Effects of High-Voltage Discharge Plasma on Drying Properties, Microstructure, and Nutrients of Oat Grass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
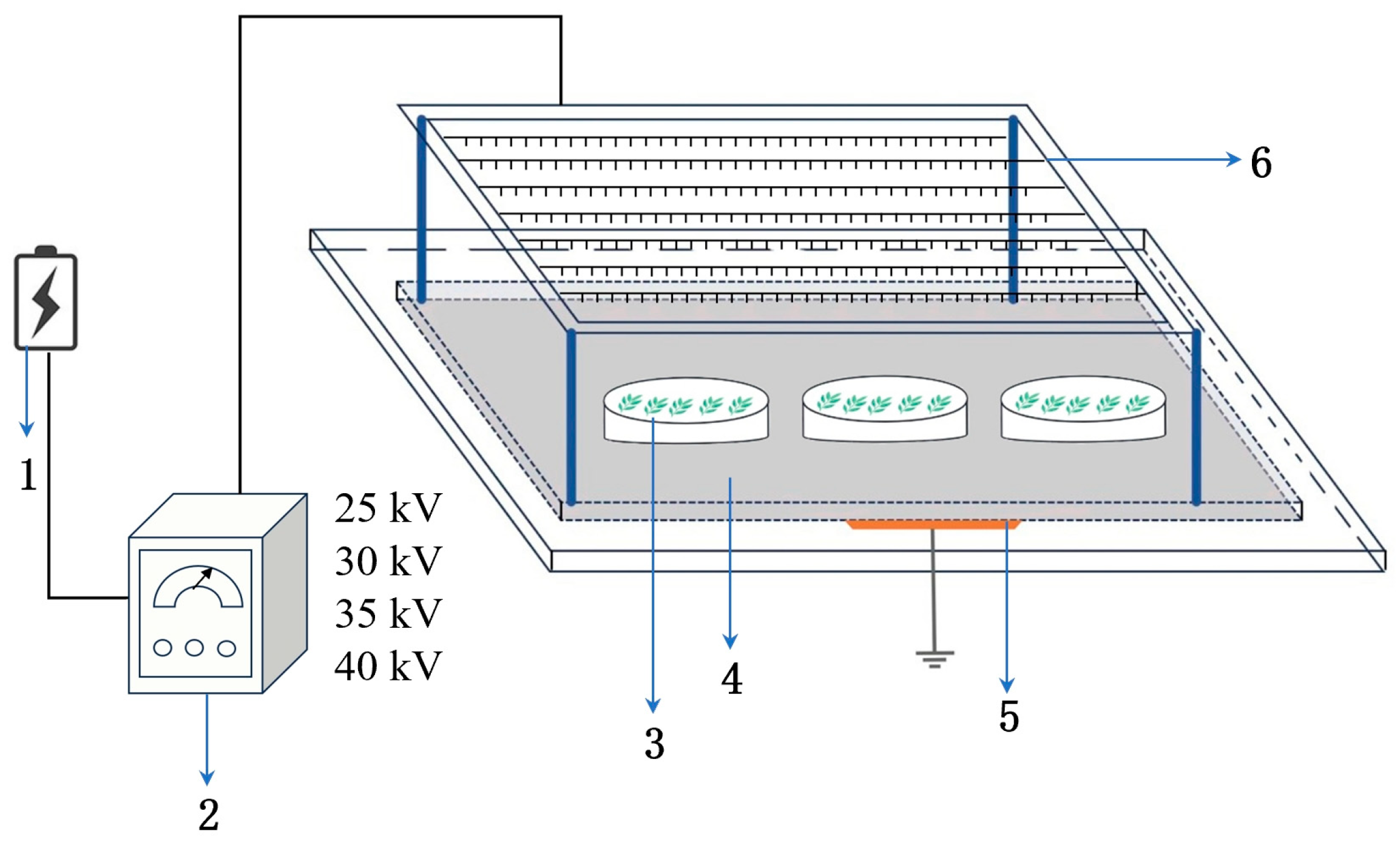
2.2. Instrument and Equipment
2.3. Experimental Methods
2.4. Voltage, Current and Power Parameters
2.5. Moisture Content Measurement
2.6. Rehydration Rate
2.7. Electrical Conductivity
2.8. Scanning Electron Microscopy (SEM)
2.9. Transmission Electron Microscopy (TEM)
2.10. Infrared Spectrum
2.11. Determination of Nutrient Composition
2.12. Statistical Analysis
3. Results and Discussion
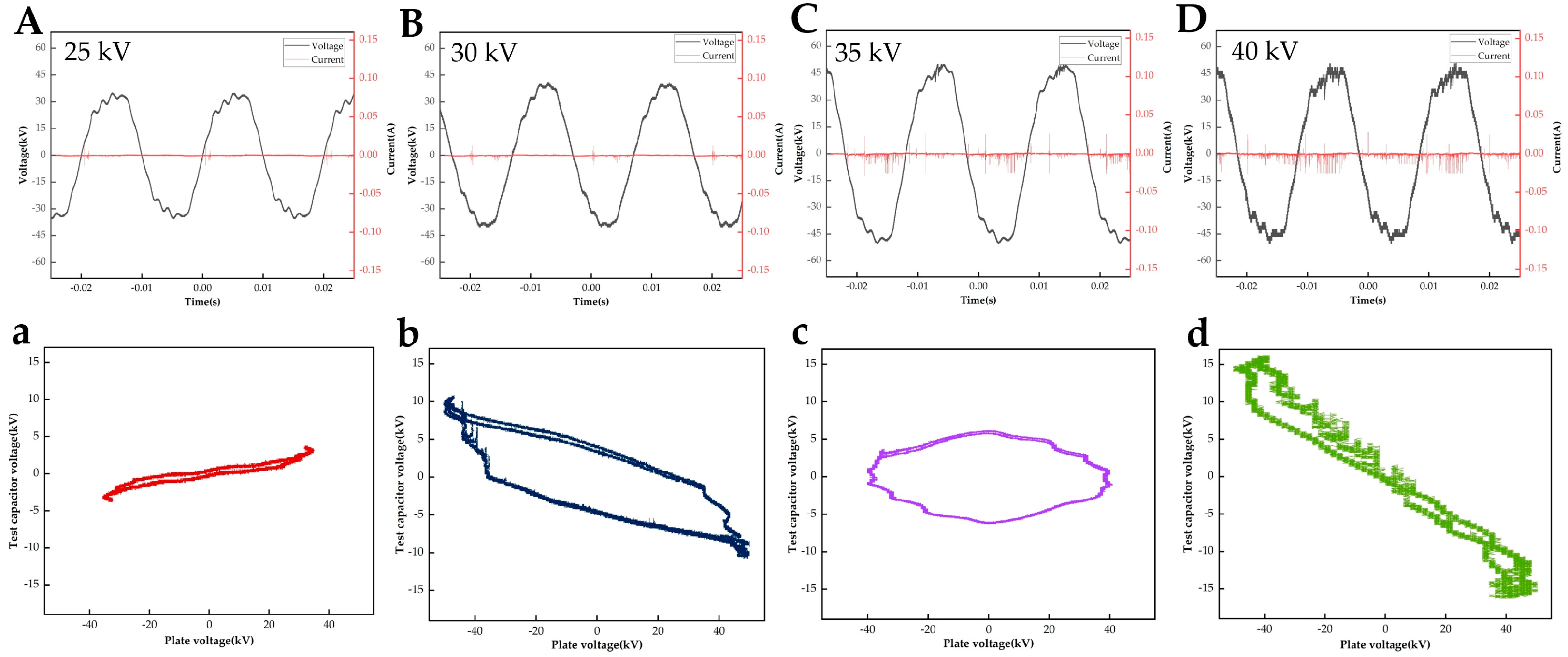
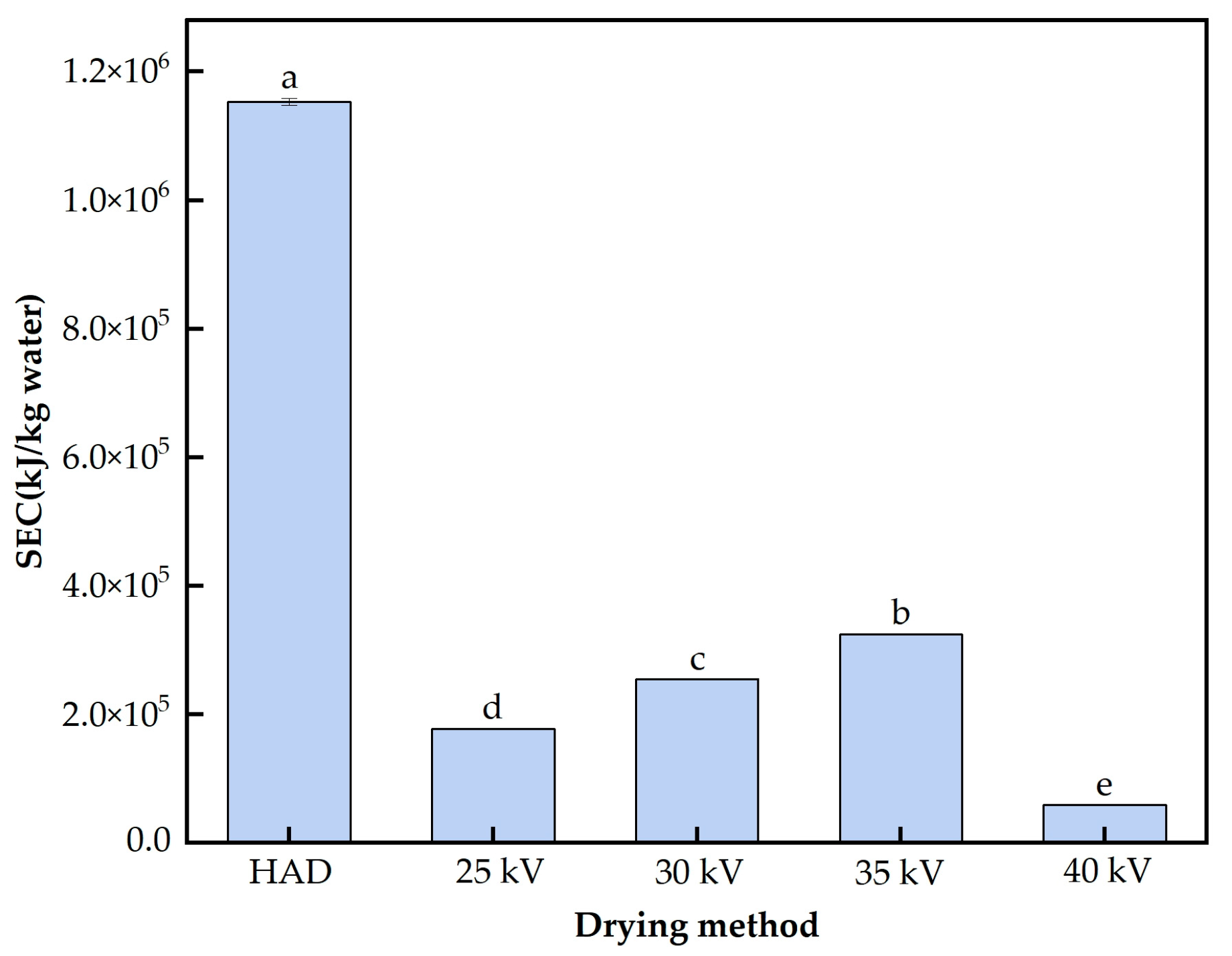
3.1. HVDP Drying Characterisation
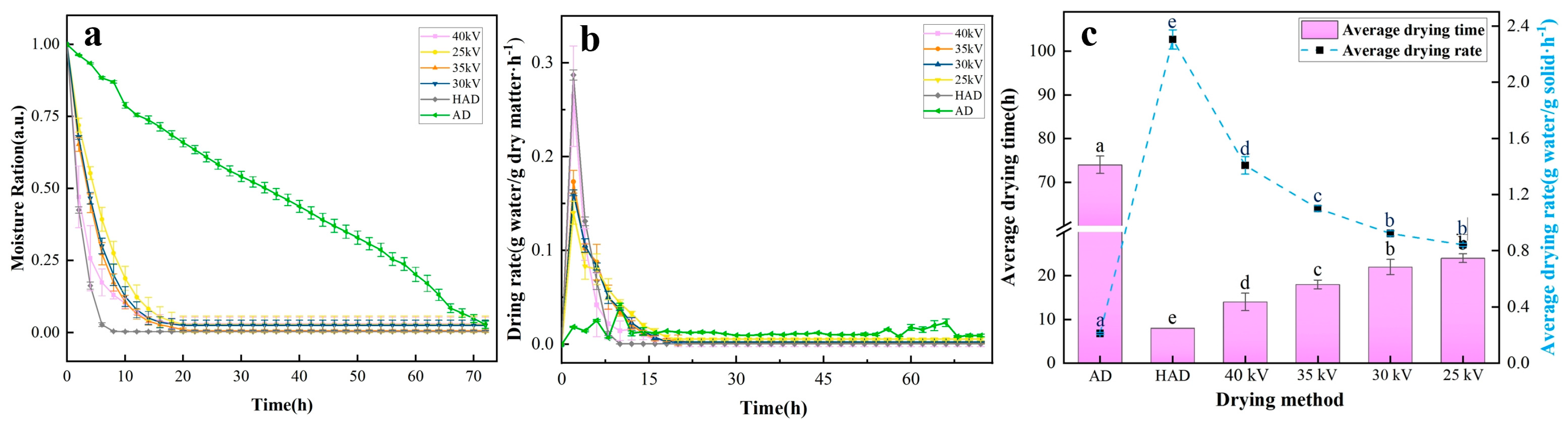
3.2. Drying Properties
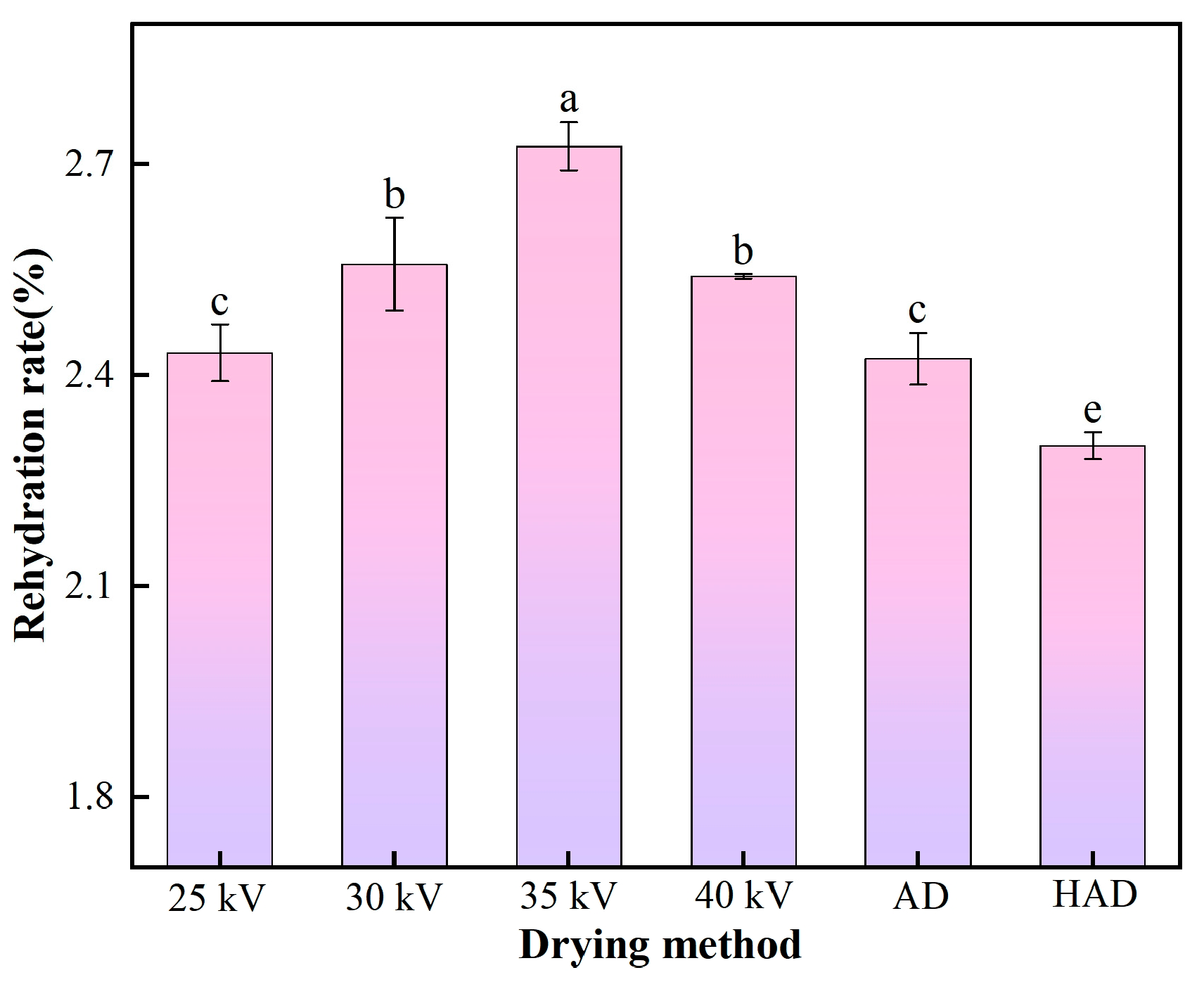
3.3. Rehydration Rate Analysis
3.4. Microstructure
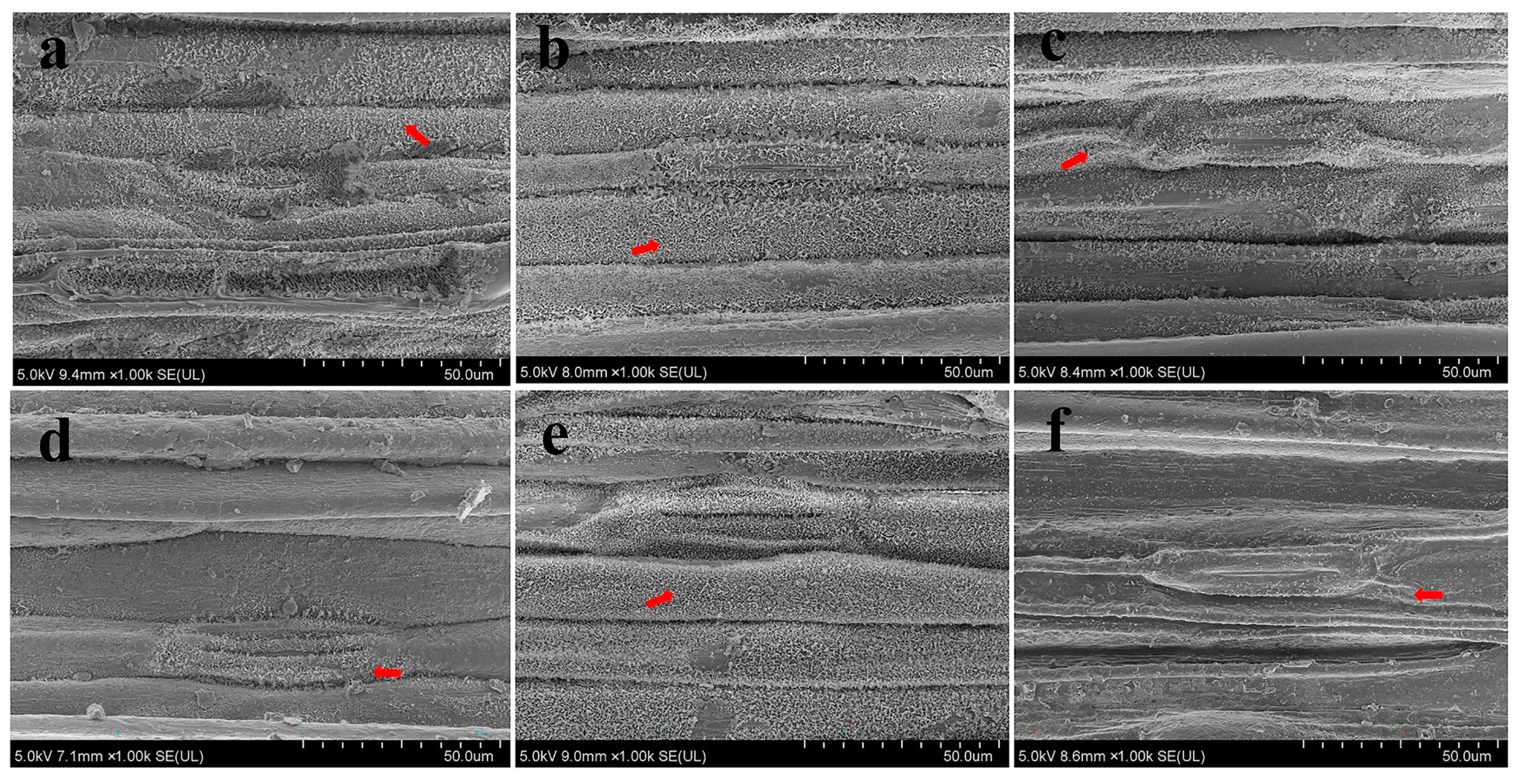
3.4.1. Scanning Electron Microscope Analysis
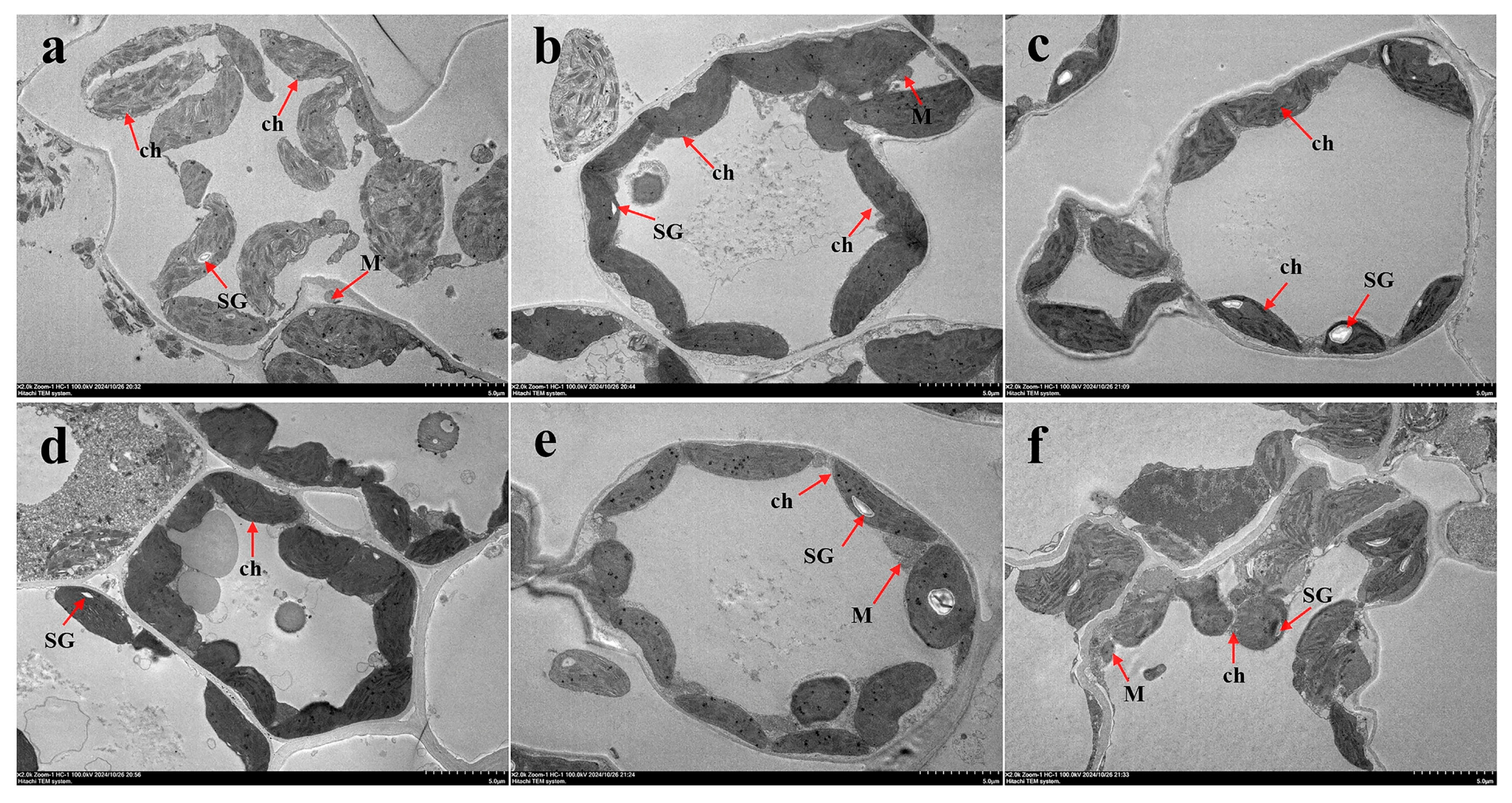
3.4.2. Transmission Electron Microscopy Analysis
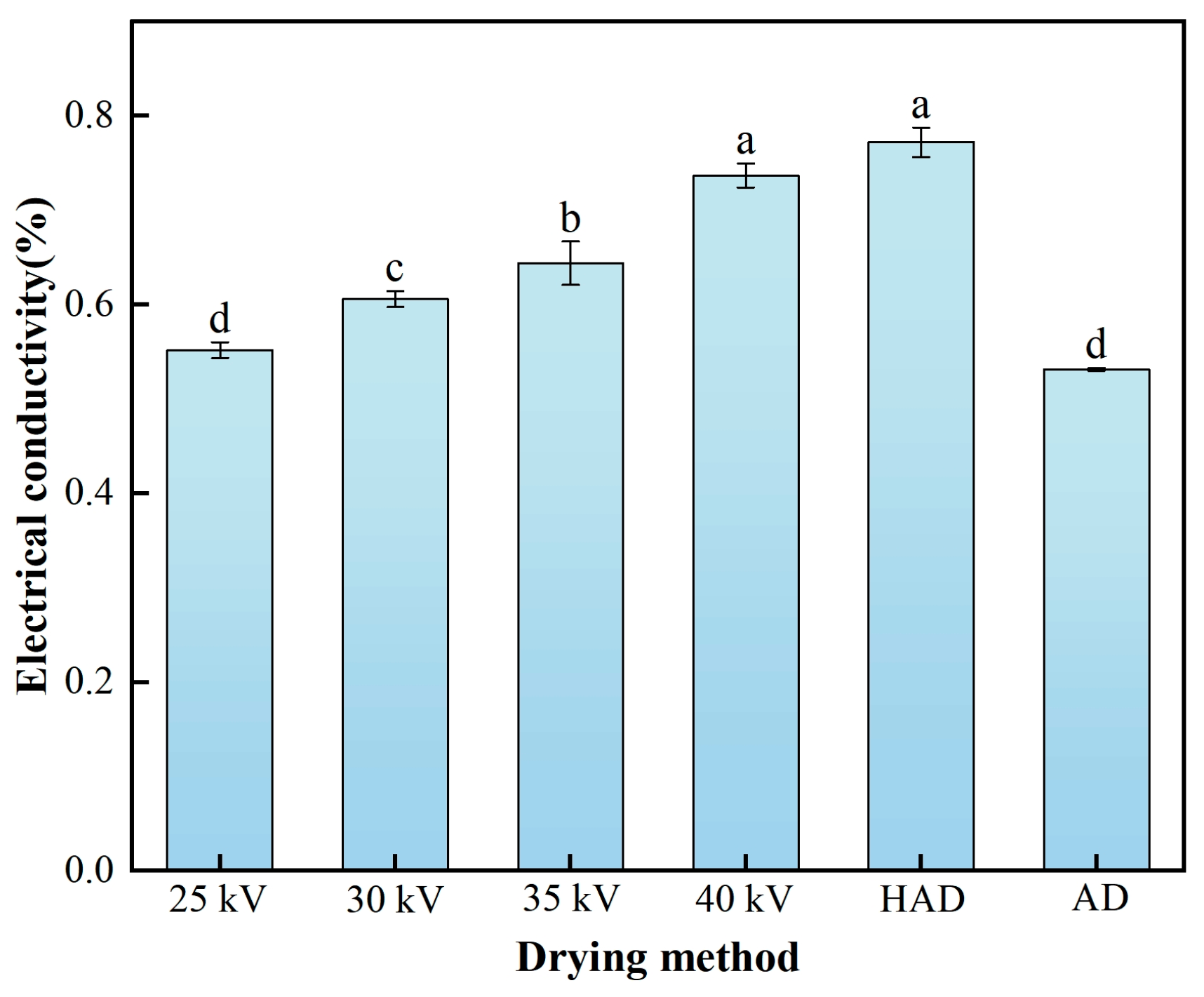
3.5. Electrical Conductivity Analysis
3.6. Nutritional Quality
3.6.1. Protein Content (CP)
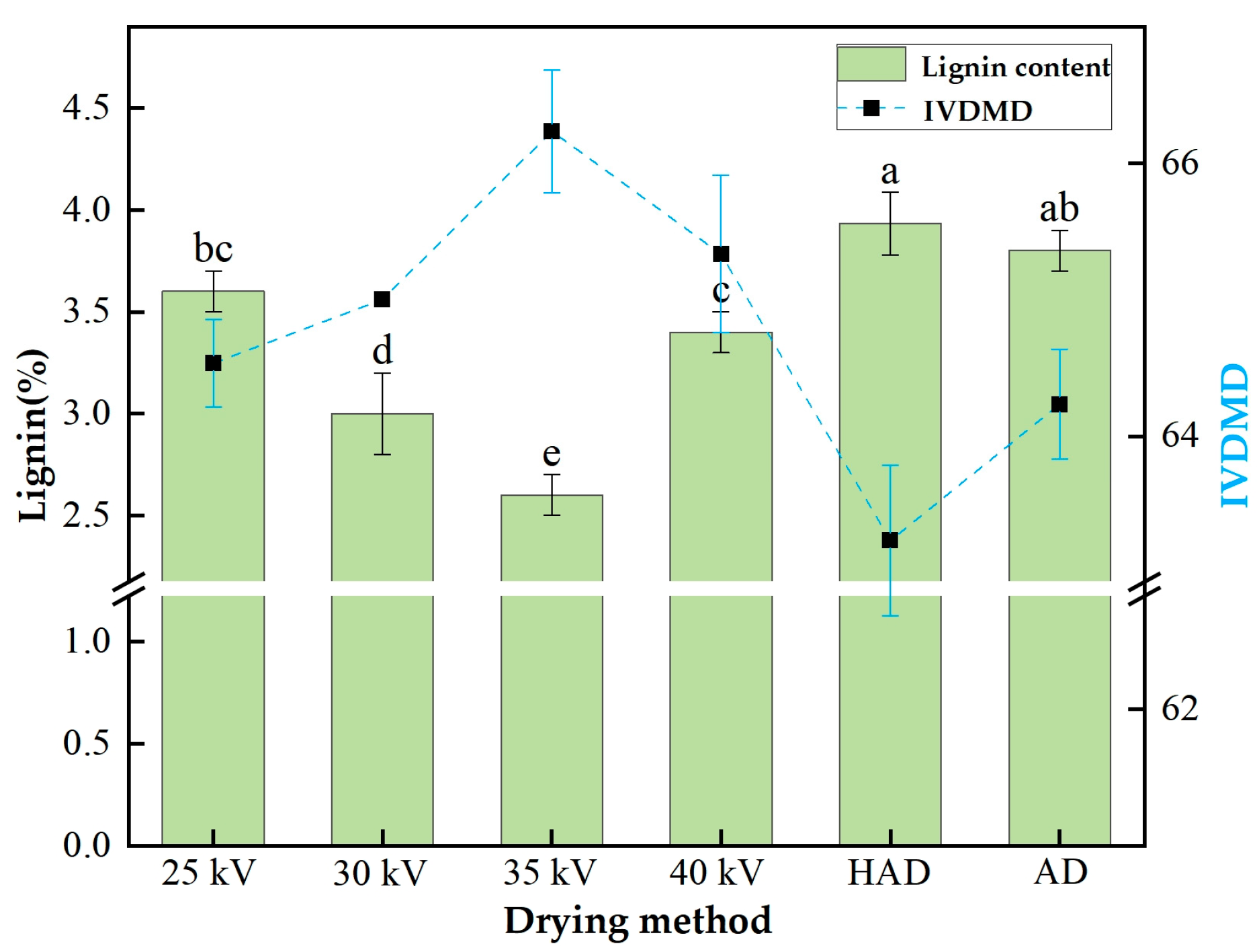
3.6.2. Lignin Content
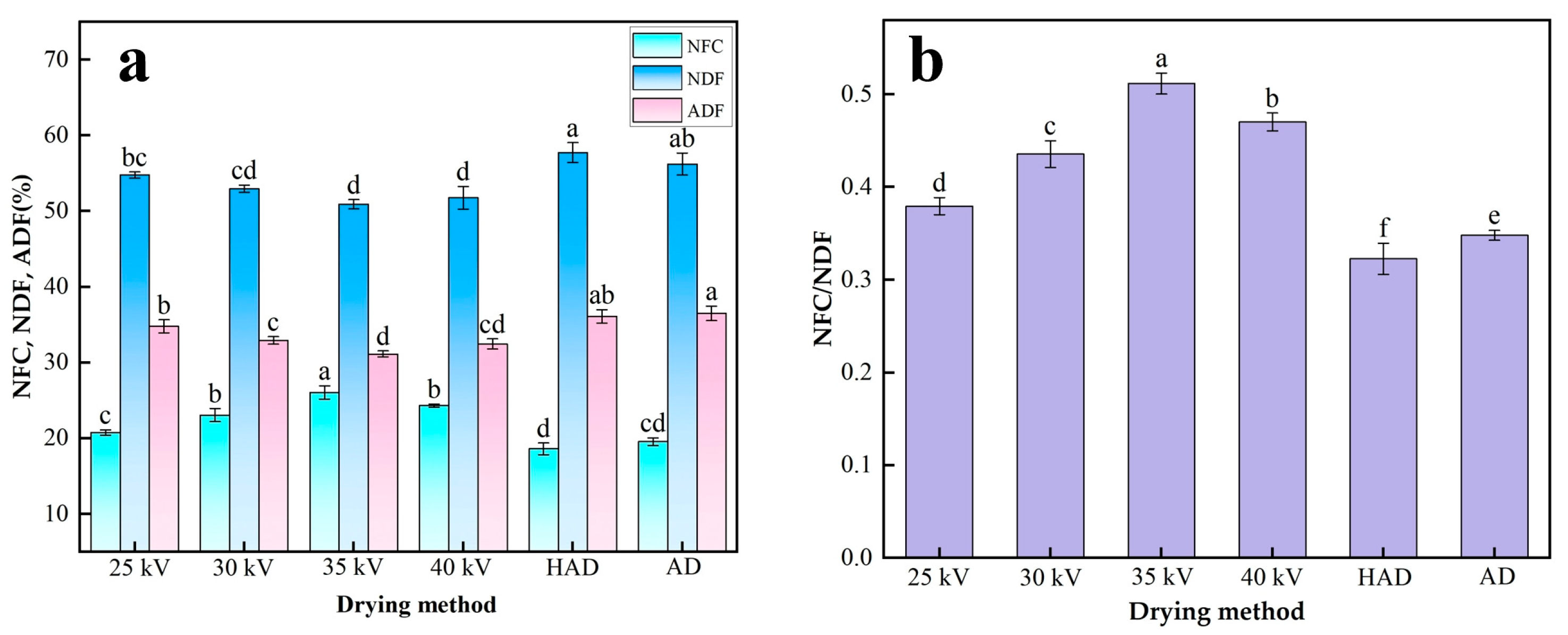
3.6.3. Contents of NFC, NDF and ADF
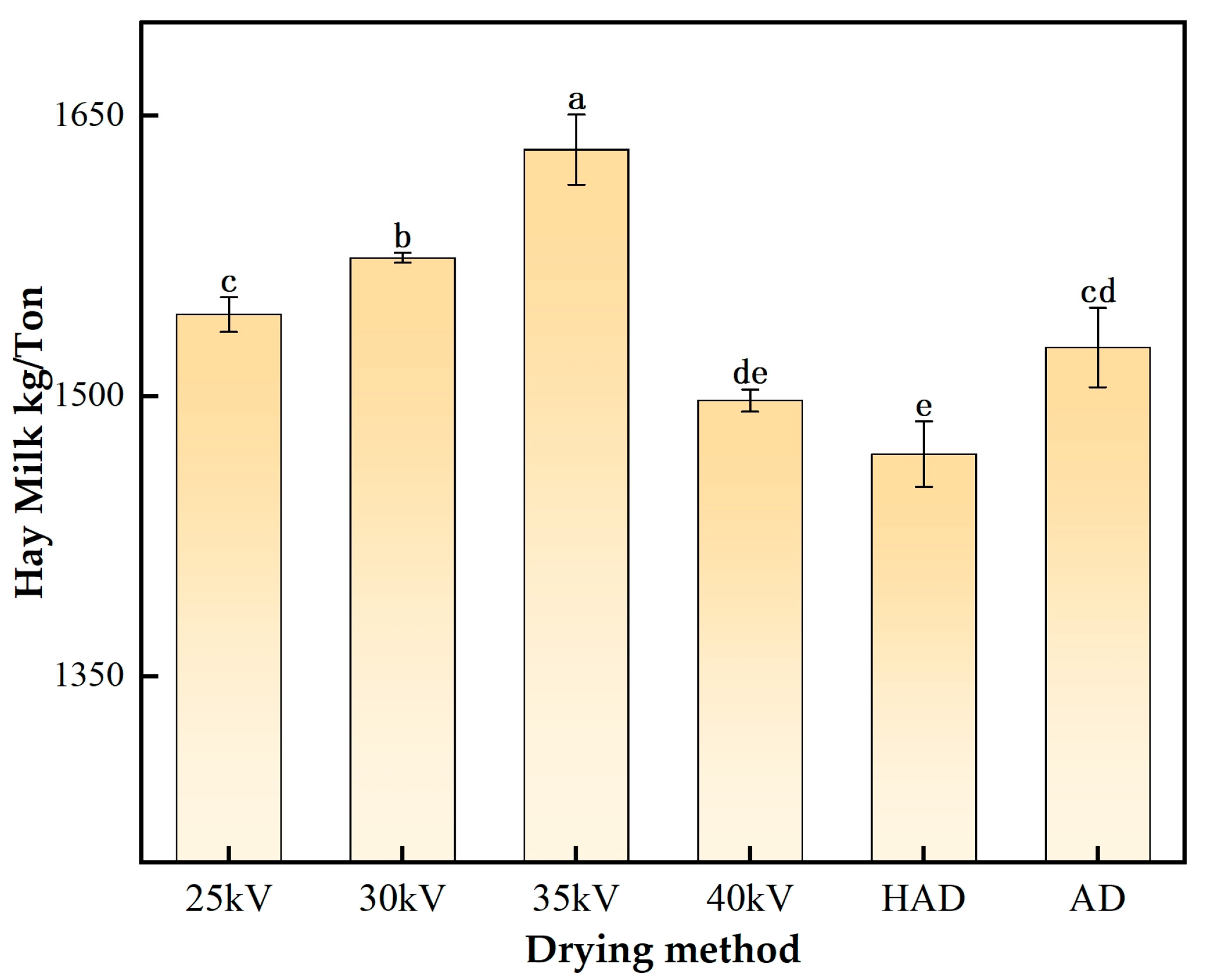
3.6.4. Milk Yield per Ton
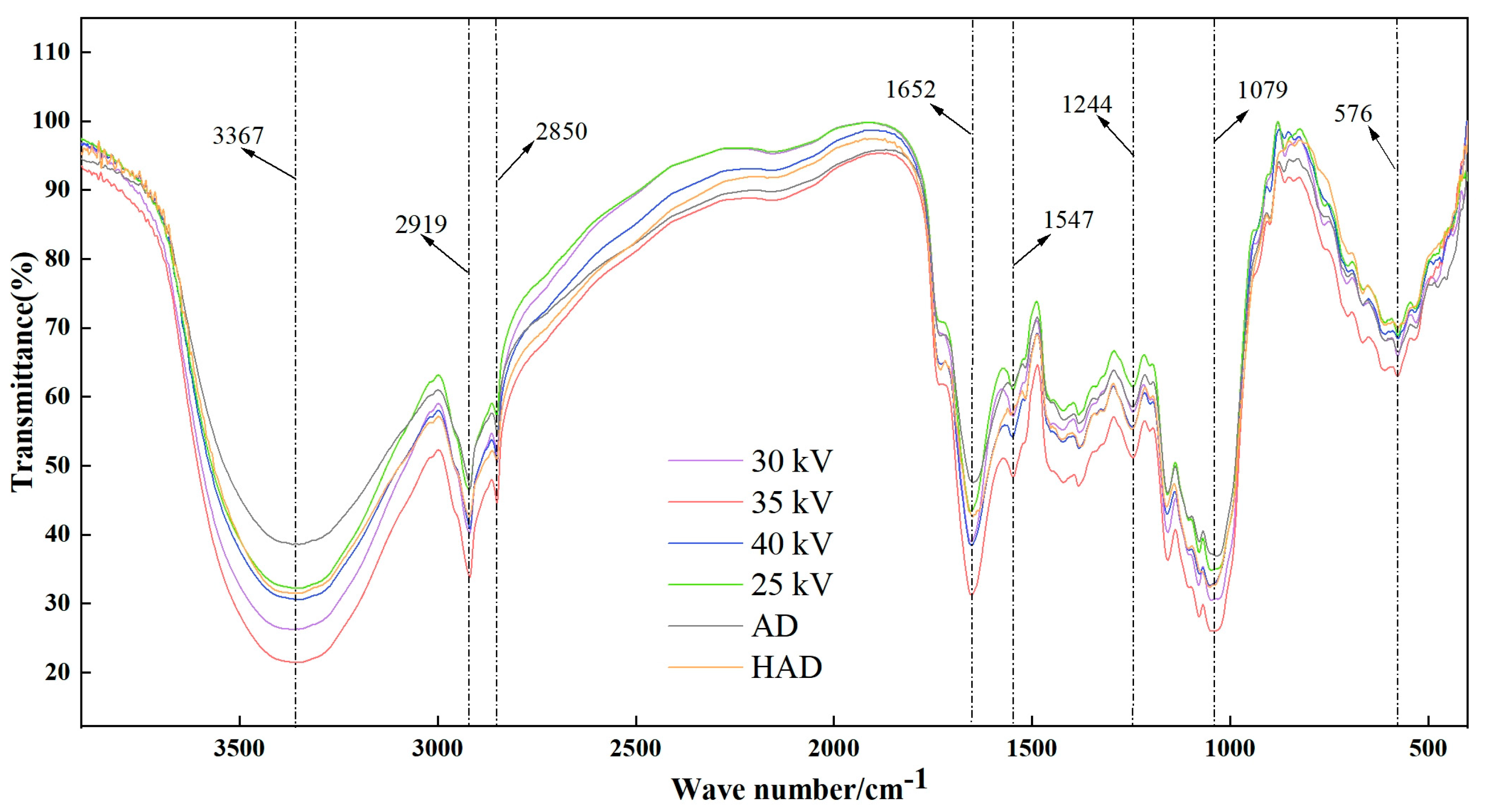
3.7. Infrared Spectrum Analysis
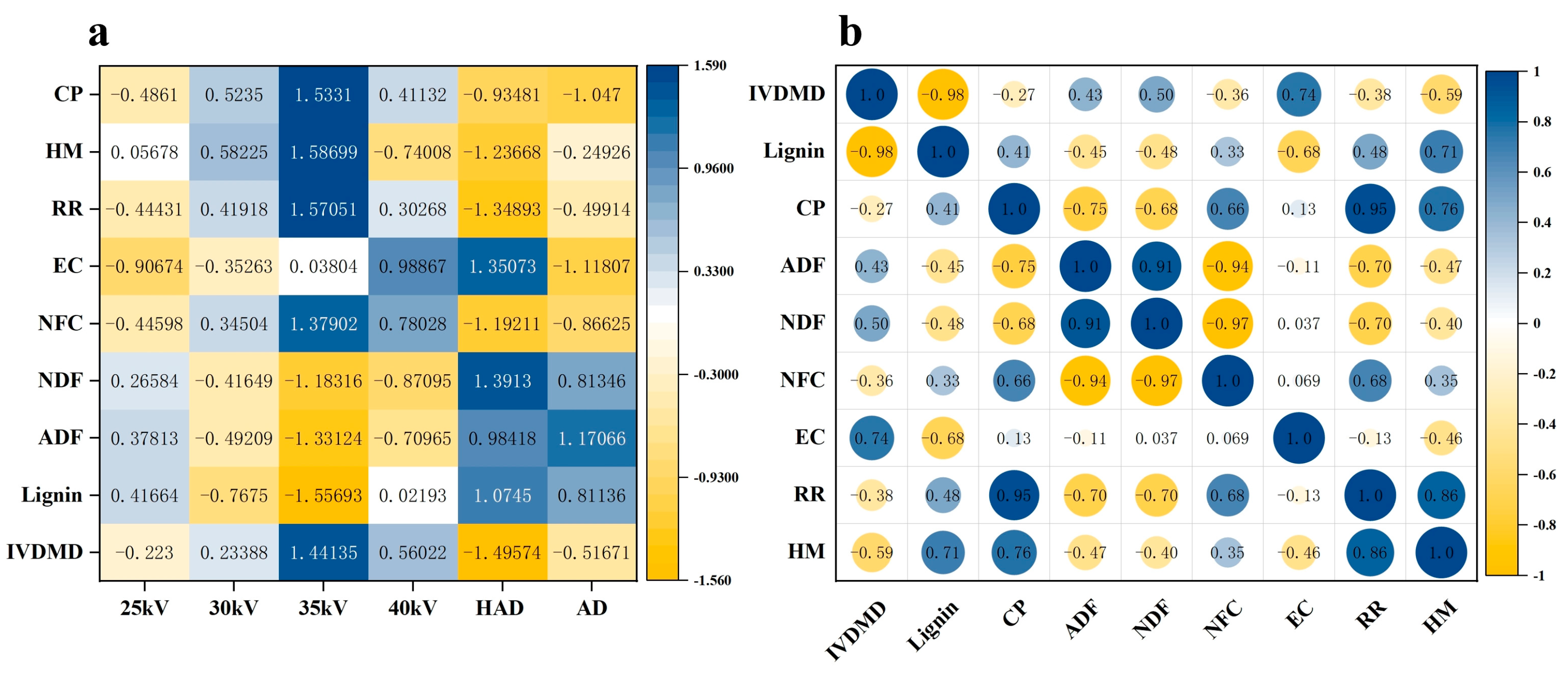
3.8. Correlation Analyse
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | air drying |
| HAD | hot-air drying |
| HVDP | high-voltage discharge plasma |
| SEC | specific energy consumption |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| RR | rehydration rate |
| EC | electrical conductivity |
| CP | crude protein |
| HM | hay milk |
| IVDMD | in vitro dry matter digestibility |
| NFC | non-fibrous carbohydrate |
| NDF | neutral detergent fibre |
