Hydroxychalcones as Herbicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Seeds
2.2. Test Solutions
2.3. Germination Experiments
2.4. Initial Growth Experiments
2.5. Evaluation of Seedling Vigor Index
2.6. Statistical Analysis
3. Results
3.1. 3′-Hydroxychalcones’ Effects on Germination
3.2. 3′-Hydroxychalcones’ Effects on Initial Growth
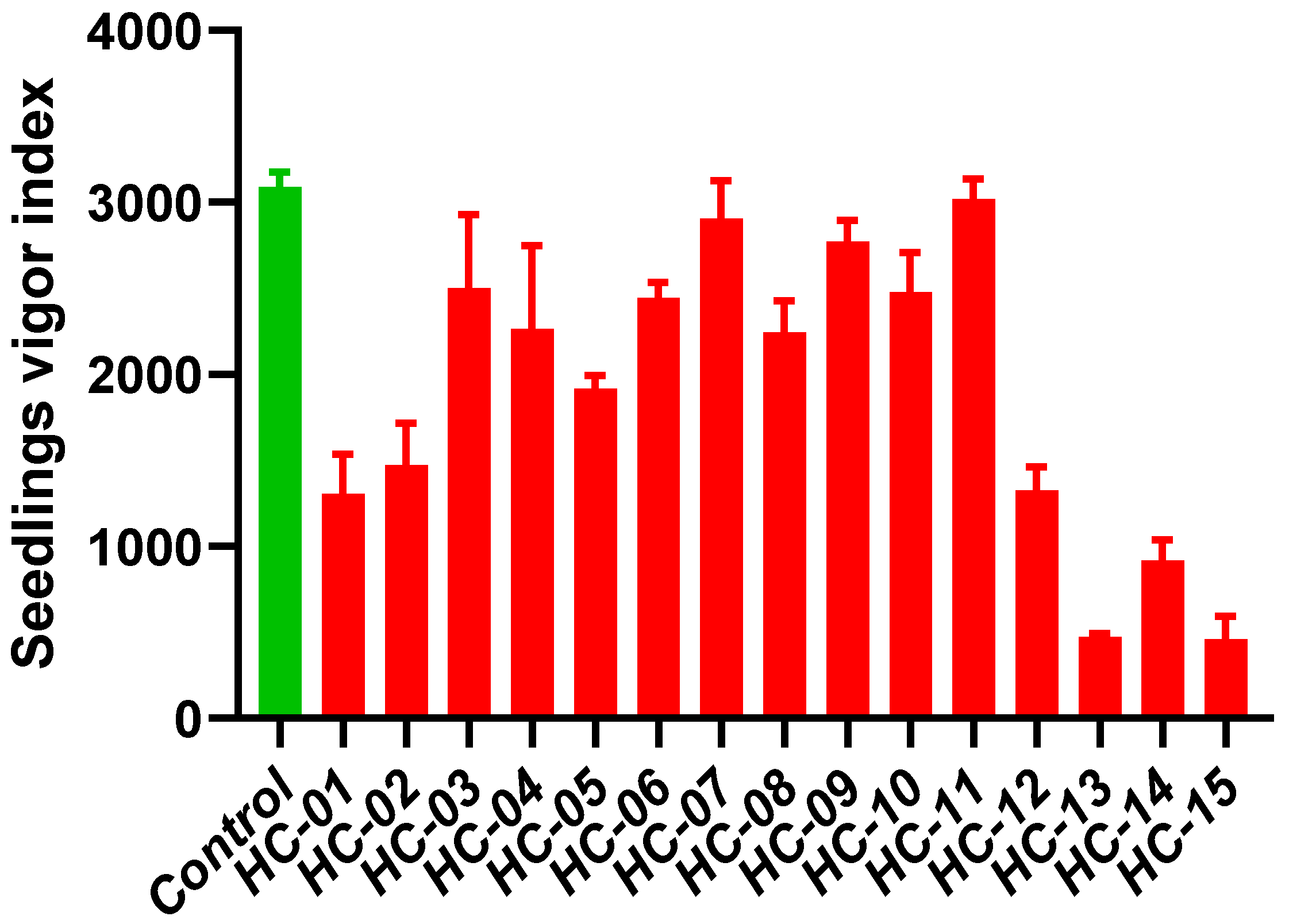
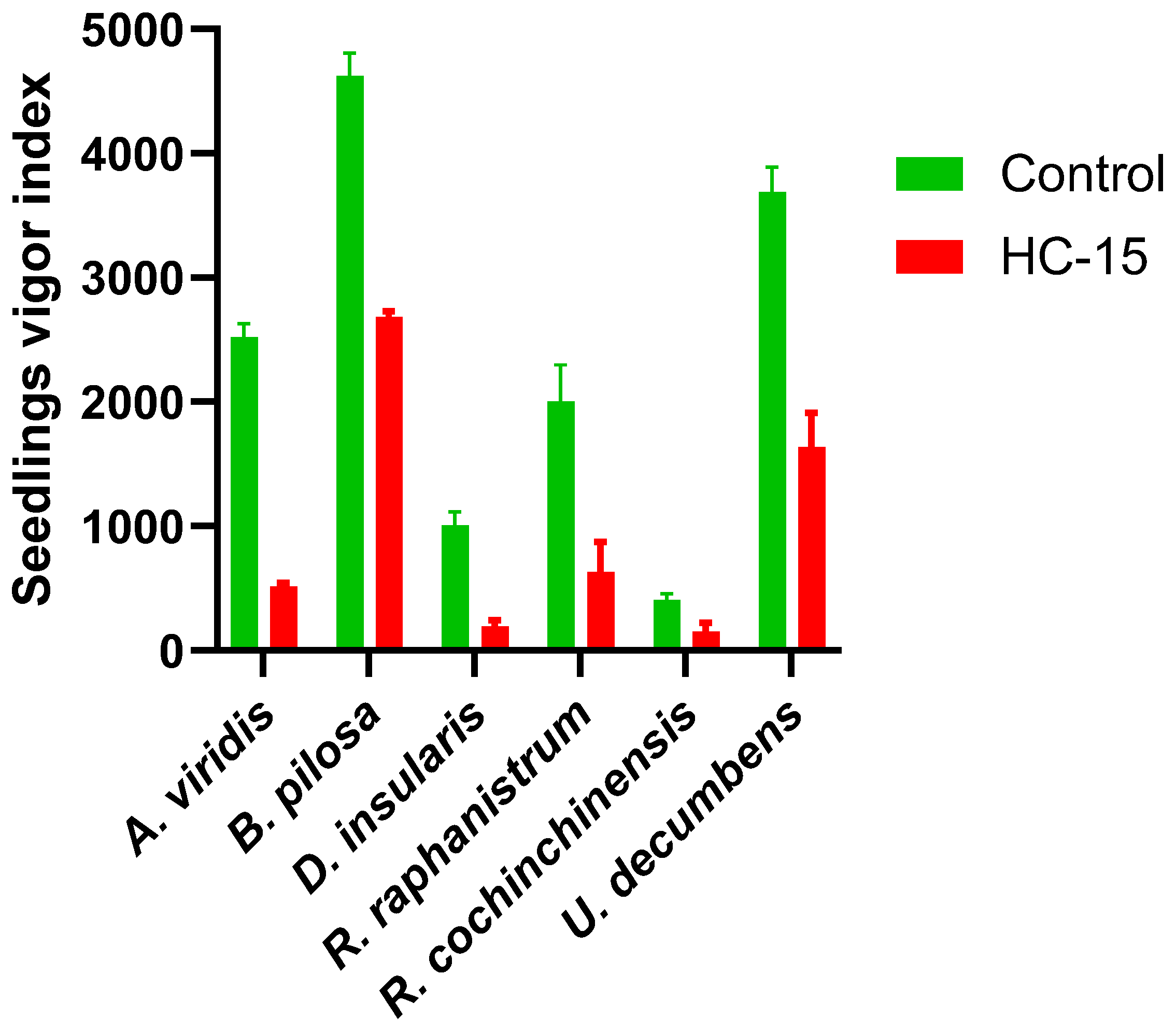
3.3. Evaluation of Seedling Vigor Index
4. Discussion
4.1. 3′-Hydroxychalcones’ Effects on Germination
4.2. 3′-Hydroxychalcones Effects’ on Initial Growth
4.3. Seedling Vigor Index
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrido, R.M.; Dayan, F.E.; Kolb, R.M. Herbicidal Activity of Smoke Water. Agronomy 2023, 13, 975. [Google Scholar] [CrossRef]
- Nguyen, G.T.T.; Erlenkamp, G.; Jäck, O.; Küberl, A.; Bott, M.; Fiorani, F.; Gohlke, H.; Groth, G. Chalcone-Based Selective Inhibitors of a C4 Plant Key Enzyme as Novel Potential Herbicides. Sci. Rep. 2016, 6, 27333–27345. [Google Scholar] [CrossRef] [PubMed]
- Dziągwa-Becker, M.; Oleszek, M.; Zielińska, S.; Oleszek, W. Chalcones—Features, Identification Techniques, Attributes and Application in Agriculture. Molecules 2024, 29, 247. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural Products in Crop Protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Kaab, S.B.; Rebey, I.B.; Hanafi, M.; Hammi, K.M.; Smaoui, A.; Fauconnier, M.L.; De Clerck, C.; Jijakli, M.H.; Ksouri, R. Screening of Tunisian Plant Extracts for Herbicidal Activity and Formulation of a Bioherbicide Based on Cynara cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Diaz-Tielas, C.; Grana, E.; Reigosa, M.J.; Sanchez-Moreiras, A.M. Biological Activities and Novel Applications of Chalcones. Planta Daninha 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Reigosa, M.; Gomes, A.S.; Ferreira, A.G.; Borghetti, F. Allelopathic Research in Brazil. Acta Bot. Brasilica 2013, 27, 629–646. