Abstract
Alternaria brown spot (ABS), caused by Alternaria alternata, is one of the main citrus diseases that causes heavy production losses and reductions in fruit quality worldwide. The application of chemical fungicides has a key role in the management of ABS. In this study, 48 isolates of A. alternata collected from citrus orchards since 2014 were tested in vitro for their sensitivity to pyraclostrobin and fludioxonil, the latter being temporarily registered in Italy since 2020. Pyraclostrobin sensitivity was determined using spore germination and mycelial growth assays. The effective concentration inhibiting 50% of fungal growth (EC50) was determined for each isolate. The sensitivity assays showed that the majority of A. alternata isolates tested were sensitive to pyraclostrobin. EC50 values of fludioxonil in a mycelial growth assay indicated that 100% of isolates were sensitive to this fungicide. The analysis of the cytochrome b gene showed that none of the 40 isolates with a different sensitivity profile had the G143A mutation, and the subgroup of 8 isolates analyzed by real-time PCR did not carry the G137R and F129L mutations. A subset of four more sensitive and two reduced-sensitive isolates was chosen to assess sensitivity on detached citrus leaves treated with pyraclostrobin at the maximum recommended label rate. Disease incidence and symptom severity were significantly reduced, with a small reduction reported in leaves inoculated with the reduced-sensitive isolates. Furthermore, there was no correlation between sensitivity and fitness parameters evaluated in vitro (mycelium growth and sporulation rate). These findings help the development of monitoring resistance programs and, consequently, set up effective anti-resistance strategies for managing ABS on citrus orchards.
1. Introduction
Alternaria brown spot (ABS), caused by Alternaria alternata (Fr.) Keissl., is one of the main citrus diseases worldwide that causes heavy production losses and reductions in fruit quality, especially in citrus-growing countries with a Mediterranean climate, such as Greece [1], Italy [2], and Spain [3]. The pathogen attacks leaves, twigs, and fruit, causing small necrotic brown to black spots. Severe infections may cause premature leaf and fruit drop, resulting in twig dieback and yield reduction [4]. Although tangerines (Citrus reticulata Blanco) and their hybrids are the major hosts of the pathogen, recent studies in Italy demonstrated that the disease could also have a serious economic impact on sweet orange and lemon [5,6]. A survey conducted over a period of eleven years (2010–2020) in representative Sicilian production districts shows a high number of susceptible citrus varieties to ABS on fruit and leaf, especially “Tarocco” and “Femminello Siracusano 2KR” [6]. Regarding control, strategies include the use of healthy nursery stock, wide tree spacing, pruning practices to reduce the canopy humidity, avoidance of overhead irrigation, and reduction of nitrogen fertilization. Although the agronomic practices reduce the inoculum level, disease control relies almost completely on fungicide application, especially in an area with a high risk of infection due to the use of susceptible citrus species and conducive environmental conditions. Formerly, mancozeb was the standard protectant fungicide used for ABS control, but a Commission Implementing Regulation (EU) 2020/2087 of 14 December 2020 [7] did not renew its approval. Thus, Cu-based compounds and pyraclostrobin are currently the only two active ingredients authorized in disease management programs. Pyraclostrobin belongs to quinone outside inhibitors (QoIs). This fungicide group is classified by the Fungicide Resistance Action Committee (FRAC) as having a high risk of resistance to its specific mode of action [8]. QoIs inhibit mitochondrial respiration by binding at the Qo site (the outer quinol oxidation site) of the cytochrome bc1 enzyme complex (complex III). This inhibition blocks the transfer of electrons between cytochrome b and cytochrome c1, leading to an energy deficiency in the fungal cells by halting the production of ATP [9,10]. Resistance to QoIs in plant pathogens is generally due to a single point mutation of the mitochondrial cytochrome b gene, which is related to an amino acid substitution (G143A) that prevents fungicide binding at the target site [11]. Moreover, two other mutations causing QoI resistance have already been identified: the substitution of glycine by arginine at position 137 (G137R) and phenylalanine by leucine at position 129 (F129L) [12,13,14,15]. The highest level of resistance has been associated with the G143A mutation [16], while the moderate levels of resistance with the F129L and G137R mutations [15,17]. Alternaria alternata isolates with the G143A mutation are associated with the resistant phenotype to QoI, with a consequent breakdown of the efficacy of these compounds in the field [18,19]. Although resistant A. alternata populations have been reported worldwide in several crops, such as citrus, pistachio, and potato [20,21,22], to the authors’ knowledge, no monitoring program has been performed in citrus orchards in Italy to detect the sensitivity of A. alternata to pyraclostrobin. Moreover, a fungicide containing fludioxonil (Geoxe®, Syngenta Italia) was authorized for a period of 120 days per year from 2021 to 2023 for emergency use on citrus to control ABS and anthracnose [23,24,25]. The extent of fludioxonil use became necessary to contain the disease, and it was not suitable for other active ingredients except for pyraclostrobin and Cu-compounds. Fludioxonil is a non-systemic fungicide belonging to the phenylpyrroles and derived from the natural antibiotic pyrrolnitrin produced by several Pseudomonas spp. [26]. Phenylpyrroles interfere with the osmoregulatory signal transmission pathway of pathogenic fungi. In detail, the target of this group is the two-component histidine kinase (HK), including the high osmolarity glycerol (HOG), which belongs to group III hybrid histidine kinases involved in an osmotic-regulatory signal transduction cascade of mitogen-activated protein kinase (MAPK) signaling pathway. MAPK pathway perceives extracellular stimuli and coordinates cellular adaptation to hyperosmotic stress [27]. Since fludioxonil may be permanently registered for the control of ABS, it is necessary to determine the baseline sensitivity for A. alternata. Thus, the aims of this study were: (i) to determine the in vitro sensitivity (EC50) to pyraclostrobin in a representative population of A. alternata isolates collected in Sicilian citrus orchards with no and/or uncertain previous exposure to pyraclostrobin; (ii) to evaluate the fitness cost for isolates with various levels of sensitivity to QoI; (iii) to determine the baseline sensitivity to fludioxonil of A. alternata isolates that have never been exposed to phenylpyrroles; (iv) to verify possible breakdown of in vivo efficacy of pyraclostrobin against infections caused by reduced-sensitive A. alternata isolates.
2. Materials and Methods
2.1. Fungal Isolates and Fungicides
A. alternata isolates used in this study were recovered from the fungal collection of the Plant Pathology section at the Department of Agriculture, Food and Environment, University of Catania. Briefly, isolates were collected from different orange, mandarin, and lemon orchards with Alternaria disease symptoms in Catania, Syracuse, and Palermo provinces (Sicily, Italy) from 2014 to 2021. Among these, 34 isolates (formerly named AA) were collected from citrus orchards whose fungicide treatment history was uncertain and identified as A. alternata based on morphological characteristics and phylogenetic analysis [5]. In addition, 14 isolates (formerly named MN, AR) were collected from citrus orchards that have never been treated with QoIs. Isolates were maintained for long-term storage on sterile filter paper at −20 °C. In total, 48 single-spore isolates were selected to establish the sensitivity to pyraclostrobin and fludioxonil. Commercial formulations were used: Cabrio® WG (pyraclostrobin 20% a.i., Basf, Cesano Maderno, Italy) and Geoxe® (fludioxonil 50% a.i., Syngenta, Milan, Italy).
2.2. DNA Extraction, PCR Amplification, and Sequencing
The genomic DNA of A. alternata isolates, formerly named MN and AR, was extracted using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA) and sent to Macrogen Inc. (Seoul, Republic of Korea) for PCR and sequencing. The following loci were amplified and sequenced: the internal transcribed space region (ITS) rDNA gene was amplified with the primers ITS5 and ITS4 [28]; the glyceraldehyde-3-phosphate dehydrogenase (gapdh) using primers gpd1/gpd2 [29] and the translation elongation factor 1-alpha (tef1) gene with primers EF1-728F/EF1-986R [30]. Chromatograms were inspected using FinchTV v.1.4.0 (Geospiza, Seattle, WA, USA; www.geospiza.com/finchtv accessed on 2 February 2024). The DNA sequences (forward and reverse) were assembled using MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms [31] and with Lasergene SeqMan Pro (DNASTAR, Madison, WI, USA).
