and Climatic Descriptors
4. Discussion
- -
- Within the range of values reported by [2], between 290 and 1333 mg/100 g of dry matter, depending on the tissues, varieties and country of cultivation. These authors also highlight that variations in the climatic parameters of the growing areas have an impact on the final Acy, with those that develop at low temperatures and in acidic conditions being more stable. Ref. [63] reported a range between 93 and 851 mg/100 g of whole grain for the pigmented phenotypes, which are the highest values among the Mexican corn phenotypes studied.
- -
- Higher than those reported by i/ [43] for Mexican purple corn varieties, which are between 9.35 and 27.04 mg/100 g of cob; ii/ Medina-Hoyos [9] for Peruvian varieties (including PMV-581), which are between 4.14 and 6.12 mg/100 g of cob core; iii/ [12] also for Peruvian varieties, between 54 and 115 mg/100 g of grain; and iv/ [64,65,66] with 43.02 (for Commercial variety, Korea), 141.58 (for KGW1, Thailand) and 304.5 (for Hybrid, China) mg/100 g of grain, respectively.
- -
- Lower than those reported by i/ [55] for the Peruvian varieties Canta, Joya and PMV-581, which are between 1336 and 2060 mg/100 g of cob core; ii/ [63] for a commercial Peruvian variety, with a very high value of 125.76 mg/g of cob core; and iii/ [7], also for Peruvian commercial varieties, determined by HPLC between 26 and 38 mg per gram of cob core extract.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pedraza, M.; Idogro, G.; Pedraza, S. Densidad de siembra y comportamiento agronómico de tres variedades de maíz morado (Zea mayz L.). Rev. ECIPeru 2017, 14, 20–40. [Google Scholar] [CrossRef]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in Metabolites of Purple Corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- MIDAGRI. Compendio Anual de “PRODUCCIÓN AGRÍCOLA”. Available online: https://www.gob.pe/institucion/midagri/informes-publicaciones/2730325-compendio-anual-de-produccion-agricola (accessed on 10 June 2024).
- Salvador-Reyes, R.; Silva, M.T.P. Peruvian Andean Maize: General Characteristics, Nutritional Properties, Bioactive Compounds, and Culinary Uses. Food Res. Int. 2020, 130, 108934. [Google Scholar] [CrossRef] [PubMed]
- MIDAGRI. Análisis de Mercado—Maíz Morado 2015–2021. Available online: https://www.gob.pe/institucion/sse/informes-publicaciones/2624383-analisis-de-mercado-maiz-morado-2015-2021 (accessed on 10 June 2024).
- Guillén-Sánchez, J.; Mori-Arismendi, S.; Paucar-Menacho, L.M. Características y propiedades funcionales del maíz morado (Zea mayz L.) var. subnigroviolaceo. Sci. Agropecu. 2010, 5, 211–217. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Purple Corn (Zea mayz L.) Pericarp Hydroalcoholic Extracts Obtained by Conventional Processes at Atmospheric Pressure and by Processes at High Pressure. Braz. J. Chem. Eng. 2020, 37, 237–248. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, K.Y.; Kim, M.; Hong, M.; Deepa, P.; Kim, S. A Review of the Biological Properties of Purple Corn (Zea mayz L.). Sci. Pharm. 2023, 91, 6. [Google Scholar] [CrossRef]
- Medina-Hoyos, A.; Narro-León, L.; Chávez-Cabrera, A. Cultivo de maíz morado (Zea mayz L.) en zona altoandina de Perú: Adaptación e identificación de cultivares de alto rendimiento y contenido de antocianina. Sci. Agropecu. 2020, 11, 291–299. [Google Scholar] [CrossRef]