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Deng, Y.; Xiao, C.; Luan, S.; Huang, Q. Novel Galactosyl Moiety-Conjugated Furylchalcones Synthesized Facilely Display Significant Regulatory Effect on Plant Growth. J. Agric. Food Chem. 2022, 70, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Twitty, A.; Baker, C.; Sands, D.; Boddy, L.; Travaini, M.L.; Sosa, G.; Polidore, A.L.A.; Jhala, A.J.; Kloeber, J.M.; et al. New Approaches to Herbicide and Bioherbicide Discovery. Weed Sci. 2024, 5, 444–464. [Google Scholar] [CrossRef]
- de Pádua, G.M.S.; Pitteri, T.S.; Ferreira Basso, M.A.; de Vasconcelos, L.G.; Ali, A.; Dall’Oglio, E.L.; Sampaio, O.M.; Curcino Vieira, L.C. Synthesis and Evaluation of New Phytotoxic Fluorinated Chalcones as Photosystem II and Seedling Growth Inhibitors. Chem. Biodivers. 2024, 21, e202301564. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Inhibitory Effects of a Variety of Aldehydes on Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. Molecules 2018, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Duke, S.O. Structure Simplification of Natural Products as a Lead Generation Approach in Agrochemical Discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Chen, K.; Li, N.; Wang, X.-K.; Wang, S.-B.; Li, P.; Hua, X.-W.; Lei, K.; Ji, L.-S. Discovery of 3-(1-Amino-2-Phenoxyethylidene)-6-Methyl-2H-Pyran-2,4(3H)-Dione Derivatives as Novel Herbicidal Leads. Agronomy 2023, 13, 202. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural Products That Have Been Used Commercially as Crop Protection Agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Sotelo, T.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Phytotoxic Potential of Trans-Chalcone on Crop Plants and Model Species. J. Plant Growth Regul. 2014, 33, 181–194. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wang, B.-X.; Yang, Y.-S.; Liang, C.; Yang, C.; Chai, H.-L. Synthesis and Self-Assembly of Chalcone-Based Organogels. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 449–455. