Dynamics of Growth and Galanthamine Biosynthesis in Hippeastrum papilio (Ravena) Van Sheepen Hydroponic Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cultivation and Experimental Design
2.3. Phytochemical Analysis
2.3.1. Sample Preparation
2.3.2. Gas Chromatography–Mass Spectrometry
2.3.3. Quantification of Galanthamine and Haemanthamine
2.4. Statistical Analysis
3. Results
3.1. The Effects of Age and Fertilizers on Galanthamine and Biomass Accumulation
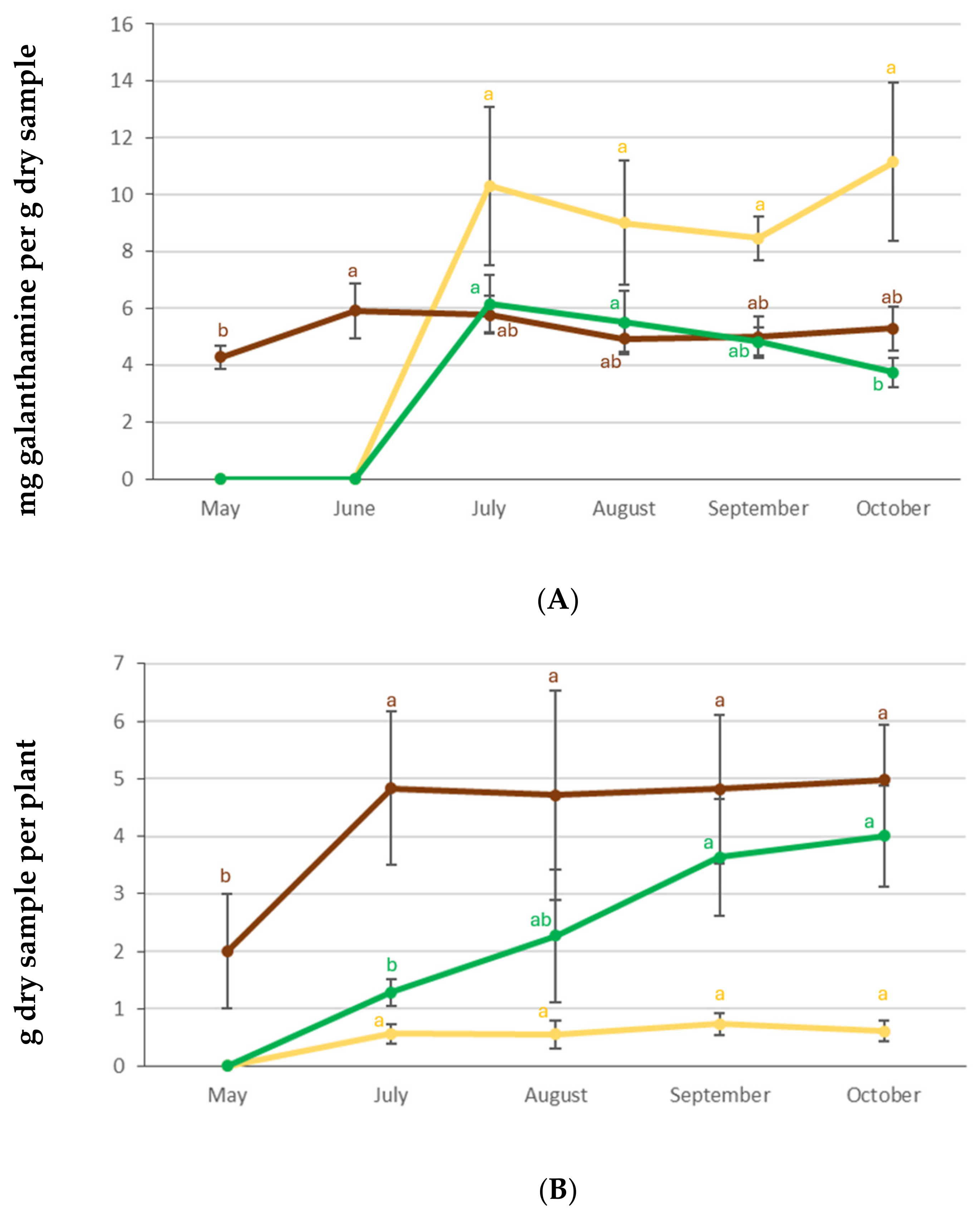
3.2. Dynamics of Galanthamine and Biomass Accumulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berkov, S.; Georgieva, L.; Sidjimova, B.; Bastida, J. Evaluation of Hippeastrum papilio (Ravenna) Van Scheepen potential as a new industrial source of galanthamine. Ind. Crops. Prod. 2022, 178, 114619. [Google Scholar] [CrossRef]
- Marco-Contelles, J.; do Carmo Carreiras, M.; Rodríguez, C.; Villarroya, M.; García, A.G. Synthesis and pharmacology of galantamine. Chem. Rev. 2006, 106, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.; Choi, Y.H.; Vreeburg, P.; Verpoorte, R. Effect of fertilizers on galanthamine and metabolite profiles in Narcissus bulbs by 1H NMR. J. Agric. Food Chem. 2011, 59, 3155–3161. [Google Scholar] [CrossRef]
- Kreh, M. Studies on galanthamine extraction from Narcissus and other Amaryllidaceae. In Medicinal and Aromatic Plants—Industrial Profiles: Narcissus and Daffodil. The Genus Narcissus; Hanks, G., Ed.; Taylor and Francis: London, UK; New York, NY, USA, 2002; pp. 256–271. [Google Scholar]
- Berkov, S.; Georgieva, L.; Kondakova, V.; Atanassov, A.; Viladomat, F.; Bastida, J.; Codina, C. Plant sources of galanthamine: Phytochemical and biotechnological aspects. Biotechnol. Biotechnol. Equip. 2009, 23, 1170–1176. [Google Scholar] [CrossRef]
- Chang, X. Lycoris, the basis of the galanthamine industry in China. Res. Rev. Int. J. Agric. Allied Sci. 2015, 4, 1–8. Available online: https://www.rroij.com/open-access/lycoris-the-basis-of-the-galanthamine-industry-in-china-.php?aid=73250 (accessed on 1 May 2024).
