Abstract
Oxidative stress results from an imbalance between the production and accumulation of reactive oxygen species (ROS), which can impede plant growth under various environmental stresses. While waterlogging is a well-known inducer of oxidative stress, the effects of oxidative stress on plant roots grown using the deep flow technique (DFT) hydroponic system remain poorly understood. In this study, we demonstrate that N-acetylcysteine (NAC) enhances the growth of lettuce seedlings transplanted into a DFT system. NAC application significantly improved both shoot and root growth, with the most pronounced effects observed at a concentration of 0.3 mM. Moreover, NAC mitigated the accumulation of hydrogen peroxide in roots following transplantation. It also reduced a temporary increase in lipid peroxidation and total phenolic content in both roots and shoots. These results suggest that NAC functions as an antioxidant, alleviating oxidative stress by scavenging hydrogen peroxide in the roots. Importantly, NAC’s protective effects may extend to other hydroponically grown crops, offering broader potential for reducing oxidative stress across various cultivation systems.
1. Introduction
The redox state of cells is maintained by a dynamic balance between the production and removal rates of reactive oxygen species (ROS) [1]. During developmental stages, such as seed germination, root growth, pollen tube growth, and leaf development, intracellular ROS fluctuate within a moderate concentration range and serve as important components of intracellular and intercellular signaling pathways [2]. However, uncontrolled elevation of ROS levels results in the accumulation of oxidative damage in cellular components. Therefore, proper regulation of the cellular redox state is required to maintain optimal cellular function.
Plant growth is impaired by various adverse environmental conditions, known as abiotic stresses [3,4]. The triggers of abiotic stress include excessive light, cold, heat, drought, injury, waterlogging, and heavy metals. These stresses cause plant cells to generate and accumulate excessive amounts of ROS. ROS damage cell membranes, proteins, and lipids, and inhibit various metabolic pathways such as photosynthesis and respiration. Consequently, oxidative stress at the cellular level reduces both crop yield and quality.
Waterlogging stress occurs when root zones are inundated with water, leading to reduced oxygen availability in the soil medium, a condition known as hypoxia [5]. Under hypoxic conditions, the mitochondrial electron transport chain primarily generates superoxide (O2−) through complexes I and III, increasing the release of ROS and resulting in cellular injury [6]. In Zea mays, waterlogging induces hypoxic stress in the root zone, leading to the production of ROS, lipid peroxidation, and increased membrane permeability [7]. Hypoxic stress in the roots caused by waterlogging also reduces shoot biomass and photosynthetic activity in Jatropha and Prunus [8,9].
N-acetylcysteine (NAC) is a thiol compound derived from the amino acid cysteine, and is widely utilized as an antioxidant [10]. In humans, NAC is considered a safe and well-tolerated drug that has been used for various diseases associated with oxidative stress [11,12]. In plants, NAC has been employed to analyze ROS signaling in processes such as cell division, seed germination, programmed cell death, and leaf abscission [13,14,15,16]. NAC is also effective as an antioxidant in counteracting oxidative stress induced by abiotic stresses, including from exposure to heavy metals, UV radiation, herbicides, and physical injury [17,18,19]. Additionally, NAC has been shown to promote the growth of plants and algae under non-stress conditions [20,21,22,23].
The Deep Flow Technique (DFT) is a hydroponic system that suspends plant roots in a nutrient-rich water solution, thereby eliminating the necessity for soil in the root zone [24]. In this system, the oxygen required for root cell respiration is supplied exclusively by dissolved oxygen in the water. Considering that the saturated dissolved oxygen concentration achieved through the aeration of hydroponic water is significantly lower than the oxygen concentration in the soil space [25], it is hypothesized that plant roots cultivated in the DFT endure continuous hypoxic stress, regardless of the level of water aeration.
In prior research, lettuce cultivated using the DFT method exhibited significantly higher rates of tipburn injury compared to cultivation in a perlite medium [26]. While increasing dissolved oxygen concentrations through microbubble aeration has been shown to enhance lettuce growth in DFT systems [27,28], severe root hypoxia induced by nitrogen gas aeration led to oxidative stress responses, such as ROS production, lipid peroxidation, and disruptions in the antioxidative system in Malus plants [29]. On the other hand, in hydroponically grown baby red chard, it has been reported that while oxidative stress is induced by ozone treatment of the nutrient solution, the improved availability of certain micronutrients leads to an increase in yield [30]. Recently, waterlogging stress in arabidopsis roots was shown to induce rapid oxidative stress throughout the plant [31]. Despite these findings, the mechanisms underlying oxidative stress in standard DFT conditions remain poorly understood. This study addresses this research gap by investigating the oxidative stress induced by transplanting into a DFT hydroponic system, with a specific focus on the effects of NAC treatment on the root zone. Our findings will provide a deeper understanding of oxidative stress in hydroponic systems, which is crucial for enhancing crop resilience and productivity.
2. Materials and Methods
2.1. Experimental Conditions
Red leaf lettuce (Lactuca sativa L. cv. Red Wave; Sakata Seed Co., Kyoto, Japan) was used in this study. This cultivar is particularly suitable for research-level analysis because it accumulates anthocyanins in response to stress when grown under artificial light conditions. Plant seeds were sown in sponge cubes at a temperature of 20 °C under a photosynthetic photon flux (PPF) of 200 μmol·m−2·s−1 provided by fluorescent lamps (FL40SBR-A; NEC Co., Tokyo, Japan) operating on a 16-h light cycle. The nutrient solution used was a half-strength formulation of the Otsuka House A-recipe (OAT Agrio Co., Ltd., Tokyo, Japan), as previously detailed [32]. Fourteen days after sowing, the seedlings were transferred to a DFT hydroponic system with continuous aeration under a PPF of 250 μmol m⁻2 s⁻1 and a 16-h light cycle. NAC was then introduced into the hydroponic solutions. In experiments with varying NAC concentrations, preliminary tests were conducted to identify the range from concentrations that began to show growth-promoting effects to those that exhibited cytotoxicity. The harvesting of the plants and subsequent growth and metabolite analyses were conducted 21 days after sowing.