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally Occurring Chalcones and Their Biological Activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- de Pádua, G.M.S.; de Souza, J.M.; Sales, M.C.M.; de Vasconcelos, L.G.; Dall’Oglio, E.L.; Faraggi, T.M.; Sampaio, O.M.; Vieira, L.C.C. Evaluation of Chalcone Derivatives as Photosynthesis and Plant Growth Inhibitors. Chem. Biodivers. 2021, 18, e2100226. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal Activity of Flavokawains and Related Trans-Chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef]
- Akamatsu, M. Importance of Physicochemical Properties for the Design of New Pesticides. J. Agric. Food Chem. 2010, 59, 2909–2917. [Google Scholar] [CrossRef]
- Macías, F.A.; Castellano, D.; Molinillo, J.M.G. Search for a Standard Phytotoxic Bioassay for Allelochemicals. Selection of Standard Target Species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef]
- Akeel, A.; Khan, M.M.A.; Jaleel, H.; Uddin, M. Smoke-Saturated Water and Karrikinolide Modulate Germination, Growth, Photosynthesis and Nutritional Values of Carrot (Daucus carota L.). J. Plant Growth Regul. 2019, 38, 1387–1401. [Google Scholar] [CrossRef]
- Bitencourt, H.R.; Santos, L.S.; Souza Filho, A.P.S. Atividade Alelopática de Chalcona Sintética, de Seus Precursores e de Cetonas e Aldeídos Relacionados. Planta Daninha 2007, 25, 747–753. [Google Scholar] [CrossRef]
- Spitters, C.J.T.; Van Den Bergh, J.P. Competition between Crop and Weeds: A System Approach. In Biology and Ecology of Weeds; Holzner, W., Numata, M., Eds.; Springer: Dordrecht, The Netherlands, 1982; Volume 2, pp. 137–148. [Google Scholar]
- Constantinescu, T.; Mihis, A.G. Two Important Anticancer Mechanisms of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2022, 23, 11595. [Google Scholar] [CrossRef]