- Rabanal-Atalaya, M.; Medina-Hoyos, A. Evaluación del rendimiento, características morfológicas y químicas de variedades del maíz morado (Zea mayz L.) en la región Cajamarca-Perú. Rev. TERRA Latinoam. 2021, 39, e829. [Google Scholar] [CrossRef]
- Grobman, A. Races of Maize in Peru: Their Origins, Evolution and Classification; National Academy of Sciences-National Research Council: Washington, DC, USA, 1961. [Google Scholar]
- Salinas Moreno, Y.; Sanchez, G.S.; Hernandez, D.R.; Lobato, N.R. Characterization of Anthocyanin Extracts from Maize Kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Pérez-Alonso, J.J.; Vázquez-Carrillo, G.; Aragón-Cuevas, F.; Velázquez-Cardelas, G.A. Antocianinas y actividad antioxidante en maíces (Zea mayz L.) de las razas chalqueño, elotes cónicos y bolita. Agrociencia 2012, 46, 693–706. [Google Scholar]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize—A Potential Source of Human Nutrition and Health: A Review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Houghton, A.; Appelhagen, I.; Martin, C. Natural Blues: Structure Meets Function in Anthocyanins. Plants 2021, 10, 726. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Akgun, B. Petunidin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In Handbook of Dietary Flavonoids; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–34. ISBN 978-3-030-94753-8. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Yoojin, L.; Ji-Young, L. Protective Actions of Polyphenols in the Development of Nonalcoholic Fatty Liver Disease. In Dietary Interventions in Liver Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 91–99. [Google Scholar]
- Thapphasaraphong, S.; Rimdusit, T.; Priprem, A.; Puthongking, P. Crops of Waxy Purple Corn: A Valuable Source of Antioxidative Phytochemicals. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 73–77. [Google Scholar] [CrossRef]
- Razgonova, M.; Zinchenko, Y.; Pikula, K.; Tekutyeva, L.; Son, O.; Zakharenko, A.; Kalenik, T.; Golokhvast, K. Spatial Distribution of Polyphenolic Compounds in Corn Grains (Zea mayz L. var. Pioneer) Studied by Laser Confocal Microscopy and High-Resolution Mass Spectrometry. Plants 2022, 11, 630. [Google Scholar] [CrossRef]
- Singh, A.A.; Agrawal, S.B.; Shahi, J.P.; Agrawal, M. Investigating the Response of Tropical Maize (Zea mayz L.) Cultivars against Elevated Levels of O3 at Two Developmental Stages. Ecotoxicology 2014, 23, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Upadhyaya, M.K. Influence of R/FR Ratio on Response of Maize, Lettuce, and Amaranthus retroflexus L. to UV-B Radiation. Weed Res. 2023, 63, 165–174. [Google Scholar] [CrossRef]
- Magaña-Cerino, J.M.; Peniche-Pavía, H.A.; Tiessen, A.; Gurrola-Díaz, C.M. Pigmented Maize (Zea mayz L.) Contains Anthocyanins with Potential Therapeutic Action Against Oxidative Stress—A Review. Pol. J. Food Nutr. Sci. 2020, 70, 85–99. [Google Scholar] [CrossRef]
- Aguilar-Hernández, Á.D.; Salinas-Moreno, Y.; Ramírez-Díaz, J.L.; Alemán-De la Torre, I.; Bautista-Ramírez, E.; Flores-López, H.E. Anthocyanins and Color in Grain and Cob of Peruvian Purple Corn Grown in Jalisco, Mexico. Rev. Mex. Cienc. Agrícolas 2019, 10, 1071–1082. [Google Scholar]