2.2. Measurement of Hydrogen Peroxide
The hydrogen peroxide (H2O2) content was spectrophotometrically measured following the method described previously [33], with slight modifications. Second true leaves or roots (100 mg) were homogenized in 600 µL of 1% trichloroacetic acid (TCA). Each sample was then centrifuged at 10,000× g for 10 min. The supernatant (250 µL) was mixed with 250 µL of 10 mM potassium phosphate buffer (pH 7.0) and 500 µL of 1 M potassium iodide (KI). The absorbance of the resulting mixture was measured at 390 nm, and a standard curve was prepared using H2O2 concentrations ranging from 25 to 500 μmol mL⁻1.
2.3. Histochemical Detection of Hydrogen Peroxide and Lipid Peroxidation
Histochemical detection of H2O2 was conducted with slight modifications to the previously described method [34]. Entire roots or root sections were treated with a 1 mg∙mL⁻1 solution of 3,3′-diaminobenzidine (DAB) at pH 3.8 and incubated for 3 h. Subsequently, the samples were observed using a light microscope.
Histochemical detection of lipid peroxidation was performed using Schiff’s reagent according to the method described in Ref. [35]. Roots were incubated in Schiff’s reagent for 20 min. Afterward, the stained roots were treated with a solution containing 0.5% (w/v) K2S2O5 (prepared in 0.05 M HCl) until the roots appeared light red. Following treating, the samples were observed using a light microscope.
2.4. Measurement of the Thiobarbituric Acid Reaction Content
The concentration of malondialdehyde (MDA), a product of lipid peroxidation, was quantified using the thiobarbituric acid reactive substances (TBARS) assay, as previously described [33]. Second true leaves or roots (100 mg) were homogenized in 0.5 mL of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 10,000× g for 5 min. Subsequently, 0.2 mL of the supernatant was combined with 0.8 mL of 0.5% thiobarbituric acid (TBA) in 20% TCA. This mixture was incubated at 95 °C for 30 min and then cooled on ice to terminate the reaction. The samples were centrifuged again at 10,000× g for 5 min at room temperature. The absorbance of the supernatant was measured at 532 nm, and the nonspecific absorbance at 600 nm was subtracted. The MDA concentration was calculated using a molar extinction coefficient of 155 mM⁻1∙cm⁻1.
2.5. Measurement of Total Phenolic Content
The total phenolic content was measured spectrophotometrically using a slightly modified version of the Folin–Ciocalteu method, as previously described [36]. Second true leaves or roots (50 mg) were homogenized with 500 µL of 90% methanol. The homogenate was then centrifuged at 10,000× g for 5 min. An aliquot of the supernatant (20 µL) was diluted with 680 µL of distilled water, followed by the addition of 50 µL of phenol reagent. Subsequently, 300 µL of 5% sodium carbonate was added, and the mixture was incubated in the dark at 25 °C for 30 min. The absorbance of the resulting solution was measured at 765 nm, and a standard curve was constructed using gallic acid.
2.6. Measurement of DPPH Radical Scavenging Capacity
The radical scavenging capacity was measured using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, following a previously described method with minor modifications [36]. Second true leaves or roots (50 mg) were homogenized in 500 µL of 90% methanol. The homogenate was centrifuged at 10,000× g for 5 min. Subsequently, 40 µL of the supernatant was mixed with 400 µL of 0.5 mM DPPH in ethanol and 360 µL of methanol. The mixture was incubated for 30 min, and the absorbance was measured at 517 nm. The DPPH radical scavenging capacity was expressed as the percentage of DPPH radicals scavenged, calculated by comparing the absorbances before and after incubation.
2.7. Measurement of Nitrate Cont
Second true leaves or roots (50 mg) were homogenized in 1 mL of deionized water. The homogenate was filtered through filter paper to remove tissue debris. The nitrate content was measured using a reflectometer (RQflex plus) and nitrate test strips (Nitrate Test, Merck, Darmstadt, Germany).
2.8. Data Analysis
Using the JMP statistical package (SAS Institute, Cary, NC, USA), the data were analyzed. An independent t-test was used to compare the means of two groups. To determine significant differences among treatments, a one-way analysis of variance (ANOVA) was performed. This was followed by the Tukey–Kramer honest significant difference (HSD) test for pairwise comparisons, with significance set at p < 0.05.
3. Results
3.1. Effect of Various NAC Concentrations on the Growth of Lettuce
Fourteen days after sowing, lettuce seedlings were transplanted into a DFT hydroponic system and subjected to treatments with or without NAC. After 7 days of transplantation, the shoot size was significantly reduced by the 3.0 mM NAC treatment (Figure 1). In contrast, lower concentrations of NAC resulted in an increase in shoot size compared to the 0 mM control plot. Similarly, the shoot fresh weight after 7 days of transplantation was significantly increased in plants treated with low concentrations of NAC (0.03, 0.1, and 0.3 mM) compared to the control plants (Figure 2A,B). In the 0.3 mM treatment—where NAC was most effective in promoting growth—shoot fresh weight increased 1.57-fold and dry matter weight increased 1.50-fold compared to the control plot. Conversely, in the 3.0 mM NAC treatment, fresh weight and dry weight decreased significantly compared to the control plot. Additionally, shoot water content remained unchanged in the lower NAC treatments, but significantly decreased in the 3.0 mM NAC treatment compared to the other treatment plots (Figure 2C). The fresh weight of roots increased in the 0.03 mM to 1.0 mM NAC treatments compared to the control plot (Figure 2D,E and Figure S1). In the 0.3 mM treatment, where NAC was most effective in promoting growth, root fresh weight increased 1.66-fold and dry matter weight increased 1.68-fold compared to the control plot. In contrast, root fresh weight and dry matter weight decreased significantly in the 3.0 mM NAC-treated plots. Root water content was significantly decreased only in the 3.0 mM NAC-treated plot compared to the control plot (Figure 2F). The growth-promoting effect of NAC persisted for up to 14 days following treatment (Supplementary Figure S2).
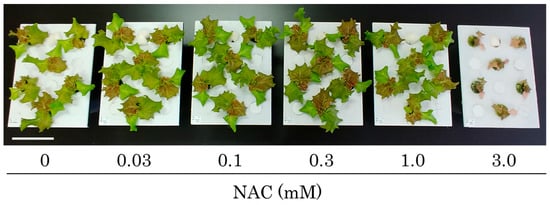
Figure 1.