| ADF | acid detergent fibre |
References
- Tang, Y.M.; Qiu, S.N.; Li, Q.Y.; Di, G.L.; Wang, J.L.; Sun, H.X. Simulated global climate change benefits the nutritive value of oat grass. Plant Biol. 2022, 24, 846–853. [Google Scholar] [CrossRef]
- Ma, Y.; Khan, M.Z.; Liu, Y.; Xiao, J.; Chen, X.; Ji, S.; Cao, Z.; Li, S. Analysis of nutrient composition, rumen degradation characteristics, and feeding value of Chinese rye grass, barley grass, and naked oat straw. Animals 2021, 11, 2486. [Google Scholar] [CrossRef]
- An, X.; Zhang, L.; Luo, J.; Zhao, S.; Jiao, T. Effects of oat hay content in diets on nutrient metabolism and the rumen microflora in sheep. Animals 2020, 10, 2341. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Dar, Z.; Habib, M. A review on oat (Avena sativa L.) as a dual-purpose crop. Sci. Res. Essays 2014, 9, 52–59. [Google Scholar]
- Morand-Fehr, P.; Fedele, V.; Decandia, M.; Le Frileux, Y. Influence of farming and feeding systems on composition and quality of goat and sheep milk. Small Ruminant Res. 2007, 68, 20–34. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Miller, A.J. Improving the yield and nutritional quality of forage crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.; Gentry, W.; Pritchard, R.; Hales, K. Evaluation of inclusion of hay, dampened hay, and silage in receiving diets of newly weaned beef calves. Appl. Anim. Sci. 2020, 36, 367–376. [Google Scholar] [CrossRef]
- Caixia, P.; Kuanhu, D. Effect of different harvest time and drying methods on nutrient as water soluble carbon hydrates of herbage. Grassl. China 2002, 24, 32–37. [Google Scholar]
- Guo, Z.; Li, C.; Wang, L.; Wang, G.; Wang, Z.; Wang, D. Effects of Triple-Pass Drum Dryer on Enzyme Activity and Quality of Forage Oats. Appl. Eng. Agric. 2022, 38, 637–644. [Google Scholar] [CrossRef]
