Smoke Compounds Compensate for Light Irrespective of Its Spectrum in Positively Photoblastic German Chamomile Seeds, Although Red Light Is Crucial
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Illumination Regimes, and SW Treatment
2.2. Germination Indicators and Morphometrical Analyses
- g2, … and g7 = the percentage of germinated seeds on day 2, …. and day 7
- n2, … and n7 = the number of germinated seeds on day 2, …. and day 7,
- d2, …d7 = the number of days,
- 7, …, 2 in GI = weights assigned to the seeds germinated on the day 2, …, and 7, respectively.
2.3. Statistical Analysis
3. Results
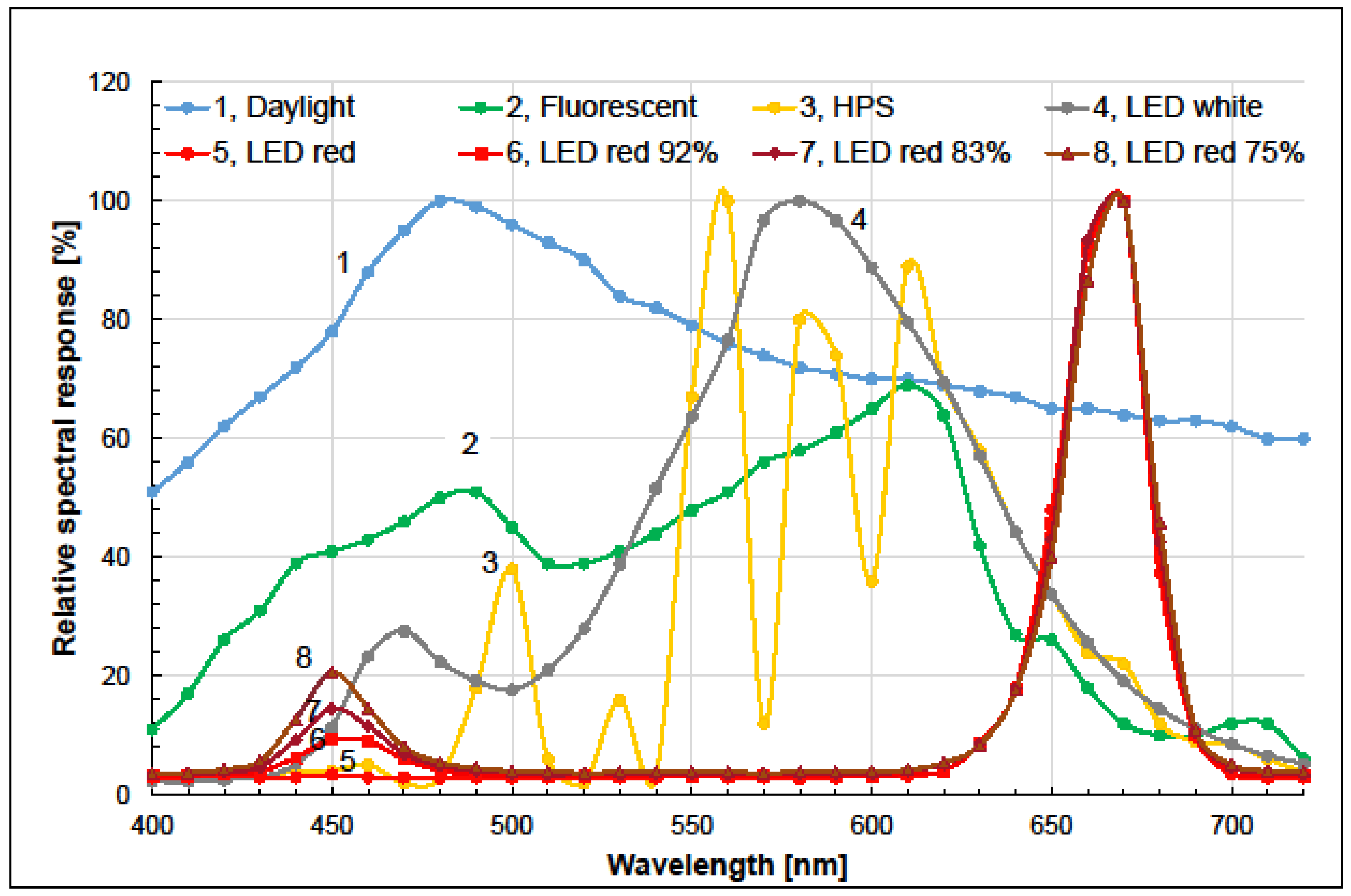
3.1. Light Spectrum Recordings
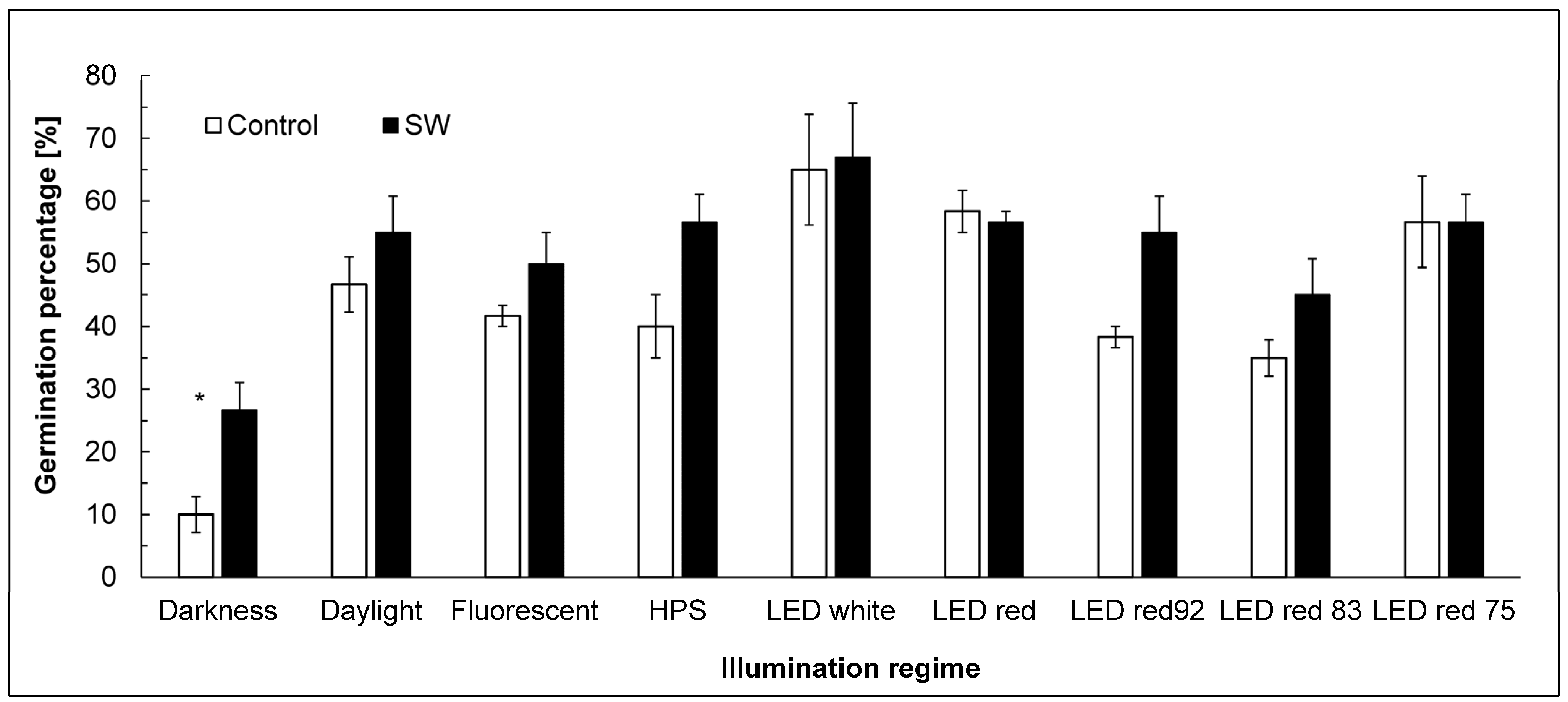
3.2. Germination Pattern
3.3. Seedling Morphometry
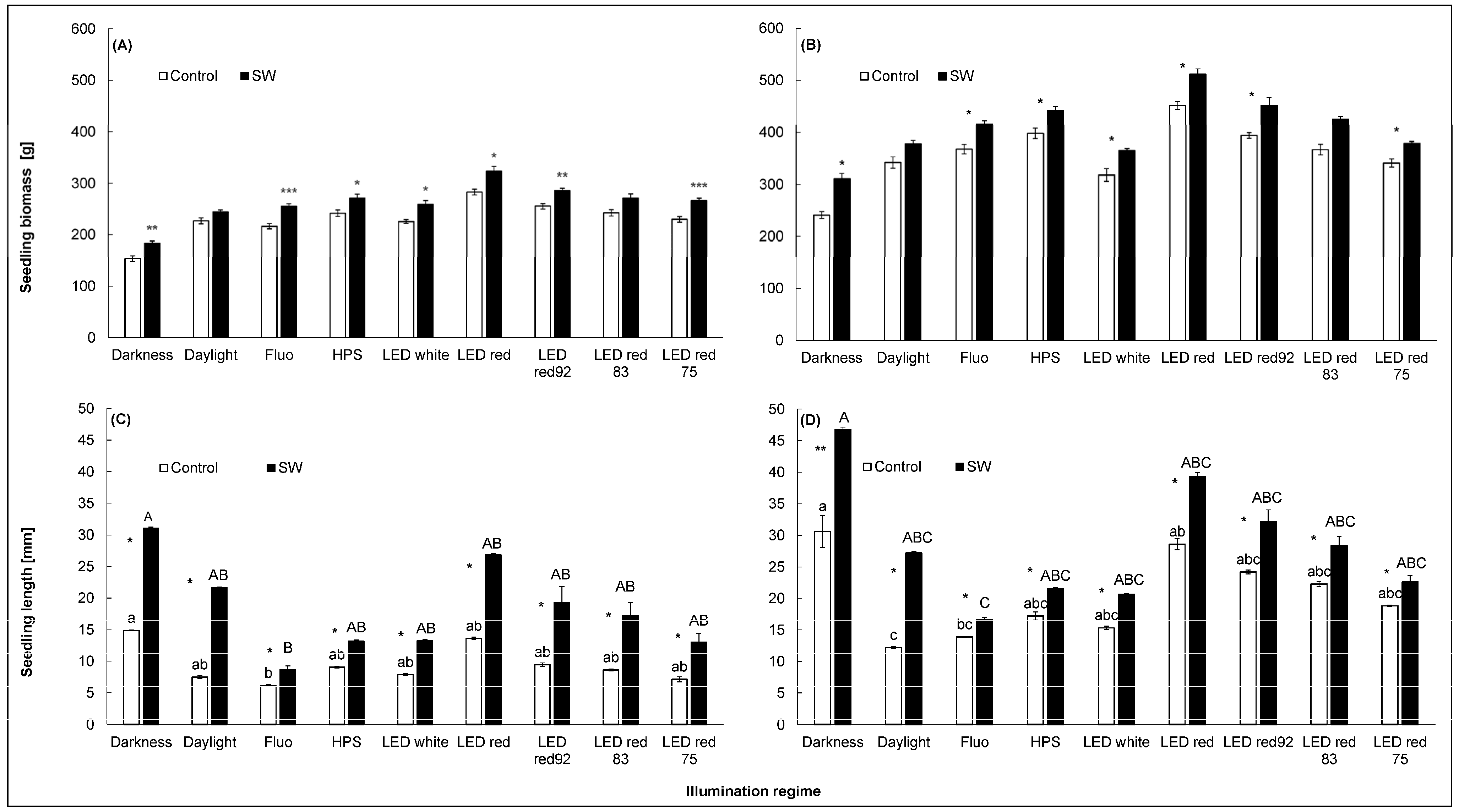
3.3.1. Seedling Biomass
3.3.2. Seedling Length
4. Discussion
4.1. Photoblastism and Smoke-Induced Germination in Chamomile Seeds—Opportunities and Limitations
4.2. Potential Mechanism of Light Signaling in Chamomile Seeds
4.3. Seed Germination Parameters Under the Influence of Light and SW
4.4. Initial Growth of Seedlings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
References
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Seidler-Lozykowska, K. Effect of the selected weather conditions on essential oil, α-bisabolol and chamazulene content in flower heads of chamomile (Chamomilla recutita (L.) Rausch.). J. Essent. Oil Res. 2010, 22, 45–48. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Kozieł, A.; Seidler-Łożykowska, K. Are the fluorescence parameters of German chamomile leaves the first indicators of the anthodia yield in drought conditions? Photosynthetica 2011, 49, 87–97. [Google Scholar] [CrossRef]
- Brabandt, H.; Ehlert, D. Chamomile harvesters: A review. Ind. Crops Prod. 2011, 34, 818–824. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R. Swailing affects seed germination of plants of European bio-and agricenosis in a different way. Open Life Sci. 2017, 12, 62–75. [Google Scholar] [CrossRef]
- Marvel, K.; Su, W.; Delgado, R.; Aarons, S.; Chatterjee, A.; Garcia, M.E.; Hausfather, Z.; Hayhoe, K.; Hence, D.A.; Jewett, E.B.; et al. Chapter 2: Climate trends. In Fifth National Climate Assessment; USGCRP (U.S. Global Change Research Program): Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Pons, T.L. Seed responses to light. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 237–260. [Google Scholar] [CrossRef]