The effect of different NAC concentrations on the shoot morphology of DFT-transplanted lettuces was assessed 7 days after treatments. NAC was applied to 14-day-old plants via the nutrient solution at concentrations of 0, 0.03, 0.1, 0.3, 1.0, and 3.0 mM. Scale bar is 10 cm.
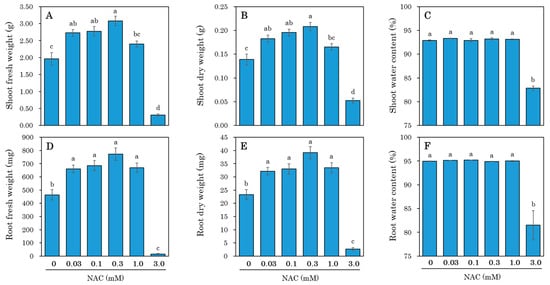
Figure 2.
The effect of different NAC concentrations on the shoot and root growth of DFT-transplanted lettuces was assessed at 7 days after treatments. NAC was applied to 14-day-old plants via the nutrient solution at concentrations of 0, 0.03, 0.1, 0.3, 1.0, and 3.0 mM. (A) Shoot fresh weight. (B) Shoot dry weight. (C) Shoot water content. (D) Root fresh weight. (E) Root dry weight. (F) Root water content. Vertical bars represent ± SE (n = 6). Different letters indicate a significant difference per Tukey’s multiple comparison test (p < 0.05).
3.2. Effect of Various Nutrient Concentrations on the Growth-Promoting Effect of NAC
Given that NAC has been reported to promote plant growth [20,21,22,23], the influence of nutrient concentration in the hydroponic solution on the growth-promoting effect of NAC was investigated after 7 days of transplantation. All NAC-treated plots exhibited increased growth of shoots and roots compared to the NAC-untreated plants, including those in nutrient-deprived plots (Figure 3). The decrease in water content induced by nutrient deficiency was also mitigated by the NAC treatment.
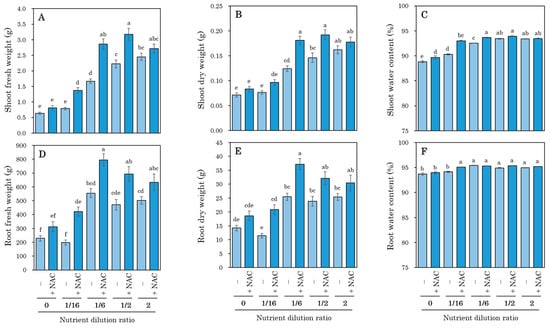
Figure 3.
The effect of various nutrient concentrations on the growth-promoting effects of NAC in DFT-transplanted lettuces was examined at 7 days after treatments. NAC (0.3 mM) and different nutrient concentrations of the Otsuka House A-recipe were applied to 14-day-old plants. (A) Shoot fresh weight. (B) Shoot dry weight. (C) Shoot water content. (D) Root fresh weight. (E) Root dry weight. (F) Root water content. Vertical bars represent ± SE (n = 6). Different letters indicate a significant difference per Tukey’s multiple comparison test (p < 0.05).
3.3. Effect of NAC on the Production of Hydrogen Peroxide in the Roots
Root flooding induces various stress responses in plants, including the production of ROS [37,38]. To observe oxidative stress in plant roots transplanted into a DFT hydroponic system, hydrogen peroxide was detected in the roots 3 days after transplantation using DAB staining. In the control plot, the entire root was stained brown, whereas in the NAC-treated plot, the staining was attenuated (Figure 4A). Microscopic observations revealed little to no brown staining on the transplanting date (0 days). In contrast, DAB brown staining was observed at both root tips and in the elongation zone at 1 and 3 days after transplantation in the control plot (Figure 4B). NAC treatment suppressed this staining at both 1 and 3 days after transplantation. Quantification of hydrogen peroxide in the roots 3 days after treatment showed a significant decrease in the NAC-treated roots compared to the control roots (Figure 4C).
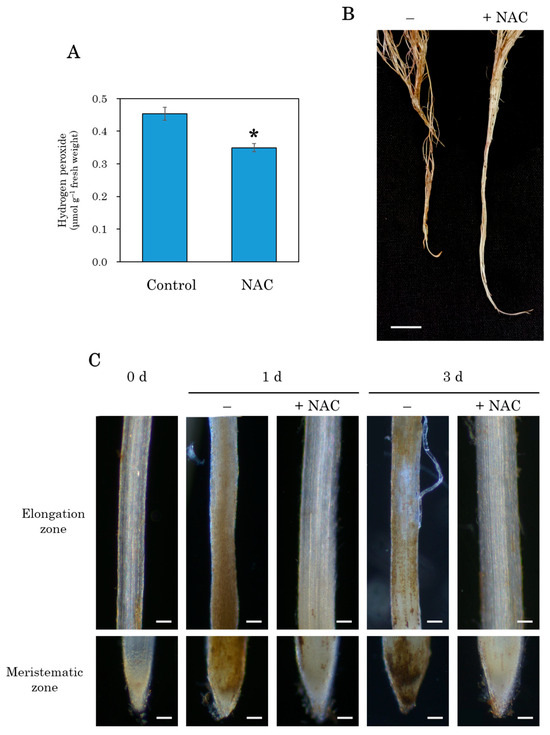
Figure 4.
The effect of NAC on hydrogen peroxide production in DFT-transplanted lettuce roots. Hydrogen peroxide content in plant roots at 3 days after treatments was quantified spectrophotometrically in roots (A). Additionally, hydrogen peroxide was visualized throughout the roots (B) and root tips using DAB staining under a microscope (C). Vertical bars in (A) represent ± SE (n = 4). An asterisk indicates that values between treatments are significantly different according to the independent t-test, with p < 0.01. Scale bars are 2 cm in (B), and 100 μm in (C).
3.4. Effect of NAC on the Lipid Peroxidation in the Roots and Leaves
The overproduction of intracellular hydrogen peroxide is closely associated with cellular oxidative damage [39]. To assess this, we determined lipid peroxidation using histological staining with Schiff’s reagent. Compared to the control roots, the NAC-treated roots exhibited a lesser extent of magenta staining at both the root tip and the elongation zone (Figure 5). MDA, a product of intracellular lipid oxidation, was measured in both roots and leaves. In the control plot, MDA levels increased 1 and 3 days after transplantation compared to the NAC-treated plot in both roots and leaves (Figure 6). After 7 days, no significant differences were observed between the two treatments.