2.3. Phylogenetic Analyses
The sequences obtained in this study were compared with the NCBI GenBank nucleotide database through the standard nucleotide Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi accessed on 19 July 2024). All isolates that had identical sequences in all the markers sequenced were deposited in GenBank (https://www.ncbi.nlm.nih.gov/ accessed on 3 September 2024) (Table 1) and used for phylogenetic analyses within a combined matrix of ITS, gapdh, and tef1 sequences. Thirty-four species of Alternaria for which sequences were available were included in the matrix, including type/ex-type strains. The GenBank accession numbers of the sequences used in these analyses are given in Table 1. The newly generated sequences of each genomic region were aligned to reference sequences of Alternaria downloaded from GenBank, selecting Alternaria alternantherae as an outgroup. Sequence alignments for phylogenetic analyses were produced with the server version of MAFFT (https://mafft.cbrc.jp/alignment/server/ accessed on 5 September 2024) and checked and refined using BioEdit Sequence Alignment Editor 7.7.1.0 [32]. All three loci (ITS, gapdh, and tef1) were concatenated to a combined matrix using Phyutility v. 2.2 [33]. The combined data matrix used for phylogenetic analyses contained 1336 characters (522 nucleotides of ITS, 573 nucleotides of gapdh, and 241 nucleotides of tef1). Maximum likelihood (ML) analyses were performed with RAxML [34], as implemented in raxmlGUI 2.0 [35], using the ML + rapid bootstrap setting and the GTRGAMMA substitution model, which was selected as the most appropriate model by Modeltest. The matrix was partitioned for the different gene regions, and bootstrap analyses were done with 1000 bootstrap replicates. For evaluation and discussion of bootstrap support, values between 60% and 90% were considered medium/moderate, above 90% high, and 100% maximum. Maximum parsimony (MP) bootstrap analyses were performed with Phylogenetic Analyses Using Parsimony (PAUP) v. 4.0a169 [36]. One thousand bootstrap replicates were implemented using five rounds of heuristic search replicates with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect, COLLAPSE command set to MINBRLEN, and each replicate limited to 1 million rearrangements) during each bootstrap replicate. All molecular characters were unordered and given equal weight, with gaps treated as missing data, and the COLLAPSE command was set to minbrlen.

Table 1.
Information on fungal isolates deposited in GenBank and used in the phylogenetic analyses.
2.4. In Vitro Sensitivity of A. alternata to Fungicides
The sensitivity of A. alternata to pyraclostrobin was assessed using the mycelial growth inhibition (on 48 isolates) and spore germination methods (on 33 isolates), whereas the baseline sensitivity of 48 isolates to fludioxonil was determined using only the mycelial growth inhibition assay. For each assay, the effective concentration inhibiting 50% of the mycelial growth and spore germination (EC50 value) was assessed for each isolate according to logit models.
2.4.1. Mycelial Growth Assay
A. alternata isolates were grown on potato dextrose agar (PDA, Oxoid, Basingstoke, UK) for 6 days at 25 ± 1 °C. Fungicides were dissolved in acetone at 10 μg mL−1, serially diluted in sterile distilled water (SDW), and added to melted PDA medium (45–50 °C) after sterilization. To block the in vitro alternative respiration that avoids the effects of the QoI fungicides on the cytochrome b system, 100 μg mL−1 of salicylhydroxamic acid (SHAM, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in methanol and incorporated into the medium [37]. The final concentrations of pyraclostrobin and fludioxonil were 0, 0.5, 1, and 10 µg mL−1 and 0, 0.01, 0.1, 1, and 10 µg mL−1, respectively. As soon as the medium was solidified, 5 mm diameter mycelial plugs were cut from the margin of the actively growing colony with a sterile cork-borer and placed mycelium-side-down at the center of a 90 mm of PDA plate amended with different concentrations of fungicide. The control consisted of PDA amended with SHAM for pyraclostrobin and PDA medium for fludioxonil. Each concentration was replicated three times. After 7 days of incubation at 25 ± 1 °C, the diameter of the fungal colony was measured across two axes, and the relative growth inhibition (RGrI) was determined using the following formula: RGrI = [(Dcontrol − Dtreatment)/Dcontrol] × 100, where D = diameter of the colony. Three replicates (PDA plates) per combination of isolate-fungicide-concentration were used.
2.4.2. Spore Germination Assay
The effect of pyraclostrobin on spore germination of A. alternata isolates was assessed according to the monitoring method reported by FRAC [38] with slight modification. Spore suspensions of 33 isolates included in the mycelium growth inhibition assay were obtained from 10-day-old fungal cultures maintained on V-8 agar media at 25 ± 1 °C. Thus, 10–15 mL of SDW was added to Petri plates, and the mycelia were gently rubbed with a sterile loop, filtered through a layer of cheesecloth, and adjusted to a final concentration of 1 × 105 conidia mL−1 using a microscope slide hemocytometer. Fungicides were dissolved in acetone at 10 μg mL−1, serially diluted in SDW, and added to molten PDA medium (45–50 °C) after sterilization. SHAM (100 μg mL−1) was added to the medium as described above. The final concentrations of pyraclostrobin were 0, 0.5, 1, and 10 µg mL−1. As soon as the medium was solidified, 100 μL of A. alternata spore suspension was added to the plates and homogeneously spread with a sterile loop. The control consisted of PDA amended with SHAM. After incubation in the dark for 14–16 h at 25 ± 1 °C, the germination rate was quantified by counting 50 conidia in each plate. A conidium was considered germinated when the germ tube was at least longer than the length of a conidium or when multiple germ tubes developed, as reported by Chitolina et al. [19] and Camiletti et al. [39]. Three replicates (PDA plates) per combination of isolate–fungicide concentration were used.
2.5. Analysis of the Cyt b Gene Region of A. alternata Isolates
A representative sub-group of A. alternata isolates (AA1, AA2, AA14, AA24, AA27, AA43, AA74, and AA119) with different sensitivity profile to pyraclostrobin based on the mycelial growth assay was selected to verify the presence of the three mutations (G143A, F129L, G137R) in the cytochrome b gene responsible of the shift in sensitivity of fungal pathogens to QoI. In total, 8 isolates were used for the analysis of the cyt b gene region, with two isolates selected from each of the four sensitivity groups based on the EC50 values (0.01–1, 1–10; 10–100, and >100) of the mycelial growth assay. Genomic DNA was extracted as previously described. The cytochrome b gene of A. alternata isolates was partially amplified using DTRcytb2 (5′-CTAGTATGAACTATTGGTAC-3′) and DTRcytb2r (5′-GAGCAAAAGATATTCTTT-3′) primers [40]. The PCR amplification condition was set as follows: an initial denaturation temperature of 94 °C for 5 min, followed by 35 cycles at the denaturation temperature of 94 °C for 1 min, annealing temperature at 52 °C for 1 min, extension at 72 °C for 2 min, and final extension at 72 °C for 5 min. All PCR products were visualized on 1.5% agarose gel (90 V for 40 min) and stained with GelRed (Biotium, Hayward, CA, USA). PCR products were purified and sequenced by Macrogen Inc. (Seoul, Republic of Korea). All the sequences were aligned and edited by MEGA X. Mutations presence was visually inspected and for comparison, sequences from the GenBank database were aligned with A. alternata isolates obtained in the current study. BLAST searches were performed against the NCBI nucleotide database. The additional group of isolates (AA12, AA16, AA25, AA28, AA36, AA62, AA63, AA64, AA71, AA72, AA73, AA88, AA89, AA90, AA92, AA93, AA94, AA99, AA100, AA102, AA103, AA107, AA108, AA118, AA143, AA144, AR1, AR2, AR3, AR6, AR7, AR8, AR9, AR10, AR11, MN1, MN3, MN4, MN5, and MN7) was selected to verify the presence of the G143A mutation and to distinguish the isolates with this mutation from the wild-type isolates using Real-time PCR. The G143A mutation, which confers QoIs resistance, was widespread and deeply investigated [19,40,41,42,43]. Real-time PCR amplifications were performed according to the methodology described in Ma and Michailides [44] and Luo et al. [45].
2.6. Detached Leaf Bioassay
In vivo efficacy of pyraclostrobin on disease control was assessed on detached citrus leaves inoculated with four more sensitive (AA1, AA28, AA62, and AA90) and two less sensitive (AA2 and AA27) isolates, selected based on the EC50 values obtained in the spore germination assay. Young leaves of mandarin (Citrus reticulata Blanco) “Tardivo di Ciaculli”, about 3 cm long, were collected from 2-year-old plants grown in a greenhouse. Citrus species used in this study were selected based on the most susceptible germplasm to ABS tested in a study conducted by Vitale et al. [6]. Twenty-four hours prior to the pathogen inoculation, pyraclostrobin was applied on the adaxial side of the leaves at the maximum-label rate recommended for citrus (75 g hL−1) by using a manual sprayer. Then, leaves were inoculated by spraying conidial suspension of each A. alternata isolate, previously grown on PDA for 10 days at 25 ± 1 °C. Thus, an aliquot of SDW was added to the plates, and the mycelia were gently rubbed with a sterile loop, filtered through a triple layer of cheesecloth, and adjusted to a final concentration of about 1 × 105 conidia mL−1 using a microscope slide hemocytometer. The control consisted of leaves treated with SDW and inoculated. Leaves were placed into 20 × 15 × 6 cm clean closed plastic boxes filled with 30 g of perlite and 150 mL of SDW to maintain humidity and then incubated at 25 ± 1 °C with a 16 h photoperiod. Fourteen days after inoculation, the disease incidence (DI) was assessed by counting the number of symptomatic leaves, and symptom severity (SS), expressed as percent diseased leaf area, was determined using the ImageJ software v.1.63b [46]. Three replications, each including 10 leaves, were tested for each isolate.
2.7. Fitness Assessment
The fitness assessment was carried out among six pyraclostrobin-sensitive (AA1, AA14, AA36, AA103, AA107, and MN3) and two reduced-sensitive (AA2 and MN5) isolates of A. alternata, based on the EC50 values previously obtained in the spore germination assay. The fitness components were evaluated according to the method reported by Karaoglanidis et al. [47], with a slight modification: mycelial growth on agar medium and spore production in vitro. The experiment was performed twice.