- Aguilera, M.; Reza, M.d.C.; Chew, R.G.; Meza, J.A. Propiedades funcionales de las antocianinas. Biotecnia 2011, 13, 16–22. [Google Scholar] [CrossRef]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of Anthocyanin Composition and Concentration in Diverse Maize Germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Raja, S.; He, J.; Paramannil, M. Functional Relevance of Bioactive Compounds in Purple Maize: A Contemporary Extraction Progressions and Prospective Applications. Cereal Res. Commun. 2023, 51, 263–282. [Google Scholar] [CrossRef]
- Bae, H.-H.; Yi, G.; Go, Y.S.; Ha, J.Y.; Choi, Y.; Son, J.-H.; Shin, S.; Jung, T.-W.; Lee, S. Measuring Antioxidant Activity in Yellow Corn (Zea mayz L.) Inbreds from Three Different Geographic Regions. Appl. Biol. Chem. 2021, 64, 56. [Google Scholar] [CrossRef]
- Mutlu, C.; Arslan-Tontul, S.; Candal, C.; Kilic, O.; Erbas, M. Physicochemical, Thermal, and Sensory Properties of Blue Corn (Zea mayz L.). J. Food Sci. 2018, 83, 53–59. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple Corn (Zea mayz L.) Phenolic Compounds Profile and Its Assessment as an Agent Against Oxidative Stress in Isolated Mouse Organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef]
- Vargas-Yana, D.; Aguilar-Morón, B.; Pezo-Torres, N.; Shetty, K.; Ranilla, L.G. Ancestral Peruvian Ethnic Fermented Beverage “Chicha” Based on Purple Corn (Zea mayz L.): Unraveling the Health-Relevant Functional Benefits. J. Ethn. Foods 2020, 7, 35. [Google Scholar] [CrossRef]
- Gálvez, L.; Christopher, A.; Sarkar, D.; Shetty, K.; Chirinos, R.; Campos, D. Phenolic Composition and Evaluation of the Antimicrobial Activity of Free and Bound Phenolic Fractions from a Peruvian Purple Corn (Zea mayz L.) Accession. J. Food Sci. 2017, 82, 2968–2976. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, M.; Chu, Z.; Gao, Y.; Guo, L.; Ji, S.; Jiang, L.; Gong, L. Effects of Temperature on Growth and Grain Maturity of Spring Maize in Northeast China: A Study of Different Sowing Dates. Atmosphere 2023, 14, 1755. [Google Scholar] [CrossRef]
- Fuentes-Cardenas, I.S.; Cuba-Puma, R.; Marcilla-Truyenque, S.; Begazo-Gutiérrez, H.; Zolla, G.; Fuentealba, C.; Shetty, K.; Galvez, L. Diversity of the Peruvian Andean Maize (Zea mayz L.) Race Cabanita: Polyphenols, Carotenoids, in Vitro Antioxidant Capacity, and Physical Characteristics. Front. Nutr. 2022, 9, 983208. [Google Scholar] [CrossRef]
- Vidal, J.P. Las ocho regiones naturales del Perú. Terra Bras. Rev. Rede Bras. História Geogr. E Geogr. Histórica 2014, 3, 1–21. [Google Scholar] [CrossRef]
- Kuti, C.; Láng, L.; Bedő, Z. Pedigree Records in Plant Breeding: From Independent Data to Interdependent Data Structures. Cereal Res. Commun. 2006, 34, 911–918. [Google Scholar] [CrossRef]
- MIDAGRI. El Maíz Morado Peruano: Un Producto con Alto Contenido de Antocianina, Poderoso Antioxidante Natural; MIDAGRI: Santiago, Chile, 2021. [Google Scholar]
- Baker, L. Corn Meets Maize: Food Movements and Markets in Mexico; Rowman & Littlefield: Lanham, MD, USA, 2013; ISBN 978-1-4422-0651-9. [Google Scholar]
- IBPGR. Descriptors for Maize/Descriptores Para Maiz/Descripteurs Pour Le Maïs; International Board for Plant Genetic Resources: Rome, Italy, 1991; ISBN 978-92-9043-189-3. [Google Scholar]
- Argimon, J.M.; Jiménez, J. Métodos de Investigacion Clinica y Epidemiologica 4 Ed. Josep Argimon Pallás | Aurelio Velasco—Academia.Edu. Available online: https://www.academia.edu/24421999/M%C3%A9todos_de_investigacion_clinica_y_epidemiologica_4_ed_Josep_Argimon_Pall%C3%A1s (accessed on 11 June 2024).