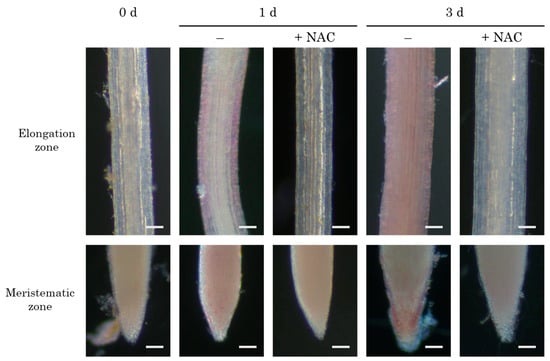
Figure 5.
The effect of NAC on lipid peroxidation in DFT-transplanted lettuce roots. Root lipid peroxidation was visualized with Schiff’s reagent. Scale bars are 100 μm.
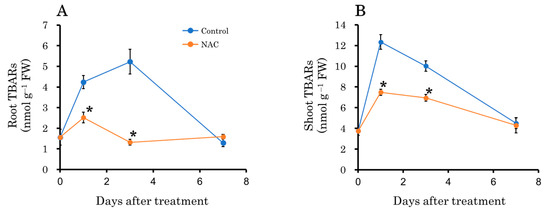
Figure 6.
The effect of NAC on lipid peroxidation in DFT-transplanted lettuce roots (A) and shoots (B) over 7 days after treatments. Lipid peroxidation was monitored by measuring the malondialdehyde (MDA) concentration. Vertical bars represent ± SE (n = 4). Asterisks indicate significant differences within each day between treatments according to the independent t-test, with p < 0.05. The absence of a symbol indicates no significant differences.
3.5. Effect of NAC on the Total Phenolic Content and DPPH Radical Scavenging Capacity in the Roots and Leaves
Plants synthesize antioxidant phenolic compounds in response to oxidative stress to enhance their antioxidant activity [34,40,41]. Therefore, we measured the total phenolic content in roots and leaves. The results indicated that the total phenolic content increased in the control plot 1 and 3 days after transplantation compared to the NAC-treated plot in both roots and leaves (Figure 7). After 7 days, no significant difference was observed in roots, but the increase in leaves persisted in the control plot. Changes in DPPH, an indicator of radical scavenging capacity, showed trends similar to those observed in total phenolic content (Figure 8).
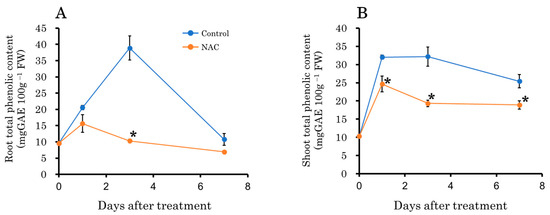
Figure 7.
The effect of NAC on total phenolic content in DFT-transplanted lettuce roots (A) and shoots (B) over 7 days after treatments. Vertical bars represent ± SE (n = 4). Asterisks indicate significant differences within each day between treatments according to the independent t-test, with p < 0.05. The absence of a symbol indicates no significant differences.
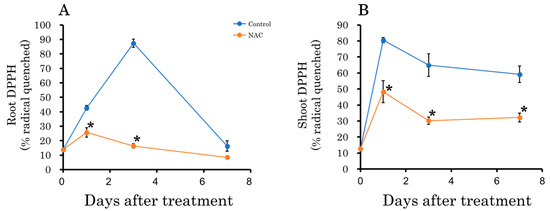
Figure 8.
The effect of NAC on DPPH radical scavenging capacity in DFT-transplanted lettuce roots (A) and shoots (B) over 7 days after treatments. Vertical bars represent ± SE (n = 4). Asterisks indicate significant differences within each day between treatments according to the independent t-test, with p < 0.05. The absence of a symbol indicates no significant differences.
3.6. Effect of NAC on the Nitrate Content in the Roots and Leaves
It is well established that the increase in plant biomass due to photosynthesis is influenced by nitrate uptake and consumption [42]. Therefore, we examined the effect of NAC on the nitrate content in roots and leaves. Three days after transplantation, the root nitrate content was higher in the NAC-treated plot compared to the control plot, but it was lower in the NAC-treated plot 7 days after transplantation (Figure 9A). Conversely, in leaves, the nitrate content was consistently lower in the control plot compared to the NAC-treated plot throughout the experimental period (Figure 9B).
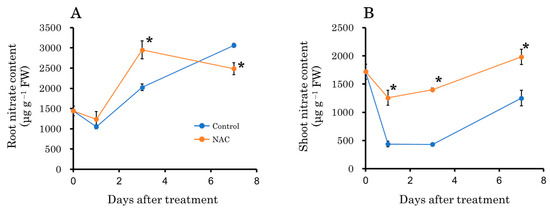
Figure 9.
The effect of NAC on nitrate content in DFT-transplanted lettuce roots (A) and shoots (B) over 7 days after treatments. Vertical bars represent ± SE (n = 4). Asterisks indicate significant differences within each day between treatments according to the independent t-test, with p < 0.05. The absence of a symbol indicates no significant differences.
4. Discussion
Plant roots undergo hypoxic stress under DFT hydroponics due to continuous submergence in nutrient solution [26]. The micro- and nano-bubble aeration of DFT hydroponic solutions is known to enhance plant growth by reducing oxygen limitation to the roots [43,44]. Similarly, the improvement of hypoxic conditions in DFT using hyper-oxygenated nutrient solutions has been shown to enhance the growth of mini-bok choy [45]. Conversely, aeration with nitrogen gas reduced dissolved oxygen in the solution and decreased the growth of hydroponic Malus plants [29]. The lack of proper aeration also restricts the growth of carrot taproots in DFT hydroponics [46]. In our study, the transplanting of lettuce seedlings to the DFT hydroponics induced the production of hydrogen peroxide in the roots and its mitigation by NAC. Considering that roots grown in DFT hydroponics are subjected to hypoxic conditions compared to roots grown in soil [25], it was suggested that the transplanting to DFT induced hypoxic stress in the roots, resulting in hydrogen peroxide production and lipid peroxidation. The application of NAC to the root zone of rice seedlings has been shown to mitigate transplanting-induced wounding injuries, such as hydrogen peroxide production and lipid peroxidation [47]. Our findings, together with this evidence, suggest that NAC treatment may be an effective strategy to alleviate growth inhibition associated with oxidative stress during seedling transplantation. Limited oxygen availability in roots is known to cause oxidative stress in both roots and shoots [48,49]. In peach seedlings, waterlogging reduces root activity and increases oxidative stress and membrane permeability in leaves [49]. Our study demonstrated that transplantation into a DFT hydroponics system increased lipid peroxidation in both roots and leaves, suggesting that transplantation stress affects shoots as well. Furthermore, total phenolic content and DPPH radical scavenging capacity in roots and shoots increased, indicating plant responses to oxidative stress in whole plants.