2.7.1. Mycelial Growth
A five-millimeter diameter mycelial plug was removed from the margin of actively growing 10-day-old colonies of A. alternata and transferred to the center of PDA plates. After incubation at 22 ± 1 °C for 6 days in the dark, the colony diameters were measured at two perpendicular points. For each isolate, four replicates were used.
2.7.2. Spore Production Rate
To determine the spore production on PDA, A. alternata isolates were grown on an agar medium for 10 days. After incubation at 25 ± 1 °C, the mycelial plug was transferred into PDA plates and incubated at 22 ± 1 °C with a 14-h photoperiod provided by cool white light. After 10 days, spores were harvested by adding 20 mL of SDW, gently rubbing the surface with a sterile loop, and filtering through double layers of sterile cheesecloth. The concentration of spore suspension was measured using a microscope slide hemocytometer and expressed as the number of conidia per square millimeter of the PDA culture. Four replicate droplets were counted for each plate and four plates were used per isolate.
2.8. Data Analyses
Statistical analysis of in vitro and in vivo data collected from each assay or experiment was performed using the Statistics package software (Statistics version 10; Statsoft Inc., Tulsa, OK, USA). The analysis of the variance was performed on raw data considering all the single replicates. Data from the repeated assays were combined for statistical analysis because variances between assays were homogeneous. Dose–response curves for single isolates exposed to each fungicide were generated by plotting all raw mycelial growth or spore germination reduction data against log10 of fungicide rate. The effective concentration to inhibit 50% and 95% of mycelial growth or spore germination, i.e., EC50 and EC95, was calculated for each isolate by linear regressions (by calculating the coefficient of determination R2) versus the active ingredient concentrations [48,49,50]. The Pearson coefficient (r) was determined for all possible coupled in vitro EC50 datasets of single fungicides. All in vivo data were subjected to variance analysis. Control efficacy (%) of fungicides on mean reduction of lesion size within a full set of A. alternata isolates. Since the in vivo assays were performed two times, F and p values were calculated to evaluate whether the effects of a single factor as treatment and assay were significant. In post hoc analyses, the means were always separated by Fisher’s least significant difference test (α = 0.05).
3. Results
3.1. Phylogenetic Analyses
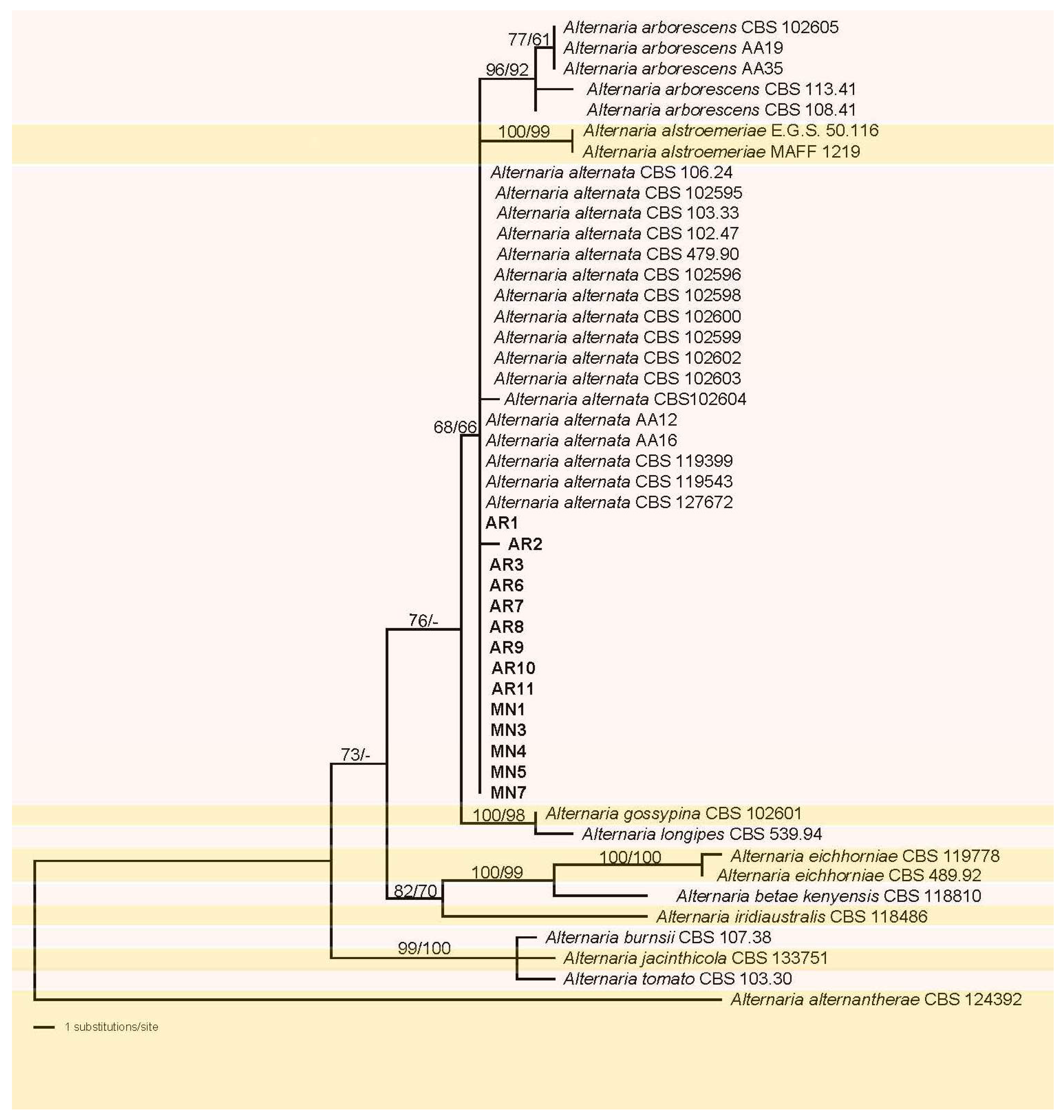
Of the 1336 characters of the combined matrix used for phylogenetic analyses, 44 were parsimony informative (3 from ITS, 26 from gapdh, and 15 from tef1), 72 were parsimony-uninformative, and 1220 were constant. The ML tree (−lnL = 2636.651053) obtained by RAxML is shown in Figure 1. Maximum likelihood analyses resulted in a tree topology similar to that revealed by MP bootstrap analysis. The fourteen isolates of this study included in the matrix formed a distinct monophyletic clade within Alternaria alternata with a sister group relationship to a medium-supported (68% ML, 66% MP) clade containing A. arborescens, and A. alstroemeriae. Most of the terminal received high to maximum support. However, support for intermediate nodes was mostly lacking.
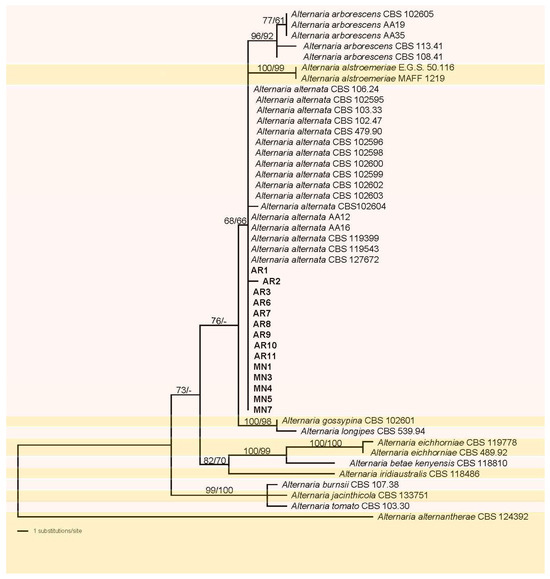
Figure 1.
Phylogram of the best ML tree (−lnL = 2636.651053) revealed by RAxML from an analysis of the combined ITS–gapdh-tef matrix of Alternaria, showing the phylogenetic position of the fourteen Alternaria alternata isolates formerly named as AR and MN (bold), with A. alternantherae selected as outgroup to root the tree. Maximum likelihood (ML) and maximum parsimony (MP) bootstrap support above 60% are given at the first and second position, respectively, above the branches.
3.2. In Vitro Sensitivity of A. alternata to Pyraclostrobin
The EC50 values detected for pyraclostrobin in mycelial growth assay were very variable (Table 2). Colony diameters and an exemplificative overview of A. alternata mycelial growth are reported in Figure 2 and Figure 3, respectively. Comprehensively, EC50 values ranged from 0.41 to a value more than 100 μg mL−1, the latter value detected for 25% of tested isolates. The EC50 values ranging from 10 to 100 μg mL−1 intercepted about 27.1% of the sample, whereas the isolates having EC50 between 1 and 10 μg mL−1 were about 35.4% of the total number. Only 12.5% of the A. alternata isolates were found to be sensitive to pyraclostrobin (EC50 ranging from 0.01 to 1 μg mL−1). The EC50 distribution observed on the spore germination assay was different from the previous one. In detail, about 54.5% and 30.3% of the tested isolates were found to be very sensitive (EC50 < 0.01 μg mL−1) and sensitive (0.01 μg mL−1 < EC50 < 1 μg mL−1), respectively, whereas about 12.1% and 3.0% were found to have high reduced (10 μg mL−1 < EC50 < 100 μg mL−1) and reduced (1 μg mL−1 < EC50 < 10 μg mL−1) QoIs sensitivity levels, respectively (Table 2).