- Wang, G.; Li, C.; He, L.; Lei, K.; Wang, F.; Pu, Y.; Yang, Z.; Cao, D.; Ma, L.; Chen, J.; et al. Design, Synthesis and Biological Evaluation of a Series of Pyrano Chalcone Derivatives Containing Indole Moiety as Novel Anti-Tubulin Agents. Bioorg. Med. Chem. 2014, 22, 2060–2079. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, W.; Gong, Z.; Huang, Y.; Li, Y.; Peng, Z. Synthesis, Biological Evaluation, and Molecular Modelling of New Naphthalene-Chalcone Derivatives as Potential Anticancer Agents on MCF-7 Breast Cancer Cells by Targeting Tubulin Colchicine Binding Site. J. Enzym. Inhib. Med. Chem. 2020, 35, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Molinillo, J.M.G.; Torres, A.; Varela, R.M.; Castellano, D. Bioactive Flavonoids from Helianthus annuus Cultivars. Phytochemistry 1997, 45, 683–687. [Google Scholar] [CrossRef]
- Chen, W.J.; Yun, M.S.; Deng, F.; Yogo, Y. Effects of Root-Applied Naringenin and Chalcone on the Growth of Annual Plants. Weed Biol. Manag. 2004, 4, 235–238. [Google Scholar] [CrossRef]
- Gomes, A.S.; Oliveira, S.C.C.; Mendonça, I.S.; da Silva, C.C.; Guiotti, N.X.; Melo, L.R.; Silva, W.A.; Borghetti, F. Potential Herbicidal Effect of Synthetic Chalcones on the Initial Growth of Sesame, Sesamum indicum L., and Brachiaria, Urochloa decumbens (Stapf) R. D. Webster. Iheringia Série Botânica 2018, 73, 46–52. [Google Scholar] [CrossRef]
- Diaz-Tielas, C.; Grana, E.; Sotelo, T.; Reigosa, M.J.; Sanchez-Moreiras, A.M. The Natural Compound Trans-Chalcone Induces Programmed Cell Death in Arabidopsis thaliana Roots. Plant Cell Environ. 2012, 35, 1500–1517. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Germination (%) 1 | MGT 2 (days) 1 |
|---|---|---|
| Negative control | 95 ± 6 | 1.3 ± 0.2 |
| Tebuthiuron | 51 ± 17 * | 2.3 ± 0.8 * |
| HC-01 | 53 ± 13 * | 2.5 ± 0.7 |
| HC-02 | 57 ± 14 * | 1.6 ± 0.4 |
| HC-03 | 68 ± 24 | 1.6 ± 0.1 |
| HC-04 | 71 ± 29 | 1.6 ± 0.3 |
| HC-05 | 55 ± 4 * | 1.6 ± 0.3 |
| HC-06 | 84 ± 6 | 3.9 ± 0.6 * |
| HC-07 | 88 ± 12 | 4.1 ± 0.6 * |
| HC-08 | 86 ± 11 | 3.8 ± 0.3 * |
| HC-09 | 93 ± 7 | 3.8 ± 0.5 * |
| HC-10 | 81 ± 13 | 3.9 ± 0.8 * |