- Corona-Terán, J.; López-Orona, C.A.; Romero-Gómez, S.d.J.; Martínez-Campos, A.R. Caracterización física, contenido de fenoles y capacidad antioxidante de maíces nativos (Zea mayz L.) del Estado de México. ITEA Inf. Téc. Económica Agrar. Rev. Asoc. Interprofesional Para El Desarro. Agrar. AIDA 2017, 113, 5–19. [Google Scholar]
- Lee, J.; Durst, R.; Wrolstad, R. AOAC 2005.02: Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines-pH Differential Method. In Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005; pp. 37–39. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F. Estadística para las Ciencias Agropecuarias, 7th ed.; Editoria Brujas: Cordoba, Argentina, 2009; ISBN 978-987-591-112-3. [Google Scholar]
- Balzarini, M.; Di Rienzo, J.; Tablada, M.; Gonzalez, L.; Bruno, C.; Córdoba, M.; Robledo, W.; Casanoves, F. Estadística y Biometría Ilustraciones Del Uso de InfoStat En Problemas de Agronomía; InfoStat: Córdoba, Argentina, 2015; ISBN 978-987-591-646-3. [Google Scholar]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. Extension to Ggplot2. Available online: https://ggobi.github.io/ggally/ (accessed on 11 June 2024).
- Valérie, D. Understanding a Structure; Elsevier: Amsterdam, The Netherlands, 2017; pp. 95–153. ISBN 978-1-78548-239-7. [Google Scholar]
- Shimizu, G.D.; Azeredo, L.S. AgroReg: Main Regression Models in Agricultural Sciences Implemented as an R Package. Sci. Agric. 2023, 80, e20220041. [Google Scholar] [CrossRef]
- Martínez, M.; Ortiz, R.; Ríos, H.; Acosta, R. Análisis de las correlaciones en poblaciones cubanas de maíz. Cultiv. Trop. 2010, 31, 82–91. [Google Scholar]
- Omar, S.; Abd, R.; Khalid, N.; Jolánkai, M.; Tarnawa, Á.; Percze, A.; Mikó, P.P.; Kende, Z. Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary. Agriculture 2023, 13, 1836. [Google Scholar] [CrossRef]
- Arsyad, F.; Basunanda, P. Identification of Agronomic Characters Effecting Cob Weight of the Families BTP1-X Purple Sweet Corn (Zea mayz L. Saccharata Sturt) Using Path Analysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012011. [Google Scholar] [CrossRef]
- Barreto, G.; Petry, C.; Silveira, D.C.; Silva, I.C.; Frizon, P. Phenotypic Characterization and Productivity of Local Varieties of Zea mayz L. in Agroecological No-Tillage Method. Rev. Em. Agronegocio. Meio Ambiente 2023, 16, e10147. [Google Scholar] [CrossRef]
- Quispe, F.; Arroyo, K.; Gorriti, A. Cultivares de maiz morado (Zea mayz L.). Rev. Soc. Quím. Perú 2011, 77, 205–217. [Google Scholar]