Table 2.
Alternaria alternata isolates grouped by fungicide sensitivity to pyraclostrobin according to the EC50 range calculated for mycelial growth and spore germination reductions with logit models.
Table 2.
Alternaria alternata isolates grouped by fungicide sensitivity to pyraclostrobin according to the EC50 range calculated for mycelial growth and spore germination reductions with logit models.
| EC50 Range (μg mL−1) for Mycelial Growth Reduction | ||||
|---|---|---|---|---|
| <0.01 | 0.01–1 | 1–10 | 10–100 | >100 |
| - | AA1, AA36, AA43, AA102, AA103, MN3 | AA16, AA24, AA63, AA64, AA74, AA92, AA93, AA94, AA108, AA118, MN1, MN7, AR2, AR6, AR7, AR8, AR11 | AA2, AA12, AA71, AA72, AA88, AA89, AA90, AA100, AA119, AA144, AR1, AR3, AR9 | AA14, AA25, AA27, AA28, AA62, AA73, AA99, AA107, AA143, MN4, MN5, AR10 |
| EC50 Range (μg mL−1) for Spore Germination Inhibition | ||||
| <0.01 | 0.01–1 | 1–10 | 10–100 | >100 |
| AA1, AA14, AA25, AA28, AA36, AA64, AA71, AA72, AA89, AA90, AA103, AA107, AA108, AA118, AA119, AA143, MN1, MN3, | AA12, AA16, AA62, AA63, AA73, AA88, AA102, AR2, AR9, AR10 | AA99 | AA2, AA27, AA144, MN5 | - |
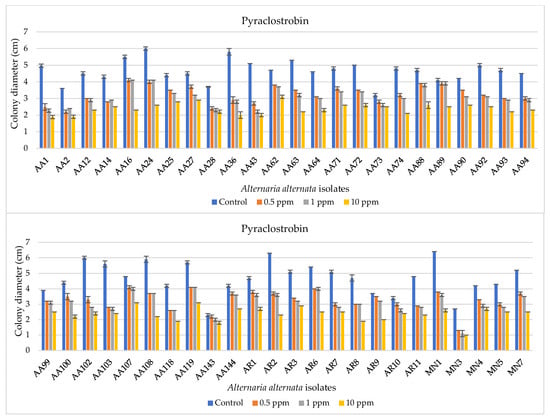
Figure 2.
Bar diagrams showing colony diameters of Alternaria alternata isolates cultured on PDA amended with pyraclostrobin at different doses (0, 0.5, 1, and 10 ppm). Diameters derive from the mean of three replicates. Error bars represent standard deviation.
Figure 2.
Bar diagrams showing colony diameters of Alternaria alternata isolates cultured on PDA amended with pyraclostrobin at different doses (0, 0.5, 1, and 10 ppm). Diameters derive from the mean of three replicates. Error bars represent standard deviation.
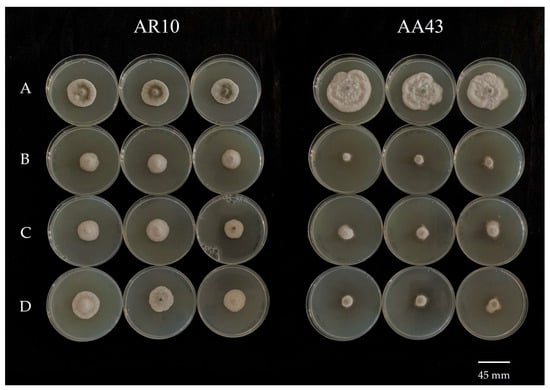
Figure 3.
Mycelial growth reduction of two Alternaria alternata isolates grown on PDA plates amended with pyraclostrobin at different concentrations. (A) 0 μg mL−1; (B) 10 μg mL−1; (C) 1 μg mL−1; (D) 0.5 μg mL−1. From left to right, a reduced-sensitive isolate (AR10) and a sensitive isolate (AA43).
Figure 3.
Mycelial growth reduction of two Alternaria alternata isolates grown on PDA plates amended with pyraclostrobin at different concentrations. (A) 0 μg mL−1; (B) 10 μg mL−1; (C) 1 μg mL−1; (D) 0.5 μg mL−1. From left to right, a reduced-sensitive isolate (AR10) and a sensitive isolate (AA43).
3.3. In Vitro Baseline Sensitivity of A. alternata to Fludioxonil
The EC50 values relative to fludioxonil sensitivity were always lower than 1 μg mL−1, ranging from 0.0003 to 0.91 μg mL−1, and the isolates were grouped for EC50 values as reported in Table 3. Most of these isolates (almost 65%) had EC50 values falling within 0.1−0.25 μg mL−1, about 31% showed an EC50 from 0.26 to 0.50 μg mL−1, no isolates showed EC50 within 0.51−0.75 μg mL−1, and only two isolates had an EC50 comprised between 0.76 and 0.99 μg mL−1. Colony diameters and an exemplificative overview of A. alternata mycelial growth are reported in Figure 4 and Figure 5, respectively.

Table 3.
Sensitive profile of Alternaria alternata isolates to fludioxonil according to EC50 range calculated for mycelial growth.
Table 3.
Sensitive profile of Alternaria alternata isolates to fludioxonil according to EC50 range calculated for mycelial growth.
| Fludioxonil EC50 Range (μg mL−1) | |||
|---|---|---|---|
| 0–0.25 | 0.26–0.50 | 0.51–0.75 | 0.76–0.99 |
| AA1, AA12, AA14, AA16, AA24, AA36, AA43, AA64, AA71, AA72, AA73, AA74, AA88, AA90, AA92, AA94, AA99, AA102, AA103, AA107, AA108, AA118, AA143, AA144, MN1, MN7, AR1, AR2, AR3, AR6, AR10 | AA2, AA25, AA28, AA62, AA63, AA89, AA93, AA100, AA119, MN3, MN4, MN5, AR7, AR8, AR9, | - | AA27, AR11 |
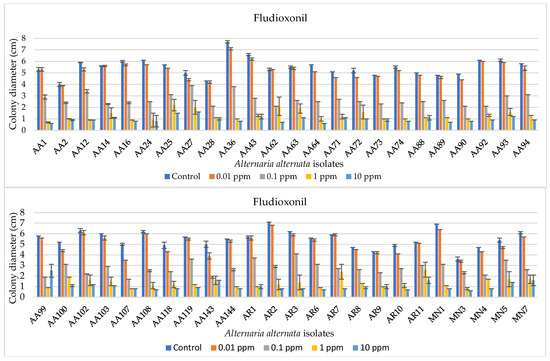
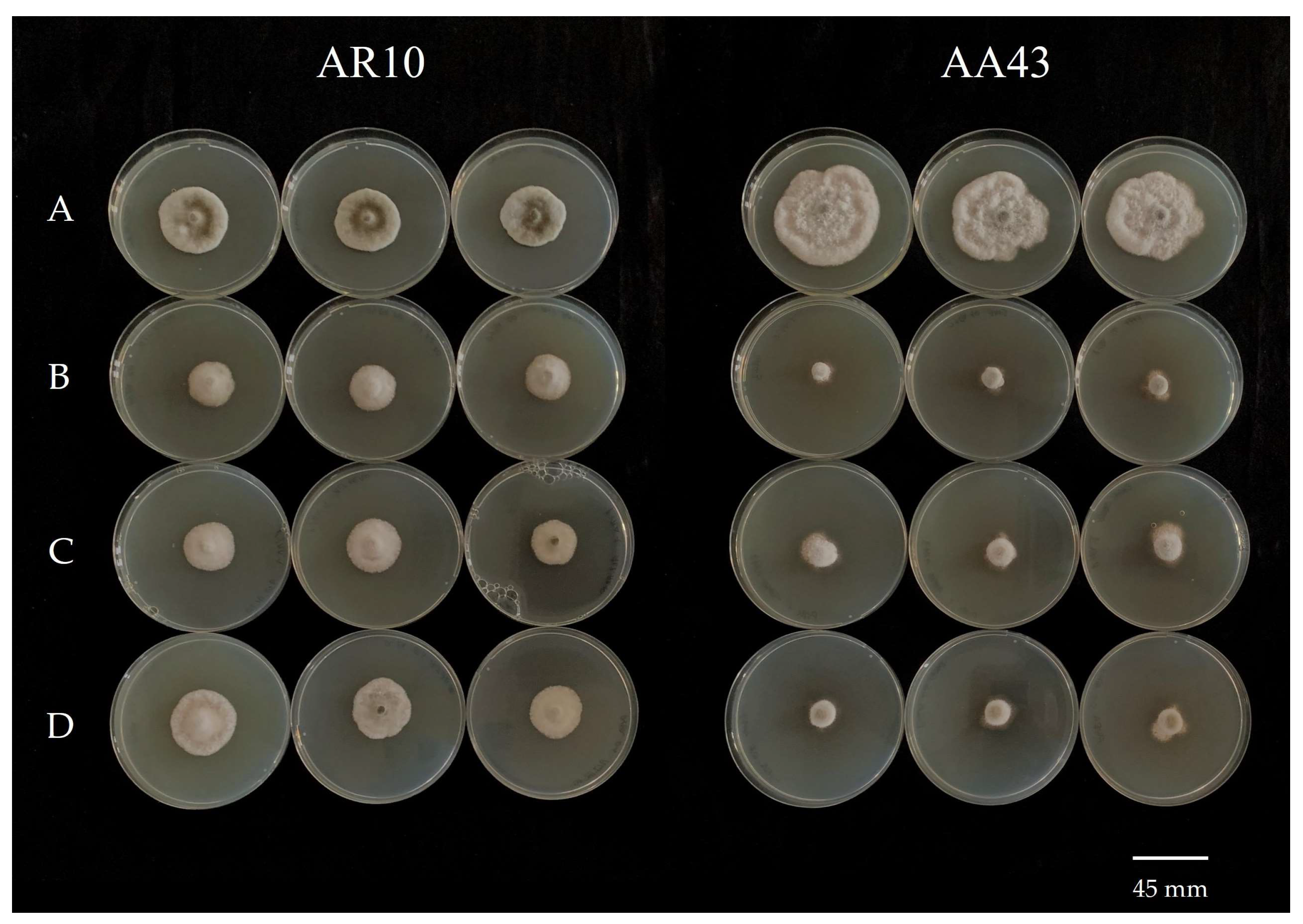
Figure 4.