| HC-11 | 86 ± 8 | 3.6 ± 0.2 * |
| HC-12 | 89 ± 8 | 4.0 ± 0.6 * |
| HC-13 | 97 ± 4 | 3.7 ± 0.3 * |
| HC-14 | 91 ± 9 | 3.7 ± 0.5 * |
| HC-15 | 64 ± 15 * | 4.1 ± 1.2 * |
| Treatment | Germination (%) 1 | MGT 2 (Days) 1 |
|---|---|---|
| A. viridis | ||
| Negative control | 53 ± 13 ab | 2.7 ± 0.4 ab |
| Tebuthiuron | 60 ± 20 a | 4.6 ± 0.5 ab |
| HC-01 | 30 ± 11 b | 7.7 ± 2.9 a |
| HC-02 | 47 ± 14 ab | 4.0 ± 0.3 ab |
| HC-05 | 28 ± 7 b | 2.5 ± 0.4 b |
| HC-15 | 37 ± 5 ab | 3.8 ± 0.8 ab |
| B. pilosa | ||
| Negative control | 73 ± 10 a | 3.0 ± 0.4 a |
| Tebuthiuron | 50 ± 10 ab | 9.4 ± 1.60 b |
| HC-01 | 50 ± 14 ab | 10.0 ± 3.0 b |
| HC-02 | 46 ± 10 b | 5.5 ± 0.7 ab |
| HC-05 | 61 ± 13 ab | 5.3 ± 0.8 ab |
| HC-15 | 57 ± 2 ab | 6.5 ± 1.8 ab |
| D. insularis | ||
| Negative control | 27 ± 15 a | 4.5 ± 0.9 a |
| Tebuthiuron | 20 ± 9 a | 8.2 ± 1.7 b |
| HC-01 | 7 ± 9 a | 5.2 ± 0.7 a |
| HC-02 | 8 ± 7 a | 4.7 ± 1.4 a |
| HC-05 | 20 ± 3 a | 4.6 ± 1.3 a |
| HC-15 | 15 ± 7 a | 3.7 ± 0.9 a |
| R. raphanistrum | ||
| Negative control | 43 ± 8 ab | 2.3 ± 0.4 a |
| Tebuthiuron | 35 ± 11 a | 3.9 ± 1.9 a |
| HC-01 | 51 ± 13 ab | 3.1 ± 0.5 a |
| HC-02 | 59 ± 4 b | 2.3 ± 0.4 a |
| HC-05 | 41 ± 9 ab | 3.0 ± 0.9 a |
| HC-15 | 50 ± 12 ab | 2.4 ± 0.2 a |
| R. cochinchinensis | ||
| Negative control | 19 ± 7 a | 3.9 ± 0.6 a |
| Tebuthiuron | 7 ± 4 a | 4.3 ± 0.5 a |
| HC-01 | 5 ± 2 a | 3.5 ± 0.6 a |
| HC-02 | 13 ± 4 a | 4.1 ± 0.4 a |
| HC-05 | 23 ± 13 a | 3.7 ± 0.6 a |
| HC-15 | 21 ± 12 a | 3.9 ± 0.2 a |
| U. decumbens | ||
| Negative control | 70 ± 8 a | 3.2 ± 0.3 ab |
| Tebuthiuron | 58 ± 17 a | 4.6 ± 1.2 a |
| HC-01 | 60 ± 7 a | 3.5 ± 0.7 ab |
| HC-02 | 66 ± 11 a | 3.4 ± 0.4 ab |
| HC-05 | 68 ± 14 a | 3.0 ± 0.2 b |
| HC-15 | 75 ± 21 a | 3.2 ± 0.5 ab |
| Treatment | Root Measurement (mm) 1 | Shoot Measurement (mm) 1 | Root + Shoot Measurement (mm) 1 |
|---|---|---|---|
| Negative control | 25.2 ± 11.3 | 7.3 ± 3.5 | 32.5 ± 12.2 |
| Glyphosate | 8.2 ± 2.2 * | 6.1 ± 2.6 | 14.3 ± 3.6 * |