- Pelletier, S.; Tremblay, G.F.; Bertrand, A.; Bélanger, G.; Castonguay, Y.; Michaud, R. Drying procedures affect non-structural carbohydrates and other nutritive value attributes in forage samples. Anim. Feed Sci. Technol. 2010, 157, 139–150. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kolb, J.F.; Holub, M.; Uhrlandt, D.; Šimek, M.; Ostrikov, K.; Hamaguchi, S.; Cvelbar, U.; Černák, M.; Locke, B. The future for plasma science and technology. Plasma Process. Polym. 2019, 16, 1800118. [Google Scholar] [CrossRef]
- Ihediwa, V.E.; Akpan, G.E.; Akuwueke, L.; Oriaku, L.; Ndubuisi, C.; Mbanasor, J.; Ndukwu, M.C. Thermal dehydration of some forage grasses for livestock feeding: Effect of different methods on moisture diffusivity and the quality of dried leaves. Energy Nexus 2022, 8, 100156. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A.C.; Cotozzolo, E.; Benincasa, P. Effect of heat-and freeze-drying treatments on phytochemical content and fatty acid profile of alfalfa and flax sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. [Google Scholar] [CrossRef]
- Zhang, A.-A.; Ni, J.-B.; Martynenko, A.; Chen, C.; Fang, X.-M.; Ding, C.-J.; Chen, J.; Zhang, J.-W.; Xiao, H.-W. Electrohydrodynamic drying of citrus (Citrus sinensis L.) peel: Comparative evaluation on the physiochemical quality and volatile profiles. Food Chem. 2023, 429, 136832. [Google Scholar] [CrossRef] [PubMed]
- Esehaghbeygi, A.; Pirnazari, K.; Sadeghi, M. Quality assessment of electrohydrodynamic and microwave dehydrated banana slices. LWT-Food Sci. Technol. 2014, 55, 565–571. [Google Scholar] [CrossRef]
- Sriariyakul, W.; Swasdisevi, T.; Devahastin, S.; Soponronnarit, S. Drying of aloe vera puree using hot air in combination with far-infrared radiation and high-voltage electric field: Drying kinetics, energy consumption and product quality evaluation. Food Bioprod. Process. 2016, 100, 391–400. [Google Scholar] [CrossRef]
- Esehaghbeygi, A.; Basiry, M. Electrohydrodynamic (EHD) drying of tomato slices (Lycopersicon esculentum). J. Food Eng. 2011, 104, 628–631. [Google Scholar] [CrossRef]