Bar diagrams showing the colony diameters of Alternaria alternata isolates cultured on PDA amended with fludioxonil at different doses (0, 0.01, 0.1, 1, and 10 ppm). Diameters derive from the mean of three replicates. Error bars represent standard deviation.
Figure 4.
Bar diagrams showing the colony diameters of Alternaria alternata isolates cultured on PDA amended with fludioxonil at different doses (0, 0.01, 0.1, 1, and 10 ppm). Diameters derive from the mean of three replicates. Error bars represent standard deviation.
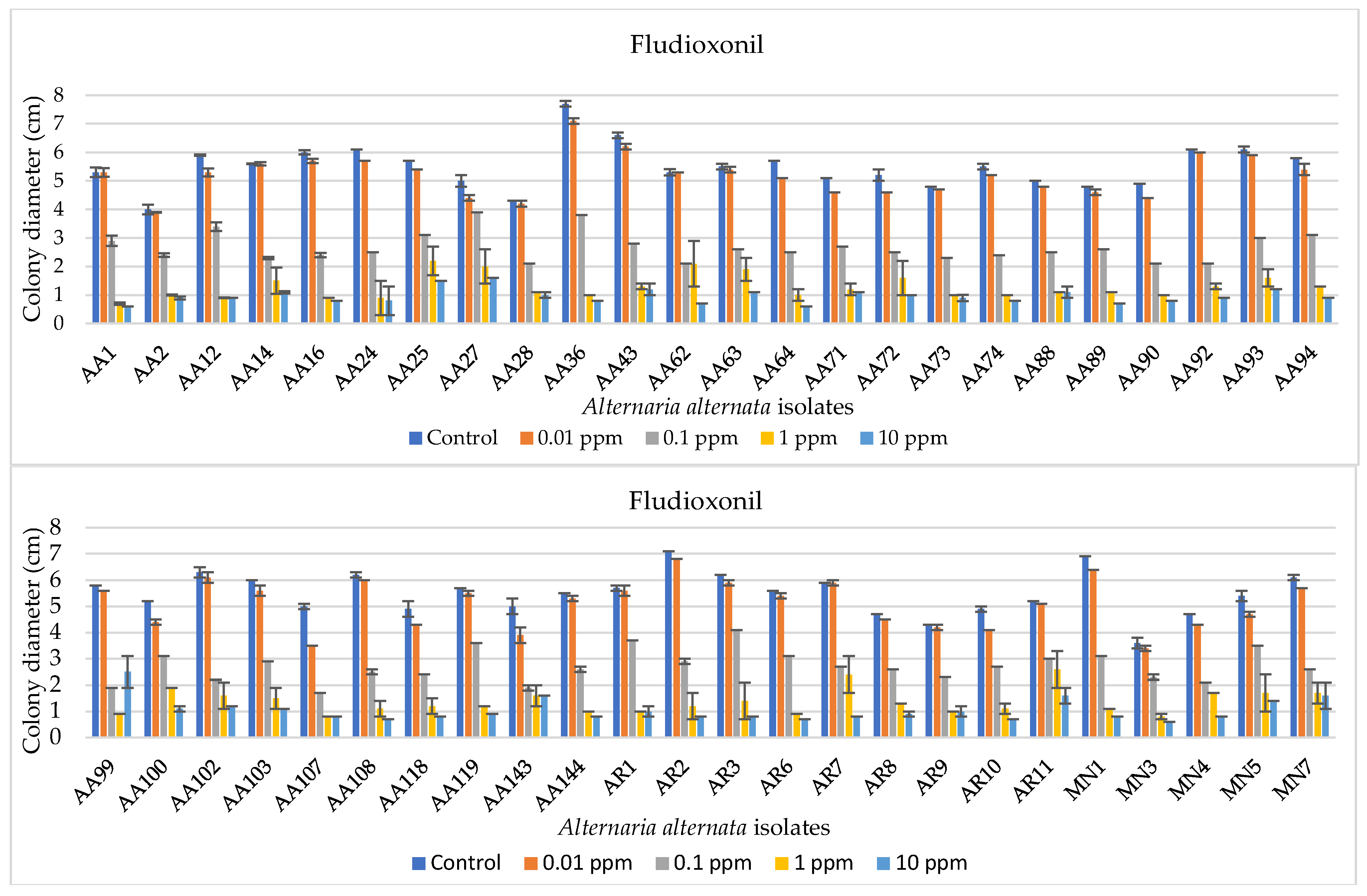
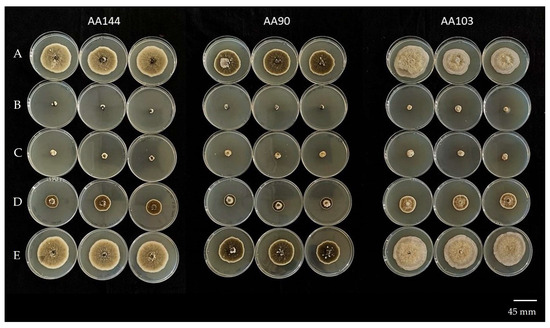
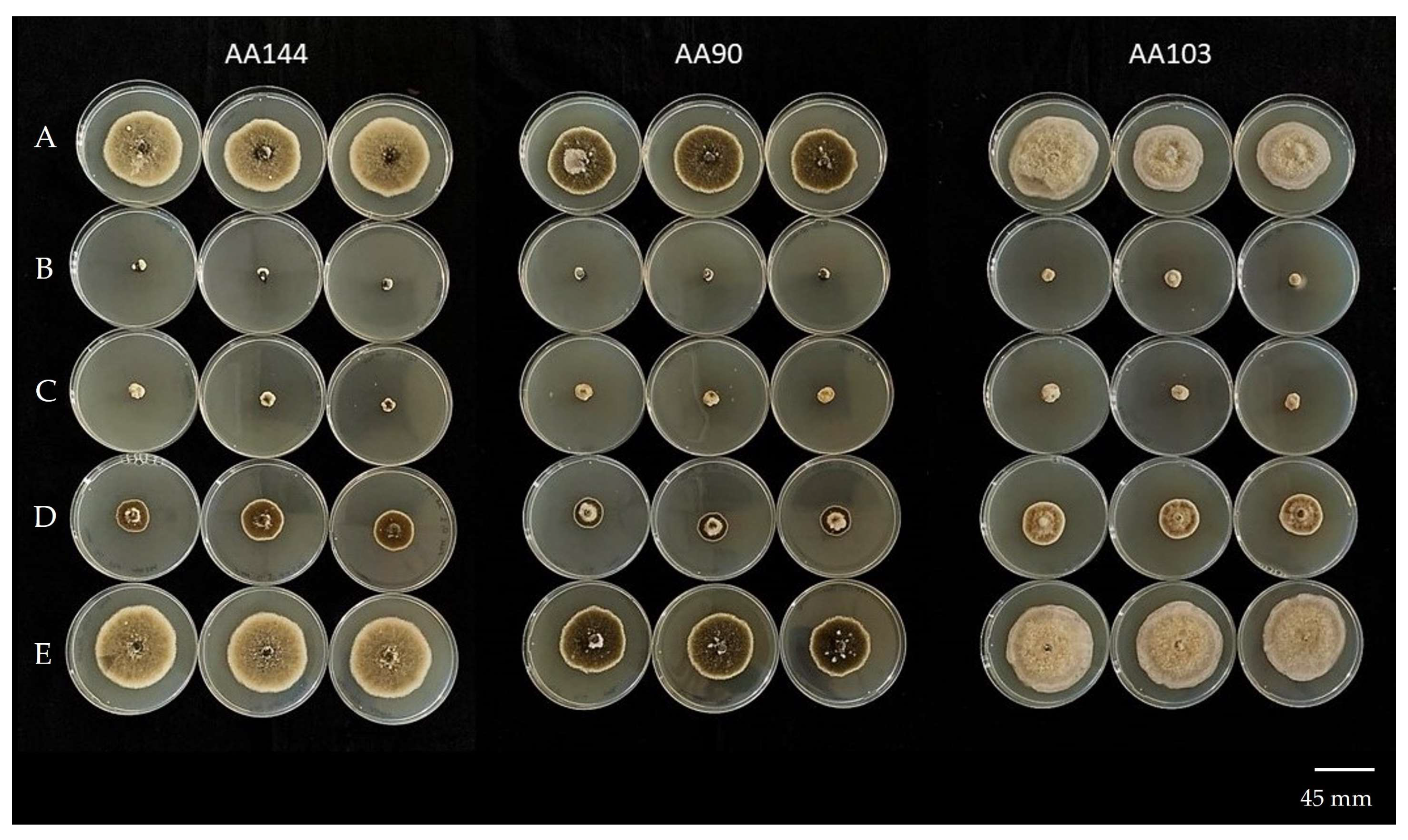
Figure 5.