Waterlogging stress induces various physiological and biochemical adjustments in whole plants, including changes in morphological structure, alterations in energy metabolism, modifications in the biosynthesis of endogenous hormones, and adaptations in signal transduction pathways [50]. Under waterlogging stress, plant roots and shoots rely on antioxidant enzyme systems and other active antioxidants to regulate ROS levels, thereby reducing oxidative damage [31,51,52]. In our study, plants transplanted to DFT hydroponics exhibited increased lipid peroxidation in the roots and shoots after 1 and 3 days; however, after 7 days, lipid peroxidation levels decreased to baseline, suggesting that acclimation to oxidative stress was achieved through the upregulation of antioxidant enzymes and antioxidants. NAC may mitigate flooding-induced oxidative stress by acting similarly to these antioxidants in the roots. Given that hydroponically grown plants are generally known to exhibit enhanced growth [24], the oxidative stress induced by transplantation into a DFT system may be a transient phenomenon occurring during the early stages of transplantation.
NAC is recognized as a precursor to the formation of reduced glutathione (GSH), which exerts antioxidant effects and mitigates free radicals in animal cells [53]. In the livers of mice, NAC treatment reduces ischemia-reperfusion-induced apoptosis by scavenging ROS through the action of NAC-converted GSH [54]. In plants, glutathione (GSH) is demonstrated as a key mediator in coping with various oxidative stresses [55,56]. GSH-deficient plants have been shown to be more sensitive to oxidative stress [57,58], whereas upregulation of GSH improves intracellular oxidative status and thus enhances oxidative stress resistance [57,59]. NAC also alleviates toxicity caused by heavy metals and herbicides, with a corresponding increase in endogenous glutathione (GSH) levels [17,18,60]. Therefore, it is suggested that the alleviation of oxidative stress in roots observed in this study is related to the enhancement of endogenous GSH levels. In animals, hypoxic stress is shown to reduce the cellular glutathione levels via mitochondrial ROS production [61]. In addition, flooding-induced hypoxia is accompanied with the reduction of reduced glutathione content in several plants [29,62,63]. Therefore, manipulating endogenous GSH levels through NAC or GSH treatment may be a useful strategy for alleviating various oxidative stresses.
The application of various amino acids is known to enhance plant growth [64,65,66]. A combination of amino acids has been reported to serve as a nitrogen source for plants, thereby promoting growth [64]. Given that NAC has been shown to promote the growth of corn, rice, and marine microalgae [20,21,22,23], the growth-promoting effects of NAC in this study may be due to its function as a nitrogen source, particularly in nutrient-deficient conditions. It has been shown that treating hydroponic lettuce with L-methionine in the culture solution increases photosynthetic activity and promotes growth [66]. Similarly, NAC treatment has been reported to enhance photosynthetic activity and promote growth in plants [20,22]. In this study, NAC treatment increased nitrate consumption in leaves compared to the control, suggesting that NAC application to roots may promote growth by enhancing photosynthetic activity in leaves.
A hypoxic condition is known to alter nitrate metabolism in plants [67]. In Arabidopsis root cultures, low oxygen conditions upregulated the expression of a nitrate/chlorate transporter gene [68]. Additionally, the high-affinity nitrate transporter gene BnNRT2.1a was upregulated under waterlogging in rapeseed [69]. Given that nitrate content increased after 3 days of DFT transplantation in our study, this suggests that DFT transplantation alters root nitrate metabolism, including transporter activation. Hypoxia also induces the reduction of root nitrate to produce nitric oxide [70]. Nitric oxide is known to act as a reactive nitrogen species, triggering oxidative stress in plants [71]. Considering that NAC is shown to decrease oxidative stress by reducing nitric oxide production [72], DFT transplantation may also induce hypoxia-triggered nitric oxide production, which can be mitigated by NAC.
Although NAC is widely used as an antioxidant, it can also induce cell death when applied in high concentrations [73,74,75]. In animal cells, high doses of NAC have been shown to increase oxidative stress via the repression of glutathione levels [74,75]. In Arabidopsis, treatment with 4 to 8 mM NAC resulted in reduced growth compared to untreated controls [76]. In our study, a 3.0 mM NAC treatment significantly inhibited shoot and root growth, suggesting that the imbalance in ROS homeostasis adversely affected plant development.
5. Conclusions
Hydroponics is known to promote plant growth by immersing the roots in a nutrient-rich solution, enabling efficient nutrient uptake. However, when plants are transplanted from seedlings to a hydroponic system, their growing environment, including the root zone, undergoes significant changes, which may cause a temporary stagnation in growth as the plants adjust. Similar growth limitations occur when plants are transferred from in vitro to ex vitro environments due to reduced photosynthetic activity and other growth restrictions [77]. These transplant-related growth limitations are commonly linked to stressors such as temperature and humidity fluctuations, physical injury, and oxygen deprivation, all of which contribute to oxidative stress. During oxidative stress, reduced glutathione serves as an endogenous antioxidant, playing a crucial role in mitigating oxidative damage in plants [78].