- Huanuqueño, H.; Zolla, G.; Jimenez, J. Selección de Líneas Estables y de Alto Rendimiento de Maíz Morado (Zea mayz L.) Var. Reventón Usando El Índice de Estabilidad de Múltiples Caracteres (MTSI). Sci. Agropecu. 2022, 13, 125–133. [Google Scholar] [CrossRef]
- Xu, H.; Ming, B.; Wang, K.; Xue, J.; Hou, P.; Li, S.; Xie, R. The Effects of Photoperiod and Temperature-Related Factors on Maize Leaf Number and Leaf Positional Distribution in the Field. Front. Plant Sci. 2023, 14, 1006245. [Google Scholar] [CrossRef]
- Austin, M.W.; Cole, P.O.; Olsen, K.M.; Smith, A.B. Climate Change Is Associated with Increased Allocation to Potential Outcrossing in a Common Mixed Mating Species. Am. J. Bot. 2022, 109, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Maccagnani, B.; Sgolastra, F. Solitary Bees As Pollinators. In Entomovectoring for Precision Biocontrol and Enhanced Pollination of Crops; Smagghe, G., Boecking, O., Maccagnani, B., Mänd, M., Kevan, P.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 63–79. ISBN 978-3-030-18917-4. [Google Scholar]
- Jauharlina, J.; Quinnell, R.J.; Robertson, H.G.; Compton, S.G. The effects of seasonal changes on the dynamics of a fig tree’s pollination. Acta Oecologica 2023, 120, 103918. [Google Scholar] [CrossRef]
- Hongping, L.; Kui, L.; Zhibin, L.; Moubiao, Z.; Yongen, Z.; Shuyan, L.; Xiuling, W.; Jinlong, Z.; Yali, Z.; Tianxue, L.; et al. Mixing trait-based corn (Zea mayz L.) cultivars increases yield through pollination synchronization and increased cross-fertilization. Crop J. 2023, 11, 291–300. [Google Scholar] [CrossRef]
- López-Martínez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Chen-Hsien, L.; Parkin, K.L.; García, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kwon, S.H.; Wang, Z.; Kim, T.H.; Kang, Y.-H.; Lee, J.-Y.; Lim, S.S. Optimization of Extraction Parameters of PTP1β (Protein Tyrosine Phosphatase 1β), Inhibitory Polyphenols, and Anthocyanins from Zea mayz L. Using Response Surface Methodology (RSM). BMC Complement. Altern. Med. 2016, 16, 317. [Google Scholar] [CrossRef] [PubMed]
- Tangwongchai, R.; Lertrat, K.; Saikaew, K. Influence of Variety and Maturity on Bioactive Compounds and Antioxidant Activity of Purple Waxy Corn (Zea mayz L. var. Ceratina). Int. Food Res. J. 2018, 25, 1985–1995. [Google Scholar]
- Zhao, X.; Corrales, M.; Zhang, C.; Hu, X.; Ma, Y.; Tauscher, B. Composition and Thermal Stability of Anthocyanins from Chinese Purple Corn (Zea mayz L.). J. Agric. Food Chem. 2008, 56, 10761–10766. [Google Scholar] [CrossRef]