- Yang, M.; Ding, C.; Zhu, J. The drying quality and energy consumption of Chinese wolfberry fruits under electrohydrodynamic system. Int. J. Appl. Electromagn. Mech. 2017, 55, 101–112. [Google Scholar] [CrossRef]
- Guo, M.; Qian, Y.; Yuan, J.; Song, Z. Influence of High-Voltage Corona Discharge on Drying Kinetics and Physicochemical Properties of Alfalfa at Various Air-Gap Spacings. Agronomy 2024, 14, 1235. [Google Scholar] [CrossRef]
- Elhussein, E.A.A.; Şahin, S. Drying behaviour, effective diffusivity and energy of activation of olive leaves dried by microwave, vacuum and oven drying methods. Heat Mass Transf. 2018, 54, 1901–1911. [Google Scholar] [CrossRef]
- Lawrence, A.; Thollander, P.; Andrei, M.; Karlsson, M. Specific energy consumption/use (SEC) in energy management for improving energy efficiency in industry: Meaning, usage and differences. Energies 2019, 12, 247. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, C. Effect of Electrohydrodynamic Drying on Drying Characteristics and Physicochemical Properties of Carrot. Foods 2023, 12, 4228. [Google Scholar] [CrossRef]
- Hu, W.; Song, Z.; Ma, Q.; Li, B.; Zhang, M.; Ding, C.; Chen, H.; Zhao, S. Study on the Drying Characteristics and Physicochemical Properties of Alfalfa under High-Voltage Discharge Plasma. Agriculture 2024, 14, 1134. [Google Scholar] [CrossRef]
- Dumančić, E.; Vojta, L.; Fulgosi, H. Beginners guide to sample preparation techniques for transmission electron microscopy. Period. Biol. 2023, 125, 123–131. [Google Scholar] [CrossRef]
- Woodcock, T.; Downey, G.; O’Donnell, C.P. Better quality food and beverages: The role of near infrared spectroscopy. J. Near Infrared Spectrosc. 2008, 16, 1–29. [Google Scholar] [CrossRef]