- Gussev, C.; Bosseva, Y.; Pandova, B.; Yanev, S.; Stanilova, M. Resource assessment of Leucojum aestivum L. (Amaryllidaceae) populations in Bulgaria. Bocconea 2007, 21, 405–411. [Google Scholar]
- Parolo, G.; Abeli, T.; Rossi, G.; Dowgiallo, G.; Matthies, D. Biological flora of Central Europe: Leucojum aestivum L. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 319–330. [Google Scholar] [CrossRef]
- Haist, G.; Sidjimova, B.; Yankova-Tsvetkova, E.; Nikolova, M.; Denev, R.; Semerdjieva, I.; Bastida, J.; Berkov, S. Metabolite profiling and histochemical localization of alkaloids in Hippeastrum papilio (Ravena) van Scheepen. J. Plant Physiol. 2024, 296, 154223. [Google Scholar] [CrossRef]
- Cahlíková, L.; Kawano, I.; Řezáčová, M.; Blunden, G.; Hulcová, D.; Havelek, R. The Amaryllidaceae alkaloids haemanthamine, haemanthidine and their semisynthetic derivatives as potential drugs. Phytochem. Rev. 2021, 20, 303–323. [Google Scholar] [CrossRef]
- Hazrati, S.; Mousavi, Z.; Nicola, S. Harvest time optimization for medicinal and aromatic plant secondary metabolites. Plant Physiol. Biochem. 2024, 212, 108735. [Google Scholar] [CrossRef]
- Mu, H.-M.; Wang, R.; Li, X.-D.; Jiang, Y.-M.; Peng, F.; Xia, B. Alkaloid accumulation in different parts and ages of Lycoris chinensis. Z. Naturforsch. 2010, 65, 458–462. [Google Scholar] [CrossRef]
- Lubbe, A.; Gude, H.; Verpoorte, R.; Choi, C.Y. Seasonal accumulation of major alkaloids in organs of pharmaceutical crop Narcissus Carlton. Phytochemistry 2013, 88, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.N.; Verpoorte, R.; Pomahačová, B. Effect of bulb age on alkaloid contents of Narcissus pseudonarcissus bulbs. S. Afr. J. Bot. 2021, 136, 182–189. [Google Scholar] [CrossRef]
- Waller, G.R.; Nowacki, E.K. Alkaloid Biology and Metabolism in Plants; Plenum Press: New York, NY, USA, 1979; pp. 85–119. [Google Scholar] [CrossRef]