| HC-01 | 18.8 ± 14.2 * | 5.8 ± 3.2 * | 24.6 ± 15.9 * |
| HC-02 | 22.0 ± 15.2 * | 3.8 ± 2.3 * | 25.8 ± 16.2 * |
| HC-03 | 31.3 ± 15.7 | 5.5 ± 2.3 * | 36.8 ± 16.9 |
| HC-04 | 26.4 ± 14.6 | 5.5 ± 2.4 * | 32.0 ± 16.0 |
| HC-05 | 29.5 ± 16.7 | 5.4 ± 3.4 * | 34.9 ± 19.0 |
| HC-06 | 24.0 ± 13.9 | 5.1 ± 2.0 * | 29.1 ± 15.1 * |
| HC-07 | 27.7 ± 15.7 | 5.3 ± 3.1 * | 33.1 ± 17.6 |
| HC-08 | 20.3 ± 13.5 * | 5.8 ± 3.8 * | 26.0 ± 15.5 * |
| HC-09 | 24.0 ± 14.9 | 5.8 ± 3.2 * | 29.8 ± 16.8 * |
| HC-10 | 25.9 ± 14.7 | 4.7 ± 3.3 * | 30.6 ± 16.8 |
| HC-11 | 29.2 ± 12.6 | 5.9 ± 2.4 * | 35.1 ± 13.6 |
| HC-12 | 10.5 ± 11.9 * | 4.4 ± 4.3 * | 14.8 ± 15.6 * |
| HC-13 | 3.3 ± 3.6 * | 1.6 ± 1.9 * | 4.9 ± 5.3 * |
| HC-14 | 7.1 ± 10.3 * | 3.0 ± 3.1 * | 10.1 ± 13.0 * |
| HC-15 | 5.3 ± 7.5 * | 1.9 ± 1.8 * | 7.1 ± 9.0 * |
| Treatment | Root Measurement (mm)1 | Shoot Measurement (mm) 1 | Root + Shoot Measurement (mm) 1 |
|---|---|---|---|
| A. viridis | |||
| Negative control | 31.2 ± 5.3 a | 16.4 ± 3.0 a | 47.6 ± 6.9 a |
| Glyphosate | 13.2 ± 3.5 b | 10.9 ± 3.7 b | 24.1 ± 6.6 b |
| HC-12 | 7.8 ± 3.3 c | 5.5 ± 2.6 c | 13.3 ± 4.9 c |
| HC-13 | 7.2 ± 3.1 c | 5.5 ± 2.2 c | 12.7 ± 4.6 c |
| HC-14 | 15.3 ± 6.6 b | 11.7 ± 5.8 b | 27.0 ± 10.3 b |
| HC-15 | 8.6 ± 3.3 c | 5.3 ± 2.5 c | 14.0 ± 5.1 c |
| B. pilosa | |||
| Negative control | 35.7 ± 12.1 a | 25.8 ± 6.4 a | 61.5 ± 15.9 a |
| Glyphosate | 18.4 ± 5.2 b | 23.1 ± 6.0 b | 41.5 ± 10.0 bd |
| HC-12 | 13.7 ± 7.4 c | 14.9 ± 6.6 c | 28.6 ± 12.9 c |
| HC-13 | 15.2 ± 8.4 bc | 14.2 ± 6.2 c | 29.4 ± 13.2 c |
| HC-14 | 38.2 ± 18.4 ad | 18.0 ± 6.4 d | 56.2 ± 23.0 ad |
| HC-15 | 31.9 ± 18.3 d | 15.2 ± 5.6 c | 47.1 ± 22.4 d |
| D. insularis | |||
| Negative control | 33.9 ± 6.2 a | 3.4 ± 0.8 a | 37.3 ± 6.5 a |
| Glyphosate | 27.9 ± 7.2 a | 3.0 ± 1.0 a | 31.0 ± 7.6 a |
| HC-12 | 8.0 ± 4.3 bc | 1.2 ± 0.5 b | 9.2 ± 4.4 bc |
| HC-13 | 7.1 ± 4.4 c | 1.1 ± 0.3 b | 8.2 ± 4.4 c |
| HC-14 | 10.7 ± 5.6 b | 1.3 ± 0.6 b | 12.0 ± 5.7 b |