The application of NAC is hypothesized to enhance antioxidant activity by replenishing intracellular reduced glutathione levels, thereby alleviating stress during transplantation. Importantly, our findings suggest that the oxidative stress-reducing effects of NAC are not limited to hydroponically grown lettuce, but could potentially be applied to a wide range of hydroponically cultivated crops. Moreover, the ability of NAC to mitigate oxidative stress may be extendable to other forms of oxidative stress encountered during plant cultivation, including stress induced by environmental factors such as drought, extreme temperatures, or excessive light exposure. In summary, NAC shows great potential in optimizing plant growth by minimizing oxidative stress, offering broader applications for improving the resilience and productivity of hydroponically grown crops and beyond.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14092112/s1, Figure S1: The effect of NAC (0.3 mM) on root morphologies in DFT-transplanted lettuce at 7 days after treatments; Figure S2: The effect of NAC (0.3 mM) on shoot morphologies (A) and shoot fresh weight (B) in DFT-transplanted lettuce at 14 days after treatments. Vertical bars represent ± SE (n = 5). An asterisk indicates that values between treatments are significantly different according to the independent t-test, with p < 0.05.
Author Contributions
Conceptualization, M.S. and T.S.; formal analysis, M.S.; investigation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, M.S. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2011, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Abiotic Stress, Generation of Reactive Oxygen Species, and Their Consequences: An Overview. In Reactive Oxygen Species in Plants; Wiley: Hoboken, NJ, USA, 2017; pp. 23–50. ISBN 978-1-119-28729-2. [Google Scholar]
- Bechtold, U.; Field, B. Molecular Mechanisms Controlling Plant Growth during Abiotic Stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging Stress in Plants: A Review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, H.; Gao, K.; Li, G.; Xu, Q.; Deng, X.; Li, J.; Mei, F.; Zhou, Z. The Opening of Mitochondrial Permeability Transition Pore (mPTP) and the Inhibition of Electron Transfer Chain (ETC) Induce Mitophagy in Wheat Roots under Waterlogging Stress. Protoplasma 2023, 260, 1179–1191. [Google Scholar] [CrossRef]
- Jamei, R.; Heidari, R.; Khara, J.; Zare, S. Hypoxia Induced Changes in the Lipid Peroxidation, Membrane Permeability, Reactive Oxygen Species Generation, and Antioxidative Response Systems in Zea mays Leaves. Turk. J. Biol. 2009, 33, 45–52. [Google Scholar] [CrossRef]
- Verma, K.; Singh, M.; Gupta, R.; Verma, C. Photosynthetic Gas Exchange, Chlorophyll Fluorescence, Antioxidant Enzymes, and Growth Responses of Jatropha Curcas during Soil Flooding. Turk. J. Bot. 2014, 38, 130–140. [Google Scholar] [CrossRef]
- Pimentel, P.; Almada, R.D.; Salvatierra, A.; Toro, G.; Arismendi, M.J.; Pino, M.T.; Sagredo, B.; Pinto, M. Physiological and Morphological Responses of Prunus Species with Different Degree of Tolerance to Long-Term Root Hypoxia. Sci. Hortic. 2014, 180, 14–23. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-Acetylcysteine for Antioxidant Therapy: Pharmacology and Clinical Utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Dhouib, E.I.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A Minireview on N-Acetylcysteine: An Old Drug with New Approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef]
- Livanos, P.; Apostolakos, P.; Galatis, B. Plant Cell Division: ROS Homeostasis Is Required. Plant Signal. Behav. 2012, 7, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Koda, Y.; Zheng, S.-H.; Yuasa, T.; Iwaya-Inoue, M. Regulation of Soybean Seed Germination through Ethylene Production in Response to Reactive Oxygen Species. Ann. Bot. 2013, 111, 95–102. [Google Scholar] [CrossRef]
- Sakamoto, M.; Tada, Y.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Two Phases of Intracellular Reactive Oxygen Species Production during Victorin-Induced Cell Death in Oats. J. Gen. Plant Pathol. 2005, 71, 387–394. [Google Scholar] [CrossRef]
- Sakamoto, M.; Munemura, I.; Tomita, R.; Kobayashi, K. Involvement of Hydrogen Peroxide in Leaf Abscission Signaling, Revealed by Analysis with an in Vitro Abscission System in Capsicum Plants. Plant J. 2008, 56, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Colak, N.; Torun, H.; Gruz, J.; Strnad, M.; Ayaz, F.A. Exogenous N-Acetylcysteine Alleviates Heavy Metal Stress by Promoting Phenolic Acids to Support Antioxidant Defence Systems in Wheat Roots. Ecotoxicol. Environ. Saf. 2019, 181, 49–59. [Google Scholar] [CrossRef]
- Iriti, M.; Castorina, G.; Picchi, V.; Faoro, F.; Gomarasca, S. Acute Exposure of the Aquatic Macrophyte Callitriche Obtusangula to the Herbicide Oxadiazon: The Protective Role of N-Acetylcysteine. Chemosphere 2009, 74, 1231–1237. [Google Scholar] [CrossRef]
- Malanga, G.; Kozak, R.G.; Puntarulo, S. N-Acetylcysteine-Dependent Protection against UV-B Damage in Two Photosynthetic Organisms. Plant Sci. 1999, 141, 129–137. [Google Scholar] [CrossRef]
- Munirah, N.; Mat, N.; Jahan, M. N-Acetylcysteine and Zn Regulate Corn Yield. Sci. Asia 2015, 41, 246–250. [Google Scholar] [CrossRef]
- Nozulaidi, M.; Jahan, M.; Khairi, M.; Khandaker, M.; Nashriyah, M.; Khanif, Y. N-Acetylcysteine Increased Rice Yield. Turk. J. Agric. For. 2015, 39, 204–211. [Google Scholar] [CrossRef]
- Syuhada, N.; Jahan, M.S. Glutathione Functions on Physiological Characters to Increase Copper-Induced Corn Production. Russ. Agricult. Sci. 2016, 42, 5–10. [Google Scholar] [CrossRef]