- Jung, S.; Fang, J.; Chadha, T.S.; Biswas, P. Atmospheric pressure plasma corona enhanced by photoionizer for degradation of VOCs. J. Phys. D Appl. Phys. 2018, 51, 445206. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Lu, J.; Zhu, J.; Bai, W.; Guan, P.; Song, Z.; Chen, H. Effect of electrohydrodynamic (EHD) drying on active ingredients, textural properties and moisture distribution of yam (Dioscorea opposita). Food Chem. X 2024, 23, 101622. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink-Kobayashi, A.; Kirschvink, J.L. Electrostatic enhancement of industrial drying processes. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 1027–1030. [Google Scholar] [CrossRef]
- Han, B.; Ding, C.; Jia, Y.; Wang, H.; Bao, Y.; Zhang, J.; Duan, S.; Song, Z.; Chen, H.; Lu, J. Influence of electrohydrodynamics on the drying characteristics and physicochemical properties of garlic. Food Chem. X 2023, 19, 100818. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Martynenko, A.; Kudra, T. Electrically-induced transport phenomena in EHD drying—A review. Trends Food Sci. Technol. 2016, 54, 63–73. [Google Scholar] [CrossRef]
- Paul, A.; Martynenko, A. Electrohydrodynamic drying: Effects on food quality. Dry. Technol. 2021, 39, 1745–1761. [Google Scholar] [CrossRef]
- Marques, L.G.; Prado, M.M.; Freire, J.T. Rehydration characteristics of freeze-dried tropical fruits. LWT-Food Sci. Technol. 2009, 42, 1232–1237. [Google Scholar] [CrossRef]
- Hu, L.; Bi, J.; Jin, X.; van der Sman, R. Microstructure evolution affecting the rehydration of dried mushrooms during instant controlled pressure drop combined hot air drying (DIC-HA). Innov. Food Sci. Emerg. Technol. 2022, 79, 103056. [Google Scholar] [CrossRef]
- Hu, L.; Bi, J.; Jin, X.; Qiu, Y.; Sman, R.v.d. Study on the rehydration quality improvement of shiitake mushroom by combined drying methods. Foods 2021, 10, 769. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakis, E. The principles of high voltage electric field and its application in food processing: A review. Food Res. Int. 2016, 89, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.; Shouse, P.; Blum, A.; Miller, F.; Monk, R. Environmental physiology of Sorghum. II. Epicuticular wax load and cuticular transpiration 1. Crop Sci. 1984, 24, 1168–1173. [Google Scholar] [CrossRef]