- Tognacca, R.S.; Botto, J.F. Post-transcriptional regulation of seed dormancy and germination: Current understanding and future directions. Plant Commun. 2021, 2, 100169. [Google Scholar] [CrossRef]
- Lariguet, P.; Ranocha, P.; De Meyer, M.; Barbier, O.; Penel, C.; Dunand, C. Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis. Planta 2013, 238, 381–395. [Google Scholar] [CrossRef]
- Nemahunguni, N.K.; Gupta, S.; Kulkarni, M.; Finnie, J.F.; van Staden, J. The effect of biostimulants and light wavelengths on the physiology of Cleome gynandra seeds. Plant Growth Regul. 2020, 90, 467–474. [Google Scholar] [CrossRef]
- Stawska, M.; Oracz, K. phyB and HY5 are Involved in the Blue Light-Mediated Alleviation of Dormancy of Arabidopsis Seeds Possibly via the Modulation of Expression of Genes Related to Light, GA, and ABA. Int. J. Mol. Sci. 2019, 20, 5882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Staden, J.; Jäger, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Jurado, E.; Márquez-Linares, M.; Flores, J. Effect of cold storage, heat, smoke and charcoal on breaking seed dormancy of Arctostaphylos pungens HBK (Ericaceae). FYTON 2011, 80, 4–11. [Google Scholar] [CrossRef]
- Hidayati, S.N.; Walck, J.L.; Merritt, D.J.; Turner, S.R.; Turner, D.W.; Dixon, K.W. Sympatric species of Hibbertia (Dilleniaceae) vary in dormancy break and germination requirements: Implications for classifying morphophysiological dormancy in Mediterranean biomes. Ann. Bot. 2012, 109, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Leperlier, C.; Riviere, J.-N.E.; Allibert, A.; Dessau, D.; Lacroix, S.; Fock-Bastide, I. Overcoming dormancy and light requirements in seeds of Heteropogon contortus, a target species for savanna restoration. Ecol. Eng. 2018, 122, 10–15. [Google Scholar] [CrossRef]