- Sauvage, J.; Wikfors, G.H.; Li, X.; Gluis, M.; Nevejan, N.; Sabbe, K.; Joyce, A. Effect of Pluronic Block Polymers and N-Acetylcysteine Culture Media Additives on Growth Rate and Fatty Acid Composition of Six Marine Microalgae Species. Appl. Microbiol. Biotechnol. 2021, 105, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Elkazzaz, A. Soilless Agriculture a New and Advanced Method for Agriculture Development: An Introduction. Agric. Res. Technol. Open Access J. 2017, 3, 63–72. [Google Scholar] [CrossRef]
- Soffer, H.; Burger, D.W. Effects of Dissolved Oxygen Concentrations in Aero-Hydroponics on the Formation and Growth of Adventitious Roots. J. Amer. Soc. Hort. Sci. 1988, 113, 218–221. [Google Scholar] [CrossRef]
- Assimakopoulou, A.; Kotsiras, A.; Nifakos, K. Incidence of Lettuce Tipburn as Related to Hydroponic System and Cultivar. J. Plant Nutr. 2013, 36, 1383–1400. [Google Scholar] [CrossRef]
- Park, J.-S.; Kurata, K. Application of Microbubbles to Hydroponics Solution Promotes Lettuce Growth. HortTechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Park, J.S.; Ohashi, K.; Kurata, K.; Lee, J.W. Promotion of Lettuce Growth by Application of Microbubbles in Nutrient Solution Using Different Rates of Electrical Conductivity and under Periodic Intermittent Generation in a Deep Flow Technique Culture System. Eur. J. Hortic. Sci. 2010, 75, 198. [Google Scholar]
- Bai, T.; Li, C.; Ma, F.; Feng, F.; Shu, H. Responses of Growth and Antioxidant System to Root-Zone Hypoxia Stress in Two Malus Species. Plant Soil 2010, 327, 95–105. [Google Scholar] [CrossRef]
- Machuca Vargas, A.; Silveira Gómez, A.C.; Hernández-Adasme, C.; Escalona Contreras, V.H. Effect of the Ozone Application in the Nutrient Solution and the Yield and Oxidative Stress of Hydroponic Baby Red Chard. Horticulturae 2023, 9, 1234. [Google Scholar] [CrossRef]
- Peláez-Vico, M.Á.; Tukuli, A.; Singh, P.; Mendoza-Cózatl, D.G.; Joshi, T.; Mittler, R. Rapid Systemic Responses of Arabidopsis to Waterlogging Stress. Plant Physiol. 2023, 193, 2215–2231. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Nutrient Solution Concentration on the Growth of Hydroponic Sweetpotato. Agronomy 2020, 10, 1708. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Synergistic Effects of a Night Temperature Shift and Methyl Jasmonate on the Production of Anthocyanin in Red Leaf Lettuce. Am. J. Plant Sci. 2017, 08, 1534. [Google Scholar] [CrossRef][Green Version]
- Sakamoto, M.; Suzuki, T. Methyl Jasmonate and Salinity Increase Anthocyanin Accumulation in Radish Sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, H.-Z.; Zheng, M.-Y.; Xian, M.; Qi, Z.-Q.; Li, Y.-Q.; Hu, L.-B.; Chen, J.; Yang, L.-F. Selenium Inhibits Root Elongation by Repressing the Generation of Endogenous Hydrogen Sulfide in Brassica Rapa. PLoS ONE 2014, 9, e110904. [Google Scholar] [CrossRef]
- Sakamoto, M.; Komatsu, Y.; Suzuki, T. Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae 2021, 7, 525. [Google Scholar] [CrossRef]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.-M. Sensing and Signalling during Plant Flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B.; Colmer, T.D. Response and Adaptation by Plants to Flooding Stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Kuźniak, E.; Urbanek, H. The Involvement of Hydrogen Peroxide in Plant Responses to Stresses. Acta Physiol. Plant. 2000, 22, 195–203. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of Salt Stress on Volatile Compounds, Total Phenolic Content and Antioxidant Activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Zeng, M.; van Dam, N.M.; Hause, B. MtEIN2 Affects Nitrate Uptake and Accumulation of Photosynthetic Pigments under Phosphate and Nitrate Deficiency in Medicago truncatula. Physiol. Plant. 2023, 175, e13899. [Google Scholar] [CrossRef]
- Ikeura, H.; Tsukada, K.; Tamaki, M. Effect of Microbubbles in Deep Flow Hydroponic Culture on Spinach Growth. J. Plant Nutr. 2017, 40, 2358–2364. [Google Scholar] [CrossRef]
- Yang, X.; Fan, J.; Ge, J.; Luo, Z. Effect of Irrigation with Activated Water on Root Morphology of Hydroponic Rice and Wheat Seedlings. Agronomy 2022, 12, 1068. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Higashi, R.; Kinugasa, T. A New Technique to Realize a Drastic Acceleration of Crop Growth in the DFT Hydroponic Cultivation with Hyper-Oxygenated Nutrient Solution. J. Hortic. 2020, 7, 1–7. [Google Scholar]
- Que, F.; Wang, G.-L.; Feng, K.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Hypoxia Enhances Lignification and Affects the Anatomical Structure in Hydroponic Cultivation of Carrot Taproot. Plant Cell Rep. 2018, 37, 1021–1032. [Google Scholar] [CrossRef]
- He, W.; Zhong, Q.; He, B.; Wu, B.; Mohi Ud Din, A.; Han, J.; Ding, Y.; Liu, Z.; Li, W.; Jiang, Y.; et al. N-Acetylcysteine Priming Alleviates the Transplanting Injury of Machine-Transplanted Rice by Comprehensively Promoting Antioxidant and Photosynthetic Systems. Plants 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.D.; Zhou, Y.F.; Yue, Z.X.; Chen, X.F.; Cao, X.; Xu, X.X.; Xing, Y.F.; Jiang, B.; Ai, X.Y.; Huang, R.D. Changes in Photosynthesis, Chloroplast Ultrastructure, and Antioxidant Metabolism in Leaves of Sorghum under Waterlogging Stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen Sulfide Alleviates Waterlogging-Induced Damage in Peach Seedlings via Enhancing Antioxidative System and Inhibiting Ethylene Synthesis. Front. Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Tang, B.; Xu, S.; Zou, X.; Zheng, Y.; Qiu, F. Changes of Antioxidative Enzymes and Lipid Peroxidation in Leaves and Roots of Waterlogging-Tolerant and Waterlogging-Sensitive Maize Genotypes at Seedling Stage. Agric. Sci. China 2010, 9, 651–661. [Google Scholar] [CrossRef]