- Al-Humaid, A.I. Effects of compound fertilization on growth and alkaloids of Datura plants. J. Plant Nutr. 2005, 27, 2203–2219. [Google Scholar] [CrossRef]
- Abdolzadeh, A.; Hosseinian, F.; Aghdasi, M.; Sadgipoor, H. Effects of Nitrogen sources and levels on growth and alkaloid content of Periwinkle. Asian J. Plant Sci. 2006, 5, 271–276. [Google Scholar] [CrossRef]
- Ullrich, S.; Rothauer, A.; Hagels, H.; Kayser, O. Influence of light, temperature, and macronutrients on growth and scopolamine biosynthesis in Duboisia species. Planta Med. 2017, 83, 937–945. [Google Scholar] [CrossRef]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Effects of nutrient fertility on growth and alkaloidal content in Mitragyna speciosa (Kratom). Front. Plant Sci. 2020, 11, 597696. [Google Scholar] [CrossRef]
- Vazquez, C.; Reed, S.; Dunn, C. Nitrogen fertilization as ammonium or nitrate-N on Hippeastrum hybridum bulb growth. Agric. Sci. 2015, 6, 1547–1554. [Google Scholar] [CrossRef]
- Inkham, C.; Panjama, K.; Sato, T.; Ruamrungsri, S. Effect of N Source on growth and N uptake of Hippeastrum using 15N tracers. J. Hortic. 2022, 91, 85–93. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical farming: Moving from genetic to environmental modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Hahm, S.; Lee, B.; Bok, G.; Kim, S.; Park, J. Diniconazole promotes the yield of female hemp (Cannabis sativa) inflorescence and cannabinoids in a vertical farming system. Agronomy 2023, 13, 1497. [Google Scholar] [CrossRef]
- Erekath, S.; Seidlitz, H.; Schreiner, M.; Dreyer, C. Food for future: Exploring cutting-edge technology and practices in vertical farm. Sustain. Cities Soc. 2024, 106, 105357. [Google Scholar] [CrossRef]
- Kroggel, M.; Kubota, C. Hydroponic nutrient solution for optimized greenhouse tomato production. Agric. Nat. Resour. 2018, HYG-1437. Available online: https://ohioline.osu.edu/factsheet/hyg-1437 (accessed on 1 May 2024).