- Merritt, D.J.; Kristiansen, M.; Flematti, G.R.; Turner, S.R.; Ghisalberti, E.L.; Trengove, R.D.; Dixon, K.W. Effects of a butanolide present in smoke on light-mediated germination of Australian Asteraceae. Seed Sci. Res. 2006, 16, 29–35. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Light, M.E.; Van Staden, J. Plant-derived smoke: Old technology with possibilities for economic applications in agriculture and horticulture. S. Afr. J. Bot. 2011, 77, 972–979. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Naidoo, D.; Pošta, M.; Finnie, J.F.; Van Staden, J. The effects of smoke derivatives on in vitro seed germination and development of the leopard orchid Ansellia africana. Plant Biol. 2016, 18, 289–294. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Antonkiewicz, J.; Łopata-Stasiak, A.; Kępka, W. Smoke compounds aggravate stress inflicted on Brassica seedlings by unfavourable soil conditions. Photosynthetica 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Elsadek, M.A.; Yousef, E.A.A. Smoke-Water Enhances Germination and Seedling Growth of Four Horticultural Crops. Plants 2019, 8, 104. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R. An Interplay of Light and Smoke Compounds in Photoblastic Seeds. Plants 2022, 11, 1773. [Google Scholar] [CrossRef]
- Solano, C.J.; Hernández, J.A.; Suardíaz, J.; Barba-Espín, G. Impacts of LEDs in the Red Spectrum on the Germination, Early Seedling Growth and Antioxidant Metabolism of Pea (Pisum sativum L.) and Melon (Cucumis melo L.). Agriculture 2020, 10, 204. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Osmenda, M.; Słowiński, K.; Latowski, D.; Tabor, S.; Woodward, S. Stratification, Scarification and Application of Phytohormones Promote Dormancy Breaking and Germination of Pelleted Scots Pine (Pinus sylvestris L.) Seeds. Forests 2021, 12, 621. [Google Scholar] [CrossRef]
- Kamath, D.; Kong, Y.; Dayboll, C.; Blom, T.; Zheng, Y. Seed germination responses to low-level narrow-band light spectra for 14 ornamental plant genotypes. Can. J. Plant Sci. 2021, 101, 933–942. [Google Scholar] [CrossRef]
- Araújo, R.C.; Rodrigues, F.A.; Dória, J.; Pasqual, M. In vitro germination of Adenium obesum under the effects of culture medium and light emitting diodes of different colors. Plant Cell Tissue Organ Cult. 2022, 149, 523–533. [Google Scholar] [CrossRef]