- Hanlon, M.; Moorby, J.; McConochie, H.; Foskolos, A. Effects of addition of nutritionally improved straw in dairy cow diets at 2 starch levels. J. Dairy Sci. 2020, 103, 10233–10244. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.-L.; Xiong, H.; Yuan, D.-Y.; Zhang, X.; Zou, F. Effect of temperature stress on leaf structure and photosynthesis of chinquapin (Castanea henryi). Acta Agric. Univ. Jiangxiensis 2020, 42, 692–699. [Google Scholar]
- Melser, S.; Lavie, J.; Bénard, G. Mitochondrial degradation and energy metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2812–2821. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Pawlak, G. Effect of drying on microstructure of plant tissue. Dry. Technol. 2003, 21, 657–683. [Google Scholar] [CrossRef]
- Coradi, P.C.; Melo, E.d.C.; Rocha, R.P.d. Evaluation of electrical conductivity as a quality parameter of lemongrass leaves (Cymbopogon citratus Stapf) submitted to drying process. Dry. Technol. 2014, 32, 969–980. [Google Scholar] [CrossRef]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Angersbach, A.; Heinz, V.; Knorr, D. Electrophysiological model of intact and processed plant tissues: Cell disintegration criteria. Biotechnol. Prog. 1999, 15, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Nogoy, K.M.C.; Sun, B.; Shin, S.; Lee, Y.; Li, X.Z.; Choi, S.H.; Park, S. Fatty acid composition of grain-and grass-fed beef and their nutritional value and health implication. Food Sci. Anim. Resour. 2022, 42, 18. [Google Scholar] [CrossRef]
- Narmada, C. Protein Content comparsion between tray dried and oven dried muskmelon (Cucumis Melo). J. Surv. Fish. Sci. 2023, 10, 2580–2592. [Google Scholar]