| HC-15 | 11.3 ± 6.6 b | 1.4 ± 0.6 b | 12.6 ± 6.9 b |
| R. raphanistrum | |||
| Negative control | 30.3 ± 26.0 a | 16.3 ± 9.5 a | 46.6 ± 31.8 a |
| Glyphosate | 9.6 ± 3.4 b | 10.9 ± 5.6 bd | 20.5 ± 7.1 b |
| HC-12 | 22.9 ± 18.4 a | 13.3 ± 7.7 ad | 36.2 ± 22.5 ad |
| HC-13 | 13.4 ± 13.7 b | 12.8 ± 7.0 ad | 26.3 ± 16.4 bd |
| HC-14 | 14.8 ± 16.8 b | 11.8 ± 7.8 bd | 26.7 ± 22.3 b |
| HC-15 | 6.4 ± 10.8 c | 6.2 ± 8.7 c | 12.6 ± 18.3 c |
| R. cochinchinensis | |||
| Negative control | 10.7 ± 2.9 a | 10.7 ± 4.6 a | 21.5 ± 7.0 a |
| Glyphosate | 9.5 ± 2.1 a | 8.1 ± 3.5 a | 17.5 ± 5.0 a |
| HC-12 | 4.7 ± 1.2 b | 1.5 ± 2.1 b | 6.2 ± 2.8 b |
| HC-13 | 5.3 ± 2.3 b | 1.9 ± 3.3 b | 7.3 ± 5.3 b |
| HC-14 | 4.9 ± 2.4 b | 2.4 ± 4.1 b | 7.3 ± 6.2 b |
| HC-15 | 5.1 ± 2.3 b | 2.1 ± 3.8 b | 7.3 ± 6.0 b |
| U. decumbens | |||
| Negative control | 47.2 ± 23.8 a | 5.5 ± 2.6 a | 52.7 ± 25.9 a |
| Glyphosate | 24.0 ± 5.9 b | 4.1 ± 1.4 a | 28.0 ± 6.9 b |
| HC-12 | 15.4 ± 10.6 c | 2.7 ± 1.4 b | 18.0 ± 6.9 c |
| HC-13 | 8.2 ± 6.5 d | 1.6 ± 1.4 c | 9.8 ± 7.4 d |
| HC-14 | 23.2 ± 12.4 be | 2.4 ± 1.5 b | 25.5 ± 13.3 be |
| HC-15 | 19.6 ± 11.7 ce | 2.2 ± 1.3 bc | 21.8 ± 12.6 ce |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, R.M.; Dayan, F.E.; Ozanique, P.R.; Regasini, L.O.; Kolb, R.M. Hydroxychalcones as Herbicides. Agronomy 2025, 15, 572. https://doi.org/10.3390/agronomy15030572
Garrido RM, Dayan FE, Ozanique PR, Regasini LO, Kolb RM. Hydroxychalcones as Herbicides. Agronomy. 2025; 15(3):572. https://doi.org/10.3390/agronomy15030572
Chicago/Turabian StyleGarrido, Raphael Mota, Franck Emmanuel Dayan, Patrick Rômbola Ozanique, Luis Octavio Regasini, and Rosana Marta Kolb. 2025. "Hydroxychalcones as Herbicides" Agronomy 15, no. 3: 572. https://doi.org/10.3390/agronomy15030572
APA StyleGarrido, R. M., Dayan, F. E., Ozanique, P. R., Regasini, L. O., & Kolb, R. M. (2025). Hydroxychalcones as Herbicides. Agronomy, 15(3), 572. https://doi.org/10.3390/agronomy15030572