- Borowski, E.; Michałek, S.; Rubinowska, K.; Hawrylak-Nowak, B.; Grudziński, W. The effects of light quality on photosynthetic parameters and yield of lettuce plants. Acta Sci. Pol. Hortorum Cultus 2015, 14, 177–188. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seeds Testing Association (ISTA): Wallisellen, Switzerland, 2017. [Google Scholar] [CrossRef]
- Pire, R.; Vargas-Simón, G. Recurrent inconsistencies in publications that involve Maguire’s germination rate formula. For. Syst. 2019, 28, eSC02. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Soufi, H.R.; Roosta, H.R.; Stępień, P.; Malekzadeh, K.; Hamidpour, M. Manipulation of light spectrum is an effective tool to regulate biochemical traits and gene expression in lettuce under different replacement methods of nutrient solution. Sci. Rep. 2023, 13, 8600. [Google Scholar] [CrossRef]
- Pavlovič, A.; Masarovičová, E.; Král’ová, K.; Kubová, J. Response of Chamomile Plants (Matricaria recutita L.) to Cadmium Treatment. Bull. Environ. Contam. Toxicol. 2006, 77, 763–771. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, F.; Shuai, H.; Luo, X.; Ding, J.; Tang, S.; Xu, S.; Liu, J.; Liu, W.; Du, J.; et al. Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci. Rep. 2016, 6, 22073. [Google Scholar] [CrossRef]
- Haliniarz, M.; Kapeluszny, J.; Michałek, S. Germination of rye brome (Bromus secalinus L.) seeds under simulated drought and different thermal conditions. Acta Agrobot. 2013, 66, 157–164. [Google Scholar] [CrossRef]
- Basu, S.; Groot, S.P.C. Seed Vigour and Invigoration. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Díaz-Rueda, P.; Cantos-Barragán, M.; Colmenero-Flores, J.M. Growth, Quality and Development of Olive Plants Cultured In-Vitro under Different Illumination Regimes. Plants 2021, 10, 2214. [Google Scholar] [CrossRef] [PubMed]
- Waters, T.W.; Scaffidi, A.; Flematti, G.R.; Smith, S.M. The origins and mechanisms of karrikin signalling. Curr. Opin. Plant Biol. 2013, 16, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Nelson, D.C. Karrikin perception and signalling. New Phytol. 2023, 237, 1525–1541. [Google Scholar] [CrossRef]
- Sawada, Y.; Aoki, M.; Nakaminami, K.; Mitsuhashi, W.; Tatematsu, K.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Inoue, Y.; Nambara, E.; et al. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol. 2008, 146, 1386–1396. [Google Scholar] [CrossRef]