- Berkov, S.; Bastid, J.; Viladomat, F.; Codina, C. Development and validation of a GC -MS method for rapid determination of galanthamine in Leucojum aestivum and Narcissus ssp.: A metabolomic approach. Talanta 2011, 83, 1455–1465. [Google Scholar] [CrossRef]
- Luanratana, O.; Griffin, W.J. Cultivation of a Duboisia hybrid. Part A. Nutritional requirements and effects of growth regulators on alkaloid content. J. Nat. Prod. 1980, 43, 546–551. [Google Scholar] [CrossRef]



| Fertilizer I | Fertilizer II | Fertilizer III | |
|---|---|---|---|
| Macronutrient | g/L | g/L | g/L |
| KNO3 | 0.0 | 22.2 | 50.1 |
| KH2PO4 | 20.7 | 20.7 | 20.7 |
| MgSO4-7H2O | 61.2 | 61.2 | 61.2 |
| K2SO4 | 20.7 | 49.6 | 24.1 |
| Ca(NO3)2 | 57.9 | 57.9 | 78.9 |
| CaCl2 | 9.4 | 13.9 | 13.9 |
| Micronutrient | mg/L | mg/L | mg/L |
| Borax | 165.9 | 165.9 | 165.9 |
| MnSO4-H2O | 177.4 | 177.4 | 177.4 |
| CuSO4-5H2O | 20.0 | 20.0 | 20.0 |
| Na2MoO4-2H2O | 12.6 | 12.6 | 12.6 |
| ZnSO4-H2O | 145.1 | 145.1 | 145.1 |
| Fe chelate (DTPA) | 2.0 | 2.0 | 2.0 |
| (A) | Age 0 | Age 1 | Age 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fertilizer | I | II | III | I | II | III | I | II | III |
| Roots | 25 ± 8 | 17 ± 8 | 21 ± 11 | 110 ± 58 | 64 ± 18 | 83 ± 23 | 116 ± 28 | 114 ± 47 | 90 ± 27 |
| Bulbs | 142 ± 49 | 125 ± 52 | 214 ± 149 | 624 ± 266 | 520 ± 99 | 655 ± 207 | 677 ± 132 | 712 ± 105 | 592 ± 142 |
| Leaves | 109 ± 36 | 125 ± 54 | 218 ± 134 | 492 ± 91 | 417 ± 91 | 403 ± 143 | 343 ± 76 | 392 ± 54 | 324 ± 92 |
| Total | 275 ± 92 | 267 ± 114 | 453 ± 292 | 1226 ± 580 | 1000 ± 196 | 1141 ± 368 | 1136 ± 227 | 1217 ± 136 | 1006 ± 235 |
| (B) | Age 0 | Age 1 | Age 2 | ||||||
| Fertilizer | I | II | III | I | II | III | I | II | III |
| Roots | 167 ± 96 | 82 ± 75 | 113 ± 40 | 983 ± 405 | 665 ± 136 | 722 ± 240 | 874 ± 313 | 869 ± 254 | 622 ± 55 |
| Bulbs | 623 ± 214 | 566 ± 286 | 1043 ± 708 | 3650 ± 2213 | 2704 ± 670 | 3510 ± 1160 | 3816 ± 988 * | 2386 ± 270 | 2452 ± 462 |
| Leaves | 598 ± 162 | 470 ± 387 | 759 ± 554 | 1558 ± 759 | 1469 ± 235 | 1567 ± 647 | 1559 ± 248 | 1268 ± 354 | 1125 ± 215 |
| Total | 1388 ± 440 | 1118 ± 691 | 1915 ± 1269 | 6191 ± 3275 | 4838 ± 724 | 5799 ± 1884 | 6249 ± 1487 | 4523 ± 559 | 4198 ± 631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haist, G.; Sidjimova, B.; Denev, R.; Bastida, J.; Berkov, S. Dynamics of Growth and Galanthamine Biosynthesis in Hippeastrum papilio (Ravena) Van Sheepen Hydroponic Culture. Agronomy 2024, 14, 2115. https://doi.org/10.3390/agronomy14092115
Haist G, Sidjimova B, Denev R, Bastida J, Berkov S. Dynamics of Growth and Galanthamine Biosynthesis in Hippeastrum papilio (Ravena) Van Sheepen Hydroponic Culture. Agronomy. 2024; 14(9):2115. https://doi.org/10.3390/agronomy14092115
Chicago/Turabian StyleHaist, Gabriela, Borjana Sidjimova, Rumen Denev, Jaume Bastida, and Strahil Berkov. 2024. "Dynamics of Growth and Galanthamine Biosynthesis in Hippeastrum papilio (Ravena) Van Sheepen Hydroponic Culture" Agronomy 14, no. 9: 2115. https://doi.org/10.3390/agronomy14092115
APA StyleHaist, G., Sidjimova, B., Denev, R., Bastida, J., & Berkov, S. (2024). Dynamics of Growth and Galanthamine Biosynthesis in Hippeastrum papilio (Ravena) Van Sheepen Hydroponic Culture. Agronomy, 14(9), 2115. https://doi.org/10.3390/agronomy14092115