- Grant, K.; Kreyling, J.; Dienstbach, L.F.; Beierkuhnlein, C.; Jentsch, A. Water stress due to increased intra-annual precipitation variability reduced forage yield but raised forage quality of a temperate grassland. Agric. Ecosyst. Environ. 2014, 186, 11–22. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sust. Energ. Rev. 2018, 90, 536–556. [Google Scholar]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Adjesiwor, A.T.; Islam, M.A.; Zheljazkov, V.D.; Ritten, J.P.; Garcia y Garcia, A. Grass-legume seed mass ratios and nitrogen rates affect forage accumulation, nutritive value, and profitability. Crop Sci. 2017, 57, 2852–2864. [Google Scholar] [CrossRef]
- Song, S.; Chen, G.; Guo, C.; Rao, K.; Gao, Y.; Peng, Z.; Zhang, Z.; Bai, X.; Wang, Y.; Wang, B. Effects of exogenous fibrolytic enzyme supplementation to diets with different NFC/NDF ratios on the growth performance, nutrient digestibility and ruminal fermentation in Chinese domesticated black goats. Anim. Feed Sci. Technol. 2018, 236, 170–177. [Google Scholar] [CrossRef]
- Bal, M.; Coors, J.; Shaver, R. Impact of the maturity of corn for use as silage in the diets of dairy cows on intake, digestion, and milk production. J. Dairy Sci. 1997, 80, 2497–2503. [Google Scholar] [CrossRef]
- Takaki, K.; Hayashi, N.; Wang, D.; Ohshima, T. High-voltage technologies for agriculture and food processing. J. Phys. D Appl. Phys. 2019, 52, 473001. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Ma, X.; Li, M.; Kong, D.; Phavady, P.; Wang, Q.; Abdelkader, T.K. Chinese medicinal materials’ drying technologies advancements—Principles, energy performance, and influence on the bioactive components. Dry. Technol. 2024, 42, 1815–1845. [Google Scholar] [CrossRef]
- Xiao, A.; Ding, C. Effect of electrohydrodynamic (EHD) on drying kinetics and quality characteristics of shiitake mushroom. Foods 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, G.F.; Schmitt, F.-J.; Kanzler, C.; Epping, J.D.; Flemig, S.; Hornemann, A. Structural characterization of melanoidin formed from d-glucose and l-alanine at different temperatures applying FTIR, NMR, EPR, and MALDI-ToF-MS. Food Chem. 2018, 245, 761–767. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, A.; Pan, Y.; Yu, S.; Jiang, L.; Xiong, B. Near infrared prediction model establishment for conventional nutrient contents of oat grass. Chin. J. Anim. Nutr. 2022, 34, 1334–1342. [Google Scholar]
- Akram, K.; Ahn, J.J.; Kwon, J.H. Identification and characterization of gamma-irradiated dried Lentinus edodes using ESR, SEM, and FTIR analyses. J. Food Sci. 2012, 77, C690–C696. [Google Scholar] [PubMed]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and quality assessment of meat products by fourier-transform infrared (FTIR) spectroscopy. Food Eng. Rev. 2021, 13, 66–91. [Google Scholar] [CrossRef]
- Tucker, M.P.; Nguyen, Q.A.; Eddy, F.P.; Kadam, K.L.; Gedvilas, L.M.; Webb, J.D. Fourier transform infrared quantitative analysis of sugars and lignin in pretreated softwood solid residues. In Twenty-Second Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2001; pp. 51–61. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Z.; Zhao, P.; Song, Z. Effects of High-Voltage Discharge Plasma on Drying Properties, Microstructure, and Nutrients of Oat Grass. Agronomy 2025, 15, 408. https://doi.org/10.3390/agronomy15020408
Wang X, Liu Z, Zhao P, Song Z. Effects of High-Voltage Discharge Plasma on Drying Properties, Microstructure, and Nutrients of Oat Grass. Agronomy. 2025; 15(2):408. https://doi.org/10.3390/agronomy15020408
Chicago/Turabian StyleWang, Xixuan, Zhidan Liu, Peishuo Zhao, and Zhiqing Song. 2025. "Effects of High-Voltage Discharge Plasma on Drying Properties, Microstructure, and Nutrients of Oat Grass" Agronomy 15, no. 2: 408. https://doi.org/10.3390/agronomy15020408
APA StyleWang, X., Liu, Z., Zhao, P., & Song, Z. (2025). Effects of High-Voltage Discharge Plasma on Drying Properties, Microstructure, and Nutrients of Oat Grass. Agronomy, 15(2), 408. https://doi.org/10.3390/agronomy15020408