- Nelson, D.C.; Flematti, G.R.; Riseborough, J.A.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 7095–7100. [Google Scholar] [CrossRef]
- Hountalas, J.E.; Bunsick, M.; Xu, Z.; Taylor, A.A.; Pescetto, G.; Ly, G.; Boyer, F.-D.; McErlean, C.S.P.; Lumba, S. HTL/KAI2 signalling substitutes for light to control plant germination. PLoS Genet. 2024, 20, e1011447. [Google Scholar] [CrossRef]
- Sanoubar, R.; Calone, R.; Noli, E.; Barbanti, L. Data on seed germination using LED versus fluorescent light under growth chamber conditions. Data Brief 2018, 19, 594–600. [Google Scholar] [CrossRef]
- Tester, M.; Morris, C. The penetration of light through soil. Plant Cell Environ. 1987, 10, 281–286. [Google Scholar] [CrossRef]
- Ciani, A.; Goss, K.U.; Schwarzenbach, R.P. Light penetration in soil and particulate minerale. Eur. J. Soil Sci. 2005, 56, 561–574. [Google Scholar] [CrossRef]
- Bustamante, J.A.; Miller, N.D.; Spalding, E.P. Separate sites of action for cry1 and phot1 blue-light receptors in the Arabidopsis hypocotyl. Curr. Biol. 2025, 35, 100–108.e4. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Rizza, A.; Tang, B.; Frommer, W.B.; Jones, A.M. GIBBERELLIN PERCEPTION SENSOR 2 reveals genesis and role of cellular GA dynamics in light-regulated hypocotyl growth. Plant Cell 2024, 36, 4426–4441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Tae, J.I.; Kim, E.A.; Shin, E.J.; Lee, S.; Lee, M.; Nam, S.Y. Evaluating the Influence of Various Light Spectra on the Growth and Morphological Responses of Air Plant (Tillandsia ionantha Planch.) Grown under Non-substrate and Restricted Irrigation Conditions in a Controlled Environment Facility. J. Agric. Life Environ. Sci. 2024, 36, 546–561. [Google Scholar] [CrossRef]
- Zhang, W.J.; Björn, L.O. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Ferruzzi, M.G.; Mitchell, C.A. Manipulating Sensory and Phytochemical Profiles of Greenhouse Tomatoes Using Environmentally Relevant Doses of Ultraviolet Radiation. J. Agric. Food Chem. 2016, 64, 6801–6808. [Google Scholar] [CrossRef]
| Factor | Germination Percentage, Day 2 | GRI (Germination Rate Index) | GI (Germination Index) | Seedling Biomass, Day 7 | Seedling Biomass, Day 14 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| Illumination regime | 19.38 | 0.000 *** | 16.48 | 0.000 *** | 14.95 | 0.000 *** | 72.00 | 0.000 *** | 86.9 | 0.000 *** |