- Napan, L.E.; Vietti-Guzmán, F.F.; Alvarez-Yanamango, E.; Huayta, F. Evaluation of Some Functional Properties of Purple Corn (Zea mayz L.) Dye, during Its Processing at Pilot Scale. In Proceedings of the 16th LACCEI International Multi-Conference for Engineering, Education, and Technology: “Innovation in Education and Inclusion”, Lima, Peru, 19–21 July 2018. [Google Scholar]





| Test Location | Variable | Average | SD | EE | CV | Mín | Max | A | K |
|---|---|---|---|---|---|---|---|---|---|
| Marabamba (M-Y) | NPS | 7.63 | 1.41 | 0.50 | 18.46 | 5.00 | 9.00 | −1.16 | −0.44 |
| CIL/cm | 105.13 | 11.28 | 3.99 | 10.73 | 80.00 | 115.00 | −1.85 | 1.22 | |
| PL/cm | 266.88 | 29.39 | 10.39 | 11.01 | 220.00 | 300.00 | −0.62 | −1.08 | |
| ND/cm | 9.83 | 0.84 | 0.30 | 8.55 | 8.50 | 10.50 | −1.23 | −0.80 | |
| CD/cm | 5.31 | 0.47 | 0.17 | 8.80 | 4.60 | 6.00 | −0.09 | −1.12 | |
| RD/cm | 2.73 | 0.68 | 0.24 | 24.79 | 2.00 | 4.00 | 0.85 | −0.43 | |
| CL/cm | 16.03 | 1.33 | 0.47 | 8.32 | 14.00 | 18.00 | −0.02 | −1.08 | |
| TCW/g | 170.38 | 15.52 | 5.49 | 9.11 | 150.00 | 200.00 | 0.71 | −0.16 | |
| GWC/g | 139.38 | 14.72 | 5.20 | 10.56 | 121.00 | 168.00 | 1.00 | −0.16 | |
| CCW/g | 31.00 | 7.69 | 2.72 | 24.81 | 17.00 | 42.00 | −0.30 | −0.21 | |
| Pistaloli (P-SA) | NPS | 6.25 | 0.46 | 0.16 | 7.41 | 6.00 | 7.00 | 1.44 | −0.67 |
| CIL/cm | 97.63 | 6.48 | 2.29 | 6.64 | 89.00 | 107.00 | −0.20 | −1.31 | |
| PL/cm | 238.38 | 9.47 | 3.35 | 3.97 | 220.00 | 250.00 | −1.02 | −0.10 | |
| ND/cm | 9.25 | 1.34 | 0.47 | 14.45 | 7.50 | 12.00 | 1.20 | 0.46 | |
| CD/cm | 3.23 | 0.65 | 0.23 | 20.01 | 2.00 | 4.00 | −0.77 | −0.32 | |
| RD/cm | 1.81 | 0.44 | 0.16 | 24.22 | 1.00 | 2.50 | −0.50 | −0.04 | |
| CL/cm | 10.26 | 2.48 | 0.88 | 24.20 | 7.80 | 14.00 | 0.28 | −1.60 | |
| TCW/g | 105.00 | 6.99 | 2.47 | 6.66 | 98.00 | 118.00 | 0.86 | −0.61 | |
| GWC/g | 79.63 | 7.13 | 2.52 | 8.95 | 70.00 | 90.00 | 0.15 | −1.17 | |
| CCW/g | 25.38 | 3.78 | 1.34 | 14.89 | 20.00 | 30.00 | −0.52 | −1.12 | |
| Winchuspata (W-Q) | NPS | 6.13 | 0.64 | 0.23 | 10.46 | 5.00 | 7.00 | −0.07 | −0.31 |
| CIL/cm | 99.38 | 5.71 | 2.02 | 5.74 | 92.00 | 110.00 | 0.90 | −0.36 | |
| PL/cm | 199.63 | 22.83 | 8.07 | 11.44 | 160.00 | 240.00 | −0.01 | 0.15 | |
| ND/cm | 8.63 | 0.69 | 0.25 | 8.05 | 8.00 | 10.00 | 1.12 | −0.14 | |
| CD/cm | 4.88 | 0.69 | 0.25 | 14.24 | 3.80 | 5.70 | −0.56 | −1.16 | |
| RD/cm | 2.79 | 0.61 | 0.22 | 21.98 | 2.00 | 3.50 | −0.28 | −1.44 | |
| CL/cm | 14.78 | 1.64 | 0.58 | 11.10 | 13.00 | 18.00 | 1.19 | −0.22 | |