In vitro sensitivity of three Alternaria alternata isolates (AA144, AA90, and AA103) to fludioxonil at different fungicide concentrations: (A) 0 μg mL−1; (B) 10 μg mL−1; (C) 1 μg mL−1; (D) 0.1 μg mL−1; (E) 0.01 μg mL−1.
Figure 5.
In vitro sensitivity of three Alternaria alternata isolates (AA144, AA90, and AA103) to fludioxonil at different fungicide concentrations: (A) 0 μg mL−1; (B) 10 μg mL−1; (C) 1 μg mL−1; (D) 0.1 μg mL−1; (E) 0.01 μg mL−1.
3.4. Analysis of the Cyt b Gene Region of A. alternata Isolates
To detect the mutations, the cytochrome b gene was partially amplified in 8 isolates with different sensitivity to pyraclostrobin. The length and the intron position were determined after comparison with the reference cytochrome b sequence (GenBank Acc. No. JQ446323.1) of A. alternata. The PCR amplification generated 1461 bp fragments. One intron was inserted after the triplet encoding for glutamine at the amino acid position in 110-1361 position (E107). Comparison of the nucleotide sequences of our selected isolates with the nucleotide sequence of A. alternata from GenBank database (Acc. No. MT559003, MF001504, AY263408, AY263409, and AY263410) revealed that all the isolates characterized in the present study did not show the G143A, F129L, and G137R mutations. Sequences from our isolates were deposited in GenBank with the following accession numbers (AA1 = OQ355015; AA2 = OQ355016; AA14 = OQ355017; AA24 = OQ355018; AA27 = OQ355019; AA43 = OQ355020; AA74 = OQ355021; AA119 = OQ35502). Regarding the group of isolates investigated for the presence of the G143A mutation with the real-time PCR, no amplification curves were observed for the primer set for mutant genotype detection, whereas all the isolates showed amplification curves for the primer set designed to detect wild-type genotype DNA.
3.5. In Vivo Fungicide Effects in Reducing Infections of A. alternata at Different Pyraclostrobin Sensitivity
Since the interactions (assay × treatment) were not significant for this experiment for all fungal isolates (data not shown), the data obtained were combined (Table 4). However, a significant effect of fungicide treatment on disease parameters (DI and SS) was found for all A. alternata isolates (Table 4). As shown in Table 4, the disease amount reductions were always significant for both sensitive- and reduced-sensitive isolates. However, the significance levels detected for the sensitive isolates were stronger than those detected for reduced sensitive ones. For the latter isolates, the disease reductions were averagely lower (Figure 6). These values ranged from about 33% to 48% for DI and 65% to 78% for SS, whereas values for sensitive isolates ranged from about 80% to 97% for DI and 90.5% to 99.4% for SS (Table 4). Based on these cumulative data, an initial breakdown of the efficacy of pyraclostrobin can be hypothesized.

Table 4.
Differential effect of pyraclostrobin on citrus leaves infected with Alternaria alternata isolates exhibiting varying levels of sensitivity in detached leaf assay.
Table 4.
Differential effect of pyraclostrobin on citrus leaves infected with Alternaria alternata isolates exhibiting varying levels of sensitivity in detached leaf assay.
| Isolates | Treatment | DI (%) y | DI (va) y | SS (%) y | SS (va) y |
|---|---|---|---|---|---|
| Sensitive | |||||
| A. alternata AA1 | Control x | 100.0 ± 0.0 a | 90.0 | 60.7 ± 4.8 a | 51.3 |
| Pyraclostrobin | 20.0 ± 10.3 b | 21.9 | 0.9 ± 0.5 b | 4.2 | |
| Reduction (%) | 80.0 | 98.6 | |||
| ** | *** | ||||
| A. alternata AA28 | Control x | 100.0 ± 0.0 a | 90.0 | 64.3 ± 2.2 a | 53.3 |
| Pyraclostrobin | 16.7 ± 7.9 b | 19.9 | 3.9 ± 1.8 b | 9.2 | |
| Reduction (%) | 83.3 | 94.0 | |||
| ** | *** | ||||
| A. alternata AA62 | Control x | 96.7 ± 3.0 a | 83.9 | 43.8 a | 41.4 |
| Pyraclostrobin | 3.3 ± 2.9 b | 6.1 | 0.28 b | 1.75 | |
| Reduction (%) | 96.6 | 99.4 | |||
| *** | *** | ||||
| A. alternata AA90 | Control x | 100.0 ± 0.0 a | 90.0 | 44.6 ± 8.4 a | 41.8 |
| Pyraclostrobin | 20.0 ± 8.9 b | 22.1 | 4.2 ± 2.1 b | 9.6 | |
| Reduction (%) | 80.0 | 90.5 | |||
| ** | *** | ||||
| Reduced sensitivity | |||||
| A. alternata AA2 | Control x | 96.7 ± 3.0 a | 83.9 | 26.6 ± 4.9 a | 30.8 |
| Pyraclostrobin | 50.0 ± 13.7 b | 44.7 | 9.4 ± 2.1 b | 17.6 | |
| Reduction (%) | 48.3 | 64.7 | |||
| * | * | ||||
| A. alternata AA27 | Control x | 100.0 ± 0.0 a | 90.0 | 70.0 ± 0.8 a | 56.8 |
| Pyraclostrobin | 66.7 ± 7.9 b | 55.1 | 15.6 ± 1.2 b | 23.2 | |
| Reduction (%) | 33.3 | 77.7 | |||
| * | ** |
x Control = untreated, inoculated leaves. y DI = disease incidence; SS = symptom severity; av = angular value transformation. Data are the means of three replicates, each with 10 young citrus leaves. Values followed by the same letter within a column are not significantly different according to Fisher’s least significance difference test for disease incidence. Arcsine square root transformation was applied to the percentage prior to data analysis. *, **, *** denote significant differences at 0.01 < p < 0.05, 0.001 < p < 0.01, and p < 0.001, respectively according to the F test.

Figure 6.
Comparison between detached citrus leaves treated with pyraclostrobin and inoculated with highly sensitive (AA1) and reduced sensitive (AA27) isolates of Alternaria alternata. Untreated leaves were displayed in the upper section of each panel, while treated leaves are shown in the lower section.
Figure 6.
Comparison between detached citrus leaves treated with pyraclostrobin and inoculated with highly sensitive (AA1) and reduced sensitive (AA27) isolates of Alternaria alternata. Untreated leaves were displayed in the upper section of each panel, while treated leaves are shown in the lower section.

3.6. Fitness Assessment
In vitro evaluation of fitness parameters of isolates was carried out for six sensitive and two reduced sensitive isolates according to EC50 values previously determined with spore germination assay. In detail, the mycelial growth significantly differed among isolates independently from sensitive or reduced phenotype (Table 5). In detail, average mycelial growth varied from 5.8 cm (AA14) to 7.8 cm (MN5). Similarly, spore production significantly varied among tested isolates independently from phenotype. However, the lowest sporulation rates were detected for two reduced sensitive isolates (AA2 and MN5) together with two sensitive isolates (AA14 and AA107) (Table 5).

Table 5.
Comparisons of fitness parameters between sensitive and reduced sensitive Alternaria alternata isolates based on sporulation germination rate (%).