- Doupis, G.; Kavroulakis, N.; Psarras, G.; Papadakis, I.E. Growth, Photosynthetic Performance and Antioxidative Response of ‘Hass’ and ‘Fuerte’ Avocado (Persea Americana Mill.) Plants Grown under High Soil Moisture. Photosynthetica 2017, 55, 655–663. [Google Scholar] [CrossRef]
- Ates, B.; Abraham, L.; Ercal, N. Antioxidant and Free Radical Scavenging Properties of N-Acetylcysteine Amide (NACA) and Comparison with N-Acetylcysteine (NAC). Free. Radic. Res. 2008, 42, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pu, L.-Y.; Lu, L.; Wang, X.-H.; Zhang, F.; Rao, J.-H. N-Acetylcysteine Attenuates Reactive-Oxygen-Species-Mediated Endoplasmic Reticulum Stress during Liver Ischemia-Reperfusion Injury. World J. Gastroenterol. 2014, 20, 15289–15298. [Google Scholar] [CrossRef] [PubMed]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione Metabolism in Plants under Stress: Beyond Reactive Oxygen Species Detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Thominet, D.; Vanacker, H.; Miginiac-Maslow, M.; Gakière, B.; Noctor, G. H2O2-Activated Up-Regulation of Glutathione in Arabidopsis Involves Induction of Genes Encoding Enzymes Involved in Cysteine Synthesis in the Chloroplast. Mol. Plant 2009, 2, 344–356. [Google Scholar] [CrossRef]
- Cheng, M.-C.; Ko, K.; Chang, W.-L.; Kuo, W.-C.; Chen, G.-H.; Lin, T.-P. Increased Glutathione Contributes to Stress Tolerance and Global Translational Changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef]
- Dubreuil-Maurizi, C.; Vitecek, J.; Marty, L.; Branciard, L.; Frettinger, P.; Wendehenne, D.; Meyer, A.J.; Mauch, F.; Poinssot, B. Glutathione Deficiency of the Arabidopsis Mutant Pad2-1 Affects Oxidative Stress-Related Events, Defense Gene Expression, and the Hypersensitive Response. Plant Physiol. 2011, 157, 2000–2012. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Bin-Umer, M.A.; Widiez, T.; Finn, D.; McCormick, S.; Tumer, N.E. A Lipid Transfer Protein Increases the Glutathione Content and Enhances Arabidopsis Resistance to a Trichothecene Mycotoxin. PLoS ONE 2015, 10, e0130204. [Google Scholar] [CrossRef]
- Deng, X.; Xia, Y.; Hu, W.; Zhang, H.; Shen, Z. Cadmium-Induced Oxidative Damage and Protective Effects of N-Acetyl-l-Cysteine against Cadmium Toxicity in Solanum Nigrum L. J. Hazard. Mater. 2010, 180, 722–729. [Google Scholar] [CrossRef]
- Mansfield, K.D.; Simon, M.C.; Keith, B. Hypoxic Reduction in Cellular Glutathione Levels Requires Mitochondrial Reactive Oxygen Species. J. Appl. Physiol. 2004, 97, 1358–1366. [Google Scholar] [CrossRef]
- Biemelt, S.; Albrecht, G.; Wiedenroth, E.-M. The Effect of Post-Hypoxia on Roots inSenecio andMyosotis Species Related to the Glutathione System. Folia Geobot. 1996, 31, 65–72. [Google Scholar] [CrossRef]
- Garnczarska, M. Response of the Ascorbate–Glutathione Cycle to Re-Aeration Following Hypoxia in Lupine Roots. Plant Physiol. Biochem. 2005, 43, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Geshnizjani, N.; Khosh-khui, M. Promoted Growth and Improved Quality of Gerbera Jamesonni L. Flowers Using Exogenous Application of Amino Acids. Int. J. Hortic. Sci. Technol. 2016, 3, 155–166. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen Metabolism in Plants under Low Oxygen Stress. Planta 2014, 239, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Klok, E.J.; Wilson, I.W.; Wilson, D.; Chapman, S.C.; Ewing, R.M.; Somerville, S.C.; Peacock, W.J.; Dolferus, R.; Dennis, E.S. Expression Profile Analysis of the Low-Oxygen Response in Arabidopsis Root Cultures. Plant Cell 2002, 14, 2481–2494. [Google Scholar] [CrossRef]
- Tong, J.; Walk, T.C.; Han, P.; Chen, L.; Shen, X.; Li, Y.; Gu, C.; Xie, L.; Hu, X.; Liao, X.; et al. Genome-Wide Identification and Analysis of High-Affinity Nitrate Transporter 2 (NRT2) Family Genes in Rapeseed (Brassica napus L.) and Their Responses to Various Stresses. BMC Plant Biol. 2020, 20, 464. [Google Scholar] [CrossRef]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 11522. [Google Scholar] [CrossRef]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.S.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric Oxide-Mediated Regulation of Oxidative Stress in Plants under Metal Stress: A Review on Molecular and Biochemical Aspects. Physiol. Plant. 2020, 168, 318–344. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Alhasan, H.; Çakir, E.; Çömelekoğlu, Ü.; Çakir, E.E. Cytotoxic Effect of N-Acetyl Cysteine in DU145 Human Prostate Cancer Cells. Sanat 2019, 23, 1123–1130. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Wang, N.; Zhang, H. N-acetylcysteine Induces Apoptosis via the Mitochondria-dependent Pathway but Not via Endoplasmic Reticulum Stress in H9c2 Cells. Mol. Med. Rep. 2017, 16, 6626–6633. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-S.; Liou, G.-G.; Liao, J.-W.; Lai, P.-Y.; Yang, D.-J.; Wu, S.-H.; Wang, S.-H. N-Acetyl Cysteine Overdose Induced Acute Toxicity and Hepatic Microvesicular Steatosis by Disrupting GSH and Interfering Lipid Metabolisms in Normal Mice. Antioxidants 2024, 13, 832. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.Y.; Kim, S.Y.; Park, H.M.; You, J.Y.; Kim, B.H.; Lee, J.S.; Nam, K.H. Modulations of AtGSTF10 Expression Induce Stress Tolerance and BAK1-Mediated Cell Death. Biochem. Biophys. Res. Commun. 2009, 379, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Seon, J.-H.; Cui, Y.-Y.; Kozai, T.; Paek, K.-Y. Influence of in Vitro Growth Conditions on Photosynthetic Competence and Survival Rate of Rehmannia Glutinosa Plantlets during Acclimatization Period. Plant Cell Tissue Organ Cult. 2000, 61, 135–142. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).