| Treatment | 17.32 | 0.000 *** | 9.77 | 0.003 ** | 8.00 | 0.008 ** | 124.30 | 0.000 *** | 148.5 | 0.000 *** |
| Illumination regime × Treatment | 1.57 | 0.167 | 0.52 | 0.831 | 0.42 | 0.900 | 0.700 | 0.727 | 0.9 | 0.518 |
| Illumination Regime | FGP [%] | GRI [%/day] | GI [Number × Day] | |||
|---|---|---|---|---|---|---|
| Control | SW | Control | SW | Control | SW | |
| Darkness | 73 | 75 | 98 | 92 | 288 | 326 |
| Daylight | 83 | 82 | 115 | 113 | 383 | 390 |
| Fluorescent | 83 | 87 | 119 | 118 | 388 | 409 |
| HPS | 80 | 82 | 114 | 115 | 372 | 397 |
| LED white | 88 | 88 | 126 | 128 | 438 | 441 |
| LED Red | 82 | 82 | 110 | 116 | 389 | 400 |
| LED Red 92% | 83 | 87 | 120 | 121 | 385 | 420 |
| LED Red 83% | 83 | 83 | 116 | 111 | 371 | 387 |
| LED Red 75% | 87 | 88 | 124 | 124 | 422 | 431 |
| Factor | FGP [%] | Seedling Length, Day 7 | Seedling Length, Day 14 | |||
|---|---|---|---|---|---|---|
| H | p | H | p | H | p | |
| Illumination regime | 26.27 | 0.0009 *** | 25.27 | 0.0014 *** | 37.72 | 0.0000 *** |
| Treatment | 0.74 | 0.3883 | 22.09 | 0.0000 *** | 10.41 | 0.0013 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bączek-Kwinta, R.; Michałek, S. Smoke Compounds Compensate for Light Irrespective of Its Spectrum in Positively Photoblastic German Chamomile Seeds, Although Red Light Is Crucial. Agronomy 2025, 15, 700. https://doi.org/10.3390/agronomy15030700
Bączek-Kwinta R, Michałek S. Smoke Compounds Compensate for Light Irrespective of Its Spectrum in Positively Photoblastic German Chamomile Seeds, Although Red Light Is Crucial. Agronomy. 2025; 15(3):700. https://doi.org/10.3390/agronomy15030700
Chicago/Turabian StyleBączek-Kwinta, Renata, and Sławomir Michałek. 2025. "Smoke Compounds Compensate for Light Irrespective of Its Spectrum in Positively Photoblastic German Chamomile Seeds, Although Red Light Is Crucial" Agronomy 15, no. 3: 700. https://doi.org/10.3390/agronomy15030700
APA StyleBączek-Kwinta, R., & Michałek, S. (2025). Smoke Compounds Compensate for Light Irrespective of Its Spectrum in Positively Photoblastic German Chamomile Seeds, Although Red Light Is Crucial. Agronomy, 15(3), 700. https://doi.org/10.3390/agronomy15030700