| TCW/g | 169.38 | 12.65 | 4.47 | 7.47 | 159.00 | 195.00 | 1.40 | 0.02 | |
| GWC/g | 130.75 | 18.52 | 6.55 | 14.17 | 105.00 | 158.00 | 0.41 | −1.08 | |
| CCW/g | 38.63 | 11.25 | 3.98 | 29.13 | 22.00 | 54.00 | −0.65 | −0.82 |
| Pearson Coefficients | 1. PL | 2. CD | 3. CL | 4. TCW | 5. GWC | |
|---|---|---|---|---|---|---|
| 1. PL/cm | ||||||
| 2. CD/cm | Corrtotal M-V P-SA W-Q | 0.151 −0.272 0.676 0.575 | ||||
| 3. CL/cm | Corrtotal M-V P-SA W-Q | 0.114 −0.399 0.413 0.505 | 0.938 *** 0.931 *** 0.849 ** 0.729 * | |||
| 4. TCW/g | Corrtotal M-V P-SA W-Q | −0.075 −0.335 0.261 0.209 | 0.904 *** 0.862 ** 0.709 * 0.649 | 0.888 *** 0.856 ** 0.806 * 0.752 * | ||
| 5. GWC/g | Corrtotal M-V P-SA W-Q | −0.010 −0.504 0.138 0.140 | 0.908 *** 0.539 0.698 0.774 * | 0.881 *** 0.605 0.801 * 0.719 * | 0.960 *** 0.872 ** 0.857 ** 0.804 * | |
| 6. CCW/g | Corrtotal M-V P-SA W-Q | −0.231 0.288 0.223 0.005 | 0.287 0.708 * −0.004 −0.545 | 0.316 0.569 −0.021 −0.338 | 0.459 * 0.350 0.233 −0.199 | 0.190 −0.154 −0.302 −0.743 * |
| Test Location | Variable | Average | SD | EE. | CV | Mín | Máx | A | K |
|---|---|---|---|---|---|---|---|---|---|
| Marabamba (M-Y) | Tmax/°C | 24.31 | 8.91 | 3.15 | 36.64 | 2.30 | 28.50 | −2.81 | 3.11 |
| Tmin/°C | 14.49 | 1.56 | 0.55 | 10.77 | 11.90 | 16.30 | −0.48 | −1.05 | |
| Tm/°C | 19.40 | 4.22 | 1.49 | 21.76 | 9.20 | 22.40 | −2.56 | 2.62 | |
| pmax/mm | 5.31 | 6.69 | 2.36 | 125.86 | 0.10 | 14.20 | 0.65 | −1.71 | |
| pac/mm | 18.25 | 26.33 | 9.31 | 144.25 | 0.20 | 70.20 | 1.30 | −0.30 | |
| RH/% | 60.13 | 2.80 | 0.99 | 4.66 | 57.00 | 66.00 | 1.44 | 0.51 | |
| v/m/s | 4.13 | 0.40 | 0.14 | 9.68 | 3.40 | 4.50 | −0.84 | −0.81 | |
| Lsun/W/m2 | 204.11 | 40.42 | 14.29 | 19.80 | 144.50 | 259.80 | −0.14 | −1.37 | |
| Pistaloli (P-SA) | Tmax/°C | 31.12 | 1.05 | 0.37 | 3.36 | 29.20 | 32.80 | −0.37 | −0.02 |
| Tmin/°C | 19.96 | 0.50 | 0.18 | 2.50 | 19.20 | 20.60 | −0.04 | −1.09 | |
| Tm/°C | 25.54 | 0.61 | 0.21 | 2.38 | 24.55 | 26.70 | 0.46 | 0.35 | |
| pmax/mm | 56.18 | 33.35 | 11.79 | 59.37 | 17.20 | 110.40 | 0.90 | −0.87 | |
| pac/mm | 237.09 | 155.14 | 54.85 | 65.43 | 50.10 | 527.80 | 1.01 | −0.42 | |
| RH/% | 88.25 | 1.91 | 0.67 | 2.16 | 84.00 | 90.00 | −1.79 | 1.25 | |
| v/m/s | 1.19 | 0.14 | 0.05 | 11.42 | 0.90 | 1.30 | −1.54 | 0.56 | |
| Lsun/W/m2 | 164.94 | 42.71 | 15.10 | 25.89 | 79.50 | 215.20 | −1.06 | 0.12 | |
| Winchuspata (W-Q) | Tmax/°C | 18.71 | 0.38 | 0.14 | 2.05 | 18.20 | 19.20 | 0.20 | −1.43 |
| Tmin/°C | 7.89 | 0.71 | 0.25 | 9.05 | 6.30 | 8.50 | −1.82 | 1.17 | |