4. Discussion
The application of fungicides represents a key strategy to control ABS worldwide. Currently, pyraclostrobin and Cu-based compounds are registered in Italy for ABS management. QoIs are considered a high potential risk for resistance development in pathogen populations, especially after years of frequent exposure [16]. Several reports showed a breakdown efficacy of disease with QoI fungicides under field conditions or a higher frequency of reduced sensitive isolates on several crops in Brazil [22], USA [37,51], Germany [43], Greece [42], and South Africa [20]. In this study, we provided insight into the sensitivity profile of pyraclostrobin within a subset of A. alternata isolates. Forty-eight isolates from untreated (MN andAR) and uncertain exposure (AA) to QoIs were collected from citrus orchards in Sicily, the national leader for citrus production [52]. To determine the phenotypic sensitivity level to QoIs, the mycelial inhibition and spore germination assays were compared [11,19,21,39,40,42,53]. All isolates exhibited different sensitivity levels to pyraclostrobin. Based on the discriminatory rate of 100 µg mL−1 [42], the majority of our isolates (75%) should be defined as sensitive to pyraclostrobin using growth inhibition. The frequency of sensitive isolates increased when the sensitivity was determined in a spore germination assay, according to the FRAC monitoring method for Alternaria solani and the most recent studies [19,39]. Our findings from the spore germination assay showed that the majority of the isolates (85%) were well controlled by pyraclostrobin, with EC50 values lower than the discriminatory doses of 1 µg mL−1 as reported by Chitolina et al. [19]. Specifically, only five of the 33 isolates can be defined as reduced-sensitive, of which only one was collected from QoIs-untreated orchards. Since this occurrence was not observed for wild-type A. alternata isolates collected prior to the fungicide registration, it may be due to a pre-existing resistance of the isolate or, more likely, to the presence of neighboring treated citrus orchards from which the isolate was collected. Ishii et al. [54] and Baroffio et al. [55] assigned the presence of less sensitive or resistant isolates in orchards with no fungicide history to the ability of the fungus to long-distance wind spread, although to our knowledge, it is not reported in any sensitivity study for A. alternata. Moreover, it is noteworthy that pyraclostrobin had different efficacy on detached citrus leaves inoculated with four sensitive and two reduced sensitive isolates. Pyraclostrobin exhibited good performance in reducing ABS infection when applied 24 h before the inoculation of all isolates, although the disease reduction was lower for the two reduced-sensitive isolates. To date, resistance to QoIs in different organisms is conferred by single or combined mutations [56]. Regarding A. alternata, it is well known that the target site mutation of the cytochrome b gene for QoI-fungicide resistance could be a specific amino acid substitution from glycine to alanine at position 143 [16]. This substitution (G143A) is associated with high levels of resistance and in the field efficacy breakdown [16,19,22,40,56,57]. None of the isolates tested in our study had undergone the G143A mutation. Moreover, we also investigated the presence of two other mutations (F129L and G137R) responsible for the QoI resistance in other fungal pathogens [58,59,60], and none of these were observed in any of the eight isolates having different levels of sensitivity to pyraclostrobin. For defining shift in sensitivity, our findings suggest that isolates with EC50 greater than 0.1 and 100 µg mL−1 in the spore germination and growth inhibition assays, respectively, should be defined as isolates with “reduced-sensitivity” rather than “resistant”. This is in accordance with other similar studies [11,19,40,42,47]. Conversely, in this study, the amino acid substitution G143A was not detected in our subset of isolates, and the in vivo assay showed that the shift in sensitivity did not result in a complete loss of disease control. Spore germination has been proven once again to be the most reliable method for determining the EC50 for A. alternata. Gene mutation is seen as a factor strongly related to fitness cost, which is a result of the absence of fungicide selection pressure [61,62]. Nevertheless, Karaoglanidis et al. [47] observed variability in fitness components among sensitive and resistant A. alternata isolates. Although few reduced-sensitive isolates were used in the current study, our fitness data are in accordance with the current knowledge. These are the first data in Europe on the sensitivity profile to pyraclostrobin of A. alternata from citrus that provide useful information for future research on the practical resistance to pyraclostrobin in citrus orchards.
This work also established the baseline sensitivity of A. alternata isolates to fludioxonil. For four years (from 2021 to 2024), emergency use of this fungicide has already been granted for 120 days/year to control Alternaria diseases of citrus as a chemical alternative. Knowledge of A. alternata baseline sensitivity to new fungicides needs to receive considerable attention for future monitoring studies on sensitivity shifts within Alternaria populations. Assessing the risk of resistance development after years of fungicide application will help avoid a control breakdown, especially if fludioxonil is permanently included in the Alternaria disease management programs. Prior studies established the sensitivity to fludioxonil of wild-type isolates of A. alternata collected from blueberry [63], mandarin [64], pistachio [21], and potato [57,65]. In this study, all isolates of A. alternata were significantly sensitive to fludioxonil, with EC50 values ranging from 0.1 to 0.99 μg mL−1. These results are in accordance with Avenot and Michailides [21], who also found that EC50 values for the baseline sensitivity of Alternaria to fludioxonil were between 0.010 and 4.875 μg mL−1. Although further research should be undertaken to investigate the sensitivity of A. alternata population after exposure, these findings may help us understand how fludioxonil could be an excellent strategic tool for managing ABS of citrus. Further work is required to monitor a likely shift in the sensitivity of A. alternata populations to fludioxonil and to implement fungicide resistance management practices. In a scenario where climate change is likely to increase the number and the severity of fungal diseases, monitoring the fungicide sensitivity of populations is crucial to avoid negative effects on human and environmental health caused by the irrational use of chemicals.
Author Contributions
G.R.L.: investigation, writing—original draft—review and editing, conceptualization, methodology, software, validation, data curation, formal analysis, and visualization; G.L.Q.: investigation; D.A.: investigation, conceptualization, methodology, and validation; B.X.C. and G.G.: investigation, methodology, and validation; A.V.: writing—original draft—review and editing, conceptualization, methodology, software, validation, data curation, formal analysis, visualization, and supervision; G.P.: writing—review and editing, conceptualization, methodology, resources, validation, funding acquisition, project administration, supervision, and visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-Generation EU (Piano Nazionale di Ripresa e Resilienza (PNRR)—Missione 4 Componente 2, Investimento 1.4—D.D. 1032 17/06/2022, CN00000022) (CUP E63C22000960006), and by the University of Catania, a PhD grant to G.R.L.
Data Availability Statement
Nucleotide sequences generated in this study are deposited with NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and individual accession numbers are listed within this text. All other data and material will be made available upon request to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Elena, K. Alternaria brown spot of Minneola in Greece; evaluation of citrus species susceptibility. Eur. J. Plant Pathol. 2006, 115, 259–262. [Google Scholar] [CrossRef]
- Bella, C.; Guarino, R.; La Rosa, A. Severe infections of Alternaria spp. on a mandarin hybrid. J. Plant Pathol. 2001, 83, 231. [Google Scholar]
- Vicent, A.; Armengol, J.; Sales, R.; García-Jiménez, J. First report of Alternaria brown spot of citrus in Spain. Plant Dis. 2000, 84, 1044. [Google Scholar] [CrossRef] [PubMed]
- Timmer, L.W.; Peever, T.L.; Solel, Z.; Akimitsu, K. Alternaria disease of citrus-novel pathosystems. Phytopathol. Mediterr. 2003, 42, 99–112. [Google Scholar] [CrossRef]
- Aiello, D.; Guarnaccia, V.; Azzaro, A.; Polizzi, G. Alternaria brown spot on new clones of sweet orange and lemon in Italy. Phytopathol. Mediterr. 2020, 59, 131–145. [Google Scholar] [CrossRef]
- Vitale, A.; Aiello, D.; Azzaro, A.; Guarnaccia, V.; Polizzi, G. An eleven-year survey on field disease susceptibility of citrus accessions to Colletotrichum and Alternaria species. Agriculture 2021, 11, 536. [Google Scholar] [CrossRef]
- European Commission. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R2087&rid=6 (accessed on 26 January 2024).
- Fungicide Resistance Action Committee (FRAC). 2023. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2023---final.pdf?sfvrsn=78bc4e9a_2 (accessed on 26 January 2024).
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int. Microbiol. 2008, 11, 1. [Google Scholar]
- Fernández-Ortuño, D.; Tores, J.A.; Pérez-García, A. The QoI fungicides, the rise and fall of a successful class of agricultural fungicides. In Fungicides; Carisse, O., Ed.; InTech: London, UK, 2010; pp. 203–220. [Google Scholar] [CrossRef]
- Vega, B.; Liberti, D.; Harmon, P.F.; Dewdney, M.M. A rapid resazurin-based microtiter assay to evaluate QoI sensitivity for Alternaria alternata isolates and their molecular characterization. Plant Dis. 2012, 96, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Sierotzki, H.; Wullschleger, J.; Gisi, U. Point-mutation in cytochrome b gene conferring resistance to strobilurin fungicides in Erysiphe graminis f. sp. tritici field isolates. Pestic. Biochem. Physiol. 2000, 68, 107–112. [Google Scholar] [CrossRef]
- Sierotzki, H.; Parisi, S.; Steinfeld, U.; Tenzer, I.; Poirey, S.; Gisi, U. Mode of resistance to respiration inhibitors at the cytochrome bc1 enzyme complex of Mycosphaerella fijiensis field isolates. Pest Manag. Sci. 2000, 56, 833–841. [Google Scholar] [CrossRef]
- Goes, A.D.; Montes de Oca, A.G.; Reis, R.F. Ocurrencia de la mancha de Alternaria en mandarina Dancy en el estado de Rio de Janeiro. Fitopatol. Bras. 2001, 26, 386. [Google Scholar]
- Sierotzki, H.; Frey, R.; Wullschleger, J.; Palermo, S.; Karlin, S.; Godwin, J.; Gisi, U. Cytochrome b gene sequence and structure of Pyrenophora teres and P. tritici-repentis and implications for QoI resistance. Pest Manag. Sci. 2007, 63, 225–233. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. Review: The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, A. Resistance risk to QoI fungicides and anti-resistance strategies. In Fungicide Resistance in Crop Protection: Risk and Management; Tarlochan, S., Ed.; CABI: Wallingford, UK, 2012; pp. 141–154. [Google Scholar] [CrossRef]
- Dewdney, M.M.; Vega, B.; Peres, N.A.; Graham, J.H. Fungicide Resistance in Citrus Pathogens. In Fungicide Resistance in North America; Stevenson, K.L., McGrath, M.T., Wyenandt, C.A., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2019; pp. 233–250. [Google Scholar] [CrossRef]
- Chitolina, G.R.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, E.G.; Amorim, L. Distribution of Alternaria alternata isolates with resistance to quinone outside inhibitor (QoI) fungicides in Brazilian orchards of tangerines and their hybrids. Crop Prot. 2021, 141, 105493. [Google Scholar] [CrossRef]
- Dube, J.P.; Truter, M.; van der Waals, J.E. first report of resistance to QoI fungicides in Alternaria alternata isolates from potato in South Africa. Plant Dis. 2014, 98, 1431. [Google Scholar] [CrossRef] [PubMed]
- Avenot, H.F.; Michailides, T.J. Detection of isolates of Alternaria alternata with multiple-resistance to fludioxonil, cyprodinil, boscalid and pyraclostrobin in California pistachio orchards. Crop Prot. 2015, 78, 214–221. [Google Scholar] [CrossRef]
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. First report on quinone outside inhibitor resistance of Alternaria alternata causing alternaria brown spot in tangerines in São Paulo, Brazil. Plant Health Prog. 2019, 20, 94. [Google Scholar] [CrossRef]
- Ministero Della Salute. 2021. Available online: https://www.salute.gov.it/portale/temi/documenti/fitosanitari/2021/28_07_2021_est_impiego_in_deroga_GEOXE.pdf (accessed on 3 November 2022).