| Tm/°C | 13.30 | 0.26 | 0.09 | 1.93 | 12.75 | 13.55 | −1.60 | 0.71 | |
| pmax/mm | 15.05 | 6.49 | 2.29 | 43.10 | 7.40 | 24.60 | 0.50 | −1.17 | |
| pac/mm | 63.05 | 59.21 | 20.93 | 93.90 | 14.80 | 196.60 | 1.95 | 1.37 | |
| RH/% | 96.25 | 0.71 | 0.25 | 0.73 | 95.00 | 97.00 | −0.40 | −0.78 | |
| v/m/s | 3.23 | 0.28 | 0.10 | 8.57 | 2.70 | 3.50 | −0.99 | −0.45 | |
| Lsun/W/m2 | 172.54 | 54.96 | 19.43 | 31.85 | 83.40 | 249.80 | −0.25 | −1.06 |
| Climatic Parameters | 1. Tmin | 2. Tm | 3. pmax | 4. RH | |
|---|---|---|---|---|---|
| 1. Tmin | |||||
| 2. Tm | Corrtotal M-V P-SA W-Q | 0.878 *** −0.213 0.522 0.855 ** | |||
| 3. pmax | Corrtotal M-V P-SA W-Q | 0.578 ** 0.831 * 0.344 0.621 | 0.510 * −0.375 −0.001 0.538 | ||
| 4. RH | Corrtotal M-V P-SA W-Q | −0.249 0.448 −0.169 0.431 | −0.224 −0.814 * −0.785 * −0.039 | 0.349 0.396 −0.343 0.053 | |
| 5. v | Corrtotal M-V P-SA W-Q | −0.612 ** 0.085 0.140 −0.519 | −0.542 ** 0.808 * 0.756 * −0.191 | −0.776 *** −0.016 −0.538 −0.484 | −0.576 ** −0.796 * −0.483 −0.694 |
| Location | Tmin a/°C | RH b/% | v c/(m/s) | GWC d/g | CCW e/g | Acy f/(mg/100 g) |
|---|---|---|---|---|---|---|
| P-SA (953 m a.s.l.) | 19.96 | 88.3 | 1.19 | 79.6 | 25.4 | 603.7 |
| M-Y (1994 m m a.s.l.) | 14.49 | 60.1 | 4.13 | 139.4 | 31.0 | 623.5 |
| W-Q (2498 m m a.s.l.) | 7.89 | 96.3 | 3.23 | 130.8 | 38.6 | 684.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Aquino, V.; Ignacio-Cárdenas, S.; Japa-Espinoza, A.J.; Campos-Félix, U.; Ciriaco-Poma, J.; Campos-Félix, A.; Pantoja-Medina, B.; Dávalos-Prado, J.Z. Influence of Climatic Parameters and Plant Morphological Characters on the Total Anthocyanin Content of Purple Maize (Zea mays L., PMV-581) Cob Core. Agronomy 2024, 14, 2021. https://doi.org/10.3390/agronomy14092021
Soto-Aquino V, Ignacio-Cárdenas S, Japa-Espinoza AJ, Campos-Félix U, Ciriaco-Poma J, Campos-Félix A, Pantoja-Medina B, Dávalos-Prado JZ. Influence of Climatic Parameters and Plant Morphological Characters on the Total Anthocyanin Content of Purple Maize (Zea mays L., PMV-581) Cob Core. Agronomy. 2024; 14(9):2021. https://doi.org/10.3390/agronomy14092021
Chicago/Turabian StyleSoto-Aquino, Víctor, Severo Ignacio-Cárdenas, Anghelo Jhosepp Japa-Espinoza, Ulda Campos-Félix, Juanita Ciriaco-Poma, Alex Campos-Félix, Benancio Pantoja-Medina, and Juan Z. Dávalos-Prado. 2024. "Influence of Climatic Parameters and Plant Morphological Characters on the Total Anthocyanin Content of Purple Maize (Zea mays L., PMV-581) Cob Core" Agronomy 14, no. 9: 2021. https://doi.org/10.3390/agronomy14092021