- Ministero Della Salute. 2022. Available online: https://www.salute.gov.it/portale/temi/documenti/fitosanitari/2022/GEOXE.pdf (accessed on 3 November 2022).
- Ministero Della Salute. 2023. Available online: https://www.salute.gov.it/portale/temi/documenti/fitosanitari/2023/13_09_2023_GEOXE.pdf (accessed on 11 January 2024).
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 years, two molecules and (nearly) no resistance. Front. Microbiol. 2016, 16, 7. [Google Scholar] [CrossRef]
- Bersching, K.; Jacob, S. The molecular mechanism of fludioxonil action is different to osmotic stress sensing. J. Fungi 2021, 7, 393. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols—A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 15–322. [Google Scholar]
- Berbee, M.L.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Smith, S.A.; Dunn, C.W. Phyutility: A phyloinformatics tool for trees, alignments and molecular data. Bioinformatics 2008, 24, 715–716. [Google Scholar] [PubMed]
- Stamatakis, E. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. RaxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar] [CrossRef]
- Mondal, S.N.; Bhatia, A.; Shilts, T.; Timmer, L.W. Baseline sensitivities of fungal pathogens of fruit and foliage of citrus to azoxystrobin, pyraclostrobin, and fenbuconazole. Plant Dis. 2015, 89, 1186–1194. [Google Scholar] [CrossRef]
- Fungicide Resistance Action Committee (FRAC). FRAC Monitoring Method. 2006. Available online: https://www.frac.info/docs/default-source/monitoring-methods/approved-methods/alteso-monitoring-method-syngenta-2006-v1.pdf?sfvrsn=619a419a_4 (accessed on 26 January 2024).
- Camiletti, B.X.; Lichtemberg, P.S.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, pathogenicity, and fungicide sensitivity of Alternaria isolates associated with preharvest fruit drop in California citrus. Fungal Biol. 2022, 126, 277–289. [Google Scholar] [CrossRef]
- Grasso, V.; Palermo, S.; Sierotzki, H.; Garibaldi, A.; Gisi, U. Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag. Sci. 2006, 62, 465–472. [Google Scholar] [CrossRef]
- Duba, A.; Goriewa, K.; Wachowska, U.; Wiwart, M. Alternaria alternata (Fr.) Keissl with mutation G143A in the cyt b gene is the source of a difficult-to-control allergen. Environ. Sci. Pollut. Res. 2018, 25, 469–478. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Apostolidou, Z.A.; Louka, D.; Markoglou, A.; Flouri, F. Biological and molecular characterization of field isolates of Alternaria alternata with single or double resistance to respiratory complex II and III inhibitors. Eur. J. Plant Pathol. 2018, 152, 199–211. [Google Scholar] [CrossRef]
- Nottensteiner, M.; Absmeier, C.; Zellner, M. QoI fungicide resistance mutations in Alternaria solani and Alternaria alternata are fully established in potato growing areas in bavaria and dual resistance against SDHI fungicides is upcoming. Gesunde Pflanz. 2019, 71, 155–164. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. A real-time PCR assay for the detection of azoxystrobin-resistant Alternaria populations from pistachio orchards in California. Crop. Prot. 2004, 23, 1259–1263. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Reyes, H.C.; Morgan, D.P.; Michailides, T.J. Using real-time PCR to survey frequency of azoxystrobin-resistant allele G143A in Alternaria populations from almond and pistachio orchards in California. Pestic. Biochem. Phys. 2007, 88, 328–336. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Karaoglanidis, G.S.; Luo, Y.; Michailides, T.J. Competitive ability and fitness of Alternaria alternata isolates resistant to QoI fungicides. Plant. Dis. 2011, 95, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, A.; Castello, I.; Cirvilleri, G.; Perrone, G.; Epifani, F.; Ferrara, M.; Polizzi, G.; Walters, D.R.; Vitale, A. Detection of Botrytis cinerea field isolates with multiple fungicide resistance from table grape in Sicily. Crop Prot. 2015, 77, 65–73. [Google Scholar] [CrossRef]
- Vitale, A.; Panebianco, A.; Polizzi, G. Baseline sensitivity and efficacy of fluopyram in Botrytis cinerea from table grape in Italy. Ann. Appl. Biol. 2016, 169, 36–45. [Google Scholar] [CrossRef]
- Fragalà, F.; Castello, I.; Puglisi, I.; Padoan, E.; Baglieri, A.; Montoneri, E.; Vitale, A. New insights into municipal biowaste derived products as promoters of seed germination and potential antifungal compounds for sustainable agriculture. Chem. Biol. Technol. Agric. 2022, 9, 69. [Google Scholar] [CrossRef]
- Ma, Z.; Felts, D.; Michailides, T.J. Resistance to azoxystrobin in Alternaria isolates from pistachio in California. Pestic. Biochem. Physiol. 2003, 77, 6–74. [Google Scholar] [CrossRef]
- ISTAT, Istituto Nazionale di Statistica. 2021. Available online: http://dati.istat.it/ (accessed on 11 January 2023).
- Avenot, H.; Morgan, D.P.; Michailides, T.J. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine® (pyraclostrobin & boscalid) fungicide in Alternaria alternata causing alternaria late blight of pistachios in California. Plant Pathol. 2008, 57, 135–140. [Google Scholar] [CrossRef]
- Ishii, H.; Fraaije, B.A.; Sugiyama, T.; Noguchi, K.; Nishimura, K.; Takeda, T.; Amano, T.; Hollomon, D.W. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 2001, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Baroffio, C.A.; Siegfried, W.; Hilber, U.W. Long-term monitoring for resistance of Botryotinia fuckeliana to anilinopyrimidine, phenylpyrrole and hydroxyanilide fungicides in Switzerland. Plant Dis. 2003, 87, 662–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yang, J.H.; Fan, F.; Luo, C.X.; Schnabel, G. Fitness and competitive ability of Alternaria alternata field isolates with resistance to SDHI, QoI, and MBC fungicides. Plant Dis. 2015, 99, 1744–1750. [Google Scholar] [CrossRef]
- Pasche, J.S.; Wharam, C.M.; Gudmestad, N.C. Shift in sensitivity of Alternaria solani in response to QoI fungicides. Plant Dis. 2004, 88, 181–187. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, G.; Wang, B.; Du, Y.; Li, Z.; Gao, X.; Zhang, C.; Liu, X. Three point-mutations in cytochrome b confer resistance to trifloxystrobin in Magnaporthe oryzae. Pest Manag. 2020, 76, 4258–4267. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhang, H.; Yang, D.; Deng, Y.; Wang, Y.; Qi, Z. Development of a LAMP method for detecting F129L mutant in azoxystrobin-resistant Pyricularia oryzae. Fungal Biol. 2022, 126, 47–53. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Slakhorst, M.; Debets, A.J.M.; Hoekstra, R.F. Comparing artificial and natural selection in rate of adaptation to genetic stress in Aspergillus nidulans. J. Evol. Biol. 2005, 18, 771–778. [Google Scholar] [CrossRef]
- Jeger, M.J.; Wijngaarden, P.J.; Hoekstra, R.F. Adaptation to the cost of resistance in a haploid clonally reproducing organism: The role of mutation, migration, and selection. J. Theor. Biol. 2008, 252, 621–632. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide resistance in Alternaria alternata from blueberry in California and its impact on control of Alternaria rot. Plant Dis. 2022, 106, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Seiya, S.; Xiao, C.L. Fungicide resistance of Alternaria alternata and A. arborescens isolates from mandarin fruit and its influence on control of postharvest Alternaria rot. Plant Dis. 2023, 107, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Budde-Rodriguez, S.; Pasche, J.S.; Mallik, I.; Gudmestad, N.C. Sensitivity of Alternaria spp. from potato to pyrimethanil, cyprodinil, and fludioxonil. Crop Prot. 2022, 152, 105855. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).